-
PDF
- Split View
-
Views
-
Cite
Cite
Andrea Scotti, Augustin Coisne, Maurizio Taramasso, Juan F Granada, Sebastian Ludwig, Josep Rodés-Cabau, Philipp Lurz, Jörg Hausleiter, Neil Fam, Susheel K Kodali, Joel Rosiene, Ari Feinberg, Alberto Pozzoli, Hannes Alessandrini, Luigi Biasco, Eric Brochet, Paolo Denti, Rodrigo Estévez-Loureiro, Christian Frerker, Edwin C Ho, Vanessa Monivas, Georg Nickenig, Fabien Praz, Rishi Puri, Horst Sievert, Gilbert H L Tang, Martin Andreas, Ralph Stephan Von Bardeleben, Karl-Philipp Rommel, Guillem Muntané-Carol, Mara Gavazzoni, Daniel Braun, Benedikt Koell, Daniel Kalbacher, Kim A Connelly, Jean-Michel Juliard, Claudia Harr, Giovanni Pedrazzini, Giulio Russo, François Philippon, Joachim Schofer, Holger Thiele, Matthias Unterhuber, Dominique Himbert, Marina Ureña Alcázar, Mirjam G Wild, Stephan Windecker, Ulrich Jorde, Francesco Maisano, Martin B Leon, Rebecca T Hahn, Azeem Latib, Sex-related characteristics and short-term outcomes of patients undergoing transcatheter tricuspid valve intervention for tricuspid regurgitation, European Heart Journal, Volume 44, Issue 10, 7 March 2023, Pages 822–832, https://doi.org/10.1093/eurheartj/ehac735
Close - Share Icon Share
Abstract
The impact of sexuality in patients with significant tricuspid regurgitation (TR) undergoing transcatheter tricuspid valve intervention (TTVI) is unknown. The aim of this study was to investigate sex-specific outcomes in patients with significant TR treated with TTVI vs. medical therapy alone.
The Transcatheter Tricuspid Valve Therapies (TriValve) registry collected data on patients with significant TR from 24 centres who underwent TTVI from 2016 to 2021. A control cohort was formed by medically managed patients with ≥severe isolated TR diagnosed in 2015–18. The primary endpoint was freedom from all-cause mortality. Secondary endpoints were heart failure (HF) hospitalization, New York Heart Association (NYHA) functional status, and TR severity. One-year outcomes were assessed for the TriValve cohort and compared with the control cohort with the inverse probability of treatment weighting (IPTW). A total of 556 and 2072 patients were included from the TriValve and control groups, respectively. After TTVI, there was no difference between women and men in 1-year freedom from all-cause mortality 80.9% vs. 77.9%, P = 0.56, nor in HF hospitalization (P = 0.36), NYHA Functional Classes III and IV (P = 0.17), and TR severity >2+ at last follow-up (P = 0.42). Multivariable Cox-regression weighted by IPTW showed improved 1-year survival after TTVI compared with medical therapy alone in both women (adjusted hazard ratio 0.45, 95% confidence interval 0.23–0.83, P = 0.01) and men (adjusted hazard ratio 0.42, 95% confidence interval 0.18–0.89, P = 0.03).
After TTVI in high-risk patients, there were no sex-related differences in terms of survival, HF hospitalization, functional status, and TR reduction up to 1 year. The IPTW analysis shows a survival benefit of TTVI over medical therapy alone in both women and men.
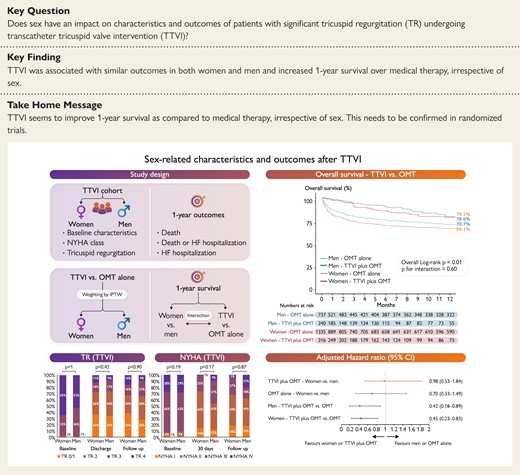
Sex-related characteristics and outcomes after transcatheter tricuspid valve intervention. CI, confidence interval; NYHA, New York Heart Association; OMT, optimal medical therapy; TR, tricuspid regurgitation; TTVI, transcatheter tricuspid valve intervention.
See the editorial comment for this article ‘Valvular heart diseases in women: facts vs. incantations’, by M. Enriquez-Sarano and J. Grapsa, https://doi.org/10.1093/eurheartj/ehac774.
Introduction
Tricuspid regurgitation (TR) is a highly prevalent valvular heart disease and is associated with increased long-term mortality and adverse clinical outcomes.1–3 The majority of patients with significant TR are deemed to be at high or prohibitive surgical risk, and surgery for isolated TR is seldom performed.4 The unmet clinical need for operative TR management led to the development of transcatheter tricuspid valve intervention (TTVI), which has been shown to be a safe and effective therapeutic option.5,6 Several studies have shown sex-related differences in the presentation and outcomes of patients with aortic stenosis or mitral regurgitation, irrespective of the medical or operative management.7–9 In particular, women have been found to be older at presentation for intervention, have less clinical benefit after mitral transcatheter edge-to-edge repair (TEER), and have markedly higher mortality after aortic valve intervention for low-flow, low-gradient aortic stenosis. Natural history studies report an increased prevalence of significant TR in women,10 and risk score to predict outcomes for isolated tricuspid valve (TV) surgery includes female sex as a risk factor.11 However, the impact of sex on the characteristics and outcomes of patients with significant TR undergoing TTVI remains unknown.
Hence, we sought to perform a comprehensive analysis of sex-related differences regarding clinical presentation, echocardiographic characteristics, and outcomes of patients undergoing TTVI enrolled in a large real-world, international registry [Transcatheter Tricuspid Valve Therapies (TriValve) registry, NCT03416166] and compare them with a control group of patients with ≥severe isolated TR under optimal medical therapy (OMT).
Methods
Transcatheter tricuspid valve intervention cohort
The details of the TriValve registry have been previously described.12 Briefly, the TriValve registry included patients with symptomatic TR who underwent TTVI across 24 centres in Europe and North America. All patients had symptomatic heart failure (HF) and significant (≥ moderate) TR according to the European and American guidelines.13,14 Patients were referred to the registry by local investigators and were deemed at prohibitive risk by the local interdisciplinary heart team. The Institutional Review Board at each participating site approved the study protocol, and informed, written consent for participation was provided by all patients. Baseline characteristics, including clinical and echocardiographic data, were collected before TTVI. Procedural success was defined as patient alive at the end of the procedure, with the device successfully implanted and delivery system retrieved, with a TR reduction of at least one grade, and an absolute residual TR ≤2+.
Medical therapy cohort
The control cohort was formed by all consecutive patients with a new diagnosis of severe or greater TR made with echocardiographic assessment at the Montefiore–Einstein Center for Heart and Vascular Care (Bronx, NY, USA) between 2015 and 2018. All data were prospectively collected in an institutional registry and further examined for the presence of the inclusion (severe or greater TR) and exclusion [age <18 years, previous TV intervention (whether surgical or transcatheter), heart valvular intervention during the follow-up period, or patients with concomitant more than moderate mitral or aortic valve disease] criteria. No transcatheter option was available for these patients in the study period. Baseline characteristics, including clinical and echocardiographic data, were collected at the time of the echocardiographic assessment. Clinical follow-up was carried out by clinical visits and/or phone consultation. The inclusion of patients in this study was approved by the local institutional review board. All the patients in both the interventional and medical therapy groups were medically treated according to guideline-directed medical therapy.
Echocardiographic evaluation
All patients underwent comprehensive two-dimensional and Doppler echocardiography. TR severity was graded into four grades: mild (1+), moderate (2+), severe (3+), and massive/torrential (4+) using a combination of semiquantitative and quantitative assessment, as described by the American Society of Echocardiography guidelines as well as the European Association of Echocardiography guidelines.15–17 TR effective regurgitant orifice area was quantified using the proximal isovelocity surface area method. Pacemaker-induced TR was diagnosed with targeted interrogation of the TV leaflets in the presence of leads and leaflet impingement, leaflet adherence, leaflet perforation, or pacing-mediated TR. Chamber sizes and functions were quantified in accordance with the most recent European and US guidelines.16,18 Specially, right ventricular (RV) function was estimated by measuring tricuspid annular plane systolic excursion (TAPSE) or Doppler tissue imaging-derived tricuspid lateral annular systolic velocity. The RV end-diastolic diameter was defined as the maximal transversal dimension in the basal one-third of the RV inflow at end-diastole and right atrial volume was calculated using single-plane area length or disk summation techniques. All right-side measurements were performed in a dedicated apical four-chamber view.
Clinical outcomes
In the absence of specific criteria and definitions for TTVI adverse outcomes, Mitral Valve Academic Research Consortium criteria were adopted to define adverse events. The primary endpoint was 1 year of freedom from all-cause death. The secondary endpoints were HF hospitalization, functional status [assessed by the New York Heart Association (NYHA) functional class], and recurrence of more than moderate TR severity. Acute kidney injury was defined as Stage 2 or 3 of the modified RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) criteria. Follow-up data were collected at discharge, at 30 days, and then according to the time frame elapsed from the index procedure to data lock for the present analysis.
