-
PDF
- Split View
-
Views
-
Cite
Cite
M Cikes, K Jering, B Claggett, O Amir, A J Cadena Bonfanti, M C Cho, C Granger, L M Gullestad, H L Kao, J Morais, J F Tanguay, M Tokmakova, P Widimsky, S D Solomon, PARADISE-MI , Atrial fibrillation in patients with high-risk acute myocardial infarction – the PARADISE-MI trial, European Heart Journal, Volume 43, Issue Supplement_2, October 2022, ehac544.1178, https://doi.org/10.1093/eurheartj/ehac544.1178
Close - Share Icon Share
Abstract
Atrial fibrillation or flutter (AFF) is common in both patients with myocardial infarction (MI) and those with heart failure (HF). However, its impact on the risk of adverse outcomes in MI complicated by either reduced LVEF and/or transient pulmonary congestion is less known.
To assess the relationship between AFF and outcomes and whether AFF modified the treatment response to sacubitril/valsartan in the PARADISE-MI (Prospective ARNI versus ACEi trial to determine superiority in reducing HF events after MI) trial.
5656 patients enrolled in the PARADISE-MI trial were divided into 3 groups: no known AFF, history of AFF without AFF at enrolment, and AFF occurring with the index MI event. We assessed outcomes and the treatment response to sacubitril/valsartan in all groups. The primary outcome of the PARADISE-MI trial was death from cardiovascular (CV) causes or incident HF. The outcome analyses were adjusted for the number of risk augmenting factors, age, pulmonary congestion, percutaneous coronary intervention, LVEF and hypertension.
259 patients (4.6%) had only a history of AFF, 525 patients (9.3%) had AFF associated with index MI. Patients with a history of AFF and AFF with index MI were older, with a higher rate of pulmonary congestion and hypertension, lower eGFR values but lower rates of diabetes, compared with those without AFF (Table 1). In unadjusted analyses, history of AFF and AFF with index MI were associated with a significant increase in the risk of the primary outcome (hazard ratio (HR): 1.76; 95% confidence interval (CI): 1.32–2.35 and HR 1.69, 95% CI 1.37–2.10, respectively), remaining significant after adjustment only in those with AFF with index MI (HR=1.40, 95% CI 1.12–1.74) (Fig. 1). This was primarily driven by an increase in the crude and adjusted risk of incident HF, both in those with a history of AFF and AFF with index MI (adjusted HR=1.56, 95% CI 1.10–2.22 and HR=1.55, 95% CI 1.18–2.03, respectively). An increase in the crude risk of CV death was present in patients with a history of AFF and AFF with index MI (HR=1.57, 95% CI 1.04–2.39 and HR=1.66, 95% CI 1.23–2.24, respectively), yet did not remain significant after adjustment. The risk of the composite outcome of death from coronary heart disease, non-fatal MI, hospitalisation for angina or coronary was not associated with either a history of AFF or AFF with index MI, in unadjusted or adjusted analyses (adjusted HR=0.83, 95% CI 0.57–1.19 and HR=1.00, 95% CI 0.78–1.29, respectively) (Fig. 1). Neither history of AFF nor AFF with index MI modified the treatment effect of sacubitril/valsartan (p>0.05).
In this post-MI cohort, history of AFF and AFF occurring with the index MI event were associated with an increased risk of CV death or incident heart failure, primarily driven by an increased risk of incident HF. However, the risk of the composite coronary outcome was not associated with AFF status, compared to other studied outcomes.
Type of funding sources: Other. Main funding source(s): The PARADISE-MI trial was funded by Novartis

Table 1. Baseline characteristics
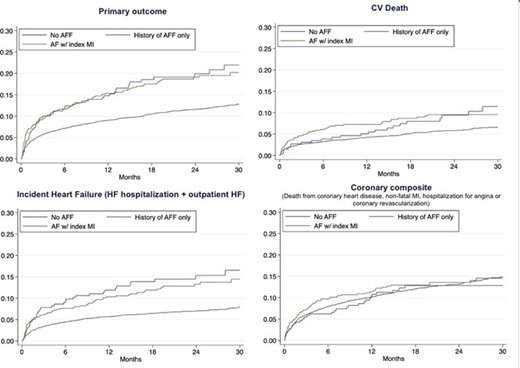
Figure 1. Kaplan-Maier curves for the key outcomes
- myocardial infarction, acute
- angina pectoris
- angiotensin-converting enzyme inhibitors
- atrial fibrillation
- myocardial infarction
- percutaneous coronary intervention
- left ventricular ejection fraction
- hypertension
- diabetes mellitus
- heart failure
- diabetes mellitus, type 2
- pulmonary congestion
- cardiovascular system
- coronary heart disease
- post myocardial infarction
- sacubitril/valsartan
- primary outcome measure
- composite outcomes
- angiotensin receptor-neprilysin inhibitors



