-
PDF
- Split View
-
Views
-
Cite
Cite
Mohamed B Jalloh, Christopher B Granger, Gregg C Fonarow, Harriette G C Van Spall, Multi-level implementation strategies to improve uptake of evidence-based therapies in heart failure, European Heart Journal, Volume 44, Issue 23, 14 June 2023, Pages 2055–2058, https://doi.org/10.1093/eurheartj/ehad150
Close - Share Icon Share
Heart failure (HF) is a leading cause of mortality, morbidity, and hospitalization in older adults.1 The combination of four classes of guideline-directed medical therapies (GDMTs) results in >70% reduction in mortality in patients with HF with reduced ejection fraction (HFrEF), yet translating this evidence into practice remains a challenge. A large proportion of eligible patients with HFrEF are under-prescribed beta-blockers (BBs), angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), angiotensin receptor–neprilysin inhibitors (ARNIs), mineralocorticoid receptor antagonists (MRAs), and sodium–glucose cotransporter 2 inhibitors (SGLT2Is).2 To save lives, there is an urgent need to implement these therapies in clinical settings.
Several strategies have been designed to close gaps in the implementation of evidence-based HF care. These implementation strategies include, but are not limited to, system-level interventions such as financial incentives or penalties; organizational-level interventions such as multidisciplinary teams, transitional care programs, and clinical algorithms or pathways; clinician-level interventions such as decision support via electronic health records (EHRs); and patient-level interventions such as education.3 We discuss these strategies and highlight effective ones in the present review (Table 1, Figure 1).
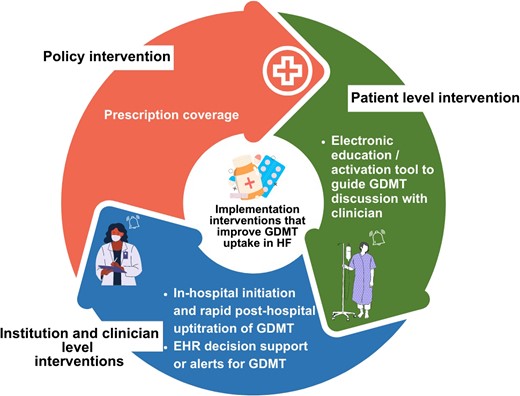
Implementation interventions that improve GDMT uptake in patients with HF based on clinical trial evidence. Interventions targeted the healthcare system (policy), institutions or clinicians, and patients
Examples of RCTs that tested implementation interventions to improve HF care
| Trial . | Trial design, population . |
|---|---|
| Policy interventions | |
| Prescription coverage policies | |
| Post-Myocardial Infarction Free Rx Event and Economic Evaluation (MI-FREEE)4 | Population: 2980 insurance-plan sponsors, 5855 patients post MI (mean age 53.7 years, 24.9% female). |
| Design: parallel cluster RCT. | |
| Intervention: full prescription coverage of insurance plan sponsors for any brand name or generic statins, BBs, ACEIs, or ARBs. | |
| Control: usual coverage paid out-of-pocket costs established by the insurance plan for prescribed medications. | |
| Interventions targeting institutions or clinicians | |
| Audit-and-feedback interventions | |
| Get With The Guidelines-Heart Failure (GWTG-HF)5 | Population: 165 hospitals, 71 829 patients hospitalized for HFrEF (median age 74.5 years, 48.6% female). |
| Design: parallel cluster RCT. | |
| Intervention: personalized quality improvement feedback, teleconferences, webinar invites, and specialized tool kits sent to hospitals every quarter, in addition to baseline information sent to hospitals in the control group. | |
| Control: access to baseline on-demand information, quality improvement resources, and open-access webinars. | |
| Transitional care interventions | |
| Patient-Centered Care Transitions in HF (PACT-HF)1 | Population: 10 hospitals, 2494 patients hospitalized for HF across the range of LVEF (mean age 77.7 years, 50.4% female). |
| Design: stepped-wedge cluster RCT. | |
| Intervention: in-hospital education, structured discharge summary, primary care visit within a week of discharge, and for high-risk patients, nurse-led home visits and heart function clinic visits following discharge. | |
| Control: usual transitional care as per clinician’s discretion. | |
| Care Optimization Through Patient and Hospital Engagement Clinical Trial for HF (CONNECT-HF)6 | Population: 161 hospitals; 5746 patients with HFrEF (mean age 62.6 years, 33.3% female). |
| Design: parallel cluster RCT. | |
| Intervention: discharge, transition, and outpatient quality improvement initiative, with regular education of clinicians by a trained group of experts and feedback on HF process measures (e.g. use of GDMT). | |
| Control: usual care. | |
| GDMT optimization algorithms or alerts | |
| PRagmatic Trial Of Messaging to Providers about outpatient Treatment of HF (PROMPT-HF)7 | Population: 93 providers, 1310 patients with HFrEF (median age 72.0 years, 30.7% female). |
| Design: parallel cluster RCT. | |