Statistical analysis
Patients were divided into two groups according to sex in both cohorts. Category variables were reported as numbers and corresponding proportions and compared with the χ2 test with continuity correction or the Fisher’s exact test, as appropriate. Continuous variables were described as mean ± standard deviation (SD) or as median (interquartile range) and compared with a two-sided Student’s t-test (parametric test) or the Wilcoxon rank sum test (non-parametric test), according to their distribution. A propensity score methodology with inverse probability of treatment weighting (IPTW) was performed to limit selection bias and balance baseline characteristics between TTVI and medical therapy groups.19,20 Propensity scores predicting each patient’s probability of undergoing TTVI or not were estimated using generalized linear models, including variables with a difference in their distribution between the two groups or considered clinically significant (age, atrial fibrillation, diabetes, and chronic kidney disease). Propensity scores were used to compute stabilized weights. IPTW was used to maintain the numbers of patients in both cohorts, contrary to traditional propensity matching that requires trimming both groups in order to create a balanced match. The balance of measured covariance between groups was compared by generating a standardized difference, and the optimal balance was determined with a value of 10% or less. Subsequent survival analyses, including both TTVI and medical therapy groups, were weighted by IPTW. Overall survival and freedom from the composite endpoint of death or unplanned HF hospitalization were estimated using the Kaplan–Meier method and compared using the log-rank test. The incidence of HF hospitalization was estimated using the cumulative incidence function, accounting for death as a competing risk. Hazard ratios (HRs) and 95% confidence intervals (CIs) were determined using Cox proportional hazards regression. Multivariable Cox proportional hazards regression models were used to explore the association of TTVI and sex with primary and secondary endpoints. A two-sided P-value of <0.05 was considered statistically significant. Statistics were performed using R, version 4.1.3 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline and procedural characteristics
A total of 556 patients underwent TTVI and were included in the TriValve registry. Among them, 316 (56.8%) were women. Baseline characteristics according to sex are depicted in Table 1. Compared with men, women were less likely to have ascites (20.3% vs. 32.1%, P < 0.01) or previous hospitalization for RV failure (65.1% vs. 75.7%, P = 0.02). Conversely, there was no difference regarding the incidence of NYHA Classes III and IV (women 93.6% vs. men 91.5%, P = 0.19), diabetes (women 29.8% vs. men 24.2%, P = 0.18), or atrial fibrillation (women 66.6% vs. men 68.5%, P = 0.70). Although men had more implanted pacemaker or intracardiac defibrillators (31.2% vs. 21.6%, P = 0.02), the TR mechanism was mainly functional (88.8%) with similar proportions between men and women (91.6% vs. 86.7%, P = 0.28). Women had higher left ventricular ejection fraction (53.8 ± 11.5% vs. 46.3 ± 14.7%, P < 0.01), with similar left ventricular and left atrial sizes, measured as left ventricular end-diastolic diameter index (P = 0.63), and left atrial volume index (P = 0.82). There were no statistical differences in RV size (i.e. RV end-diastolic diameter) and function (i.e. TAPSE; Table 2).
| . | Overall (n = 556) . | Women (n = 316) . | Men (n = 240) . | P-value . |
|---|---|---|---|---|
| Age (years) | 76.0 ± 9.6 | 76.1 ± 10.5 | 75.9 ± 8.2 | 0.82 |
| BMI (kg/m²) | 26.2 ± 5.2 | 26.3 ± 5.8 | 26.1 ± 4.4 | 0.68 |
| BSA (m2) | 1.8 ± 0.2 | 1.8 ± 0.2 | 2.0 ± 0.2 | <0.001 |
| Diabetes | 148 (27.4) | 92 (29.8) | 56 (24.2) | 0.18 |
| COPD | 121 (22.0) | 60 (19.0) | 61 (25.8) | 0.07 |
| Atrial fibrillation | 370 (67.4) | 209 (66.6) | 161 (68.5) | 0.70 |
| Prior myocardial infarction | 89 (16.2) | 35 (11.12) | 54 (23.1) | <0.01 |
| PM/ICD | 140 (25.7) | 67 (21.6) | 73 (31.2) | 0.02 |
| NYHA Classes III and IV | 509 (92.7) | 294 (93.6) | 215 (91.5) | 0.19 |
| Ascites | 127 (25.5) | 57 (20.3) | 70 (32.1) | <0.01 |
| Peripheral oedema | 396 (77.3) | 222 (76.3) | 174 (78.7) | 0.59 |
| Previous RV failure | 341 (69.6) | 185 (65.1) | 156 (75.7) | 0.02 |
| CKD | 427 (76.8) | 239 (75.6) | 188 (78.3) | 0.52 |
| Previous left-side valve intervention | 168 (30.4) | 108 (34.2) | 60 (25.3) | 0.03 |
| TR aetiology | – | – | – | 0.28 |
| ȃFunctional | 492 (88.8) | 274 (86.7) | 218 (91.6) | – |
| ȃDegenerative | 27 (4.9) | 17 (5.4) | 10 (4.2) | – |
| ȃMixed | 26 (4.7) | 19 (6.0) | 7 (2.9) | – |
| ȃOther | 9 (1.6) | 6 (1.9) | 3 (1.3) | – |
| EuroSCORE II (%) | 6.3 (3.7–12.4) | 6.7 (4.1–13.2) | 6.0 (3.3–11.0) | 0.11 |
| STS mortality (%) | 4.1 (2.6–6.9) | 4.3 (2.7–6.7) | 4.0 (2.3–7.4) | 0.51 |
| Haemoglobin (g/dL) | 10.7 ± 2.3 | 11.0 ± 2.3 | 10.2 ± 2.3 | <0.01 |
| eGFR (mL/min/1.73 m²) | 45.7 ± 20.5 | 46.6 ± 21.1 | 44.5 ± 19.8 | 0.25 |
| NT-proBNP (pg/mL) | 2656 (1309–5632) | 2482 (1154–4830) | 3038 (1640–6985) | <0.01 |
| AST (U/L) | 28.2 (23.0–36.0) | 29.0 (22.0–37.8) | 28.0 (23.9–33.0) | 0.67 |
| ALT (U/L) | 19.0 (14.0–26.0) | 20.0 (14.0–28.0) | 18.6 (13.0–24.0) | 0.05 |
| . | Overall (n = 556) . | Women (n = 316) . | Men (n = 240) . | P-value . |
|---|---|---|---|---|
| Age (years) | 76.0 ± 9.6 | 76.1 ± 10.5 | 75.9 ± 8.2 | 0.82 |
| BMI (kg/m²) | 26.2 ± 5.2 | 26.3 ± 5.8 | 26.1 ± 4.4 | 0.68 |
| BSA (m2) | 1.8 ± 0.2 | 1.8 ± 0.2 | 2.0 ± 0.2 | <0.001 |
| Diabetes | 148 (27.4) | 92 (29.8) | 56 (24.2) | 0.18 |
| COPD | 121 (22.0) | 60 (19.0) | 61 (25.8) | 0.07 |
| Atrial fibrillation | 370 (67.4) | 209 (66.6) | 161 (68.5) | 0.70 |
| Prior myocardial infarction | 89 (16.2) | 35 (11.12) | 54 (23.1) | <0.01 |
| PM/ICD | 140 (25.7) | 67 (21.6) | 73 (31.2) | 0.02 |
| NYHA Classes III and IV | 509 (92.7) | 294 (93.6) | 215 (91.5) | 0.19 |
| Ascites | 127 (25.5) | 57 (20.3) | 70 (32.1) | <0.01 |
| Peripheral oedema | 396 (77.3) | 222 (76.3) | 174 (78.7) | 0.59 |
| Previous RV failure | 341 (69.6) | 185 (65.1) | 156 (75.7) | 0.02 |
| CKD | 427 (76.8) | 239 (75.6) | 188 (78.3) | 0.52 |
| Previous left-side valve intervention | 168 (30.4) | 108 (34.2) | 60 (25.3) | 0.03 |
| TR aetiology | – | – | – | 0.28 |
| ȃFunctional | 492 (88.8) | 274 (86.7) | 218 (91.6) | – |
| ȃDegenerative | 27 (4.9) | 17 (5.4) | 10 (4.2) | – |
| ȃMixed | 26 (4.7) | 19 (6.0) | 7 (2.9) | – |
| ȃOther | 9 (1.6) | 6 (1.9) | 3 (1.3) | – |
| EuroSCORE II (%) | 6.3 (3.7–12.4) | 6.7 (4.1–13.2) | 6.0 (3.3–11.0) | 0.11 |
| STS mortality (%) | 4.1 (2.6–6.9) | 4.3 (2.7–6.7) | 4.0 (2.3–7.4) | 0.51 |
| Haemoglobin (g/dL) | 10.7 ± 2.3 | 11.0 ± 2.3 | 10.2 ± 2.3 | <0.01 |
| eGFR (mL/min/1.73 m²) | 45.7 ± 20.5 | 46.6 ± 21.1 | 44.5 ± 19.8 | 0.25 |
| NT-proBNP (pg/mL) | 2656 (1309–5632) | 2482 (1154–4830) | 3038 (1640–6985) | <0.01 |
| AST (U/L) | 28.2 (23.0–36.0) | 29.0 (22.0–37.8) | 28.0 (23.9–33.0) | 0.67 |
| ALT (U/L) | 19.0 (14.0–26.0) | 20.0 (14.0–28.0) | 18.6 (13.0–24.0) | 0.05 |
Data are mean ± SD, median (interquartile range), or n (%).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter defibrillator; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PM, pacemaker; RV, right ventricular; STS, Society of Thoracic Surgeons; TR, tricuspid regurgitation.
