-
PDF
- Split View
-
Views
-
Cite
Cite
Rod S Taylor, Suzanne Fredericks, Ian Jones, Lis Neubeck, Julie Sanders, Noemi De Stoutz, David R Thompson, Deepti N Wadhwa, Sherry L Grace, Global perspectives on heart disease rehabilitation and secondary prevention: a scientific statement from the Association of Cardiovascular Nursing and Allied Professions, European Association of Preventive Cardiology, and International Council of Cardiovascular Prevention and Rehabilitation, European Heart Journal, Volume 44, Issue 28, 21 July 2023, Pages 2515–2525, https://doi.org/10.1093/eurheartj/ehad225
Close - Share Icon Share
Abstract
Cardiovascular disease is a leading cause of death, morbidity, disability, and reduced health-related quality of life, as well as economic burden worldwide, with some 80% of disease burden occurring in the low- and middle-income country (LMIC) settings. With increasing numbers of people living longer with symptomatic disease, the effectiveness and accessibility of secondary preventative and rehabilitative health services have never been more important. Whilst LMICs experience the highest prevalence and mortality rates, the global approach to secondary prevention and cardiac rehabilitation, which mitigates this burden, has traditionally been driven from clinical guidelines emanating from high-income settings. This state-of-the art review provides a contemporary global perspective on cardiac rehabilitation and secondary prevention, contrasting the challenges of and opportunities for high vs. lower income settings. Actionable solutions to overcome system, clinician, programme, and patient level barriers to cardiac rehabilitation access in LMICs are provided.
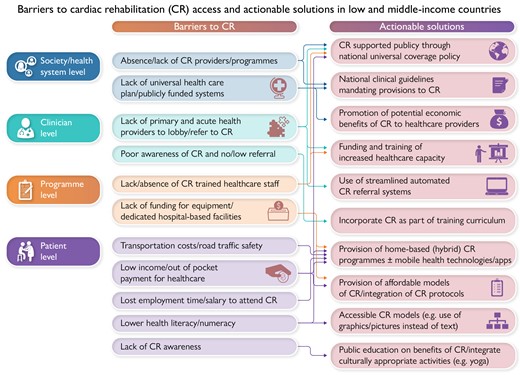
Barriers to cardiac rehabilitation (CR) access and actionable solutions in low and middle-income countries.
Introduction
Cardiovascular disease (CVD) has been the most prevalent non-communicable disease, leading cause of global mortality, and a major contributor of premature disability and ill-health over the last two decades. Heart disease is a main contributor to the burden of CVD.1 However, whilst low- and middle-income countries (LMICs) now experience the highest prevalence and mortality rates of both, our global approach to cardiac secondary prevention and rehabilitation, which mitigates this burden, has traditionally been driven from clinical guidelines emanating from high-income countries (HICs).2
We also know that there are significant gaps in real-world delivery of secondary prevention and cardiac rehabilitation (CR), even in the highest-resourced settings such as Europe and the United States, where, despite high level of evidence, inequalities in such care and patient outcomes remain an important challenge.3,4 Health systems and care barriers vary considerably between different regions of the world and particularly contrasting economic settings,5 yet to our knowledge, there has been no recent review of cardiac secondary prevention and CR around the globe, with a specific focus on the contrasting challenges of high vs. low-income country settings. Indeed whilst, certainly, there are gaps in knowledge, there is very recent and rapidly emerging evidence regarding the benefits of rehabilitation in lower-resource settings as well as novel delivery models, to ensure what we know works reaches more patients in need. This state-of-the-art review seeks to provide a contemporary global perspective on CR and secondary prevention, specifically considering issues in HICs vs. LMICs in relation to:
disease burden and socioeconomic impact
evidence and availability of CR and secondary prevention
key challenges and opportunities for CR and secondary prevention
global practice and policy priorities.
In the World Heart Federation’s Cardiovascular Roadmap, secondary prevention is defined as ‘any strategy aimed to reduce the probability of a recurrent cardiovascular event [(e.g. heart attack or stroke or death)] in patients with known atherosclerotic cardiovascular disease….’.6 Whilst we recognize the overlap in their definition and objectives, for the purposes of this review, we use the term ‘secondary prevention’ to include the use of medical interventions to improve prognosis through the reduction of future cardiovascular events and improved survival. For CR, we apply the World Health Organization’s definition that emphasizes measures aimed at developing patient (and family) self-management strategies and maximizing their function and quality of life (i.e. ‘the sum of activities required to influence favourably the underlying cause of the disease, as well as to provide the best possible physical, mental and social conditions, so that the patients may, by their own efforts, preserve or resume when lost as normal a place as possible in the community’.7)
Search strategy and selection criteria
We searched for existing systematic reviews using PubMed/MEDLINE (1 November 2016 to 1 November 2021). The following search strategy that combines key terms and Cochrane search filter for systematic reviews was used: ‘ ((Cardiovascular) NOT (Pulmonary)) NOT (stroke) AND rehabilitation[Ti] OR secondary prevention[Ti] (((systematic review[ti] OR systematic literature review[ti] OR systematic scoping review[ti] OR systematic narrative review[ti] OR systematic qualitative review[ti] OR systematic evidence review[ti] OR systematic quantitative review[ti] OR systematic meta-review[ti] OR systematic critical review[ti] OR systematic mixed studies review[ti] OR systematic mapping review[ti] OR systematic cochrane review[ti] OR systematic search and review[ti] OR systematic integrative review[ti]) NOT comment[pt] NOT (protocol[ti] OR protocols[ti])) NOT MEDLINE [subset]) OR (Cochrane Database Syst Rev[ta] AND review[pt]) OR systematic review[pt]’.
Publications that were part of personal databases or familiar to the co-author team were also included.
