-
PDF
- Split View
-
Views
-
Cite
Cite
Dan Haberman, Rodrigo Estévez-Loureiro, Tomas Benito-Gonzalez, Paolo Denti, Dabit Arzamendi, Marianna Adamo, Xavier Freixa, Luis Nombela-Franco, Pedro Villablanca, Lian Krivoshei, Neil Fam, Konstantinos Spargias, Andrew Czarnecki, Isaac Pascual, Fabien Praz, Doron Sudarsky, Arthur Kerner, Vlasis Ninios, Marco Gennari, Ronen Beeri, Leor Perl, Yishay Wasserstrum, Haim Danenberg, Lion Poles, Jacob George, Berenice Caneiro-Queija, Salvatore Scianna, Igal Moaraf, Davide Schiavi, Claudia Scardino, Noé Corpataux, Julio Echarte-Morales, Michael Chrissoheris, Estefanía Fernández-Peregrina, Mattia Di Pasquale, Ander Regueiro, Carlos Vergara-Uzcategui, Andres Iñiguez-Romo, Felipe Fernández-Vázquez, Danny Dvir, Francesco Maisano, Maurizio Taramasso, Mony Shuvy, Conservative, surgical, and percutaneous treatment for mitral regurgitation shortly after acute myocardial infarction, European Heart Journal, Volume 43, Issue 7, 14 February 2022, Pages 641–650, https://doi.org/10.1093/eurheartj/ehab496
Close - Share Icon Share
Abstract
Severe mitral regurgitation (MR) following acute myocardial infarction (MI) is associated with high mortality rates and has inconclusive recommendations in clinical guidelines. We aimed to report the international experience of patients with secondary MR following acute MI and compare the outcomes of those treated conservatively, surgically, and percutaneously.
Retrospective international registry of consecutive patients with at least moderate-to-severe MR following MI treated in 21 centres in North America, Europe, and the Middle East. The registry included patients treated conservatively and those having surgical mitral valve repair or replacement (SMVR) or percutaneous mitral valve repair (PMVR) using edge-to-edge repair. The primary endpoint was in-hospital mortality. A total of 471 patients were included (43% female, age 73 ± 11 years): 205 underwent interventions, of whom 106 were SMVR and 99 PMVR. Patients who underwent mitral valve intervention were in a worse clinical state (Killip class ≥3 in 60% vs. 43%, P < 0.01), but yet had lower in-hospital and 1-year mortality compared with those treated conservatively [11% vs. 27%, P < 0.01 and 16% vs. 35%, P < 0.01; adjusted hazard ratio (HR) 0.28, 95% confidence interval (CI) 0.18–0.46, P < 0.01]. Surgical mitral valve repair or replacement was performed earlier than PMVR [median of 12 days from MI date (interquartile range 5–19) vs. 19 days (10–40), P < 0.01]. The immediate procedural success did not differ between SMVR and PMVR (92% vs. 93%, P = 0.53). However, in-hospital and 1-year mortality rates were significantly higher in SMVR than in PMVR (16% vs. 6%, P = 0.03 and 31% vs. 17%, P = 0.04; adjusted HR 3.75, 95% CI 1.55–9.07, P < 0.01).
Early intervention may mitigate the poor prognosis associated with conservative therapy in patients with post-MI MR. Percutaneous mitral valve repair can serve as an alternative for surgery in reducing MR for high-risk patients.
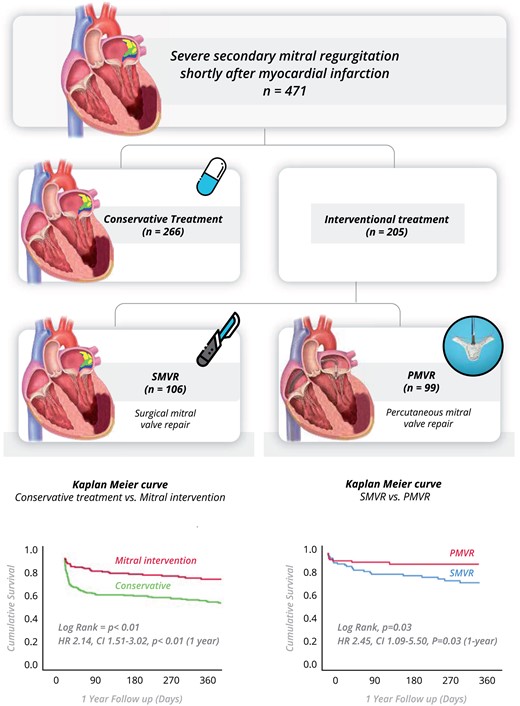
The registry included 471 patients with significant mitral regurgitation within 90-days after acute myocardial infarction who remained symptomatic on optimal medical therapy. Overall, patients who treated by mitral intervention had better survival over patients treated conservatively. Among patients treated with mitral intervention, Surgical mitral valve repair or replacement (SMVR) was associated with a higher mortality rate compared with percutaneous mitral valve repair (PMVR).
Introduction
Acute or sub-acute mitral regurgitation (MR) may develop in the setting of myocardial infarction (MI) as a result of papillary muscle rupture that requires urgent surgical intervention or due to the rapid remodelling of the infarcted left ventricle (LV) causing apical and inferior displacement of the papillary muscles that leads to secondary MR. It is often accompanied by haemodynamic instability and has been linked to poor prognosis.1–4 The relatively acute onset of MR can lead to pulmonary oedema and even cardiogenic shock. Moderate or severe MR is found in 12% of all ST-elevation MI patients at 30-day follow-up.5 Although even mild MR was associated with excess morbidity and mortality, patients who presented with moderate to severe or severe (+3 or +4) MR faced the worst outcomes with high mortality rates of 24%, 42%, and 52% at 30 days, 6 months, and 1 year, respectively.6 These rates could be even higher among patients with acute decompensation that require mechanical ventilation, intravenous diuretics, intravenous inotropes, or mechanic circulatory support. Most of these patients are deemed as high risk for mitral valve surgery or even considered inoperable, and thus managed conservatively with a grim prognosis. Until recently, the only possible intervention targeting MR was surgery. However, in this setting, it is associated with high mortality rates early after MI, reaching 25%.7
Previous literature is limited to a small case series, reporting that percutaneous mitral valve repair (PMVR) using the MitraClip (Abbott; Menlo Park, CA, USA) device after MI complicated by severe MR, is safe and effective. Our group published two case series, showing that, in the majority of patients, salvage PMVR procedure significantly decreased MR and improved haemodynamic parameters, leading to rapid clinical improvement.8 , 9 However, the descriptive data were not compared with any reference groups. To address this gap in knowledge, we aimed to collect the largest experience worldwide of acute MR following MI treated with PMVR and compare the characteristics and outcomes of such patients with patients treated surgically or conservatively.
Methods
The International Registry of MitraClip in Acute Mitral Regurgitation following acute Myocardial Infarction (IREMMI) was established to assess the safety and outcomes of patients who underwent PMVR in an acute setting following MI. We approached all participating centres to review the data of patients who developed symptomatic post-MI MR.
Our current study cohort included patients who had at least symptomatic MR grade +3 within 90 days after acute MI (both ST-elevation and non-ST-elevation) between December 2009 and March 2020, in 21 centres in North America, Europe, and the Middle East, which had been managed by the surgical mitral intervention (repair or replacement, SMVR), PMVR with the MitraClip system, or without intervention (conservative group). As patients with papillary muscle rupture have different prognoses and are often treated with urgent surgical intervention, they were excluded from the analysis.
All participating centres are capable of performing both surgical and percutaneous mitral valve treatment based on multidisciplinary clinical team decisions. Medical care in intensive care units (intravenous diuretic, vasoactive medications, mechanical ventilation, and mechanical support devices) and decisions about treatment approaches were taken by a local multidisciplinary team and conducted based on clinical assessment.