| Intervention: EHR-based decision support and alerting system for clinicians managing outpatients with HFrEF. The EHR alert notified providers of personalized GDMT recommendations along with patient characteristics. | |
| Control: no alerts. | |
| Safety, Tolerability and efficacy of Rapid Optimization, helped by NT-proBNP and GDF-15, of HF therapies (STRONG-HF)8 | Population: 1078 patients hospitalized with HF (mean age 63.0 years, 38.6% female). |
| Design: parallel individual RCT. | |
| Intervention: initiation of GDMT in patients hospitalized with HF and post-discharge optimization of GDMT, with the goal of achieving 100% of the target GDMT doses within 2 weeks of discharge. Four outpatient appointments over the 2 post-discharge months to monitor clinical status, laboratory parameters, and NT-proBNP levels. | |
| Control: usual care as per local physician follow-up. | |
| Interventions targeting patients | |
| Electronically Delivered, Patient-Activation Tool for Intensification of Medications for Chronic HFrEF (EPIC-HF)9 | Population: 306 patients (median age: 65.0 years, 28.9% female). |
| Design: parallel individual RCT. | |
| Intervention: patient activation tool comprising a 3-min video and a 1-page checklist delivered electronically before a cardiology clinic appointment. | |
| Control: usual care; no implementation intervention. | |
| Trial . | Trial design, population . |
|---|---|
| Policy interventions | |
| Prescription coverage policies | |
| Post-Myocardial Infarction Free Rx Event and Economic Evaluation (MI-FREEE)4 | Population: 2980 insurance-plan sponsors, 5855 patients post MI (mean age 53.7 years, 24.9% female). |
| Design: parallel cluster RCT. | |
| Intervention: full prescription coverage of insurance plan sponsors for any brand name or generic statins, BBs, ACEIs, or ARBs. | |
| Control: usual coverage paid out-of-pocket costs established by the insurance plan for prescribed medications. | |
| Interventions targeting institutions or clinicians | |
| Audit-and-feedback interventions | |
| Get With The Guidelines-Heart Failure (GWTG-HF)5 | Population: 165 hospitals, 71 829 patients hospitalized for HFrEF (median age 74.5 years, 48.6% female). |
| Design: parallel cluster RCT. | |
| Intervention: personalized quality improvement feedback, teleconferences, webinar invites, and specialized tool kits sent to hospitals every quarter, in addition to baseline information sent to hospitals in the control group. | |
| Control: access to baseline on-demand information, quality improvement resources, and open-access webinars. | |
| Transitional care interventions | |
| Patient-Centered Care Transitions in HF (PACT-HF)1 | Population: 10 hospitals, 2494 patients hospitalized for HF across the range of LVEF (mean age 77.7 years, 50.4% female). |
| Design: stepped-wedge cluster RCT. | |
| Intervention: in-hospital education, structured discharge summary, primary care visit within a week of discharge, and for high-risk patients, nurse-led home visits and heart function clinic visits following discharge. | |
| Control: usual transitional care as per clinician’s discretion. | |
| Care Optimization Through Patient and Hospital Engagement Clinical Trial for HF (CONNECT-HF)6 | Population: 161 hospitals; 5746 patients with HFrEF (mean age 62.6 years, 33.3% female). |
| Design: parallel cluster RCT. | |
| Intervention: discharge, transition, and outpatient quality improvement initiative, with regular education of clinicians by a trained group of experts and feedback on HF process measures (e.g. use of GDMT). | |
| Control: usual care. | |
| GDMT optimization algorithms or alerts | |
| PRagmatic Trial Of Messaging to Providers about outpatient Treatment of HF (PROMPT-HF)7 | Population: 93 providers, 1310 patients with HFrEF (median age 72.0 years, 30.7% female). |
| Design: parallel cluster RCT. | |
| Intervention: EHR-based decision support and alerting system for clinicians managing outpatients with HFrEF. The EHR alert notified providers of personalized GDMT recommendations along with patient characteristics. | |
| Control: no alerts. | |
| Safety, Tolerability and efficacy of Rapid Optimization, helped by NT-proBNP and GDF-15, of HF therapies (STRONG-HF)8 | Population: 1078 patients hospitalized with HF (mean age 63.0 years, 38.6% female). |
| Design: parallel individual RCT. | |