Burden and socioeconomic impact of heart disease
Cardiovascular disease is the leading cause of death globally, taking an estimated 17.9 million lives each year, representing 32% of all global deaths.5Epidemiological data on socioeconomic impact of CVD have traditionally been obtained from studies in HICs.1 Whilst this knowledge has driven a range of highly effective interventions, a substantial burden of CVD persists, which is much higher in LMICs than in HICs with nearly 80% of all CVD deaths occurring in low-income settings, ∼40% of which are premature.1,2 As subsequently discussed in this paper, traditionally, the two populations recommended to receive CR are patients with coronary heart disease (CHD) [myocardial infarction (MI), post-revascularization, and stable angina] and heart failure (HF).8
The burden of CHD from a direct health, healthcare delivery, and financial perspective is considerable. The CHD is also the leading cause of disability and life years lost globally. The CHD is the primary cause of premature death in both men and women, and accounts for 182 million disability and life years lost globally.5,9 Global disparities in CHD are considerable. While CHD mortality is falling globally, mortality rates in many countries, particularly those in LMICs, remain very high.10 Globalization seems to have contributed to a higher prevalence of CVD risk factors in developing countries,11 and these are not often detected and even less frequently controlled. Globally, age-standardized disability and life years lost are highest in Eastern Europe, Central Asia, Oceania, and the Middle East/North Africa regions.5
Although CHD-associated hospital admissions have decreased, rates of acute CHD revascularization approaches (which accrue costs but can also provide secondary benefits) such as percutaneous coronary intervention (PCI) have increased in most countries including LMICs, whereas coronary artery bypass graft surgery has generally fallen.12 The cost-effectiveness of CHD interventions in LMICs is not known, and potentially not transferable from high-income settings due to variations in a range of factors (demographic, socio-cultural, and financial resources)13 as well as other health priorities. For example, especially in an LMIC, the provision of CR may be a more affordable approach to secondary CHD prevention than PCI.
Despite the current impact of CHD on health and healthcare systems globally, the situation is expected to continue to worsen, especially in LMICs due to the increasing life expectancy and continued globalization.9 It is estimated that by 2030, more than 80% of CVD-related disability and death will occur in the 139 LMICs owing to increasing prevalence of risk factors, such as tobacco use, hypertension, obesity, and diabetes.5 Thus, healthcare systems, particularly those in LMICs, will need to adapt to meet the increasing demands. Indeed, to aid the reduction of the global burden of CVD, World Health Organization member states are committed to providing 50% of eligible people with counselling and drug therapy to prevent heart attacks and strokes by 2025.14
Heart failure also contributes significantly to the global burden of disease in terms of high risk of mortality and hospitalization (and associated costs), and substantive negative impact on patient’s health-related quality of life.5 However, unlike CHD, there are few large population studies of the epidemiology and socio-economic impact of HF outside of North America and Europe.15 Despite advances in HF management in the past three decades, data from HICs suggest that 5-year survival after the diagnosis of HF is still only 50%–60%, with mortality rates being substantially higher in LMICs.16
Secondary prevention
Overall, although the risk factor burden is lowest in low-income countries, the incidence of major CVD events and subsequent case fatality are substantially higher than in HICs.17 Reduced access to secondary prevention drugs and PCI is likely to lead to further morbidity and increased risk of death. In LMICs, a major challenge is overcoming this care gap and barriers to the implementation of evidence-based secondary preventive therapies.
Recently updated clinical guidelines from Europe and the USA both recommend several evidence-based interventions for secondary CVD prevention.18,19 These interventions include the use of four proven medications [aspirin, angiotensin-converting enzyme (ACE) inhibitors, or angiotensin receptor blockers if ACE inhibitors are not tolerated, beta-blockers and statins], as well as lifestyle interventions like tobacco cessation, increased physical activity, and healthy diet. However, there is much inequity in secondary prevention uptake globally.
The Prospective Urban Rural Epidemiology (PURE) cohort study enrolling 153 996 adults from 628 urban and rural communities/countries across all economic strata included assessment of the use of CVD secondary prevention drugs.20 The investigators reported low uptake of most secondary prevention drugs, including antiplatelets (25.3%), beta-blockers (17.4%), ACE inhibitors or angiotensin receptor blockers (19.5%), and statins (14.6%). Whilst individual risk factors such as sex, age, tobacco use, body mass index, hypertension, and diabetes status affected medication use, the economic status of the country accounted for two-thirds of this variation. Specifically, usage was highest in HICs, and decreased in line with a countries’ socio-economic status: 80% of patients in low-income countries received no medical treatment compared with 69% in LMICs, and 45% in upper-middle income countries.20,21 Availability and affordability have been shown to effect uptake with some drugs less likely to be stocked in poorer communities, access to pharmacy being limited by geography in some rural communities and a lack of universal health insurance, limiting people’s access to costly medications.21,22
Some more limited data are available on access to other secondary preventative treatments. For example, a registry study conducted in India found that only 6% of patients with ST-segment elevation MI underwent PCI.23 PURE also identified low rates of lifestyle changes in patients with CVD, as well as low rates of adherence to diet, physical activity, and tobacco cessation, particularly in LMICs.21