All patients or their legal guardians provided written informed consent prior to the intervention. The study was conducted in accordance with the Declaration of Helsinki Ethical Principles and was approved by individual local ethics committees. Missing information in the dataset was resolved with the investigators after direct contact from registry personnel.
Echocardiographic evaluation
Routine echocardiographic exams were performed in all patients. The severity of MR, left ventricular ejection fraction (LVEF), systolic pulmonary artery pressure (sPAP), and mitral valve gradient was measured and graded according to the American Society of Echocardiography guidelines.10 , 11 The severity of MR was assessed by integrated multiparametric visual evaluation tools in accordance with standard clinical practice (incorporating 2D, spectral, and colour Doppler images), using an ordinal scale (grading 0 no MR, 1+ mild MR, 2+ moderate MR, 3+ moderate-to-severe MR, 4+ severe MR). Significant MR was considered as grades 3+ and 4+. Mitral regurgitation mechanism was thoroughly evaluated and classified as primary mechanical papillary muscle rupture or secondary MR.
Surgical mitral valve intervention
Mitral valve repair or replacement was performed under general anaesthesia in an operating theatre. Procedural success was defined as successful repair or replacement of the mitral valve with reduction of MR grade to ≤2+ and successful weaning from cardiopulmonary bypass.
Percutaneous mitral valve intervention
Percutaneous mitral valve intervention was performed using the MitraClip system. The procedure was performed under general anaesthesia, with fluoroscopy and transoesophageal echocardiography routinely used for guidance. The implantation procedure was performed as previously described.12 Immediate procedural success was defined as successful reduction of MR grade to ≤2+.
Outcomes
Procedural and clinical adverse events during follow-up were defined according to the Mitral Valve Academic Research Consortium (MVARC).13 The primary outcome of this study was in-hospital all-cause mortality. Secondary endpoints were immediate procedural success, 1-year mortality, echocardiographic (MR grade and sPAP), and clinical [New York Heart Association (NYHA) class] outcomes. Safety outcomes included procedural and peri-procedural complications. Major procedural complications were defined as MI, stroke, cardiac tamponade, reintervention for adverse events or failed procedure, renal failure requiring renal replacement therapy, septicaemia, or transfusion of two or more blood units after the procedure.
Clinical status was assessed using NYHA functional class.
Hospitalization duration was measured from index MI to discharge or in-hospital mortality.
Statistical analysis
Our main analyses compared conservative vs. intervention approaches and SMVR vs. PMVR. We repeated the analysis after excluding patients who died in-hospital and in subgroups according to patients’ median European System for Cardiac Operative Risk Evaluation (EUROSCORE II) to potentially generate additional insights.
Patient characteristics are reported according to variable properties. Categorical variables are reported as number (%), and differences between subgroups were tested using the χ2 test or Fisher’s exact test where appropriate. Continuous variables are reported according to their distribution. Those with a normal distribution are reported as mean (±standard deviation), and differences between subgroups were tested using Student’s t-test. Those without a normal distribution are reported as median [interquartile range (IQR)], and differences between subgroups were tested using Mann–Whitney U test. A P-value <0.05 was considered statistically significant.
Kaplan–Meier estimates were used to calculate survival curves, which were compared using the log-rank test. All clinical events were analysed by time to the first event for Kaplan–Meier analysis.
We performed a univariate and multivariable logistic regression method using backward elimination method (likelihood ratio was used as removal criteria) for the primary outcome, in-hospital mortality, using a myriad of clinically significant co-factors: age, gender, S/P MI, renal failure (grade 2+), anterior wall involvement, Killip class 3+, LVEF, and use of mechanical support device. The analysis was performed separately for conservative vs. intervention groups and SMVR vs. PMVR. We used a univariate Cox regression model for 1-year mortality using potential predictors; those found to be significant in the univariate model were then included in a subsequent multivariable model.
To further validate our findings, we performed a propensity score matching to create two groups with similar baseline characteristics. The first propensity score was calculated as the probability of a patient to underwent intervention and the second propensity score to underwent PMVR. Propensity score was calculated using logistic regression. Based on theoretical knowledge, age, gender, body mass index, hypertension, diabetes, dyslipidaemia, chronic obstructive pulmonary disease, prior stroke, chronic kidney disease—grade 2+, multivessel coronary artery disease, EuroSCORE II, ST-elevation MI presentation, Killip class >3, LVEF, cardiogenic shock, vasoactive medication, and use of any mechanical support device were included in the multivariable logistic regression. For matching SMVR vs. PMVR, the same set of parameters were used not including prior MI, prior coronary artery bypass grafting (CABG) and percutaneous coronary intervention in the balancing analysis due to inherent differences.
The matching ratios for the treatment groups were 1:1. After matching, both groups were confirmed to be similar in baseline characteristics using absolute standardized mean difference (SMD). Absolute SMD <0.1 was considered as neglect difference.
In-hospital mortality was compared between groups using McNemar’s test or Fisher’s exact test if not applicable. Stratified Cox regression was used to study the association between type of treatment and 1-year mortality. The IBM Statistical Package for the Social Sciences (SPSS) Statistics 26.0 (IBM Corp., Armonk, NY, USA) was utilized to perform the analyses.
Results
After excluding 46 patients who had papillary muscle rapture, the final study cohort included 471 patients with significant secondary MR shortly after MI. Two hundred and sixty-six patients were treated conservatively, and 205 underwent mitral valve interventions (SMVR or PMVR) (Figure 1). The median follow-up was 239 days (IQR 42–418).
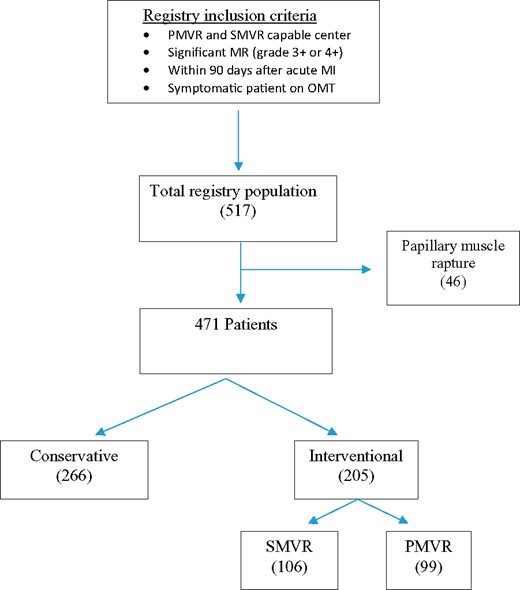
Study flow chart. MI, myocardial infarction; MR, mitral regurgitation; OMT, optimal medical therapy; PMVR, percutaneous mitral valve repair; SMVR, surgical mitral valve repair or replacement.
The mean age was 73 ± 11 years, 43% were female, 38% had diabetes mellitus, and 26% had previous MI. Overall, patients in the conservative management group were older compared with patients who underwent mitral valve intervention (75 ± 12 vs. 70 ± 10 years, P < 0.01). Patients in the intervention group had higher rates of multivessel coronary artery disease (77% vs. 65%, P = 0.02), previous MI (34% vs. 20%, P < 0.01), and were more likely to present with cardiogenic shock (41% vs. 30%, P = 0.01). Anterior wall involvement was not significantly different between the conservative and intervention groups (37% vs. 28%, P = 0.08). Ultimately, the surgical risk was higher in the intervention group [EUROSCORE II of 10% (IQR 5–19) vs. 8% (IQR 3–16), P < 0.01]. Baseline characteristics are presented in Table 1.