| . | Overall (n = 556) . | Women (n = 316) . | Men (n = 240) . | P-value . |
|---|---|---|---|---|
| Age (years) | 76.0 ± 9.6 | 76.1 ± 10.5 | 75.9 ± 8.2 | 0.82 |
| BMI (kg/m²) | 26.2 ± 5.2 | 26.3 ± 5.8 | 26.1 ± 4.4 | 0.68 |
| BSA (m2) | 1.8 ± 0.2 | 1.8 ± 0.2 | 2.0 ± 0.2 | <0.001 |
| Diabetes | 148 (27.4) | 92 (29.8) | 56 (24.2) | 0.18 |
| COPD | 121 (22.0) | 60 (19.0) | 61 (25.8) | 0.07 |
| Atrial fibrillation | 370 (67.4) | 209 (66.6) | 161 (68.5) | 0.70 |
| Prior myocardial infarction | 89 (16.2) | 35 (11.12) | 54 (23.1) | <0.01 |
| PM/ICD | 140 (25.7) | 67 (21.6) | 73 (31.2) | 0.02 |
| NYHA Classes III and IV | 509 (92.7) | 294 (93.6) | 215 (91.5) | 0.19 |
| Ascites | 127 (25.5) | 57 (20.3) | 70 (32.1) | <0.01 |
| Peripheral oedema | 396 (77.3) | 222 (76.3) | 174 (78.7) | 0.59 |
| Previous RV failure | 341 (69.6) | 185 (65.1) | 156 (75.7) | 0.02 |
| CKD | 427 (76.8) | 239 (75.6) | 188 (78.3) | 0.52 |
| Previous left-side valve intervention | 168 (30.4) | 108 (34.2) | 60 (25.3) | 0.03 |
| TR aetiology | – | – | – | 0.28 |
| ȃFunctional | 492 (88.8) | 274 (86.7) | 218 (91.6) | – |
| ȃDegenerative | 27 (4.9) | 17 (5.4) | 10 (4.2) | – |
| ȃMixed | 26 (4.7) | 19 (6.0) | 7 (2.9) | – |
| ȃOther | 9 (1.6) | 6 (1.9) | 3 (1.3) | – |
| EuroSCORE II (%) | 6.3 (3.7–12.4) | 6.7 (4.1–13.2) | 6.0 (3.3–11.0) | 0.11 |
| STS mortality (%) | 4.1 (2.6–6.9) | 4.3 (2.7–6.7) | 4.0 (2.3–7.4) | 0.51 |
| Haemoglobin (g/dL) | 10.7 ± 2.3 | 11.0 ± 2.3 | 10.2 ± 2.3 | <0.01 |
| eGFR (mL/min/1.73 m²) | 45.7 ± 20.5 | 46.6 ± 21.1 | 44.5 ± 19.8 | 0.25 |
| NT-proBNP (pg/mL) | 2656 (1309–5632) | 2482 (1154–4830) | 3038 (1640–6985) | <0.01 |
| AST (U/L) | 28.2 (23.0–36.0) | 29.0 (22.0–37.8) | 28.0 (23.9–33.0) | 0.67 |
| ALT (U/L) | 19.0 (14.0–26.0) | 20.0 (14.0–28.0) | 18.6 (13.0–24.0) | 0.05 |
| . | Overall (n = 556) . | Women (n = 316) . | Men (n = 240) . | P-value . |
|---|---|---|---|---|
| Age (years) | 76.0 ± 9.6 | 76.1 ± 10.5 | 75.9 ± 8.2 | 0.82 |
| BMI (kg/m²) | 26.2 ± 5.2 | 26.3 ± 5.8 | 26.1 ± 4.4 | 0.68 |
| BSA (m2) | 1.8 ± 0.2 | 1.8 ± 0.2 | 2.0 ± 0.2 | <0.001 |
| Diabetes | 148 (27.4) | 92 (29.8) | 56 (24.2) | 0.18 |
| COPD | 121 (22.0) | 60 (19.0) | 61 (25.8) | 0.07 |
| Atrial fibrillation | 370 (67.4) | 209 (66.6) | 161 (68.5) | 0.70 |
| Prior myocardial infarction | 89 (16.2) | 35 (11.12) | 54 (23.1) | <0.01 |
| PM/ICD | 140 (25.7) | 67 (21.6) | 73 (31.2) | 0.02 |
| NYHA Classes III and IV | 509 (92.7) | 294 (93.6) | 215 (91.5) | 0.19 |
| Ascites | 127 (25.5) | 57 (20.3) | 70 (32.1) | <0.01 |
| Peripheral oedema | 396 (77.3) | 222 (76.3) | 174 (78.7) | 0.59 |
| Previous RV failure | 341 (69.6) | 185 (65.1) | 156 (75.7) | 0.02 |
| CKD | 427 (76.8) | 239 (75.6) | 188 (78.3) | 0.52 |
| Previous left-side valve intervention | 168 (30.4) | 108 (34.2) | 60 (25.3) | 0.03 |
| TR aetiology | – | – | – | 0.28 |
| ȃFunctional | 492 (88.8) | 274 (86.7) | 218 (91.6) | – |
| ȃDegenerative | 27 (4.9) | 17 (5.4) | 10 (4.2) | – |
| ȃMixed | 26 (4.7) | 19 (6.0) | 7 (2.9) | – |
| ȃOther | 9 (1.6) | 6 (1.9) | 3 (1.3) | – |
| EuroSCORE II (%) | 6.3 (3.7–12.4) | 6.7 (4.1–13.2) | 6.0 (3.3–11.0) | 0.11 |
| STS mortality (%) | 4.1 (2.6–6.9) | 4.3 (2.7–6.7) | 4.0 (2.3–7.4) | 0.51 |
| Haemoglobin (g/dL) | 10.7 ± 2.3 | 11.0 ± 2.3 | 10.2 ± 2.3 | <0.01 |
| eGFR (mL/min/1.73 m²) | 45.7 ± 20.5 | 46.6 ± 21.1 | 44.5 ± 19.8 | 0.25 |
| NT-proBNP (pg/mL) | 2656 (1309–5632) | 2482 (1154–4830) | 3038 (1640–6985) | <0.01 |
| AST (U/L) | 28.2 (23.0–36.0) | 29.0 (22.0–37.8) | 28.0 (23.9–33.0) | 0.67 |
| ALT (U/L) | 19.0 (14.0–26.0) | 20.0 (14.0–28.0) | 18.6 (13.0–24.0) | 0.05 |
Data are mean ± SD, median (interquartile range), or n (%).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter defibrillator; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PM, pacemaker; RV, right ventricular; STS, Society of Thoracic Surgeons; TR, tricuspid regurgitation.