Cardiac rehabilitation
Cardiac Rehabilitation is a model of care that involves provision of internationally agreed core components (including secondary prevention) by a multidisciplinary team.8 These include initial assessment, medical risk factor management, structured exercise, patient education, lifestyle counselling (e.g. tobacco cessation and diet), and psychosocial management (e.g. stress, depression screening, and support in returning to life roles).8,24,25 Cardiac Rehabilitation is offered across the continuum of care from inpatient (phase I), early outpatient (phase II), to maintenance (phase III/IV). Recommended protocols to deliver CR in high and low-resource settings are available elsewhere.25,26
Participation in CR reduces the risk of cardiovascular mortality and hospital admissions, as well as results in clinically meaningful improvements in patient health-related quality of life and functional capacity. Systematic reviews and meta-analysis of randomized controlled trials of CR have consistently demonstrated these outcome benefits in patients with acute coronary syndrome, following coronary revascularization, and HF with reduced ejection fraction (Table 1).27–29
Evidence for cardiac rehabilitation: summary of Cochrane review cardiac rehabilitation findings
| . | Totality of evidence . | Meta-analysis findingsa . | ||||
|---|---|---|---|---|---|---|
| . | Number of RCTs Median follow-up . | Number of patients . | Mortality . | CV events . | Hospitalizations . | Health-related quality of life . |
| CHD (2021) (27) | 85 trials 12 months | 23 430—primarily post-MI/revascularization | All-cause RR 0.87, 95% CI 0.73–1.04 (25 trials/8823 participants/good certainty) CVD RR 0.88, 95% CI 0.68–1.14 (15 trials/5360 participants/moderate certainty) | CABG RR 0.99, 95% CI 0.78–1.27 (20 trials/4473 participants/high certainty) PCI RR 0.86, 95% CI 0.63–1.19 (13 trials/3465 participants/moderate certainty) Fatal/non-fatal MI RR 0.72, 95% CI 0.55–0.93 (22 trials/7423 participants/moderate certainty) | All-cause RR 0.58, 95% CI 0.43–0.77 (14 trials/2030 participants/moderate certainty) CVD-specific RR 0.80, 95% CI 0.41–1.59 (6 trials/1087 participants/low certainty) | SF-12/36 PCS MD: 1.70, 95% CI −0.08 to 3.47 (6 trials/1741 participants/no GRADE assessment) SF-12/36 MCS MD: 2.14, 95% CI 1.07–3.22 (6 trials/1741 participants/no GRADE assessment) |
| HF (2019) (28) | 44 trials, 6 months | 5783—primarily HFrEF | All-cause RR 0.89, 95% CI 0.66–1.21 (27 trials/2596 participants/low certainty) | Not reported | All cause RR 0.70; 95% CI 0.60–0.83 (21 trials/2182 participants/moderate certainty) HF-specific RR 0.59, 95% CI 0.42–0.84 (14 trials/1114 participants/low certainty) | MLWHF MD: −7.1, 95% CI −10.5 to −3.7 (17 trials/1995 participants/low certainty) |
| . | Totality of evidence . | Meta-analysis findingsa . | ||||
|---|---|---|---|---|---|---|
| . | Number of RCTs Median follow-up . | Number of patients . | Mortality . | CV events . | Hospitalizations . | Health-related quality of life . |
| CHD (2021) (27) | 85 trials 12 months | 23 430—primarily post-MI/revascularization | All-cause RR 0.87, 95% CI 0.73–1.04 (25 trials/8823 participants/good certainty) CVD RR 0.88, 95% CI 0.68–1.14 (15 trials/5360 participants/moderate certainty) | CABG RR 0.99, 95% CI 0.78–1.27 (20 trials/4473 participants/high certainty) PCI RR 0.86, 95% CI 0.63–1.19 (13 trials/3465 participants/moderate certainty) Fatal/non-fatal MI RR 0.72, 95% CI 0.55–0.93 (22 trials/7423 participants/moderate certainty) | All-cause RR 0.58, 95% CI 0.43–0.77 (14 trials/2030 participants/moderate certainty) CVD-specific RR 0.80, 95% CI 0.41–1.59 (6 trials/1087 participants/low certainty) | SF-12/36 PCS MD: 1.70, 95% CI −0.08 to 3.47 (6 trials/1741 participants/no GRADE assessment) SF-12/36 MCS MD: 2.14, 95% CI 1.07–3.22 (6 trials/1741 participants/no GRADE assessment) |
| HF (2019) (28) | 44 trials, 6 months | 5783—primarily HFrEF | All-cause RR 0.89, 95% CI 0.66–1.21 (27 trials/2596 participants/low certainty) | Not reported | All cause RR 0.70; 95% CI 0.60–0.83 (21 trials/2182 participants/moderate certainty) HF-specific RR 0.59, 95% CI 0.42–0.84 (14 trials/1114 participants/low certainty) | MLWHF MD: −7.1, 95% CI −10.5 to −3.7 (17 trials/1995 participants/low certainty) |
CABG, coronary artery bypass graft; CHD, coronary heart disease; CI, confidence interval; CV, cardiovascular; CVD, cardiovascular disease; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; MCS, mental component summary; MD, mean difference; MI, myocardial infarction; MLWHF, Minnesota Living With Heart Failure questionnaire; PCI, percutaneous coronary intervention; PCS, physical component summary; RR, relative risk; RCT, randomized controlled trial; SF-12/36, Short-Form 12/36; SMD, standardized mean difference.
All pooled outcomes at 6–12-month follow-up and Grading of Recommendations, Assessment, Development and Evaluations (GRADE) quality assessment, unless otherwise stated.
Evidence for cardiac rehabilitation: summary of Cochrane review cardiac rehabilitation findings
| . | Totality of evidence . | Meta-analysis findingsa . | ||||
|---|---|---|---|---|---|---|
| . | Number of RCTs Median follow-up . | Number of patients . | Mortality . | CV events . | Hospitalizations . | Health-related quality of life . |
| CHD (2021) (27) | 85 trials 12 months | 23 430—primarily post-MI/revascularization | All-cause RR 0.87, 95% CI 0.73–1.04 (25 trials/8823 participants/good certainty) CVD RR 0.88, 95% CI 0.68–1.14 (15 trials/5360 participants/moderate certainty) | CABG RR 0.99, 95% CI 0.78–1.27 (20 trials/4473 participants/high certainty) PCI RR 0.86, 95% CI 0.63–1.19 (13 trials/3465 participants/moderate certainty) Fatal/non-fatal MI RR 0.72, 95% CI 0.55–0.93 (22 trials/7423 participants/moderate certainty) | All-cause RR 0.58, 95% CI 0.43–0.77 (14 trials/2030 participants/moderate certainty) CVD-specific RR 0.80, 95% CI 0.41–1.59 (6 trials/1087 participants/low certainty) | SF-12/36 PCS MD: 1.70, 95% CI −0.08 to 3.47 (6 trials/1741 participants/no GRADE assessment) SF-12/36 MCS MD: 2.14, 95% CI 1.07–3.22 (6 trials/1741 participants/no GRADE assessment) |