Baseline patient characteristics in the Conservative and Intervention groups (SMVR and PMVR)
| Variable . | Conservative group . | Intervention group . | P-value . | SMVR . | PMVR . | P-value . |
|---|---|---|---|---|---|---|
| (n = 266) . | (n = 205) . | (n = 106) . | (n = 99) . | |||
| Age, years | 75 ± 12 | 70 ± 10 | <0.01 | 68 ± 10 | 71 ± 10 | 0.03 |
| Female sex | 124 (47) | 79 (39) | 0.09 | 28 (26) | 51 (51) | <0.01 |
| BMI, kg/m2 | 27 ± 4 | 27 ± 5 | 0.98 | 27 ± 4 | 26 ± 5 | 0.33 |
| Hypertension | 197 (74) | 137 (67) | 0.10 | 67 (63) | 70 (71) | 0.30 |
| Diabetes | 97 (36) | 83 (40) | 0.39 | 36 (34) | 47 (47) | 0.06 |
| Dyslipidaemia | 122 (46) | 124 (60) | 0.02 | 59 (56) | 65 (66) | 0.16 |
| COPD | 17 (6) | 25 (12) | 0.03 | 9 (8) | 16 (16) | 0.13 |
| Prior stroke | 15 (6) | 18 (9) | 0.20 | 6 (6) | 12 (12) | 0.14 |
| CKD grade ≥2 | 82 (31) | 43 (24) | 0.03 | 13 (12) | 30 (30) | 0.01 |
| Multivessel CAD | 174 (65) | 158 (77) | 0.02 | 78 (74) | 80 (81) | 0.25 |
| Prior MI | 52 (20) | 70 (34) | <0.01 | 15 (14) | 55 (56) | <0.01 |
| Prior CABG | 19 (7) | 29 (12) | 0.01 | 1 (<1%) | 28 (27) | <0.01 |
| EuroSCORE II, % | 8 [3–16] | 10 [5–19] | <0.01 | 10 [5–16] | 10 [7–21] | 0.03 |
| STEMI presentation | 161 (61) | 116 (57) | 0.40 | 45 (42) | 71 (72) | <0.01 |
| Involved wall—anterior | 97 (37) | 58 (28) | 0.08 | 22 (21) | 35 (36) | 0.02 |
| PCI | 199 (75) | 131 (65) | 0.02 | 37 (35) | 94 (94) | <0.01 |
| Killip class ≥3 | 114 (43) | 123 (60) | <0.01 | 57 (54) | 66 (67) | 0.07 |
| MR grade 4+ | 124 (47) | 150 (73) | <0.01 | 70 (66) | 80 (81) | 0.02 |
| sPAP, mmHg | 40 ± 17 | 47 ± 19 | <0.01 | 40 ± 17 | 54 ± 19 | <0.01 |
| LVEF, % | 40 ± 12 | 40 ± 11 | 0.95 | 45 ± 10 | 35 ± 11 | <0.01 |
| Cardiogenic shock | 79 (30) | 84 (41) | 0.01 | 33 (31) | 51 (52) | <0.01 |
| Mechanical ventilation | 47 (18) | 65 (33) | <0.01 | 26 (25) | 39 (39) | 0.07 |
| Vasoactive medication | 86 (33) | 84 (43) | 0.05 | 45 (43) | 39 (39) | 0.39 |
| IABP | 35 (13) | 77 (38) | <0.01 | 44 (47) | 33 (33) | 0.25 |
| ECMO | 0 (0) | 5 (2) | 0 (0) | 5 (5) | ||
| MCS | 36 (14) | 78 (38) | <0.01 | 44 (42) | 34 (34) | 0.32 |
| Variable . | Conservative group . | Intervention group . | P-value . | SMVR . | PMVR . | P-value . |
|---|---|---|---|---|---|---|
| (n = 266) . | (n = 205) . | (n = 106) . | (n = 99) . | |||
| Age, years | 75 ± 12 | 70 ± 10 | <0.01 | 68 ± 10 | 71 ± 10 | 0.03 |
| Female sex | 124 (47) | 79 (39) | 0.09 | 28 (26) | 51 (51) | <0.01 |
| BMI, kg/m2 | 27 ± 4 | 27 ± 5 | 0.98 | 27 ± 4 | 26 ± 5 | 0.33 |
| Hypertension | 197 (74) | 137 (67) | 0.10 | 67 (63) | 70 (71) | 0.30 |
| Diabetes | 97 (36) | 83 (40) | 0.39 | 36 (34) | 47 (47) | 0.06 |
| Dyslipidaemia | 122 (46) | 124 (60) | 0.02 | 59 (56) | 65 (66) | 0.16 |
| COPD | 17 (6) | 25 (12) | 0.03 | 9 (8) | 16 (16) | 0.13 |
| Prior stroke | 15 (6) | 18 (9) | 0.20 | 6 (6) | 12 (12) | 0.14 |
| CKD grade ≥2 | 82 (31) | 43 (24) | 0.03 | 13 (12) | 30 (30) | 0.01 |
| Multivessel CAD | 174 (65) | 158 (77) | 0.02 | 78 (74) | 80 (81) | 0.25 |
| Prior MI | 52 (20) | 70 (34) | <0.01 | 15 (14) | 55 (56) | <0.01 |
| Prior CABG | 19 (7) | 29 (12) | 0.01 | 1 (<1%) | 28 (27) | <0.01 |
| EuroSCORE II, % | 8 [3–16] | 10 [5–19] | <0.01 | 10 [5–16] | 10 [7–21] | 0.03 |
| STEMI presentation | 161 (61) | 116 (57) | 0.40 | 45 (42) | 71 (72) | <0.01 |
| Involved wall—anterior | 97 (37) | 58 (28) | 0.08 | 22 (21) | 35 (36) | 0.02 |
| PCI | 199 (75) | 131 (65) | 0.02 | 37 (35) | 94 (94) | <0.01 |
| Killip class ≥3 | 114 (43) | 123 (60) | <0.01 | 57 (54) | 66 (67) | 0.07 |
| MR grade 4+ | 124 (47) | 150 (73) | <0.01 | 70 (66) | 80 (81) | 0.02 |
| sPAP, mmHg | 40 ± 17 | 47 ± 19 | <0.01 | 40 ± 17 | 54 ± 19 | <0.01 |
| LVEF, % | 40 ± 12 | 40 ± 11 | 0.95 | 45 ± 10 | 35 ± 11 | <0.01 |
| Cardiogenic shock | 79 (30) | 84 (41) | 0.01 | 33 (31) | 51 (52) | <0.01 |
| Mechanical ventilation | 47 (18) | 65 (33) | <0.01 | 26 (25) | 39 (39) | 0.07 |
| Vasoactive medication | 86 (33) | 84 (43) | 0.05 | 45 (43) | 39 (39) | 0.39 |
| IABP | 35 (13) | 77 (38) | <0.01 | 44 (47) | 33 (33) | 0.25 |
| ECMO | 0 (0) | 5 (2) | 0 (0) | 5 (5) | ||
| MCS | 36 (14) | 78 (38) | <0.01 | 44 (42) | 34 (34) | 0.32 |
Values are given as mean ± standard deviation, n (%), or median [interquartile range].
BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; EuroSCORE II, European System for Cardiac Operative Risk Evaluation; IABP, intra-aortic balloon pump; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MSD, mechanical circulatory support; PCI, percutaneous coronary intervention; PMVR, percutaneous mitral valve repair; sPAP, systolic pulmonary artery pressure; SMVR, surgical mitral valve repair or replacement; STEMI, ST-elevation myocardial infarction. Values in bold are statistically significant p-values.