| . | Overall (n = 556) . | Women (n = 316) . | Men (n = 240) . | P-value . |
|---|---|---|---|---|
| LVEF (%) | 50.6 ± 13.5 | 53.8 ± 11.5 | 46.3 ± 14.7 | <0.01 |
| LVEDD index (mm/m2) | 27.3 ± 5.0 | 27.2 ± 5.1 | 27.4 ± 4.9 | 0.63 |
| Left atrial volume index (mL/m2) | 58.0 ± 28.2 | 57.7 ± 27.2 | 58.0 ± 29.7 | 0.82 |
| Concomitant MR ≥3+ | 181 (33.2) | 97 (31.2) | 84 (35.9) | 0.29 |
| TR jet location | – | – | – | 0.07 |
| ȃCentral | 362 (65.1) | 205 (64.9) | 157 (65.4) | – |
| ȃAnteroseptal | 63 (11.3) | 39 (12.3) | 24 (10.0) | – |
| ȃAnteroposterior | 11 (2.0) | 2 (0.6) | 9 (3.8) | – |
| ȃPosteroseptal | 21 (3.8) | 10 (3.2) | 11 (4.6) | – |
| ȃUnknown | 99 (17.8) | 60 (19.0) | 39 (16.2) | – |
| TR vena contracta (mm) | 10.5 ± 4.2 | 10.4 ± 4.2 | 10.6 ± 4.2 | 0.50 |
| TR EROA (cm²) | 0.68 ± 0.53 | 0.70 ± 0.57 | 0.65 ± 0.47 | 0.41 |
| TR regurgitant volume (mL) | 51.5 ± 30.5 | 51.0 ± 32.0 | 52.1 ± 28.8 | 0.80 |
| Tricuspid annulus diameter index (mm/m2) | 25.8 ± 5.0 | 26.0 ± 5.4 | 25.5 ± 4.3 | 0.37 |
| Tricuspid coaptation gap (mm) | 5.54 ± 2.96 | 5.33 ± 2.87 | 5.73 ± 3.04 | 0.28 |
| Tricuspid tenting area (cm²) | 2.42 ± 1.56 | 2.38 ± 1.62 | 2.46 ± 1.51 | 0.67 |
| RVEDD index (mm/m2) | 21.3 ± 6.9 | 22.4 ± 6.9 | 20.4 ± 6.0 | 0.05 |
| Right atrial volume index (mL/m2) | 58.1 ± 37.9 | 57.8 ± 36.5 | 58.5 ± 39.9 | 0.90 |
| TAPSE (mm) | 16.6 ± 4.9 | 16.8 ± 5.2 | 16.3 ± 4.6 | 0.28 |
| S-TDI (cm/s) | 9.80 ± 3.12 | 9.73 ± 3.18 | 9.98 ± 3.02 | 0.66 |
| SPAP (mmHg) | 40.7 ± 15.2 | 42.5 ± 15.7 | 38.4 ± 14.2 | <0.01 |
| . | Overall (n = 556) . | Women (n = 316) . | Men (n = 240) . | P-value . |
|---|---|---|---|---|
| LVEF (%) | 50.6 ± 13.5 | 53.8 ± 11.5 | 46.3 ± 14.7 | <0.01 |
| LVEDD index (mm/m2) | 27.3 ± 5.0 | 27.2 ± 5.1 | 27.4 ± 4.9 | 0.63 |
| Left atrial volume index (mL/m2) | 58.0 ± 28.2 | 57.7 ± 27.2 | 58.0 ± 29.7 | 0.82 |
| Concomitant MR ≥3+ | 181 (33.2) | 97 (31.2) | 84 (35.9) | 0.29 |
| TR jet location | – | – | – | 0.07 |
| ȃCentral | 362 (65.1) | 205 (64.9) | 157 (65.4) | – |
| ȃAnteroseptal | 63 (11.3) | 39 (12.3) | 24 (10.0) | – |
| ȃAnteroposterior | 11 (2.0) | 2 (0.6) | 9 (3.8) | – |
| ȃPosteroseptal | 21 (3.8) | 10 (3.2) | 11 (4.6) | – |
| ȃUnknown | 99 (17.8) | 60 (19.0) | 39 (16.2) | – |
| TR vena contracta (mm) | 10.5 ± 4.2 | 10.4 ± 4.2 | 10.6 ± 4.2 | 0.50 |
| TR EROA (cm²) | 0.68 ± 0.53 | 0.70 ± 0.57 | 0.65 ± 0.47 | 0.41 |
| TR regurgitant volume (mL) | 51.5 ± 30.5 | 51.0 ± 32.0 | 52.1 ± 28.8 | 0.80 |
| Tricuspid annulus diameter index (mm/m2) | 25.8 ± 5.0 | 26.0 ± 5.4 | 25.5 ± 4.3 | 0.37 |
| Tricuspid coaptation gap (mm) | 5.54 ± 2.96 | 5.33 ± 2.87 | 5.73 ± 3.04 | 0.28 |
| Tricuspid tenting area (cm²) | 2.42 ± 1.56 | 2.38 ± 1.62 | 2.46 ± 1.51 | 0.67 |
| RVEDD index (mm/m2) | 21.3 ± 6.9 | 22.4 ± 6.9 | 20.4 ± 6.0 | 0.05 |
| Right atrial volume index (mL/m2) | 58.1 ± 37.9 | 57.8 ± 36.5 | 58.5 ± 39.9 | 0.90 |
| TAPSE (mm) | 16.6 ± 4.9 | 16.8 ± 5.2 | 16.3 ± 4.6 | 0.28 |
| S-TDI (cm/s) | 9.80 ± 3.12 | 9.73 ± 3.18 | 9.98 ± 3.02 | 0.66 |
| SPAP (mmHg) | 40.7 ± 15.2 | 42.5 ± 15.7 | 38.4 ± 14.2 | <0.01 |
Data are mean ± SD or n (%).
EROA, effective regurgitant orifice area; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; RVEDD, right ventricular end-diastolic diameter; S-TDI, S-tissue Doppler imaging; SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
| . | Overall (n = 556) . | Women (n = 316) . | Men (n = 240) . | P-value . |
|---|---|---|---|---|
| LVEF (%) | 50.6 ± 13.5 | 53.8 ± 11.5 | 46.3 ± 14.7 | <0.01 |
| LVEDD index (mm/m2) | 27.3 ± 5.0 | 27.2 ± 5.1 | 27.4 ± 4.9 | 0.63 |
| Left atrial volume index (mL/m2) | 58.0 ± 28.2 | 57.7 ± 27.2 | 58.0 ± 29.7 | 0.82 |
| Concomitant MR ≥3+ | 181 (33.2) | 97 (31.2) | 84 (35.9) | 0.29 |
| TR jet location | – | – | – | 0.07 |
| ȃCentral | 362 (65.1) | 205 (64.9) | 157 (65.4) | – |
| ȃAnteroseptal | 63 (11.3) | 39 (12.3) | 24 (10.0) | – |
| ȃAnteroposterior | 11 (2.0) | 2 (0.6) | 9 (3.8) | – |
| ȃPosteroseptal | 21 (3.8) | 10 (3.2) | 11 (4.6) | – |
| ȃUnknown | 99 (17.8) | 60 (19.0) | 39 (16.2) | – |
| TR vena contracta (mm) | 10.5 ± 4.2 | 10.4 ± 4.2 | 10.6 ± 4.2 | 0.50 |
| TR EROA (cm²) | 0.68 ± 0.53 | 0.70 ± 0.57 | 0.65 ± 0.47 | 0.41 |
| TR regurgitant volume (mL) | 51.5 ± 30.5 | 51.0 ± 32.0 | 52.1 ± 28.8 | 0.80 |
| Tricuspid annulus diameter index (mm/m2) | 25.8 ± 5.0 | 26.0 ± 5.4 | 25.5 ± 4.3 | 0.37 |
| Tricuspid coaptation gap (mm) | 5.54 ± 2.96 | 5.33 ± 2.87 | 5.73 ± 3.04 | 0.28 |
| Tricuspid tenting area (cm²) | 2.42 ± 1.56 | 2.38 ± 1.62 | 2.46 ± 1.51 | 0.67 |
| RVEDD index (mm/m2) | 21.3 ± 6.9 | 22.4 ± 6.9 | 20.4 ± 6.0 | 0.05 |
| Right atrial volume index (mL/m2) | 58.1 ± 37.9 | 57.8 ± 36.5 | 58.5 ± 39.9 | 0.90 |
| TAPSE (mm) | 16.6 ± 4.9 | 16.8 ± 5.2 | 16.3 ± 4.6 | 0.28 |
| S-TDI (cm/s) | 9.80 ± 3.12 | 9.73 ± 3.18 | 9.98 ± 3.02 | 0.66 |
| SPAP (mmHg) | 40.7 ± 15.2 | 42.5 ± 15.7 | 38.4 ± 14.2 | <0.01 |
| . | Overall (n = 556) . | Women (n = 316) . | Men (n = 240) . | P-value . |
|---|---|---|---|---|
| LVEF (%) | 50.6 ± 13.5 | 53.8 ± 11.5 | 46.3 ± 14.7 | <0.01 |
| LVEDD index (mm/m2) | 27.3 ± 5.0 | 27.2 ± 5.1 | 27.4 ± 4.9 | 0.63 |
| Left atrial volume index (mL/m2) | 58.0 ± 28.2 | 57.7 ± 27.2 | 58.0 ± 29.7 | 0.82 |
| Concomitant MR ≥3+ | 181 (33.2) | 97 (31.2) | 84 (35.9) | 0.29 |
| TR jet location | – | – | – | 0.07 |
| ȃCentral | 362 (65.1) | 205 (64.9) | 157 (65.4) | – |
| ȃAnteroseptal | 63 (11.3) | 39 (12.3) | 24 (10.0) | – |
| ȃAnteroposterior | 11 (2.0) | 2 (0.6) | 9 (3.8) | – |
| ȃPosteroseptal | 21 (3.8) | 10 (3.2) | 11 (4.6) | – |
| ȃUnknown | 99 (17.8) | 60 (19.0) | 39 (16.2) | – |
| TR vena contracta (mm) | 10.5 ± 4.2 | 10.4 ± 4.2 | 10.6 ± 4.2 | 0.50 |
| TR EROA (cm²) | 0.68 ± 0.53 | 0.70 ± 0.57 | 0.65 ± 0.47 | 0.41 |
| TR regurgitant volume (mL) | 51.5 ± 30.5 | 51.0 ± 32.0 | 52.1 ± 28.8 | 0.80 |
| Tricuspid annulus diameter index (mm/m2) | 25.8 ± 5.0 | 26.0 ± 5.4 | 25.5 ± 4.3 | 0.37 |
| Tricuspid coaptation gap (mm) | 5.54 ± 2.96 | 5.33 ± 2.87 | 5.73 ± 3.04 | 0.28 |
| Tricuspid tenting area (cm²) | 2.42 ± 1.56 | 2.38 ± 1.62 | 2.46 ± 1.51 | 0.67 |
| RVEDD index (mm/m2) | 21.3 ± 6.9 | 22.4 ± 6.9 | 20.4 ± 6.0 | 0.05 |
| Right atrial volume index (mL/m2) | 58.1 ± 37.9 | 57.8 ± 36.5 | 58.5 ± 39.9 | 0.90 |
| TAPSE (mm) | 16.6 ± 4.9 | 16.8 ± 5.2 | 16.3 ± 4.6 | 0.28 |
| S-TDI (cm/s) | 9.80 ± 3.12 | 9.73 ± 3.18 | 9.98 ± 3.02 | 0.66 |
| SPAP (mmHg) | 40.7 ± 15.2 | 42.5 ± 15.7 | 38.4 ± 14.2 | <0.01 |
Data are mean ± SD or n (%).
EROA, effective regurgitant orifice area; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; RVEDD, right ventricular end-diastolic diameter; S-TDI, S-tissue Doppler imaging; SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Transcatheter tricuspid valve intervention and procedural outcomes
Procedural characteristics and outcomes are shown in Table 3. Overall, the duration of the procedure was similar between women and men (132.4 ± 66.4 vs. 132.0 ± 60.4 min, P = 0.95). Women were less frequently treated with TEER than men (74.4% vs. 83.3%, P < 0.01), and in the case of TEER, fewer clips were implanted in women compared with men (P < 0.01). The rates of procedural success were similar between the two groups (79.5% vs. 77.1%, P = 0.56) as well as the risk of acute kidney injury (10.8% vs. 14.6%, P = 0.32), conversion to surgery (1.2% vs. 2.1%, P = 0.46), or in-hospital death (3.5% vs. 2.1%, P = 0.57).