| HF (2019) (28) | 44 trials, 6 months | 5783—primarily HFrEF | All-cause RR 0.89, 95% CI 0.66–1.21 (27 trials/2596 participants/low certainty) | Not reported | All cause RR 0.70; 95% CI 0.60–0.83 (21 trials/2182 participants/moderate certainty) HF-specific RR 0.59, 95% CI 0.42–0.84 (14 trials/1114 participants/low certainty) | MLWHF MD: −7.1, 95% CI −10.5 to −3.7 (17 trials/1995 participants/low certainty) |
| . | Totality of evidence . | Meta-analysis findingsa . | ||||
|---|---|---|---|---|---|---|
| . | Number of RCTs Median follow-up . | Number of patients . | Mortality . | CV events . | Hospitalizations . | Health-related quality of life . |
| CHD (2021) (27) | 85 trials 12 months | 23 430—primarily post-MI/revascularization | All-cause RR 0.87, 95% CI 0.73–1.04 (25 trials/8823 participants/good certainty) CVD RR 0.88, 95% CI 0.68–1.14 (15 trials/5360 participants/moderate certainty) | CABG RR 0.99, 95% CI 0.78–1.27 (20 trials/4473 participants/high certainty) PCI RR 0.86, 95% CI 0.63–1.19 (13 trials/3465 participants/moderate certainty) Fatal/non-fatal MI RR 0.72, 95% CI 0.55–0.93 (22 trials/7423 participants/moderate certainty) | All-cause RR 0.58, 95% CI 0.43–0.77 (14 trials/2030 participants/moderate certainty) CVD-specific RR 0.80, 95% CI 0.41–1.59 (6 trials/1087 participants/low certainty) | SF-12/36 PCS MD: 1.70, 95% CI −0.08 to 3.47 (6 trials/1741 participants/no GRADE assessment) SF-12/36 MCS MD: 2.14, 95% CI 1.07–3.22 (6 trials/1741 participants/no GRADE assessment) |
| HF (2019) (28) | 44 trials, 6 months | 5783—primarily HFrEF | All-cause RR 0.89, 95% CI 0.66–1.21 (27 trials/2596 participants/low certainty) | Not reported | All cause RR 0.70; 95% CI 0.60–0.83 (21 trials/2182 participants/moderate certainty) HF-specific RR 0.59, 95% CI 0.42–0.84 (14 trials/1114 participants/low certainty) | MLWHF MD: −7.1, 95% CI −10.5 to −3.7 (17 trials/1995 participants/low certainty) |
CABG, coronary artery bypass graft; CHD, coronary heart disease; CI, confidence interval; CV, cardiovascular; CVD, cardiovascular disease; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; MCS, mental component summary; MD, mean difference; MI, myocardial infarction; MLWHF, Minnesota Living With Heart Failure questionnaire; PCI, percutaneous coronary intervention; PCS, physical component summary; RR, relative risk; RCT, randomized controlled trial; SF-12/36, Short-Form 12/36; SMD, standardized mean difference.
All pooled outcomes at 6–12-month follow-up and Grading of Recommendations, Assessment, Development and Evaluations (GRADE) quality assessment, unless otherwise stated.
Cardiac Rehabilitation has also been shown to be cost-effective in these populations.30 Reflective of this clinical and economic evidence base, current international clinical guidelines in HICs all recommend referral to CR for post-MI, revascularization, and HF patients.31–38 Both the ESC and AHA/ACC give CR a class I/1 (respectively), level A recommendation given the strong level of evidence.31–34 Table 2 provides an overview of international clinical guidelines for secondary prevention and CR and their recommendations.18,24,31–53
Summary clinical practice guidelines with recommendations for secondary prevention and/or cardiac rehabilitation for people with CHD and HF globallyb
| . | Clinical guideline . | Year . | SP . | CR . | Reference . |
|---|---|---|---|---|---|
| HICsc | |||||
| Australia and New Zealand | National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the management of acute coronary syndromes | 2016 | a | 37 | |
| National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: guidelines for the prevention, detection, and management of heart failure in Australia | 2018 | a | 38 | ||
| Canada | Comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure | 2017 | a | 39 | |
| Society Guidelines for the diagnosis and management of stable ischaemic heart disease | 2014 | a | 40 | ||
| Europe | ESC Guidelines for the diagnosis and management of chronic coronary syndromes | 2019 | a | a | 31 |
| ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure | 2021 | a | a | 32 | |
| ESC Guidelines on cardiovascular disease prevention in clinical practice | 2021 | a | a | 18 | |
| French Society of Cardiology guidelines for cardiac rehabilitation in adults | 2012 | a | 41 | ||
| Slovakia Ministry of Health standard for ambulatory cardiovascular rehabilitation | 2021 | a | 42 | ||
| Japan | JCS guidelines for rehabilitation in patients with cardiovascular disease | 2012 | a | 43 | |
| Korea | Clinical practice guideline for cardiac rehabilitation in Korea | 2019 | a | 44 | |
| UK | NICE guideline: acute coronary syndrome | 2020 | a | a | 35 |
| NICE Guideline: chronic heart failure in adults: diagnosis and management | 2018 | a | 36 | ||
| IACR: cardiac rehabilitation guidelinese | 2013 | a | 45 | ||
| SIGN cardiac rehabilitation: a national clinical guideline | 2017 | a | 46 | ||
| USA | AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease | 2011 | a | 33 | |
| ACCF/AHA guideline for the management of heart failure | 2013 | a | 34 | ||
| AACVPR Guidelines for Cardiac Rehabilitation Programs, 6th Edition | 2021 | a | 47 | ||
| LMICsc | |||||
| Brazil | Brazilian cardiovascular rehabilitation guideline | 2020 | a | 48 | |
| India | Cardiological society of India: position statement for the management of ST elevation myocardial infarction in India | 2017 | a | 49 | |
| South America | South American guidelines for cardiovascular disease prevention and rehabilitation | 2014 | a | 50 | |
| Thailand | Heart rehabilitation club, heart association of Thailand CR Guideline | 1996 | a | 51 | |
| International | ICCPR consensus statement: Cardiac rehabilitation delivery model for low-resource settings | 2016 | a | 24,52 | |
| Rehabilitation after cardiovascular diseases, with special emphasis on developing countries: report of WHO Expert Committeed | 1993 | a | 53 | ||
| . | Clinical guideline . | Year . | SP . | CR . | Reference . |
|---|---|---|---|---|---|
| HICsc | |||||
| Australia and New Zealand | National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the management of acute coronary syndromes | 2016 | a | 37 | |