Baseline patient characteristics in the Conservative and Intervention groups (SMVR and PMVR)
| Variable . | Conservative group . | Intervention group . | P-value . | SMVR . | PMVR . | P-value . |
|---|---|---|---|---|---|---|
| (n = 266) . | (n = 205) . | (n = 106) . | (n = 99) . | |||
| Age, years | 75 ± 12 | 70 ± 10 | <0.01 | 68 ± 10 | 71 ± 10 | 0.03 |
| Female sex | 124 (47) | 79 (39) | 0.09 | 28 (26) | 51 (51) | <0.01 |
| BMI, kg/m2 | 27 ± 4 | 27 ± 5 | 0.98 | 27 ± 4 | 26 ± 5 | 0.33 |
| Hypertension | 197 (74) | 137 (67) | 0.10 | 67 (63) | 70 (71) | 0.30 |
| Diabetes | 97 (36) | 83 (40) | 0.39 | 36 (34) | 47 (47) | 0.06 |
| Dyslipidaemia | 122 (46) | 124 (60) | 0.02 | 59 (56) | 65 (66) | 0.16 |
| COPD | 17 (6) | 25 (12) | 0.03 | 9 (8) | 16 (16) | 0.13 |
| Prior stroke | 15 (6) | 18 (9) | 0.20 | 6 (6) | 12 (12) | 0.14 |
| CKD grade ≥2 | 82 (31) | 43 (24) | 0.03 | 13 (12) | 30 (30) | 0.01 |
| Multivessel CAD | 174 (65) | 158 (77) | 0.02 | 78 (74) | 80 (81) | 0.25 |
| Prior MI | 52 (20) | 70 (34) | <0.01 | 15 (14) | 55 (56) | <0.01 |
| Prior CABG | 19 (7) | 29 (12) | 0.01 | 1 (<1%) | 28 (27) | <0.01 |
| EuroSCORE II, % | 8 [3–16] | 10 [5–19] | <0.01 | 10 [5–16] | 10 [7–21] | 0.03 |
| STEMI presentation | 161 (61) | 116 (57) | 0.40 | 45 (42) | 71 (72) | <0.01 |
| Involved wall—anterior | 97 (37) | 58 (28) | 0.08 | 22 (21) | 35 (36) | 0.02 |
| PCI | 199 (75) | 131 (65) | 0.02 | 37 (35) | 94 (94) | <0.01 |
| Killip class ≥3 | 114 (43) | 123 (60) | <0.01 | 57 (54) | 66 (67) | 0.07 |
| MR grade 4+ | 124 (47) | 150 (73) | <0.01 | 70 (66) | 80 (81) | 0.02 |
| sPAP, mmHg | 40 ± 17 | 47 ± 19 | <0.01 | 40 ± 17 | 54 ± 19 | <0.01 |
| LVEF, % | 40 ± 12 | 40 ± 11 | 0.95 | 45 ± 10 | 35 ± 11 | <0.01 |
| Cardiogenic shock | 79 (30) | 84 (41) | 0.01 | 33 (31) | 51 (52) | <0.01 |
| Mechanical ventilation | 47 (18) | 65 (33) | <0.01 | 26 (25) | 39 (39) | 0.07 |
| Vasoactive medication | 86 (33) | 84 (43) | 0.05 | 45 (43) | 39 (39) | 0.39 |
| IABP | 35 (13) | 77 (38) | <0.01 | 44 (47) | 33 (33) | 0.25 |
| ECMO | 0 (0) | 5 (2) | 0 (0) | 5 (5) | ||
| MCS | 36 (14) | 78 (38) | <0.01 | 44 (42) | 34 (34) | 0.32 |
| Variable . | Conservative group . | Intervention group . | P-value . | SMVR . | PMVR . | P-value . |
|---|---|---|---|---|---|---|
| (n = 266) . | (n = 205) . | (n = 106) . | (n = 99) . | |||
| Age, years | 75 ± 12 | 70 ± 10 | <0.01 | 68 ± 10 | 71 ± 10 | 0.03 |
| Female sex | 124 (47) | 79 (39) | 0.09 | 28 (26) | 51 (51) | <0.01 |
| BMI, kg/m2 | 27 ± 4 | 27 ± 5 | 0.98 | 27 ± 4 | 26 ± 5 | 0.33 |
| Hypertension | 197 (74) | 137 (67) | 0.10 | 67 (63) | 70 (71) | 0.30 |
| Diabetes | 97 (36) | 83 (40) | 0.39 | 36 (34) | 47 (47) | 0.06 |
| Dyslipidaemia | 122 (46) | 124 (60) | 0.02 | 59 (56) | 65 (66) | 0.16 |
| COPD | 17 (6) | 25 (12) | 0.03 | 9 (8) | 16 (16) | 0.13 |
| Prior stroke | 15 (6) | 18 (9) | 0.20 | 6 (6) | 12 (12) | 0.14 |
| CKD grade ≥2 | 82 (31) | 43 (24) | 0.03 | 13 (12) | 30 (30) | 0.01 |
| Multivessel CAD | 174 (65) | 158 (77) | 0.02 | 78 (74) | 80 (81) | 0.25 |
| Prior MI | 52 (20) | 70 (34) | <0.01 | 15 (14) | 55 (56) | <0.01 |
| Prior CABG | 19 (7) | 29 (12) | 0.01 | 1 (<1%) | 28 (27) | <0.01 |
| EuroSCORE II, % | 8 [3–16] | 10 [5–19] | <0.01 | 10 [5–16] | 10 [7–21] | 0.03 |
| STEMI presentation | 161 (61) | 116 (57) | 0.40 | 45 (42) | 71 (72) | <0.01 |
| Involved wall—anterior | 97 (37) | 58 (28) | 0.08 | 22 (21) | 35 (36) | 0.02 |
| PCI | 199 (75) | 131 (65) | 0.02 | 37 (35) | 94 (94) | <0.01 |
| Killip class ≥3 | 114 (43) | 123 (60) | <0.01 | 57 (54) | 66 (67) | 0.07 |
| MR grade 4+ | 124 (47) | 150 (73) | <0.01 | 70 (66) | 80 (81) | 0.02 |
| sPAP, mmHg | 40 ± 17 | 47 ± 19 | <0.01 | 40 ± 17 | 54 ± 19 | <0.01 |
| LVEF, % | 40 ± 12 | 40 ± 11 | 0.95 | 45 ± 10 | 35 ± 11 | <0.01 |
| Cardiogenic shock | 79 (30) | 84 (41) | 0.01 | 33 (31) | 51 (52) | <0.01 |
| Mechanical ventilation | 47 (18) | 65 (33) | <0.01 | 26 (25) | 39 (39) | 0.07 |
| Vasoactive medication | 86 (33) | 84 (43) | 0.05 | 45 (43) | 39 (39) | 0.39 |
| IABP | 35 (13) | 77 (38) | <0.01 | 44 (47) | 33 (33) | 0.25 |
| ECMO | 0 (0) | 5 (2) | 0 (0) | 5 (5) | ||
| MCS | 36 (14) | 78 (38) | <0.01 | 44 (42) | 34 (34) | 0.32 |
Values are given as mean ± standard deviation, n (%), or median [interquartile range].
BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; EuroSCORE II, European System for Cardiac Operative Risk Evaluation; IABP, intra-aortic balloon pump; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MSD, mechanical circulatory support; PCI, percutaneous coronary intervention; PMVR, percutaneous mitral valve repair; sPAP, systolic pulmonary artery pressure; SMVR, surgical mitral valve repair or replacement; STEMI, ST-elevation myocardial infarction. Values in bold are statistically significant p-values.