Procedural characteristics and post-procedural outcomes in the device group according to sex
| . | Overall (n = 556) . | Women (n = 316) . | Men (n = 240) . | P-value . |
|---|---|---|---|---|
| Procedure | ||||
| ȃDuration of procedure (min) | 132.2 ± 63.7 | 132.4 ± 66.4 | 132.0 ± 60.4 | 0.95 |
| ȃConcomitant mitral or aortic intervention | 127 (33.0) | 69 (30.3) | 58 (36.9) | 0.21 |
| Type of TTVI | – | – | – | <0.01 |
| ȃTEER | 435 (78.2) | 235 (74.4) | 200 (83.3) | – |
| TTVR | 13 (2.3) | 11 (3.5) | 2 (0.8) | – |
| ȃAnnuloplasty | 52 (9.4) | 40 (12.7) | 12 (5.0) | – |
| Others | 56 (10.1) | 30 (9.5) | 26 (10.8) | – |
| Number of clips | – | – | – | <0.01 |
| 1 | 20 (4.7) | 8 (3.4) | 12 (6.2) | – |
| ȃ2 | 105 (24.6) | 67 (28.9) | 38 (19.6) | – |
| 3 | 199 (46.7) | 115 (49.6) | 84 (43.3) | – |
| ȃ4 | 87 (20.4) | 39 (16.8) | 48 (24.7) | – |
| 5 | 13 (3.1) | 3 (1.3) | 10 (5.2) | – |
| ȃ6 | 2 (0.5) | 0 (0.0) | 2 (1.0) | – |
| Post-procedure outcomes | ||||
| ȃProcedural success | 415 (78.4) | 237 (79.5) | 178 (77.1) | 0.56 |
| AKI | 51 (12.4) | 26 (10.8) | 25 (14.6) | 0.32 |
| ȃNew-onset atrial fibrillation | 6 (1.4) | 5 (2.1) | 1 (0.6) | 0.41 |
| Stroke | 4 (0.9) | 3 (1.2) | 1 (0.5) | 0.64 |
| ȃLength of stay (days) | 4 (2–7) | 4 (2–7) | 4 (3–7) | 0.59 |
| Conversion to surgery | 7 (1.6) | 3 (1.2) | 4 (2.1) | 0.46 |
| ȃIn-hospital death | 13 (2.9) | 9 (3.5) | 4 (2.1) | 0.57 |
| 30-day outcomes | ||||
| ȃTAPSE (mm) | 15.7 ± 4.5 | 15.6 ± 4.5 | 15.8 ± 4.7 | 0.79 |
| SPAP (mmHg) | 43.3 ± 14.8 | 44.2 ± 14.1 | 41.9 ± 15.8 | 0.22 |
| ȃAll-cause mortality | 20 (4.9) | 11 (4.5) | 9 (5.6) | 0.82 |
| . | Overall (n = 556) . | Women (n = 316) . | Men (n = 240) . | P-value . |
|---|---|---|---|---|
| Procedure | ||||
| ȃDuration of procedure (min) | 132.2 ± 63.7 | 132.4 ± 66.4 | 132.0 ± 60.4 | 0.95 |
| ȃConcomitant mitral or aortic intervention | 127 (33.0) | 69 (30.3) | 58 (36.9) | 0.21 |
| Type of TTVI | – | – | – | <0.01 |
| ȃTEER | 435 (78.2) | 235 (74.4) | 200 (83.3) | – |
| TTVR | 13 (2.3) | 11 (3.5) | 2 (0.8) | – |
| ȃAnnuloplasty | 52 (9.4) | 40 (12.7) | 12 (5.0) | – |
| Others | 56 (10.1) | 30 (9.5) | 26 (10.8) | – |
| Number of clips | – | – | – | <0.01 |
| 1 | 20 (4.7) | 8 (3.4) | 12 (6.2) | – |
| ȃ2 | 105 (24.6) | 67 (28.9) | 38 (19.6) | – |
| 3 | 199 (46.7) | 115 (49.6) | 84 (43.3) | – |
| ȃ4 | 87 (20.4) | 39 (16.8) | 48 (24.7) | – |
| 5 | 13 (3.1) | 3 (1.3) | 10 (5.2) | – |
| ȃ6 | 2 (0.5) | 0 (0.0) | 2 (1.0) | – |
| Post-procedure outcomes | ||||
| ȃProcedural success | 415 (78.4) | 237 (79.5) | 178 (77.1) | 0.56 |
| AKI | 51 (12.4) | 26 (10.8) | 25 (14.6) | 0.32 |
| ȃNew-onset atrial fibrillation | 6 (1.4) | 5 (2.1) | 1 (0.6) | 0.41 |
| Stroke | 4 (0.9) | 3 (1.2) | 1 (0.5) | 0.64 |
| ȃLength of stay (days) | 4 (2–7) | 4 (2–7) | 4 (3–7) | 0.59 |
| Conversion to surgery | 7 (1.6) | 3 (1.2) | 4 (2.1) | 0.46 |
| ȃIn-hospital death | 13 (2.9) | 9 (3.5) | 4 (2.1) | 0.57 |
| 30-day outcomes | ||||
| ȃTAPSE (mm) | 15.7 ± 4.5 | 15.6 ± 4.5 | 15.8 ± 4.7 | 0.79 |
| SPAP (mmHg) | 43.3 ± 14.8 | 44.2 ± 14.1 | 41.9 ± 15.8 | 0.22 |
| ȃAll-cause mortality | 20 (4.9) | 11 (4.5) | 9 (5.6) | 0.82 |
Data are mean ± SD, median (interquartile range), or n (%).
AKI, acute kidney injury; SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; TEER, transcatheter edge-to-edge repair; TTVI, transcatheter tricuspid valve intervention; TTVR, transcatheter tricuspid valve replacement.
Procedural characteristics and post-procedural outcomes in the device group according to sex
| . | Overall (n = 556) . | Women (n = 316) . | Men (n = 240) . | P-value . |
|---|---|---|---|---|
| Procedure | ||||
| ȃDuration of procedure (min) | 132.2 ± 63.7 | 132.4 ± 66.4 | 132.0 ± 60.4 | 0.95 |
| ȃConcomitant mitral or aortic intervention | 127 (33.0) | 69 (30.3) | 58 (36.9) | 0.21 |
| Type of TTVI | – | – | – | <0.01 |
| ȃTEER | 435 (78.2) | 235 (74.4) | 200 (83.3) | – |
| TTVR | 13 (2.3) | 11 (3.5) | 2 (0.8) | – |
| ȃAnnuloplasty | 52 (9.4) | 40 (12.7) | 12 (5.0) | – |
| Others | 56 (10.1) | 30 (9.5) | 26 (10.8) | – |
| Number of clips | – | – | – | <0.01 |
| 1 | 20 (4.7) | 8 (3.4) | 12 (6.2) | – |
| ȃ2 | 105 (24.6) | 67 (28.9) | 38 (19.6) | – |
| 3 | 199 (46.7) | 115 (49.6) | 84 (43.3) | – |
| ȃ4 | 87 (20.4) | 39 (16.8) | 48 (24.7) | – |
| 5 | 13 (3.1) | 3 (1.3) | 10 (5.2) | – |
| ȃ6 | 2 (0.5) | 0 (0.0) | 2 (1.0) | – |
| Post-procedure outcomes | ||||
| ȃProcedural success | 415 (78.4) | 237 (79.5) | 178 (77.1) | 0.56 |
| AKI | 51 (12.4) | 26 (10.8) | 25 (14.6) | 0.32 |
| ȃNew-onset atrial fibrillation | 6 (1.4) | 5 (2.1) | 1 (0.6) | 0.41 |
| Stroke | 4 (0.9) | 3 (1.2) | 1 (0.5) | 0.64 |
| ȃLength of stay (days) | 4 (2–7) | 4 (2–7) | 4 (3–7) | 0.59 |
| Conversion to surgery | 7 (1.6) | 3 (1.2) | 4 (2.1) | 0.46 |
| ȃIn-hospital death | 13 (2.9) | 9 (3.5) | 4 (2.1) | 0.57 |
| 30-day outcomes | ||||
| ȃTAPSE (mm) | 15.7 ± 4.5 | 15.6 ± 4.5 | 15.8 ± 4.7 | 0.79 |
| SPAP (mmHg) | 43.3 ± 14.8 | 44.2 ± 14.1 | 41.9 ± 15.8 | 0.22 |
| ȃAll-cause mortality | 20 (4.9) | 11 (4.5) | 9 (5.6) | 0.82 |
| . | Overall (n = 556) . | Women (n = 316) . | Men (n = 240) . | P-value . |
|---|---|---|---|---|
| Procedure | ||||
| ȃDuration of procedure (min) | 132.2 ± 63.7 | 132.4 ± 66.4 | 132.0 ± 60.4 | 0.95 |
| ȃConcomitant mitral or aortic intervention | 127 (33.0) | 69 (30.3) | 58 (36.9) | 0.21 |
| Type of TTVI | – | – | – | <0.01 |
| ȃTEER | 435 (78.2) | 235 (74.4) | 200 (83.3) | – |
| TTVR | 13 (2.3) | 11 (3.5) | 2 (0.8) | – |
| ȃAnnuloplasty | 52 (9.4) | 40 (12.7) | 12 (5.0) | – |
| Others | 56 (10.1) | 30 (9.5) | 26 (10.8) | – |
| Number of clips | – | – | – | <0.01 |
| 1 | 20 (4.7) | 8 (3.4) | 12 (6.2) | – |
| ȃ2 | 105 (24.6) | 67 (28.9) | 38 (19.6) | – |
| 3 | 199 (46.7) | 115 (49.6) | 84 (43.3) | – |
| ȃ4 | 87 (20.4) | 39 (16.8) | 48 (24.7) | – |
| 5 | 13 (3.1) | 3 (1.3) | 10 (5.2) | – |
| ȃ6 | 2 (0.5) | 0 (0.0) | 2 (1.0) | – |
| Post-procedure outcomes | ||||
| ȃProcedural success | 415 (78.4) | 237 (79.5) | 178 (77.1) | 0.56 |
| AKI | 51 (12.4) | 26 (10.8) | 25 (14.6) | 0.32 |
| ȃNew-onset atrial fibrillation | 6 (1.4) | 5 (2.1) | 1 (0.6) | 0.41 |
| Stroke | 4 (0.9) | 3 (1.2) | 1 (0.5) | 0.64 |
| ȃLength of stay (days) | 4 (2–7) | 4 (2–7) | 4 (3–7) | 0.59 |
| Conversion to surgery | 7 (1.6) | 3 (1.2) | 4 (2.1) | 0.46 |
| ȃIn-hospital death | 13 (2.9) | 9 (3.5) | 4 (2.1) | 0.57 |
| 30-day outcomes | ||||
| ȃTAPSE (mm) | 15.7 ± 4.5 | 15.6 ± 4.5 | 15.8 ± 4.7 | 0.79 |
| SPAP (mmHg) | 43.3 ± 14.8 | 44.2 ± 14.1 | 41.9 ± 15.8 | 0.22 |
| ȃAll-cause mortality | 20 (4.9) | 11 (4.5) | 9 (5.6) | 0.82 |
Data are mean ± SD, median (interquartile range), or n (%).