| National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: guidelines for the prevention, detection, and management of heart failure in Australia | 2018 | a | 38 | ||
| Canada | Comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure | 2017 | a | 39 | |
| Society Guidelines for the diagnosis and management of stable ischaemic heart disease | 2014 | a | 40 | ||
| Europe | ESC Guidelines for the diagnosis and management of chronic coronary syndromes | 2019 | a | a | 31 |
| ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure | 2021 | a | a | 32 | |
| ESC Guidelines on cardiovascular disease prevention in clinical practice | 2021 | a | a | 18 | |
| French Society of Cardiology guidelines for cardiac rehabilitation in adults | 2012 | a | 41 | ||
| Slovakia Ministry of Health standard for ambulatory cardiovascular rehabilitation | 2021 | a | 42 | ||
| Japan | JCS guidelines for rehabilitation in patients with cardiovascular disease | 2012 | a | 43 | |
| Korea | Clinical practice guideline for cardiac rehabilitation in Korea | 2019 | a | 44 | |
| UK | NICE guideline: acute coronary syndrome | 2020 | a | a | 35 |
| NICE Guideline: chronic heart failure in adults: diagnosis and management | 2018 | a | 36 | ||
| IACR: cardiac rehabilitation guidelinese | 2013 | a | 45 | ||
| SIGN cardiac rehabilitation: a national clinical guideline | 2017 | a | 46 | ||
| USA | AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease | 2011 | a | 33 | |
| ACCF/AHA guideline for the management of heart failure | 2013 | a | 34 | ||
| AACVPR Guidelines for Cardiac Rehabilitation Programs, 6th Edition | 2021 | a | 47 | ||
| LMICsc | |||||
| Brazil | Brazilian cardiovascular rehabilitation guideline | 2020 | a | 48 | |
| India | Cardiological society of India: position statement for the management of ST elevation myocardial infarction in India | 2017 | a | 49 | |
| South America | South American guidelines for cardiovascular disease prevention and rehabilitation | 2014 | a | 50 | |
| Thailand | Heart rehabilitation club, heart association of Thailand CR Guideline | 1996 | a | 51 | |
| International | ICCPR consensus statement: Cardiac rehabilitation delivery model for low-resource settings | 2016 | a | 24,52 | |
| Rehabilitation after cardiovascular diseases, with special emphasis on developing countries: report of WHO Expert Committeed | 1993 | a | 53 | ||
Addressed in clinical guideline.
Where applicable, latest guideline versions listed.
According to World Bank: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
This will soon be superseded by WHO’s Package of Rehabilitation Interventions for Ischemic Heart Disease (expected 2022). See: https://www.who.int/activities/integrating-rehabilitation-into-health-systems/service-delivery/package-of-interventions-for-rehabilitation.
Currently being updated (personal communication, J. Gallagher, 14 December 2021).
HIC, high-income country; LMIC, low- and middle-income country; CR, cardiac rehabilitation; SP, secondary prevention; ESC, European Society of Cardiology; CHD, Coronary Heart Disease; HF, heart failure; AHA, American Heart Association; ACCF, American College of Cardiology Foundation; NICE, National Institute for Health and Care Excellence; AACVPR, American Association of Cardiovascular and Pulmonary Rehabilitation; ICCPR, International Council of Cardiovascular Prevention and Rehabilitation; IACR, Irish Association of Cardiac Rehabilitation; JCS, Japanese Circulation Society; WHO, World Health Organization; SIGN, Scottish Intercollegiate Guideline Network.
CR standards and core components, or other scientific statements such as those pertaining to only one component of secondary prevention or cardiac rehabilitation not shown, but many can be found here: https://globalcardiacrehab.com/CR-Standard/Core-Components-&-Quality-Indicators and https://globalcardiacrehab.com/CR-Guidelines.
Summary clinical practice guidelines with recommendations for secondary prevention and/or cardiac rehabilitation for people with CHD and HF globallyb
| . | Clinical guideline . | Year . | SP . | CR . | Reference . |
|---|---|---|---|---|---|
| HICsc | |||||
| Australia and New Zealand | National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the management of acute coronary syndromes | 2016 | a | 37 | |
| National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: guidelines for the prevention, detection, and management of heart failure in Australia | 2018 | a | 38 | ||
| Canada | Comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure | 2017 | a | 39 | |
| Society Guidelines for the diagnosis and management of stable ischaemic heart disease | 2014 | a | 40 | ||
| Europe | ESC Guidelines for the diagnosis and management of chronic coronary syndromes | 2019 | a | a | 31 |
| ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure | 2021 | a | a | 32 | |
| ESC Guidelines on cardiovascular disease prevention in clinical practice | 2021 | a | a | 18 | |
| French Society of Cardiology guidelines for cardiac rehabilitation in adults | 2012 | a | 41 | ||
| Slovakia Ministry of Health standard for ambulatory cardiovascular rehabilitation | 2021 | a | 42 | ||
| Japan | JCS guidelines for rehabilitation in patients with cardiovascular disease | 2012 | a | 43 | |
| Korea | Clinical practice guideline for cardiac rehabilitation in Korea | 2019 | a | 44 | |
| UK | NICE guideline: acute coronary syndrome | 2020 | a | a | 35 |
| NICE Guideline: chronic heart failure in adults: diagnosis and management | 2018 | a | 36 | ||
| IACR: cardiac rehabilitation guidelinese | 2013 | a | 45 | ||
| SIGN cardiac rehabilitation: a national clinical guideline | 2017 | a | 46 | ||
| USA | AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease | 2011 | a | 33 | |
| ACCF/AHA guideline for the management of heart failure | 2013 | a | 34 | ||
| AACVPR Guidelines for Cardiac Rehabilitation Programs, 6th Edition | 2021 | a | 47 | ||
| LMICsc | |||||
| Brazil | Brazilian cardiovascular rehabilitation guideline | 2020 | a | 48 | |
| India | Cardiological society of India: position statement for the management of ST elevation myocardial infarction in India | 2017 | a | 49 | |