Intervention: surgical mitral valve repair or replacement and percutaneous mitral valve repair
A total of 106 patients were treated with SMVR and 99 were treated with PMVR. Patients treated with PMVR were older (71 ± 10 vs. 68 ± 10, P = 0.03), had a higher prevalence of previous cardiac events, history of prior MI (56% vs. 14%, P < 0.01), and prior CABG (27% vs. 1%, P < 0.01), compared with patients that underwent surgery. Furthermore, patients treated with PMVR were more likely to present in severe clinical condition; 52% of them had a cardiogenic shock, compared with 31% in the surgical group (P < 0.01), had a significantly lower LVEF (35% ± 11% vs. 45% ± 10%, P < 0.01) and higher anterior wall involvement (36% vs. 21%, P = 0.02) (Table 1). Of those treated surgically, 60 (57%) underwent mitral valve replacement, whilst the other 45 (43%) mitral valve repair. Patients who underwent surgical intervention also had CABG performed in 87 (82%) of cases, and 3 (3%) underwent combined procedures with other valvular interventions.
The time period between the index MI event and the mitral valve procedure was directly related to the type of intervention. Overall, although patients treated with PMVR were clinically worse, SMVR was performed earlier than PMVR [median of 12 days from MI date (IQR 5–19) vs. 19 days (10–40), P < 0.01] (Table 2). Immediate procedural success was high in mitral interventions, reaching 92% of patients (98 of 106) in SMVR and 93% (92 of 99) in PMVR (P = 0.53). However, peri-procedural major complication rates were significantly higher in the surgical group (34% vs. 6%, P < 0.01).
Procedural details and patient outcomes of surgical mitral valve repair or replacement and percutaneous mitral valve repair
| Variable . | SMVR . | PMVR . | P-value . |
|---|---|---|---|
| (n = 106) . | (n = 99) . | ||
| Procedure | |||
| Procedure time, min | 150 [118–240] | 90 [60–136] | <0.01 |
| MI to Procedure, days | 12 [5–19] | 19 [10–40] | <0.01 |
| MR >2 at discharge | 9 (8) | 8 (8) | 0.80 |
| Major complications | 36 (34) | 6 (6) | <0.01 |
| Outcomes | |||
| Procedure success | 98 (92) | 92 (93) | 0.53 |
| In-hospital mortality | 17 (16) | 6 (6) | 0.03 |
| Mortality at 3 months | 21 (20) | 10 (10) | 0.13 |
| Rehospitalization at 3 months | 6 (6) | 13 (13) | 0.14 |
| 1-year mortality | 32 (31) | 16 (17) | 0.04 |
| Variable . | SMVR . | PMVR . | P-value . |
|---|---|---|---|
| (n = 106) . | (n = 99) . | ||
| Procedure | |||
| Procedure time, min | 150 [118–240] | 90 [60–136] | <0.01 |
| MI to Procedure, days | 12 [5–19] | 19 [10–40] | <0.01 |
| MR >2 at discharge | 9 (8) | 8 (8) | 0.80 |
| Major complications | 36 (34) | 6 (6) | <0.01 |
| Outcomes | |||
| Procedure success | 98 (92) | 92 (93) | 0.53 |
| In-hospital mortality | 17 (16) | 6 (6) | 0.03 |
| Mortality at 3 months | 21 (20) | 10 (10) | 0.13 |
| Rehospitalization at 3 months | 6 (6) | 13 (13) | 0.14 |
| 1-year mortality | 32 (31) | 16 (17) | 0.04 |
Values are given as median [interquartile range], or n (%).
MI, myocardial Infarction; MR, mitral regurgitation; PMVR, percutaneous mitral valve repair; SMVR, surgical mitral valve repair or replacement. Values in bold are statistically significant p-values.
Procedural details and patient outcomes of surgical mitral valve repair or replacement and percutaneous mitral valve repair
| Variable . | SMVR . | PMVR . | P-value . |
|---|---|---|---|
| (n = 106) . | (n = 99) . | ||
| Procedure | |||
| Procedure time, min | 150 [118–240] | 90 [60–136] | <0.01 |
| MI to Procedure, days | 12 [5–19] | 19 [10–40] | <0.01 |
| MR >2 at discharge | 9 (8) | 8 (8) | 0.80 |
| Major complications | 36 (34) | 6 (6) | <0.01 |
| Outcomes | |||
| Procedure success | 98 (92) | 92 (93) | 0.53 |
| In-hospital mortality | 17 (16) | 6 (6) | 0.03 |
| Mortality at 3 months | 21 (20) | 10 (10) | 0.13 |
| Rehospitalization at 3 months | 6 (6) | 13 (13) | 0.14 |
| 1-year mortality | 32 (31) | 16 (17) | 0.04 |
| Variable . | SMVR . | PMVR . | P-value . |
|---|---|---|---|
| (n = 106) . | (n = 99) . | ||
| Procedure | |||
| Procedure time, min | 150 [118–240] | 90 [60–136] | <0.01 |
| MI to Procedure, days | 12 [5–19] | 19 [10–40] | <0.01 |
| MR >2 at discharge | 9 (8) | 8 (8) | 0.80 |
| Major complications | 36 (34) | 6 (6) | <0.01 |
| Outcomes | |||
| Procedure success | 98 (92) | 92 (93) | 0.53 |
| In-hospital mortality | 17 (16) | 6 (6) | 0.03 |
| Mortality at 3 months | 21 (20) | 10 (10) | 0.13 |
| Rehospitalization at 3 months | 6 (6) | 13 (13) | 0.14 |
| 1-year mortality | 32 (31) | 16 (17) | 0.04 |
Values are given as median [interquartile range], or n (%).
MI, myocardial Infarction; MR, mitral regurgitation; PMVR, percutaneous mitral valve repair; SMVR, surgical mitral valve repair or replacement. Values in bold are statistically significant p-values.
Procedural outcomes
In both intervention groups, when compared with baseline, echocardiographic evaluation showed a marked reduction in MR at discharge (Figure 2A). Compared with baseline, NYHA functional class improved significantly at discharge (Figure 2B) in both intervention groups. Systolic PAP was significantly reduced after PMVR (54 ± 20 to 43 ± 20 mmHg, P < 0.01) and non-significantly reduced after SMVR (39 ± 15 to 35 ± 14 mmHg, P = 0.14) (Figure 2C).
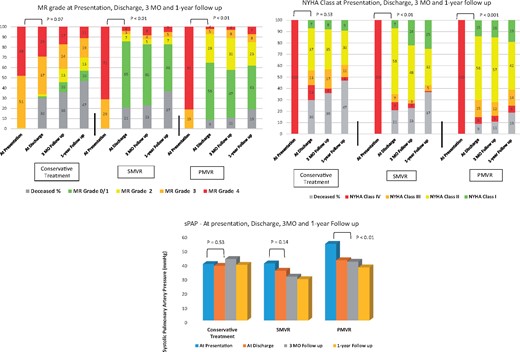
Clinical and echocardiographic evaluation in the study groups at presentation, discharge, 3-month (MO) follow-up and 1-year follow-up. (A) Mitral regurgitation (MR) was significantly reduced from presentation to discharge in the intervention groups (surgical mitral valve repair or replacement and percutaneous mitral valve repair) but not in the conservative group. (B) New York Heart Association (NYHA) class was significantly improved from presentation to discharge in the intervention groups (surgical mitral valve repair or replacement and percutaneous mitral valve repair) but not in the conservative group. (C) Systolic pulmonary artery pressure (sPAP) was significantly reduced from presentation to discharge in percutaneously treated patients. PMVR, percutaneous mitral valve repair; SMVR, surgical mitral valve repair or replacement.
Mortality analysis—conservative vs. intervention
In-hospital mortality was highest among patients treated conservatively, reaching 27%, compared with 11% following mitral valve intervention (P < 0.01). Survival curves for mortality are shown in Figure 3A and predictors for in-hospital mortality are reported in Table 3.