AKI, acute kidney injury; SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; TEER, transcatheter edge-to-edge repair; TTVI, transcatheter tricuspid valve intervention; TTVR, transcatheter tricuspid valve replacement.
Sex-related outcomes following transcatheter tricuspid valve intervention
At 1 year after TTVI, all-cause mortality occurred in 66 (20.4%) patients, HF hospitalization in 81 (25.4%), and the composite endpoint of all-cause mortality and HF hospitalization in 118 (35.4%). At 1 year, no differences between women and men were observed in the Kaplan–Meier analyses for the freedom from all-cause mortality and the composite endpoint of all-cause mortality or HF hospitalization, nor in the cumulative incidence function of HF hospitalization (Figure 1). After adjustment for left ventricular ejection fraction, previous myocardial infarction, and hospitalization for RV failure on multivariable Cox-regression analysis, results remained consistent with the unadjusted Kaplan–Meier method: freedom from all-cause mortality (adjusted HR: 1.02; 95% CI: 0.59–1.74; P = 0.95), HF hospitalization (adjusted HR: 1.28; 95% CI: 0.79–2.09; P = 0.31), and all-cause mortality or HF hospitalization (adjusted HR: 1.11; 95% CI: 0.74–1.65; P = 0.62). In addition, there were no differences between women and men in NYHA Functional Classes III and IV nor in TR severity >2+ at 30 days (P = 0.17 and P = 0.42, respectively), and at last follow-up (P = 0.87 and P = 0.90, respectively; Figure 2).
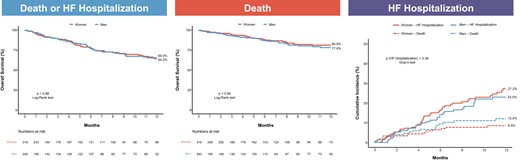
Kaplan–Meier curves of clinical outcomes after transcatheter tricuspid valve intervention according to sex. There was no difference at 1 year in the Kaplan–Meier curves for death or heart failure hospitalization and death, nor in the cumulative incidence of heart failure hospitalization after transcatheter tricuspid valve intervention between women and men. HF, heart failure.

Changes in New York Heart Association functional class and tricuspid regurgitation severity from baseline to last follow-up after transcatheter tricuspid valve intervention. No significant differences in New York Heart Association Classes III and IV or tricuspid regurgitation severity >2+ were observed between women and men at each time point. *Comparison of New York Heart Association Classes III and IV and tricuspid regurgitation severity >2+ between women and men. NYHA, New York Heart Association; TR, tricuspid regurgitation.
Transcatheter tricuspid valve intervention plus optimal medical therapy vs. optimal medical therapy alone
A total of 2072 patients formed the control group and were compared with those undergoing TTVI in the TriValve registry (Table 4). After IPTW, baseline characteristics of the weighted groups were more balanced between TTVI and OMT patients, in particular with regard to age (73.9 ± 11.5 vs. 73.4 ± 15.2 years, standardized difference = 3.8%), atrial fibrillation (48.6% vs. 42.8%, standardized difference = 5.8%), and chronic kidney disease (52.3% vs. 51.6%, standardized difference = 0.7%; Supplementary material online, Figure S1). Differences persisted in the weighted groups, with the TTVI group having a higher left ventricular end-diastolic diameter index, a left atrial volume index, and a lower TAPSE. Similar findings were observed when comparing the two treatment groups within each sex category (Supplementary material online, Tables S1 and S2). IPTW-weighted Kaplan–Meier analyses at 1 year showed a lower overall survival for women in the OMT group (women 66.1% vs. men 70.7%, log-rank P = 0.01), that was no longer evident after Cox-regression adjustment for age, body mass index, left ventricular ejection fraction, and TAPSE (adjusted HR: 0.70, 95% CI: 0.33–1.49, P = 0.35; Figure 3). In the TTVI cohort, overall survival weighted by IPTW was not affected by sex (women 79.1% vs. men 78.6%, log-rank P = 0.74; adjusted HR: 0.98, 95% CI: 0.53–1.84, P = 0.96). Finally, the benefit of TTVI plus OMT over OMT alone was consistently observed in women (TTVI plus OMT 79.1% vs. OMT alone 66.1%, log-rank P < 0.01; adjusted HR: 0.45, 95% CI: 0.23–0.83, P = 0.01), and men (TTVI plus OMT 78.6% vs. OMT alone 70.7%, log-rank P < 0.01; adjusted HR: 0.42, 95% CI: 0.18–0.89, P = 0.03, adjusted pinteraction = 0.74; Figure 3).
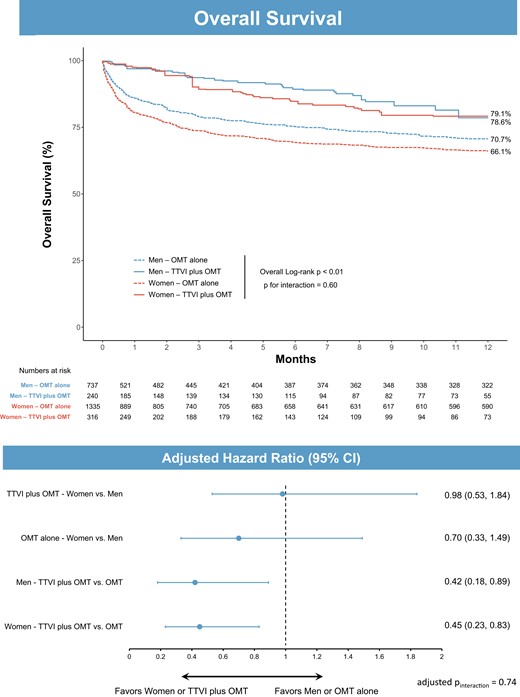
Overall survival at 1 year according to treatment group and sex after inverse probability of treatment weighting. Above: unadjusted Kaplan–Meier analysis at 1 year. Below: forest plot from multivariable Cox-regression analysis including age, body mass index, left ventricular ejection fraction, tricuspid annular plane systolic excursion, sex, and treatment. CI, confidence interval; OMT, optimal medical therapy; TTVI, transcatheter tricuspid valve intervention.