| South America | South American guidelines for cardiovascular disease prevention and rehabilitation | 2014 | a | 50 | |
| Thailand | Heart rehabilitation club, heart association of Thailand CR Guideline | 1996 | a | 51 | |
| International | ICCPR consensus statement: Cardiac rehabilitation delivery model for low-resource settings | 2016 | a | 24,52 | |
| Rehabilitation after cardiovascular diseases, with special emphasis on developing countries: report of WHO Expert Committeed | 1993 | a | 53 | ||
| . | Clinical guideline . | Year . | SP . | CR . | Reference . |
|---|---|---|---|---|---|
| HICsc | |||||
| Australia and New Zealand | National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the management of acute coronary syndromes | 2016 | a | 37 | |
| National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: guidelines for the prevention, detection, and management of heart failure in Australia | 2018 | a | 38 | ||
| Canada | Comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure | 2017 | a | 39 | |
| Society Guidelines for the diagnosis and management of stable ischaemic heart disease | 2014 | a | 40 | ||
| Europe | ESC Guidelines for the diagnosis and management of chronic coronary syndromes | 2019 | a | a | 31 |
| ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure | 2021 | a | a | 32 | |
| ESC Guidelines on cardiovascular disease prevention in clinical practice | 2021 | a | a | 18 | |
| French Society of Cardiology guidelines for cardiac rehabilitation in adults | 2012 | a | 41 | ||
| Slovakia Ministry of Health standard for ambulatory cardiovascular rehabilitation | 2021 | a | 42 | ||
| Japan | JCS guidelines for rehabilitation in patients with cardiovascular disease | 2012 | a | 43 | |
| Korea | Clinical practice guideline for cardiac rehabilitation in Korea | 2019 | a | 44 | |
| UK | NICE guideline: acute coronary syndrome | 2020 | a | a | 35 |
| NICE Guideline: chronic heart failure in adults: diagnosis and management | 2018 | a | 36 | ||
| IACR: cardiac rehabilitation guidelinese | 2013 | a | 45 | ||
| SIGN cardiac rehabilitation: a national clinical guideline | 2017 | a | 46 | ||
| USA | AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease | 2011 | a | 33 | |
| ACCF/AHA guideline for the management of heart failure | 2013 | a | 34 | ||
| AACVPR Guidelines for Cardiac Rehabilitation Programs, 6th Edition | 2021 | a | 47 | ||
| LMICsc | |||||
| Brazil | Brazilian cardiovascular rehabilitation guideline | 2020 | a | 48 | |
| India | Cardiological society of India: position statement for the management of ST elevation myocardial infarction in India | 2017 | a | 49 | |
| South America | South American guidelines for cardiovascular disease prevention and rehabilitation | 2014 | a | 50 | |
| Thailand | Heart rehabilitation club, heart association of Thailand CR Guideline | 1996 | a | 51 | |
| International | ICCPR consensus statement: Cardiac rehabilitation delivery model for low-resource settings | 2016 | a | 24,52 | |
| Rehabilitation after cardiovascular diseases, with special emphasis on developing countries: report of WHO Expert Committeed | 1993 | a | 53 | ||
Addressed in clinical guideline.
Where applicable, latest guideline versions listed.
According to World Bank: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
This will soon be superseded by WHO’s Package of Rehabilitation Interventions for Ischemic Heart Disease (expected 2022). See: https://www.who.int/activities/integrating-rehabilitation-into-health-systems/service-delivery/package-of-interventions-for-rehabilitation.
Currently being updated (personal communication, J. Gallagher, 14 December 2021).
HIC, high-income country; LMIC, low- and middle-income country; CR, cardiac rehabilitation; SP, secondary prevention; ESC, European Society of Cardiology; CHD, Coronary Heart Disease; HF, heart failure; AHA, American Heart Association; ACCF, American College of Cardiology Foundation; NICE, National Institute for Health and Care Excellence; AACVPR, American Association of Cardiovascular and Pulmonary Rehabilitation; ICCPR, International Council of Cardiovascular Prevention and Rehabilitation; IACR, Irish Association of Cardiac Rehabilitation; JCS, Japanese Circulation Society; WHO, World Health Organization; SIGN, Scottish Intercollegiate Guideline Network.
CR standards and core components, or other scientific statements such as those pertaining to only one component of secondary prevention or cardiac rehabilitation not shown, but many can be found here: https://globalcardiacrehab.com/CR-Standard/Core-Components-&-Quality-Indicators and https://globalcardiacrehab.com/CR-Guidelines.
Whilst the evidence demonstrating the beneficial effect of CR to date has been collected in randomized controlled trials (RCTs) largely conducted in HICs, there is a growing literature from developing countries. A recently published systematic review and meta-analysis identified 26 RCTs of CR in 6380 patients (primarily: post-MI, revascularization, and HF) conducted across eight LMICs (i.e. Bangladesh, Brazil, China, Egypt, India, Iran, Nigeria, and Pakistan).54 Given their small size and short follow-up, few of these trials reported sufficient clinical events to judge impact on mortality and hospital admission. However, most studies assessed the change in short-term exercise capacity, which was found to increase following CR [mean increase in peak oxygen uptake: 3.1 mL/kg/min, 95% confidence interval (CI) 2.6–3.6] compared to no CR control (Figure 1); this is very similar to figures from CR trials in HICs (i.e. 3.3 mL/kg/min, 95% CI 2.6–4.0).55
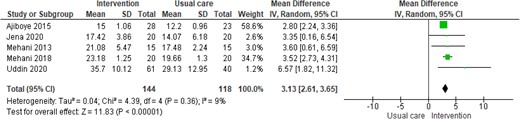
Impact of cadiac rehabilitation on exercise capacity (peak oxygen uptake, mL/kg/min)—summary of results from systematic review/meta-analysis in low- and middle-income countries39.
A systematic review of economic evaluations of CR in LMICs found no studies from low-income countries.56 However, five studies in middle-income settings of Latin America indicated that CR could be cost-effective. In Brazil, mean cost per patient was US$503 for a 3-month CR programme, with a mean monthly saving in healthcare costs of US$190 for CR, compared with an increase of US$48 in the non-CR control group.