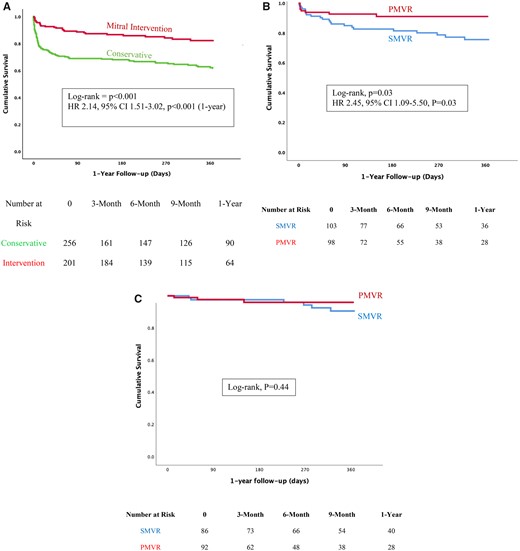
Kaplan–Meier curves for survival in the study groups. (A) Conservative treatment was associated with a higher mortality rate compared with the interventional treatment. (B) Surgical mitral valve repair or replacement was associated with a higher mortality rate compared with percutaneous mitral valve repair. (C) No significant survival difference was observed between surgical mitral valve repair or replacement and percutaneous mitral valve repair after excluding patients who died in hospital. CI, confidence interval; HR, hazard ratio; PMVR, percutaneous mitral valve repair; SMVR, surgical mitral valve repair or replacement.
| . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| Variable . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Age | 1.04 (1.03–1.06) | <0.01 | 1.05 (1.02–1.07) | <0.01 |
| Sex | 1.18 (0.83–1.69) | 0.35 | ||
| Hypertension | 1.62 (1.05–2.50) | 0.03 | 1.09 (0.69–1.73) | 0.70 |
| Diabetes mellitus | 1.52 (1.07–2.17) | 0.02 | 1.33 (0.91–1.96) | 0.15 |
| Dyslipidaemia | 0.94 (0.66–1.34) | 0.94 | ||
| Prior stroke | 1.01 (0.51–1.99) | 0.97 | ||
| Prior MI | 1.26 (0.82–1.91) | 0.29 | ||
| CKD >2 | 1.63 (1.13–2.35) | 0.01 | 1.03 (0.68–1.57) | 0.88 |
| EuroSCORE II | 1.04 (1.03–1.05) | <0.01 | 1.05 (1.03–1.05) | <0.01 |
| STEMI presentation | 1.08 (0.60–1.96) | 0.80 | ||
| Involved vall—anterior | 1.58 (1.09–2.30) | 0.06 | 1.03 (0.69–1.53) | 0.90 |
| Killip class ≥3 | 2.24 (1.54–3.25) | <0.01 | 2.31 (1.42–3.74) | <0.01 |
| Multivessel CAD | 1.17 (0.80–1.71) | 0.41 | ||
| PCI | 0.75 (0.51–1.11) | 0.15 | ||
| LVEF | 0.97 (0.96–0.99) | <0.01 | 0.974 (0.98–1.01) | 0.71 |
| Mechanical ventilation | 2.27 (1.56–3.31) | <0.01 | Represented in Killip | |
| Cardiogenic shock | 3.04 (2.13–4.35) | <0.01 | Represented in Killip | |
| Any MCS | 1.38 (0.93–2.04) | 0.11 | 2.12 (1.28–3.50) | <0.01 |
| Any intervention | 0.37 (0.25–0.56) | <0.01 | 0.26 (0.17–0.45) | <0.01 |
| . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| Variable . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Age | 1.04 (1.03–1.06) | <0.01 | 1.05 (1.02–1.07) | <0.01 |
| Sex | 1.18 (0.83–1.69) | 0.35 | ||
| Hypertension | 1.62 (1.05–2.50) | 0.03 | 1.09 (0.69–1.73) | 0.70 |
| Diabetes mellitus | 1.52 (1.07–2.17) | 0.02 | 1.33 (0.91–1.96) | 0.15 |
| Dyslipidaemia | 0.94 (0.66–1.34) | 0.94 | ||
| Prior stroke | 1.01 (0.51–1.99) | 0.97 | ||
| Prior MI | 1.26 (0.82–1.91) | 0.29 | ||
| CKD >2 | 1.63 (1.13–2.35) | 0.01 | 1.03 (0.68–1.57) | 0.88 |
| EuroSCORE II | 1.04 (1.03–1.05) | <0.01 | 1.05 (1.03–1.05) | <0.01 |
| STEMI presentation | 1.08 (0.60–1.96) | 0.80 | ||
| Involved vall—anterior | 1.58 (1.09–2.30) | 0.06 | 1.03 (0.69–1.53) | 0.90 |
| Killip class ≥3 | 2.24 (1.54–3.25) | <0.01 | 2.31 (1.42–3.74) | <0.01 |
| Multivessel CAD | 1.17 (0.80–1.71) | 0.41 | ||
| PCI | 0.75 (0.51–1.11) | 0.15 | ||
| LVEF | 0.97 (0.96–0.99) | <0.01 | 0.974 (0.98–1.01) | 0.71 |
| Mechanical ventilation | 2.27 (1.56–3.31) | <0.01 | Represented in Killip | |
| Cardiogenic shock | 3.04 (2.13–4.35) | <0.01 | Represented in Killip | |
| Any MCS | 1.38 (0.93–2.04) | 0.11 | 2.12 (1.28–3.50) | <0.01 |
| Any intervention | 0.37 (0.25–0.56) | <0.01 | 0.26 (0.17–0.45) | <0.01 |
CAD, coronary artery disease; CKD, chronic kidney disease; EuroSCORE II, European System for Cardiac Operative Risk Evaluation; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MCS, mechanical circulatory support; PCI, percutaneous coronary intervention; PMVR, percutaneous mitral valve repair; SMVR, surgical mitral valve repair or replacement; STEMI, ST-elevation myocardial infarction. Values in bold are statistically significant p-values.
| . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| Variable . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Age | 1.04 (1.03–1.06) | <0.01 | 1.05 (1.02–1.07) | <0.01 |
| Sex | 1.18 (0.83–1.69) | 0.35 | ||
| Hypertension | 1.62 (1.05–2.50) | 0.03 | 1.09 (0.69–1.73) | 0.70 |
| Diabetes mellitus | 1.52 (1.07–2.17) | 0.02 | 1.33 (0.91–1.96) | 0.15 |
| Dyslipidaemia | 0.94 (0.66–1.34) | 0.94 | ||
| Prior stroke | 1.01 (0.51–1.99) | 0.97 | ||
| Prior MI | 1.26 (0.82–1.91) | 0.29 | ||
| CKD >2 | 1.63 (1.13–2.35) | 0.01 | 1.03 (0.68–1.57) | 0.88 |
| EuroSCORE II | 1.04 (1.03–1.05) | <0.01 | 1.05 (1.03–1.05) | <0.01 |
| STEMI presentation | 1.08 (0.60–1.96) | 0.80 | ||
| Involved vall—anterior | 1.58 (1.09–2.30) | 0.06 | 1.03 (0.69–1.53) | 0.90 |
| Killip class ≥3 | 2.24 (1.54–3.25) | <0.01 | 2.31 (1.42–3.74) | <0.01 |
| Multivessel CAD | 1.17 (0.80–1.71) | 0.41 | ||
| PCI | 0.75 (0.51–1.11) | 0.15 | ||
| LVEF | 0.97 (0.96–0.99) | <0.01 | 0.974 (0.98–1.01) | 0.71 |
| Mechanical ventilation | 2.27 (1.56–3.31) | <0.01 | Represented in Killip | |
| Cardiogenic shock | 3.04 (2.13–4.35) | <0.01 | Represented in Killip | |
| Any MCS | 1.38 (0.93–2.04) | 0.11 | 2.12 (1.28–3.50) | <0.01 |
| Any intervention | 0.37 (0.25–0.56) | <0.01 | 0.26 (0.17–0.45) | <0.01 |
| . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| Variable . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Age | 1.04 (1.03–1.06) | <0.01 | 1.05 (1.02–1.07) | <0.01 |
| Sex | 1.18 (0.83–1.69) | 0.35 | ||
| Hypertension | 1.62 (1.05–2.50) | 0.03 | 1.09 (0.69–1.73) | 0.70 |
| Diabetes mellitus | 1.52 (1.07–2.17) | 0.02 | 1.33 (0.91–1.96) | 0.15 |
| Dyslipidaemia | 0.94 (0.66–1.34) | 0.94 | ||
| Prior stroke | 1.01 (0.51–1.99) | 0.97 | ||
| Prior MI | 1.26 (0.82–1.91) | 0.29 | ||
| CKD >2 | 1.63 (1.13–2.35) | 0.01 | 1.03 (0.68–1.57) | 0.88 |
| EuroSCORE II | 1.04 (1.03–1.05) | <0.01 | 1.05 (1.03–1.05) | <0.01 |
| STEMI presentation | 1.08 (0.60–1.96) | 0.80 | ||
| Involved vall—anterior | 1.58 (1.09–2.30) | 0.06 | 1.03 (0.69–1.53) | 0.90 |
| Killip class ≥3 | 2.24 (1.54–3.25) | <0.01 | 2.31 (1.42–3.74) | <0.01 |
| Multivessel CAD | 1.17 (0.80–1.71) | 0.41 | ||
| PCI | 0.75 (0.51–1.11) | 0.15 | ||
| LVEF | 0.97 (0.96–0.99) | <0.01 | 0.974 (0.98–1.01) | 0.71 |
| Mechanical ventilation | 2.27 (1.56–3.31) | <0.01 | Represented in Killip | |
| Cardiogenic shock | 3.04 (2.13–4.35) | <0.01 | Represented in Killip | |
| Any MCS | 1.38 (0.93–2.04) | 0.11 | 2.12 (1.28–3.50) | <0.01 |
| Any intervention | 0.37 (0.25–0.56) | <0.01 | 0.26 (0.17–0.45) | <0.01 |
CAD, coronary artery disease; CKD, chronic kidney disease; EuroSCORE II, European System for Cardiac Operative Risk Evaluation; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MCS, mechanical circulatory support; PCI, percutaneous coronary intervention; PMVR, percutaneous mitral valve repair; SMVR, surgical mitral valve repair or replacement; STEMI, ST-elevation myocardial infarction. Values in bold are statistically significant p-values.
As shown in Supplementary material online, Table S1, age, EUROSCORE II, anterior wall involvement, Killip class ≥3, LVEF, cardiogenic shock, mechanical ventilation, use of mechanical circulatory support (MCS), and mitral intervention were all predictors of in-hospital mortality. Following adjustment for age, Killip class ≥3, and use of MCS, conservative treatment was associated with an increased risk for in-hospital mortality (odds ratio 4.52, 95% CI 2.38–8.60, P < 0.01).
In Cox regression analysis, following adjustment for age, Killip class ≥3, LVEF, and use of MCS, conservative treatment was associated with increased risk for mortality at 1-year follow-up (crude HR 2.14, 95% CI 1.51–3.02, P < 0.001; adjusted HR 3.53, 95% CI 2.18–5.73, P < 0.01).
Mortality analysis—surgical mitral valve repair or replacement vs. percutaneous mitral valve repair
In-hospital mortality was significantly higher in patients following SMVR when compared with patients treated with PMVR (16% vs. 6%, P < 0.01) (Table 2).
As shown in Supplementary material online, Table S2, age, EUROSCORE II, cardiogenic shock, and use of vasoactive medication and type of intervention were all predictors of mortality.
The difference in mortality between groups was also consistent at 1-year follow-up (31% vs. 17%, P = 0.04). Kaplan–Meier curves are shown in Figure 3B. In Cox regression analysis, SMVR was associated with a higher risk of mortality compared with PMVR (crude HR 2.45, 95% CI 1.09–5.50, P = 0.03; adjusted HR 3.75, 95% CI 1.55–9.07, P < 0.01).
When patients who died in-hospital were excluded from the analysis, there was no significant difference in 1-year mortality between SMVR and PMVR (log-rank, P = 0.44) (Figure 3C).
The median EUROSCORE II of patients who underwent mitral intervention was 10% (IQR 5–19). The mortality rates according to EUROSCORE II are presented in Figure 4. The survival benefit of PMVR over SMVR was observed in the low EUROSCORE II group (P = 0.04) and in the high EUROSCORE II groups (P = 0.07). Patients with EURSCORE II above than 10% in the SMVR group had the worst outcomes with extremely high in-hospital mortality reaching 23% and 1-year mortality reaching 38%.
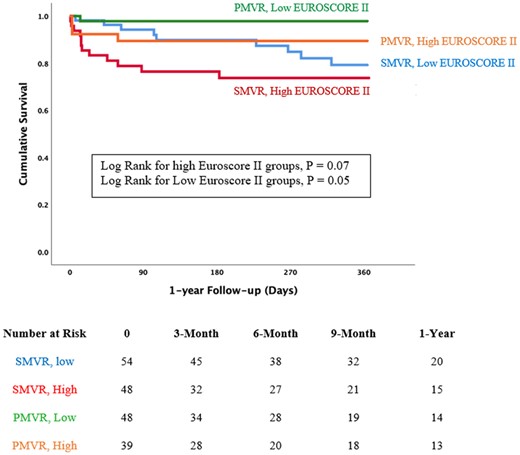
Kaplan–Meier curve comparing 1-year survival of surgical mitral valve repair or replacement and percutaneous mitral valve repair according to the median EUROSCORE II. The survival benefit of percutaneous mitral valve repair over surgical mitral valve repair or replacement was observed in the low EUROSCORE II group (P = 0.04) and in the high EUROSCORE II groups (P = 0.07). EUROSCORE II, European System for Cardiac Operative Risk Evaluation; PMVR, percutaneous mitral valve repair; SMVR, surgical mitral valve repair or replacement
Propensity score matching
Matching on estimated propensity score made available a matched cohort of 113 patients for each of the intervention and conservative groups, and 38 for each of the PMVR and SMVR groups with similar demographic, clinical, and angiographic clinical profiles (Supplementary material online, Tables S3 and S4). The in-hospital mortality was higher in the conservative group (35% vs. 12%, P < 0.01) and conservative treatment was associated with increased mortality risk (HR 2.63, 95% CI 1.48–4.67, P < 0.01).
In propensity-matched patients, in the PMVR group, the rate of in-hospital mortality was lower compared with the SMVR group (0% vs. 18%, P = 0.04).
Discussion
This current analysis is the largest and most comprehensive evaluation of different therapeutic strategies in patients with acute MI complicated by severe MR. To the best of our knowledge, this is the first and only analysis that compares outcomes of PMVR with other clinical strategies in this acute and complex setting. Patients who were treated conservatively had the worst prognosis. Mitral valve interventions, both percutaneous and surgical, had high success rates and were associated with better survival outcomes than conservative therapy. Nevertheless, patients who underwent SMVR had high perioperative mortality, and more than 15% died during hospitalization. Our findings suggest that PMVR can serve as an alternative for surgery in reducing MR for high-risk patients with significant post-MI MR (Graphical abstract).