| Intervention: initiation of GDMT in patients hospitalized with HF and post-discharge optimization of GDMT, with the goal of achieving 100% of the target GDMT doses within 2 weeks of discharge. Four outpatient appointments over the 2 post-discharge months to monitor clinical status, laboratory parameters, and NT-proBNP levels. | |
| Control: usual care as per local physician follow-up. | |
| Interventions targeting patients | |
| Electronically Delivered, Patient-Activation Tool for Intensification of Medications for Chronic HFrEF (EPIC-HF)9 | Population: 306 patients (median age: 65.0 years, 28.9% female). |
| Design: parallel individual RCT. | |
| Intervention: patient activation tool comprising a 3-min video and a 1-page checklist delivered electronically before a cardiology clinic appointment. | |
| Control: usual care; no implementation intervention. | |
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BBs, beta-blockers; EHR, electronic health record; GDMT, guideline-directed medical therapy; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal-proB-type natriuretic peptide; RCT, randomized controlled trial.
Examples of RCTs that tested implementation interventions to improve HF care
| Trial . | Trial design, population . |
|---|---|
| Policy interventions | |
| Prescription coverage policies | |
| Post-Myocardial Infarction Free Rx Event and Economic Evaluation (MI-FREEE)4 | Population: 2980 insurance-plan sponsors, 5855 patients post MI (mean age 53.7 years, 24.9% female). |
| Design: parallel cluster RCT. | |
| Intervention: full prescription coverage of insurance plan sponsors for any brand name or generic statins, BBs, ACEIs, or ARBs. | |
| Control: usual coverage paid out-of-pocket costs established by the insurance plan for prescribed medications. | |
| Interventions targeting institutions or clinicians | |
| Audit-and-feedback interventions | |
| Get With The Guidelines-Heart Failure (GWTG-HF)5 | Population: 165 hospitals, 71 829 patients hospitalized for HFrEF (median age 74.5 years, 48.6% female). |
| Design: parallel cluster RCT. | |
| Intervention: personalized quality improvement feedback, teleconferences, webinar invites, and specialized tool kits sent to hospitals every quarter, in addition to baseline information sent to hospitals in the control group. | |
| Control: access to baseline on-demand information, quality improvement resources, and open-access webinars. | |
| Transitional care interventions | |
| Patient-Centered Care Transitions in HF (PACT-HF)1 | Population: 10 hospitals, 2494 patients hospitalized for HF across the range of LVEF (mean age 77.7 years, 50.4% female). |
| Design: stepped-wedge cluster RCT. | |
| Intervention: in-hospital education, structured discharge summary, primary care visit within a week of discharge, and for high-risk patients, nurse-led home visits and heart function clinic visits following discharge. | |
| Control: usual transitional care as per clinician’s discretion. | |
| Care Optimization Through Patient and Hospital Engagement Clinical Trial for HF (CONNECT-HF)6 | Population: 161 hospitals; 5746 patients with HFrEF (mean age 62.6 years, 33.3% female). |
| Design: parallel cluster RCT. | |
| Intervention: discharge, transition, and outpatient quality improvement initiative, with regular education of clinicians by a trained group of experts and feedback on HF process measures (e.g. use of GDMT). | |
| Control: usual care. | |
| GDMT optimization algorithms or alerts | |
| PRagmatic Trial Of Messaging to Providers about outpatient Treatment of HF (PROMPT-HF)7 | Population: 93 providers, 1310 patients with HFrEF (median age 72.0 years, 30.7% female). |
| Design: parallel cluster RCT. | |
| Intervention: EHR-based decision support and alerting system for clinicians managing outpatients with HFrEF. The EHR alert notified providers of personalized GDMT recommendations along with patient characteristics. | |
| Control: no alerts. | |
| Safety, Tolerability and efficacy of Rapid Optimization, helped by NT-proBNP and GDF-15, of HF therapies (STRONG-HF)8 | Population: 1078 patients hospitalized with HF (mean age 63.0 years, 38.6% female). |
| Design: parallel individual RCT. | |
| Intervention: initiation of GDMT in patients hospitalized with HF and post-discharge optimization of GDMT, with the goal of achieving 100% of the target GDMT doses within 2 weeks of discharge. Four outpatient appointments over the 2 post-discharge months to monitor clinical status, laboratory parameters, and NT-proBNP levels. | |
| Control: usual care as per local physician follow-up. | |
| Interventions targeting patients | |