Unweighted and weighted patient characteristics by treatment cohort (TTVI plus OMT vs. OMT alone)
| . | Unweighted study population, n (%) . | Weighted study population, % . | ||||
|---|---|---|---|---|---|---|
| . | TTVI plus OMT (n = 556) . | OMT alone (n = 2072) . | Standardized difference, % . | TTVI plus OMT . | OMT alone . | Standardized difference, % . |
| Age (years) | 76.8 ± 10.3 | 72.4 ± 15.6 | 33.1 | 73.9 ± 11.5 | 73.4 ± 15.2 | 3.8 |
| Women | 316 (56.8) | 1335 (64.4) | −7.6 | 61.2 | 64.2 | −3.0 |
| BMI (kg/m²) | 26.2 ± 5.2 | 28.5 ± 8.6 | −31.6 | 26.6 ± 5.4 | 28.3 ± 8.4 | −24.9 |
| Atrial fibrillation | 370 (67.4) | 752 (36.3) | 31.1 | 48.6 | 42.8 | 5.8 |
| COPD | 121 (22.0) | 468 (22.6) | −0.6 | 21.4 | 23.7 | −2.3 |
| CKD | 427 (76.8) | 935 (45.1) | 31.7 | 52.3 | 51.6 | 0.7 |
| Diabetes | 148 (27.4) | 724 (34.9) | −7.5 | 39.8 | 33.5 | 6.2 |
| LVEF (%) | 50.6 ± 13.5 | 50.4 ± 18.2 | 1.3 | 50.4 ± 13.6 | 50.5 ± 18.1 | −0.8 |
| LVEDD index (mm/m2) | 27.3 ± 5.0 | 25.7 ± 5.2 | 30.9 | 26.9 ± 5.5 | 25.7 ± 5.2 | 22.5 |
| Left atrial volume index (mL/m2) | 58.0 ± 28.2 | 46.5 ± 18.7 | 48.2 | 56.3 ± 27.3 | 47.1 ± 18.7 | 38.4 |
| Right atrial volume index (mL/m2) | 58.1 ± 37.9 | 52.3 ± 25.2 | 18.2 | 55.2 ± 30.4 | 53.0 ± 25.4 | 6.7 |
| TAPSE (mm) | 16.6 ± 4.9 | 17.6 ± 5.5 | −20.5 | 16.5 ± 4.9 | 17.7 ± 5.5 | −23.0 |
| . | Unweighted study population, n (%) . | Weighted study population, % . | ||||
|---|---|---|---|---|---|---|
| . | TTVI plus OMT (n = 556) . | OMT alone (n = 2072) . | Standardized difference, % . | TTVI plus OMT . | OMT alone . | Standardized difference, % . |
| Age (years) | 76.8 ± 10.3 | 72.4 ± 15.6 | 33.1 | 73.9 ± 11.5 | 73.4 ± 15.2 | 3.8 |
| Women | 316 (56.8) | 1335 (64.4) | −7.6 | 61.2 | 64.2 | −3.0 |
| BMI (kg/m²) | 26.2 ± 5.2 | 28.5 ± 8.6 | −31.6 | 26.6 ± 5.4 | 28.3 ± 8.4 | −24.9 |
| Atrial fibrillation | 370 (67.4) | 752 (36.3) | 31.1 | 48.6 | 42.8 | 5.8 |
| COPD | 121 (22.0) | 468 (22.6) | −0.6 | 21.4 | 23.7 | −2.3 |
| CKD | 427 (76.8) | 935 (45.1) | 31.7 | 52.3 | 51.6 | 0.7 |
| Diabetes | 148 (27.4) | 724 (34.9) | −7.5 | 39.8 | 33.5 | 6.2 |
| LVEF (%) | 50.6 ± 13.5 | 50.4 ± 18.2 | 1.3 | 50.4 ± 13.6 | 50.5 ± 18.1 | −0.8 |
| LVEDD index (mm/m2) | 27.3 ± 5.0 | 25.7 ± 5.2 | 30.9 | 26.9 ± 5.5 | 25.7 ± 5.2 | 22.5 |
| Left atrial volume index (mL/m2) | 58.0 ± 28.2 | 46.5 ± 18.7 | 48.2 | 56.3 ± 27.3 | 47.1 ± 18.7 | 38.4 |
| Right atrial volume index (mL/m2) | 58.1 ± 37.9 | 52.3 ± 25.2 | 18.2 | 55.2 ± 30.4 | 53.0 ± 25.4 | 6.7 |
| TAPSE (mm) | 16.6 ± 4.9 | 17.6 ± 5.5 | −20.5 | 16.5 ± 4.9 | 17.7 ± 5.5 | −23.0 |
Data are mean ± SD or n (%).
BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; OMT, optimal medical therapy; TAPSE, tricuspid annular plane systolic excursion; TTVI, transcatheter tricuspid valve intervention.
Unweighted and weighted patient characteristics by treatment cohort (TTVI plus OMT vs. OMT alone)
| . | Unweighted study population, n (%) . | Weighted study population, % . | ||||
|---|---|---|---|---|---|---|
| . | TTVI plus OMT (n = 556) . | OMT alone (n = 2072) . | Standardized difference, % . | TTVI plus OMT . | OMT alone . | Standardized difference, % . |
| Age (years) | 76.8 ± 10.3 | 72.4 ± 15.6 | 33.1 | 73.9 ± 11.5 | 73.4 ± 15.2 | 3.8 |
| Women | 316 (56.8) | 1335 (64.4) | −7.6 | 61.2 | 64.2 | −3.0 |
| BMI (kg/m²) | 26.2 ± 5.2 | 28.5 ± 8.6 | −31.6 | 26.6 ± 5.4 | 28.3 ± 8.4 | −24.9 |
| Atrial fibrillation | 370 (67.4) | 752 (36.3) | 31.1 | 48.6 | 42.8 | 5.8 |
| COPD | 121 (22.0) | 468 (22.6) | −0.6 | 21.4 | 23.7 | −2.3 |
| CKD | 427 (76.8) | 935 (45.1) | 31.7 | 52.3 | 51.6 | 0.7 |
| Diabetes | 148 (27.4) | 724 (34.9) | −7.5 | 39.8 | 33.5 | 6.2 |
| LVEF (%) | 50.6 ± 13.5 | 50.4 ± 18.2 | 1.3 | 50.4 ± 13.6 | 50.5 ± 18.1 | −0.8 |
| LVEDD index (mm/m2) | 27.3 ± 5.0 | 25.7 ± 5.2 | 30.9 | 26.9 ± 5.5 | 25.7 ± 5.2 | 22.5 |
| Left atrial volume index (mL/m2) | 58.0 ± 28.2 | 46.5 ± 18.7 | 48.2 | 56.3 ± 27.3 | 47.1 ± 18.7 | 38.4 |
| Right atrial volume index (mL/m2) | 58.1 ± 37.9 | 52.3 ± 25.2 | 18.2 | 55.2 ± 30.4 | 53.0 ± 25.4 | 6.7 |
| TAPSE (mm) | 16.6 ± 4.9 | 17.6 ± 5.5 | −20.5 | 16.5 ± 4.9 | 17.7 ± 5.5 | −23.0 |
| . | Unweighted study population, n (%) . | Weighted study population, % . | ||||
|---|---|---|---|---|---|---|
| . | TTVI plus OMT (n = 556) . | OMT alone (n = 2072) . | Standardized difference, % . | TTVI plus OMT . | OMT alone . | Standardized difference, % . |
| Age (years) | 76.8 ± 10.3 | 72.4 ± 15.6 | 33.1 | 73.9 ± 11.5 | 73.4 ± 15.2 | 3.8 |
| Women | 316 (56.8) | 1335 (64.4) | −7.6 | 61.2 | 64.2 | −3.0 |
| BMI (kg/m²) | 26.2 ± 5.2 | 28.5 ± 8.6 | −31.6 | 26.6 ± 5.4 | 28.3 ± 8.4 | −24.9 |
| Atrial fibrillation | 370 (67.4) | 752 (36.3) | 31.1 | 48.6 | 42.8 | 5.8 |
| COPD | 121 (22.0) | 468 (22.6) | −0.6 | 21.4 | 23.7 | −2.3 |
| CKD | 427 (76.8) | 935 (45.1) | 31.7 | 52.3 | 51.6 | 0.7 |
| Diabetes | 148 (27.4) | 724 (34.9) | −7.5 | 39.8 | 33.5 | 6.2 |
| LVEF (%) | 50.6 ± 13.5 | 50.4 ± 18.2 | 1.3 | 50.4 ± 13.6 | 50.5 ± 18.1 | −0.8 |
| LVEDD index (mm/m2) | 27.3 ± 5.0 | 25.7 ± 5.2 | 30.9 | 26.9 ± 5.5 | 25.7 ± 5.2 | 22.5 |
| Left atrial volume index (mL/m2) | 58.0 ± 28.2 | 46.5 ± 18.7 | 48.2 | 56.3 ± 27.3 | 47.1 ± 18.7 | 38.4 |
| Right atrial volume index (mL/m2) | 58.1 ± 37.9 | 52.3 ± 25.2 | 18.2 | 55.2 ± 30.4 | 53.0 ± 25.4 | 6.7 |
| TAPSE (mm) | 16.6 ± 4.9 | 17.6 ± 5.5 | −20.5 | 16.5 ± 4.9 | 17.7 ± 5.5 | −23.0 |
Data are mean ± SD or n (%).
BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; OMT, optimal medical therapy; TAPSE, tricuspid annular plane systolic excursion; TTVI, transcatheter tricuspid valve intervention.
Discussion
In this study, we investigated the sex-related differences in characteristics and outcomes of patients undergoing TTVI for TR in the large, international real-world TriValve registry. After TTVI, women and men showed similar improvements in terms of survival, HF hospitalization, functional status, and sustained TR reduction up to 1 year follow-up. Compared with a control group of patients with isolated TR under OMT weighting by IPTW and adjusting with Cox-regression analyses, TTVI plus OMT was associated with substantial and consistent increase in 1-year survival in both women and men (Structured graphical abstract).
Sex-related differences in valvular disease epidemiology and ventricular responses to changes in loading conditions lead to differences in disease prevalence and clinical manifestations.8 Despite a predominance of males with aortic stenosis, several studies reported a higher prevalence and incidence, ranging from 53% to 75%, of TR among women.10,21–24 Our results are consistent with these findings, with 57% of women with significant TR referred for TTVI and 64% present in the OMT group. Besides, the clinical manifestations of patients with significant TR are different between women and men. We showed that, compared with men, women were less likely to have ascites or previous hospitalization for RV failure, and less left ventricular systolic dysfunction, which is in line with recent findings from Dietz et al. and Gual-Capllonch et al.25 In their study, Dietz et al.23 investigated the sex-specific differences in prognosis in patients with significant TR. In a cohort of 1569 patients (51% females), women had better 10-year survival rates compared with men (49% vs. 39%, P = 0.001). However, after propensity score matching, there was no significant difference in mortality (P = 0.23). Accordingly, our analyses with IPTW and Cox-regression adjustments for baseline characteristics show that women and men with TR under medical management had similar overall survival.