Models of care and setting
Phase II CR has traditionally been delivered in hospital settings, where physician champions developed and instituted programmes to reduce the recurrence of events they were seeing in their patients. While there is significant regional variation, globally a median CR dose (operationalized as duration of CR programme × number of sessions/weeks) of 24 is offered (equivalent to three sessions a week over eight weeks).57,58 Although this continuity of care and proximity to acute care in the rare instance of a cardiac emergency can be beneficial for patients, such settings are not ideal for several reasons. These include high cost for space, risk of nosocomial infection (e.g. SARS-CoV-2), lack of environment of ‘wellness’ or ‘recovery’, and often access issues, such as lack of or expensive parking and traffic density. Cost and travel have been identified consistently by survey of patient barriers to CR participation (see data presented in Supplementary data online, eTable S1).59–82 This has led to low global CR utilization rates (see ‘Global variation in availability’ section below), which CR programmes have attempted to mitigate through delivery of CR components or full programmes outside of hospital settings.
In contrast to HICs that typically fund healthcare including CR programmes from taxation, private health insurance and social health insurance, the International Council of Cardiovascular Prevention and Rehabilitation’s (ICCPR) 2016/7 global CR audit showed patient out-of-pocket payment to be the main form of CR funding in LMICs.58 Although the CR delivery cost was lower (as with much of healthcare provision) in LMICs compared with HICs, importantly it does not appear affordable (i.e. in LMICs the average CR cost to patients of US$570 despite an average annual income of US$833, based on 2013 purchasing power parity).58
Cardiac Rehabilitation has also been offered in clinical settings outside hospitals, as well as community settings (often phase III programmes). Home-based CR refers to delivery of all the same core CR components but remotely and/or unsupervised.83 While often some in-person sessions are required at the beginning of a programme and the end for re-assessment, generally this involves CR staff supporting patients to implement needed lifestyle changes via technology.84 This can range from one-on-one coaching calls where a CR provider reviews a patient’s exercise diary and education area for a given week, to group video-based exercise sessions with remote telemetry monitoring.
Globally, the number of CR sessions offered to patients in home-based programmes is relatively low (median of six sessions, with CR dose significantly lower in LMICs vs. HICs),57 which may be insufficient to offer a fully comprehensive programme (exercise training plus health education and psychosocial support) to achieve patient outcome benefit.85 Questions around CR quality assurance also pertain to unsupervised exercise intensity.86
Risk stratification is necessary to ensure patient safety prior to unsupervised delivery; the recent Scientific Statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology suggests that home-based models may be a reasonable option for selected clinically stable low to moderate-risk patients who are eligible for CR but cannot attend a traditional centre-based CR program.83 However, since COVID-19, more evidence is emerging regarding safety even in some higher-risk populations.87
The benefits of CR delivered in the setting of the patient’s home (with or without digital support) has been shown to be similar to those achieved in the more traditional centre-based setting.88,89 Indeed, the recently updated Cochrane review including 24 head-to-head RCTs including 3046 patients with HF, post-MI, and revascularization reported no significant difference between home vs. centre-based CR in the primary outcomes of total mortality (relative risk: 1.18, 95% CI 0.64–2.19), exercise capacity (standardized mean difference: −0.10, 95% CI −0.24 to 0.04), or health-related quality of life, with no difference in cost.8,88 A systematic review and meta-analysis of 30 telehealth RCTs of secondary prevention in CHD patients reported lower re-hospitalization and cardiac events (RR 0.56, 95% CI 0.39–0.81) as well as risk factor levels with CR vs. usual care.89
The ICCPR’s global audit of CR found that of the countries globally with any CR, in 77% of countries, programmes offer traditional supervised CR. In almost half of countries with CR, at least some programmes offer alternative models (this corresponds to 25% of all countries globally; see below), including 34% of countries with CR offering home-based CR and 23% community-based, with no difference in HICs vs. LMICs.57,58,90 Where home-based is offered, ∼20% of a programme’s patients participate in that model. Finally, almost 9% of programmes globally (from 24 countries) offer some form of hybrid model, where patients transition from hospital-based CR to some other form of delivery,90 which can be highly patient-centred.
To improve access, telehealth CR interventions could be offered to patients who cannot attend traditional centre-based CR programme as well as an adjunct to CR programmes to improve long-term adherence. Detailed reviews of digital approaches to CR provision and challenges to implementation in low-income setting are presented elsewhere.89,91,92
Global variation in cardiac rehabilitation density
As has been touched on above, despite a robust quality and quantity of evidence of benefit, CR is insufficiently available in every country of the world. The ICCPR’s 2020 global audit update revealed 5848 programmes in 111 (54.7%) countries globally.93 Given the annual capacity in each programme (where available) and incidence of CHD, there is only one CR spot per 12 patients in need each year globally (these figures do not consider HF).58,93 There is wide regional variation in CR density, ranging from one CR spot for every four patients in the Americas, to for every 283 patients in South-East Asia and every 529 in Africa. In terms of unmet need on a national level, the greatest need exists in India and China (>3 million more CR spots/country needed per year), followed by Russia (additional 1.2 million CR spots/year needed).58,93 Unfortunately, there are few CR guidelines available in these settings, despite a need to be tailored to local context (Table 2; collated via ICCPR’s network in addition to the literature search.94)
Challenges/opportunities
Improving access— high-income countries and low- and middle-income country settings
Even in countries with the highest CR density, such as the UK and the USA,93,95 CR utilization rates are low and gross inequalities exist (e.g. lower in women and those with lower socioeconomic status).93,94 When comparing degree of implementation of all guideline-recommended CVD secondary prevention strategies in inpatients or outpatients, CR referral is least-well implemented.96,97 Patients cannot access CR without a physician referral in most countries, but physicians do need programmes to which they can refer.
Rates of CR utilization in countries with national or registry data are summarized elsewhere.98 Although the barriers to CR access are complex,99 they can be broadly summarized at four levels: the health system (i.e. lack of capacity and resources), clinician (e.g. lack of CR education and referral by physicians,100) programme (e.g. not offering sufficient home-based programming, or CR outside of business hours), and patient levels101 (Graphical Abstract). Barriers reported by patients in studies conducted in LMICs and HICs using the validated CR Barriers Scale are shown in Supplementary data online, eTable S1.59 While there are some commonalities, in LMICs, the most common barriers are more fundamental such as out-of-pocket cost, distance/transportation, work conflicts, and severe weather, whereas in HICs lack of perceived need (e.g. already exercise, heart problem not that serious, and take care of on own) is paramount.101,102 Whilst home-based CR is key to overcoming many of these patient barriers in both high- and low-income setting, other existential barriers to CR access remain in low-income settings (Graphical Abstract).