| Electronically Delivered, Patient-Activation Tool for Intensification of Medications for Chronic HFrEF (EPIC-HF)9 | Population: 306 patients (median age: 65.0 years, 28.9% female). |
| Design: parallel individual RCT. | |
| Intervention: patient activation tool comprising a 3-min video and a 1-page checklist delivered electronically before a cardiology clinic appointment. | |
| Control: usual care; no implementation intervention. | |
| Trial . | Trial design, population . |
|---|---|
| Policy interventions | |
| Prescription coverage policies | |
| Post-Myocardial Infarction Free Rx Event and Economic Evaluation (MI-FREEE)4 | Population: 2980 insurance-plan sponsors, 5855 patients post MI (mean age 53.7 years, 24.9% female). |
| Design: parallel cluster RCT. | |
| Intervention: full prescription coverage of insurance plan sponsors for any brand name or generic statins, BBs, ACEIs, or ARBs. | |
| Control: usual coverage paid out-of-pocket costs established by the insurance plan for prescribed medications. | |
| Interventions targeting institutions or clinicians | |
| Audit-and-feedback interventions | |
| Get With The Guidelines-Heart Failure (GWTG-HF)5 | Population: 165 hospitals, 71 829 patients hospitalized for HFrEF (median age 74.5 years, 48.6% female). |
| Design: parallel cluster RCT. | |
| Intervention: personalized quality improvement feedback, teleconferences, webinar invites, and specialized tool kits sent to hospitals every quarter, in addition to baseline information sent to hospitals in the control group. | |
| Control: access to baseline on-demand information, quality improvement resources, and open-access webinars. | |
| Transitional care interventions | |
| Patient-Centered Care Transitions in HF (PACT-HF)1 | Population: 10 hospitals, 2494 patients hospitalized for HF across the range of LVEF (mean age 77.7 years, 50.4% female). |
| Design: stepped-wedge cluster RCT. | |
| Intervention: in-hospital education, structured discharge summary, primary care visit within a week of discharge, and for high-risk patients, nurse-led home visits and heart function clinic visits following discharge. | |
| Control: usual transitional care as per clinician’s discretion. | |
| Care Optimization Through Patient and Hospital Engagement Clinical Trial for HF (CONNECT-HF)6 | Population: 161 hospitals; 5746 patients with HFrEF (mean age 62.6 years, 33.3% female). |
| Design: parallel cluster RCT. | |
| Intervention: discharge, transition, and outpatient quality improvement initiative, with regular education of clinicians by a trained group of experts and feedback on HF process measures (e.g. use of GDMT). | |
| Control: usual care. | |
| GDMT optimization algorithms or alerts | |
| PRagmatic Trial Of Messaging to Providers about outpatient Treatment of HF (PROMPT-HF)7 | Population: 93 providers, 1310 patients with HFrEF (median age 72.0 years, 30.7% female). |
| Design: parallel cluster RCT. | |
| Intervention: EHR-based decision support and alerting system for clinicians managing outpatients with HFrEF. The EHR alert notified providers of personalized GDMT recommendations along with patient characteristics. | |
| Control: no alerts. | |
| Safety, Tolerability and efficacy of Rapid Optimization, helped by NT-proBNP and GDF-15, of HF therapies (STRONG-HF)8 | Population: 1078 patients hospitalized with HF (mean age 63.0 years, 38.6% female). |
| Design: parallel individual RCT. | |
| Intervention: initiation of GDMT in patients hospitalized with HF and post-discharge optimization of GDMT, with the goal of achieving 100% of the target GDMT doses within 2 weeks of discharge. Four outpatient appointments over the 2 post-discharge months to monitor clinical status, laboratory parameters, and NT-proBNP levels. | |
| Control: usual care as per local physician follow-up. | |
| Interventions targeting patients | |
| Electronically Delivered, Patient-Activation Tool for Intensification of Medications for Chronic HFrEF (EPIC-HF)9 | Population: 306 patients (median age: 65.0 years, 28.9% female). |
| Design: parallel individual RCT. | |
| Intervention: patient activation tool comprising a 3-min video and a 1-page checklist delivered electronically before a cardiology clinic appointment. | |
| Control: usual care; no implementation intervention. | |
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BBs, beta-blockers; EHR, electronic health record; GDMT, guideline-directed medical therapy; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal-proB-type natriuretic peptide; RCT, randomized controlled trial.