Pathogenesis and epidemiology of post-myocardial infarction mitral regurgitation
Ischaemic MR complicating acute MI can be caused by a mechanical complication of papillary muscle rupture or by secondary mechanism due to global or regional LV remodelling.14 The prevalence of this condition varies between different studies and peaks at 50% of all MI patients in some case series, while more than 10% of all ST-elevation MI patients develop significant MR (defined as moderate to severe or severe).15 Mitral regurgitation causes volume overload and increased ventricular wall stress, causing further LV dilatation and worsening MR.14 Mitral regurgitation on presentation or persistent ischaemic MR is known to be a negative prognostic factor, associated with poor short- and long-term prognosis.16
In our cohort, in-hospital and 1-year mortality rates were 20% and 36%, respectively, among patients with severe MR who were conservatively treated. These mortality rates are consistent with current literature. The mortality of MI patients according to MR level was described by Tcheng et al.6 The 30-day mortality rates of patients with moderate-to-severe MR and severe MR were 19.8% and 26.1%, respectively, and at 1 year almost 40% in both groups died. While several studies pointed to the fact that post-MI MR is an independent risk factor for reduced long-term survival, the management of this condition is undetermined, and no studies have compared medical therapy to SMVR or any other intervention early after MI.17 Furthermore, the effects of MR correction combined with CABG are not yet defined and the choice of the mitral valve procedure is still debated. The Randomized Ischaemic Mitral Evaluation (RIME) trial showed that adding mitral annuloplasty to CABG in patients with ischaemic MR improved functional capacity and promoted reverse remodelling.18 However, large-scale trials and meta-analysis suggested that the addition of mitral valve repair to CABG did not result in reverse remodelling or clinical benefits.19 , 20 In all of these studies, SMVR was performed in relatively stable patients and not in an acute setting.
Management of post-myocardial infarction acute mitral regurgitation
Besides surgical intervention, current guidelines recommend intravenous diuretic, vasodilator, inotropic support, or MCS in order to stabilize patients with post-MI acute MR.7 However, SMVR in an acute setting is often high risk. Our data suggest that short-term mortality after SMVR is high. These findings also correlate with previous observations. Lorusso et al.21 evaluated the outcomes of 279 patients who underwent emergency surgery for acute severe MR and found that the overall 30-day mortality was 22.5% and that recent MI was a predictor for mortality. In the SHOCK (SHould We Emergently Revascularize Occluded Coronaries for Cardiogenic ShocK?) trial registry, most patients with post-MI cardiogenic shock and severe MR were treated conservatively. Patients treated medically were often deemed too sick to be operated on and had an in-hospital mortality rate of 55%. Among patients who underwent SMVR, the in-hospital mortality rate was 39%.22 Similar findings were reported by Chevalier et al. 23
Surgical mitral valve repair or replacement vs. percutaneous mitral valve repair
The high surgical mortality in this population could be attributed to patients’ haemodynamic instability, complications of cardiopulmonary bypass, recent MI, and potent antiplatelet therapy. Importantly, our findings suggest that patients who survive the acute phase and the early postoperative period have relatively good long-term outcomes. In fact, after excluding in-hospital mortality, the survival rates of PMVR and SMVR were similar. The in-hospital survival benefit of PMVR suggests that it can serve as a salvage therapy for patients that are at high risk for surgery allowing recovery in an acute setting.
When compared with SMVR, patients who underwent PMVR were older, had more comorbidities and prior cardiac conditions. For example, 27% of patients who underwent PMVR had a prior history for CABG compared with <1% in the surgical group. In addition, 67% of patients who underwent PMVR presented with Killip class ≥3, 39% required mechanical ventilation and all patients remained symptomatic despite optimal medical therapy.
Although patients treated with PMVR had more comorbidities and more of them were in cardiogenic shock, the timing between index MI and PMVR was delayed when compared with patients who had SMVR. This finding may be attributed to several factors. First, PMVR in an acute setting is still not common practice, and the availability for this therapy, especially in an emergency context, is limited when compared with surgical intervention. Therefore, in many cases, PMVR was performed late in the course of the disease and only after other mechanical and medical therapy failed; it was also offered to patients that were excluded from surgery. Second, although patients in the PMVR group had higher risk scores, it is possible that other clinical unmeasured confounders led to earlier intervention in the surgical intervention.
Patients with a high EUROSCORE II who underwent surgery had high in-hospital mortality of almost 25% and therefore might be considered for PMVR strategy. Our findings may imply that intervention should not be delayed, especially considering the high safety profile of this approach and that PMVR does not preclude a future surgical intervention in case of device failure.
Limitations
Several potential limitations of this study merit consideration. First, all participating centres were requested to include all the patients who were eligible according to the inclusion criteria. Although the case number is high, the included procedures are spread over 21 centres, a long period of time and by the nature of this multicentre retrospective analysis, it is possible that not all patients were enrolled. Second, despite multivariable adjustment and matching, the associations between baseline characteristics and outcomes may be confounded by unmeasured variables. In addition, patient allocation to each therapy may be biased due to clinical factors that affected physician decision-making. Third, laboratory data, medications, detailed and Corelab echocardiographic evaluation is lacking, and therefore, the effect on LV remodelling has not been evaluated. However, the study was designed for evaluating the impact of intervention on survival in high-risk populations as a proof-of-concept study. Fourth, PMVR procedures were performed at highly experienced centres and it should be acknowledged that PMVR early after MI might be technically challenging due to valve complexity, a small left atrium, and the clinical condition of patients. Fifth, in all our cases, PMVR was performed as a salvage procedure in critically ill patients. Therefore, the findings cannot be generalized for mildly symptomatic patients who develop ischaemic MR after MI.
Finally, our analysis should be considered hypothesis generating. Therefore, randomized trials are required to validate our findings and determine the optimal treatment for post-MI acute MR.
Conclusions
Post-MI severe MR is associated with poor outcomes. Patients were often in extreme clinical condition but interventions, both surgical and percutaneous, resulted in favourable outcomes. Percutaneous mitral valve repair was successful in decreasing MR and improved haemodynamic parameters and should be considered as an alternative for patients that are deemed high risk for surgical intervention.
Supplementary material
Supplementary material is available at European Heart Journal online.
Acknowledgements
Dr Mony Shuvy would like to thank Prof. Chaim Lotan for his great vision, support, and guidance.
Funding
The authors report no specific funding related to this article.
Conflict of interest: D.H. is a consultant for Whiteswell and Abbott CEC member. R.E.-L. receives speaker fee for Abbott, Boston Scientific, and Edwards Lifescience. D.A. receives speaker fee from Abbott, Boston Scientific, and Edwards Lifescience. P.D. is a consultant for InnovHeart and receives speaker fee from Abbott and Edwards Lifescience. X.F. is a consultant and proctor for Abbott. K.S. is a consultant for Abbott and Edwards Lifesciences. A.C. received a speaker fee from Abbott. M.G. is a consultant for Medtronic. L.N.-F. has served as a proctor of Abbott Vascular and received speakers’ honoraria or consulting fee from Edwards Lifescience, Boston Scientific, and Abbott Vascular. N.F. is a consultant for Abbott Vascular and Edwards Lifesciences and received speakers’ honoraria from Abbott. F.P. receives travel expenses from Edwards Lifescience, Abbott Vascular, and Polares Medical. M.C.sevred as a proctor for Abbott Vascular and Edwards Lifesciences.
I.P has sevred as a proctor of Abbott Vacular. R.B. serve as a consultant and proctor for Abbott and Mitralix. L.P. is an Abbott CEC member and holds stock or stock options of Vectorious Medical Technologies and Powerful Medical. F.M. has served as a consultant for Abbott Vascular, Edwards Lifesciences, Cardiovalve, Xeltis, Occufit, Simulands, and Medtronic. F.M. holds stock or stock options of Cardiogard, Cardiovalve, Magenta, SwissVortex, Transseptalsolutions, 4Tech, and Perifect. M.T. is a consultant for Abbott, Edwards Lifescience, Boston Scientific, 4Tech, Shenqi Medical, Simulands, MTEx, and Occlufit and receives speaker fees from Edwards Lifescience, Mitraltech. M.S. is a proctor and consultant for Abbott and Edwards Lifesciences. All other authors have reported that they have no relationship relevant to the contents of this paper to disclose.
References
Author notes
Dan Haberman, Rodrigo Estévez-Loureiro contributed equally to this study.