There are proven approaches to address these barriers, at every level. We must advocate for more CR programmes, and reimbursement of CR services.103,104 CR programmes could explore leveraging technology as has been rapidly adopted during the SARS-CoV-19 pandemic,90 to utilize the same resources to treat more patients, while maintaining quality and safety of course. With more programmes, proven systematic referral mechanisms should be more widely implemented to overcome referral failures.105 Finally, a Cochrane review on interventions to promote patient enrolment and adherence to CR identified that working with cardiac care providers empower them to encourage inpatients to participate works.106,107
Much of this research stems from HICs, yet CR barriers are even greater in LMICs. Many are the same, but more severe, such as lack of programmes, lack of referral, prohibitive cost, transportation barriers, and time conflicts (i.e. work obligations).99,108 According to the ICCPR consensus statement, lower-cost CR models should be considered,52 and we must advocate for service coverage/reimbursement.109 For example, reconfiguring cardiology services so that CR is routinely offered to patients ahead of PCI, may provide similar health outcomes at lower costs.110,111
Multimorbidity
The management of multimorbidity (defined as the presence of two or more long-term health conditions) is a growing challenge facing health care systems globally.112 Levels of multimorbidity are predicted to grow with population demographic changes and improved survival rates resulting in increased numbers of older individuals.112 Importantly, patients with multimorbidity are at higher risk of dying prematurely, being admitted to hospital, having longer stays in hospital, and having reduced health-related quality of life113,114 than patients with only one chronic medical condition.
Although referred to CR for a specific cardiac indication (e.g. post-MI or revascularization or with HF), patients do not typically present with this single index disease alone. For example, through the 2019 National Audit for CR (NACR) in the UK, it was identified that ∼50% of the 6502 patients referred to CR had two or more comorbidities.115 In low-resource settings, the challenge of multimorbidity often includes communicable as well as non-communicable conditions. Moreover, this presence of multimorbidity may negatively impact utilization of CR. Indeed, cardiac patients with multimorbidity are less likely to be referred to a CR programme. Other data from NACR report showed that multimorbidity was a strong risk factor for both non-enrollment in CR and programme non-completion.115 For example, a higher proportion of non-completers have symptoms of anxiety and depression than completers.
While CR programs commonly identify and manage diabetes as well as arthritis in their patients, it has been argued that the traditional (single index) CR model needs to be revamped to better cater for the needs of patients with multimorbidity,116 and indeed in the USA, there is fairly extensive collaboration with pulmonary rehabilitation services. The increasing burden and complexity of multimorbidity may challenge the traditional model of CR. Personnel may not have the needed expertise, nor additional time needed to appropriately manage such patients; programs could potentially partner with other specialties to ensure comprehensive chronic care for their patients. Indeed, there are not often available comprehensive rehabilitation services for common chronic conditions such as kidney disease, and CR may be an appropriate model. However, whilst a move to a model of CR delivery that more comprehensively addresses the needs of patients with heart disease and their multimorbidity might be warranted and is indeed the model often being developed in Africa,117 the evidence base for rehabilitation for patients with multiple chronic diseases remains limited.118,119
Conclusions
The CVD is a leading cause of death, morbidity, disability, and loss of health-related quality of life, as well as economic cost burden worldwide, with some 80% of disease occurring in the LMIC setting. With increasing numbers of people living longer with disease, accessibility of high-quality secondary preventative and rehabilitative health services has never been more important.
The CR is a clinically effective and cost-effective comprehensive model of secondary preventive care for patients with CHD and HF that improves functional capacity, well-being, and health-related quality of life, as well as reduces the risk of hospital admission and CV morbidity. Although an intervention with a robust evidence-based and strong clinical guideline recommendation as a key pillar of CVD secondary prevention, compared to other evidence-based drug and device interventions, the global uptake of CR remains stubbornly poor. Barriers to CR access are complex. In the HIC setting, despite a relatively high density of CR programmes, access barriers include suboptimal clinician referral rates and a lack of reimbursement of alternative models of CR that may better suit patient’s needs. In LMICs, lack of CR capacity and infrastructure are key barriers.
Improvement in global CR access requires a multi-factorial approach to overcoming barriers that operate at system, clinician, programme, and patient levels, which often depend on the specific national and local context. Key to improve access and uptake in high- and low-income settings is the development and evaluation of innovative low-cost and scalable alternatives to traditional centre-based CR programmes including home with digitally based models of delivery. Multi-level strategies must be implemented to augment global CR capacity, especially in LMICs where the mismatch of demand and supply are greatest. Policies should promote political commitment to service delivery, supportive public health policies and financing, systematic referral strategies, and alternative and affordable models of delivery, so we may ameliorate the lives of the many people around the world suffering chronically with CVD.
Author contributions
R.S.T. conceived the idea for paper, drafted sections of the text, and provided editorial oversight. S.L.G. drafted sections of the text and provided editorial oversight. D.F., I.J., L.N., and J.S. each drafted sections of the text. N.D.S., D.R.T., and D.N.W. reviewed and commented on a final draft of the paper. All authors agreed.
Supplementary data
Supplementary data is available at European Heart Journal online.
Acknowledgement
The authors wish to acknowledge the contribution of Naomi DeStouz, ESC Patient forum representative, who contributed to conception and planning of this review article and sadly passed away during its drafting.
Funding
No specific funding was received for the conduct of this review.
Data availability
No new data were generated or analysed in support of this research.
References
Author notes
Deceased.
Co-lead authors.
Conflicts of interest R.S.T. reports membership of ESC ACNAP Scientific committee and investigator on the following ongoing externally funded research projects of cardiac rehabilitation: REACH-HFpEF randomized trial funded by UK National Institute of Health Research (NIHR130487); DK:REACH:HF study funded by Danish Heart Foundation (20-R145-A9654-22157), and SCOT:REACH-HF study funded Heart Research UK, and Director of Cardiac Rehabilitation Cochrane Centre. I.J., L.N., N.D.S., S.L.G., J.S., D.R.T., and D.N.W. report no conflicts of interest.