Policy interventions
Financial penalties
Healthcare policies are rarely tested in a scientifically rigorous manner prior to implementation and can have unintended consequences. The US Hospital Readmissions Reduction Program (HRRP)—implemented in 2010 to reduce avoidable rehospitalizations—penalized hospitals with high HF readmission rates without accounting for death as a competing risk. While HRRP was associated with a reduction in Medicare fee-for-service rehospitalization rates from 23.8% in 2010 to 20.6% in 2016, it was associated with a 0.52% temporal increase in mortality, representing a small increase relative to the baseline trend.10 This trend in increased mortality was primarily faced by patients who were not readmitted. The policy may have had unintended consequences such as premature emergency department discharges and the initiation of short-stay units to avoid coding in-hospital care following discharge as readmissions.
Prescription coverage
Reducing costs of prescriptions may increase GDMT adherence. In the Post-Myocardial Infarction-Free Rx Event and Economic Evaluation (MI-FREEE) randomized controlled trial (RCT), insurance-plan sponsors were cluster-randomized to either full prescription coverage or usual prescription coverage for statins, BBs, ACEIs, or ARBs (Table 1).4 The intervention did not improve the primary composite outcome of first readmission for a major vascular event or coronary revascularization (HR, 0.93; 95% CI, 0.82–1.04; P = 0.21), although the number of such events decreased and the odds of full medication adherence to ACEIs, BBs, and statins increased with the intervention (OR, 1.41; 95% CI, 1.18–1.67) (P < 0.001).4
Interventions targeting institutions or clinicians
Audit and feedback
The Get With The Guidelines-Heart Failure (GWTG-HF) cluster trial randomized 165 hospitals to audit-and-feedback of quality metrics vs. no feedback (Table 1).5 The targeted quality metrics in patients with HFrEF included ACEI/ARB and BB prescription at discharge. The mean change in percentage points of the primary outcome of the quality-of-care score was not different between the intervention and comparator groups at 1 year [+0.31 standard error (SE, 1.51) vs. +3.18 (SE, 1.68)]. There were no between-group differences in other quality measures, including ACEI/ARB and BB prescriptions.5
Transitional care
The Patient-Centered Care Transitions in HF (PACT-HF) stepped-wedge cluster RCT randomized 10 hospitals to transitional care services or usual care for patients hospitalized for HF (Table 1). The intervention incorporated services that were shown in prior explanatory trials to reduce death and readmission in patients hospitalized for HF.1 However, in this pragmatic trial, the intervention did not reduce the primary composite endpoint of all-cause emergent visits, hospitalizations, or death (HR, 0.99; 95% CI, 0.83–1.19) at 3 months following discharge. There was no difference in the uptake of ACEI/ARB, BB, MRA, or diuretics at 7 or 30 days.1 The intervention may have had a ceiling effect given the use of similar services in the usual care group, and the high-risk patients that it targeted may not have had modifiable clinical risk.1
The Care Optimization Through Patient and Hospital Engagement Clinical Trial for HF (CONNECT-HF) RCT—in which 161 hospitals were randomized to a transitional care quality improvement initiative vs. usual care6—demonstrated no improvement in the co-primary outcomes of composite HF readmission or all-cause death at 3 years (adjusted HR, 0.92; 95% CI, 0.81–1.05) or composite HF care quality score (difference of 3.3%; 95% CI, −0.8–7.3%) in patients hospitalized for HFrEF.6 The utilization of RAASIs, BBs, ACEIs/ARBs/ARNIs, and MRAs at >50% of target doses was low and did not improve with the intervention. A challenge was a lack of workflow integration between the inpatient and outpatient clinicians, such that the plan to start and titrate GDMT was not implemented following discharge.