Exploring gender differences in Medicare beneficiaries undergoing mitral valve operations, women were found to have higher operative mortality and lower long-term survival.26 However, these findings were largely driven by older age, a higher number of comorbidities, and a later presentation with more advanced disease for women. In the subgroup of patients undergoing mitral valve replacement, the survival benefit over medical therapy was consistent, irrespective of sex. In the case of TEER for mitral regurgitation, two studies from the randomized COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation) trial and the EuroSMR registry found that women had a less pronounced reduction in HF hospitalizations compared with men, with overall survival and improvement in clinical outcomes being similar in both sexes.27,28
Few studies have investigated the sex-related differences in postoperative outcomes after TV surgery. Exploring 92 patients who underwent isolated TV surgery, Pfannmueller et al.29 did not show significant differences in postoperative mortality between women and men. Using the National Inpatient Sample to identify 5005 patients who underwent isolated TV surgery from 2004 to 2013, Chandrashekar et al.30 compared outcomes in 366 paired patients after propensity matching. They found that overall in-hospital mortality was similar for matched women and men. However, no assessment was available after discharge.
To date, there are no data regarding the impact of sex in patients with advanced TR undergoing transcatheter interventions. In our study, we showed that after TTVI, clinical outcomes are similar in both women and men, with 1-year survival rates of 81% and 78%, respectively. Similarly, the survival benefit of TTVI over medical therapy was significant irrespective of sex. These findings are in line with previous reports on the transcatheter treatment of mitral regurgitation.27,28 In the TriValve registry, there were no marked differences in baseline characteristics between women and men. This may explain the discrepancies with surgical series, where women were at much higher risk compared with the male candidates. Also, this stresses the importance of timely referral and management of TV disease.
In the absence of any randomized controlled trial, our results suggest that the benefits of transcatheter interventional treatment of TR are substantial and not affected by gender. With increasing numbers of patients and TTVI options, further studies should explore the impact of sex according to the type of procedure and the patient’s risk profile.
Study limitations
The most relevant limitations of this study are inherent to its non-randomized, observational design with no centralized echocardiographic core-lab or clinical event adjudication committee. However, it still provides the most comprehensive information on sex-related characteristics and outcomes of patients undergoing TTVI for TR. Although several statistical methods, such as propensity IPTW and multivariable Cox-regression analyses, have been applied, we cannot exclude the impact on outcomes of unknown/unmeasured variables (e.g. TR aetiology) that could not be corrected. RV basal diameter and TAPSE may not be accurate measurements of RV size and function in presence of different TR aetiology (i.e. atrial vs. ventricular)31 and previous cardiac surgery. Longer term follow-up is required to determine if the observed outcomes with no differences between women and men are maintained or whether any new interactions may become apparent over time. Finally, our results have to be considered as hypothesis generating; randomized controlled trials are needed to validate these findings and define the ideal candidates and timing of transcatheter interventions for TR.
Conclusions
In the TriValve registry, after TTVI in high-risk patients with significant TR, there were no sex-related differences in terms of survival, HF hospitalization, functional status, and TR reduction up to 1 year. The IPTW analysis suggests that TTVI may be associated with a substantial and consistent increase in survival in both women and men compared with medical therapy alone. Future studies are needed to assess whether sex-related differences in outcomes may emerge at longer term follow-up.
Supplementary data
Supplementary data are available at European Heart Journal online.
Pre-registered Clinical Trial Number
Trial Name: International Multisite Transcatheter Tricuspid Valve Therapies Registry (TriValve).
ClinicalTrial.gov Identifier: NCT03416166
URL: https://clinicaltrials.gov/ct2/show/NCT03416166
Funding
All authors declare no funding for this contribution.
Data availability
The data underlying this article will be shared on reasonable request with the corresponding author.
References
Author notes
Andrea Scotti and Augustin Coisne contributed equally to this work and are joint first authors.
Conflict of Interest: A.S. has served as a consultant and received consulting fees from NeoChord Inc. A.C. has served as a consultant for Abbott and received speaker fees from Abbott and GE Healthcare. M.T. has served as a consultant for Abbott Vascular, Boston Scientific, 4Tech, and CoreMedic; and has received speaker honoraria from Edwards Lifesciences. S.L. has received travel compensation from Edwards Lifesciences. J.R.C. has received institutional research grants from Edwards Lifesciences. P.L. has received speaker fees from Abbott. J.H. has received speaker honoraria from Abbott Vascular and Edwards Lifesciences. S.K.K. has served on the scientific advisory board for Microinterventional Devices, Dura Biotech, Thubrikar Aortic Valve, and Supira; has served as a consultant for Meril Lifesciences, Admedus, Medtronic, and Boston Scientific; has served on the steering committee for Edwards Lifesciences and Abbott Vascular; has received honoraria from Meril Lifesciences, Admedus, Abbott Vascular, and Dura Biotech; and owns equity in Dura Biotech, Thubrikar Aortic Valve, Supira, and MID. H.A. has received consulting fees from Abbott and Edwards LifeSciences. E.B. has received speaker fees from Abbott Vascular. P.D. has served as a consultant for Abbott Vascular, 4Tech, Neovasc, and InnovHeart; and has received honoraria from Abbott and Edwards Lifesciences. R.E.L. has received speaker fees from Abbott, Boston, and Edwards Lifesciences. E.C.H. has served as a consultant and received consulting fees from NeoChord Inc. F.P. has received travel expenses from Edwards Lifesciences, Abbott Vascular, and Polares Medical. H.S. has received study honoraria, travel expenses, and consulting fees from 4Tech Cardio, Abbott, Ablative Solutions, Ancora Heart, Bavaria Medizin Technologie, Bioventrix, Boston Scientific, Carag, Cardiac Dimensions, Celonova, Comed BV, Contego, CVRx, Edwards Lifesciences, Endologix, Hemoteq, Lifetech, Maquet Getinge Group, Medtronic, Mitralign, Nuomao Medtech, Occlutech, PFM Medical, ReCor, Renal Guard, Rox Medical, Terumo, Vascular Dynamics, and Vivasure Medical. G.H.L.T. has served as a consultant, physician advisory board member, and faculty trainer for Abbott Structural Heart; has served as a consultant for Medtronic and NeoChord; and has served as a physician advisory board member for JenaValve. M.A. has served as a proctor/consultant for and has received speaking fees from Abbott, Edwards LifeSciences, Boston, Zoll, and Medtronic; and has received institutional grants from Edwards Lifesciences, Abbott, Medtronic, and LSI Solutions. M.G. has served as a consultant for Abbott Vascular. D.B. has received speaker honoraria and travel support from Abbott Vascular. D.K. has received lecture fees from Abbott and Edwards Lifesciences. K.A.C. has received honoraria from Abbott. J.S. has served as a consultant for Edwards Lifesciences. S.W. reports research, travel, or educational grants to the institution from Abbott, Abiomed, Amgen, Astra Zeneca, Bayer, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardinal Health, CardioValve, Corflow Therapeutics, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Guerbet, InfraRedx, Janssen-Cilag, Johnson & Johnson, Medicure, Medtronic, Merck Sharp & Dohm, Miracor Medical, Novartis, Novo Nordisk, Organon, OrPha Suisse, Pfizer, Polares, Regeneron, Sanofi-Aventis, Servier, Sinomed, Terumo, Vifor, V-Wave. S.W. serves as unpaid advisory board member and/or unpaid member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, Astra Zeneca, Bayer, Boston Scientific, Biotronik, Bristol Myers Squibb, Edwards Lifesciences, Janssen, MedAlliance, Medtronic, Novartis, Polares, Recardio, Sinomed, Terumo, V-Wave, and Xeltis, but has not received personal payments by pharmaceutical companies or device manufacturers. He is also member of the steering/executive committee group of several investigator-initiated trials that receive funding by industry without impact on his personal remuneration. F.M. has served as a consultant for and received consulting fees and honoraria from Abbott Vascular, Edwards Lifesciences, Cardiovalve, SwissVortex, Perifect, Xeltis, Transseptal Solutions, Magenta, Valtech, and Medtronic; has reported being a cofounder of 4Tech; has received research grant support from Abbott, Medtronic, Edwards Lifesciences, Biotronik, Boston Scientific, NVT, and Terumo; has received royalties and owns intellectual property rights from Edwards Lifesciences (FMR surgical annuloplasty); and has reported being a shareholder in Cardiovalve, Swiss Vortex, Magenta, Transseptal Solutions, Occlufit, 4Tech, and Perifect. M.B.L. has received institutional clinical research grants from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. R.T.H. has served as a consultant for Abbott Vascular, Abbott Structural, NaviGate, Philips Healthcare, Medtronic, Edwards Lifesciences, and GE Healthcare; has been the Chief Scientific Officer for the Echocardiography Core Laboratory at the Cardiovascular Research Foundation for multiple industry-supported trials, for which she receives no direct industry compensation; has received speaker fees from Boston Scientific and Baylis Medical; and has received nonfinancial support from 3mensio. A.L. has served on the advisory board for Medtronic, Abbott Vascular Boston Scientific, Edwards Lifesciences, Shifamed, NeoChord Inc, V-dyne, and Philips. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.