GDMT titration algorithms
The Rapid Optimization, helped by NT-proBNP and GDF-15, of HF therapies (STRONG-HF) RCT assessed the effect of early and frequent up-titration of GDMT in patients hospitalized with HF on suboptimal therapies (Table 1).8 Patients were randomized to in-hospital initiation followed by rapid up-titration of BBs, ACEIs/ARBs/ARNIs, and MRAs in HF clinics vs. usual care. The primary endpoint, a composite of all-cause death or HF readmission at 6 months, was reduced in the treatment group [risk ratio (RR), 0.66; 95% CI, 0·50–0.86]. At 3 months, a greater proportion of patients in the intervention group achieved >50% of the target doses for all three GDMT classes.8
Financial incentives or penalties
Financial incentives or penalties at the clinician level have not shown significant improvements in GDMT uptake in systematic reviews.3
Decision support or electronic alerts in electronic health records (EHRs)
Decision support within EHRs can facilitate clinician-level optimization of GDMT. In the PRagmatic Trial Of Messaging to Providers about outpatient Treatment of HF (PROMPT-HF) trial (Table 1), which randomized 93 clinicians to EHR alerts regarding GDMT candidacy vs. no alerts, prescription of GDMT among outpatients with HFrEF increased more in the alerts vs. no alerts group (25.7% vs. 18.7% at 30 days; adjusted RR, 1.41; 95% CI, 1.03–1.93), this was largely driven by increased prescriptions of BBs and less so by increases in the other three GDMT classes.7
Interventions targeting patients
In the Electronically Delivered Patient-Activation Tool for Intensification of Medications for Chronic HFrEF (EPIC-HF) RCT of outpatients with HFrEF (Table 1), a 3-min video and a 1-page checklist distributed to patients prior to cardiology clinic appointments resulted in an increase (RR, 1.6; 95% CI, 1.2–2.2) in GDMT intensification over the ensuing month, primarily due to up-titration of BBs.9
Role of trial design
Interventions—including implementation interventions—proven effective in RCTs are often not implemented at the healthcare system level following the trial. We propose that trials be designed for implementation at the outset, rather than as an afterthought. Phase III trials should have an implementation plan identified at the outset and consider access, scalability, and sustainability of the intervention and its delivery in the design. Hybrid effectiveness–implementation trials simultaneously test the effect of interventions while measuring implementation processes and addressing contextual factors that may impact the uptake of the intervention in clinical practice. The PACT-HF trial is an example.1 In addition to clinical outcomes, the trial collected implementation process measures and tailored the intervention to local context for better uptake. Eligibility criteria were broad, and the intervention was delivered pragmatically within usual care settings by existing healthcare personnel; this allowed for all participating hospitals to learn to deliver the intervention and continue it should it be proven effective.
Summary
Implementation strategies that increase the use of GDMT classes in HF include prescription subsidies, EHR alerts aimed at clinicians, digital education aimed at patients, and frequent algorithmic up-titration of GDMT during and following hospitalization for HF. Financial disincentives, audit and feedback alone, and transitional care services without algorithm-driven GDMT changes do not seem to be effective. The adoption of hybrid effectiveness–implementation and pragmatic trial design elements can facilitate end-of-trial implementation of proven-effective strategies.
References
Author notes
Conflict of interest H.G.C.V. receives grant support from the Canadian Institutes of Health Research and Heart and Stroke Foundation of Canada and has received educational program grants from Novartis and Boehringer Ingelheim. G.C.F. reports consulting for Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Eli Lilly, Janssen, Medtronic, Merck, Novartis, and Pfizer. C.B.G. receives consulting fees for the following companies: Abbvie, Abiomed, Alnylam Pharmaceuticals, Anthos, Bayer Corporation, Boehringer Ingelheim, Boston Scientific Corporation, Bristol Myers Squibb, Cardionomics, CeleCor Therapeutics, Janssen Pharmaceutical, Merck, Novo Nordisk, Novartis Pharmaceutical Company, Pfizer, Philips, REATA, NephroSynergy. He also has salary funded by Duke grants sponsored by: Boehinger Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Food and Drug Administration, Janssen Pharmaceuticals, Novartis Pharmaceutical Company, Pfizer, and Philips. He has Equity in Tenac.io. M.B.J. reports no conflict of interest.



