-
PDF
- Split View
-
Views
-
Cite
Cite
Karina Gasbarrino, Diana Di Iorio, Stella S Daskalopoulou, Importance of sex and gender in ischaemic stroke and carotid atherosclerotic disease, European Heart Journal, Volume 43, Issue 6, 7 February 2022, Pages 460–473, https://doi.org/10.1093/eurheartj/ehab756
Close - Share Icon Share
Abstract
Stroke is a leading cause of death and disability worldwide. Women are disproportionately affected by stroke, exhibiting higher mortality and disability rates post-stroke than men. Clinical stroke research has historically included mostly men and studies were not properly designed to perform sex- and gender-based analyses, leading to under-appreciation of differences between men and women in stroke presentation, outcomes, and response to treatment. Reasons for these differences are likely multifactorial; some are due to gender-related factors (i.e. decreased social support, lack of stroke awareness), yet others result from biological differences between sexes. Unlike men, women often present with ‘atypical’ stroke symptoms. Lack of awareness of ‘atypical’ presentation has led to delays in hospital arrival, diagnosis, and treatment of women. Differences also extend to carotid atherosclerotic disease, a cause of stroke, where plaques isolated from women are undeniably different in morphology/composition compared to men. As a result, women may require different treatment than men, as evidenced by the fact that they derive less benefit from carotid revascularization than men but more benefit from medical management. Despite this, women are less likely than men to receive medical therapy for cardiovascular risk factor management. This review focuses on the importance of sex and gender in ischaemic stroke and carotid atherosclerotic disease, summarizing the current evidence with respect to (i) stroke incidence, mortality, awareness, and outcomes, (ii) carotid plaque prevalence, morphology and composition, and gene connectivity, (iii) the role of sex hormones and sex chromosomes in atherosclerosis and ischaemic stroke risk, and (iv) carotid disease management.
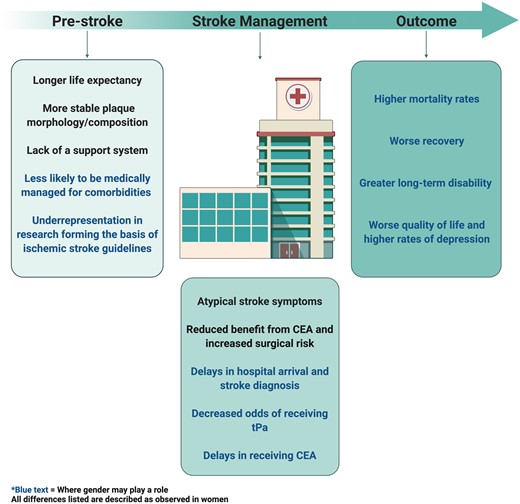
Summary of differences between women and men at the pre-stroke, stroke management, and outcome level. All differences listed are described as observed in women. Blue text indicates where gender may play a role. CEA, carotid endarterectomy; tPA, tissue plasminogen activator. Created with BioRender.com.
Introduction
Stroke is among the leading causes of death and disability worldwide, killing ∼6 million people annually and leaving another 5 million permanently disabled.1 Although men have a higher incidence of stroke than women, they have a lower rank of death due to stroke compared to women.2 In the USA, stroke is considered the fifth leading cause of death for men, but the third for women.3 Women also have worse recovery and higher disability rates than men post-stroke; women face more challenges during their recovery due to activity limitations and lower overall levels of mental and physical health.2 , 4 Decades of stroke research and clinical trials have consistently under-represented women, particularly older women who face the greatest cardiovascular disease (CVD) burden. This limitation in research is reflected in the current guidelines for stroke prevention and carotid disease management that are sex agnostic. Luckily, the scientific and medical communities have come to realize that this ‘one-size fits all’ approach has had a significant negative impact on women’s health, leading to women being ‘under-diagnosed, under-treated, and under-aware of their risks’.5
The majority of strokes are caused by cerebral ischaemia, accounting for 87% of all stroke cases.6 A major cause of ischaemic stroke is the rupture of atherosclerotic plaques and subsequent thrombus formation that occurs in the carotid artery. This review will focus on the importance of sex and gender in ischaemic stroke and carotid atherosclerotic disease to encourage change in research and clinical guidelines. Specifically, it will summarize the current evidence and knowledge gaps with respect to (i) stroke incidence, mortality, awareness, and outcomes, (ii) carotid atherosclerotic plaque prevalence, morphology and composition, and gene connectivity, (iii) the role of sex hormones and sex chromosomes in atherosclerosis and ischaemic stroke risk, and (iv) carotid disease management (Graphical Abstract). For the purposes of this review, biological sex considers chromosome/gene expression, hormone profiles, and reproductive anatomy, while gender refers to socially and culturally constructed roles, behaviours, and identities that influence self-perception and self-expression.
Stroke incidence, mortality, awareness, and outcomes
Sex differences in stroke incidence and mortality
Age is a powerful risk factor for stroke incidence and mortality. Within most age strata, men have a higher lifetime risk of ischaemic stroke than women.7 , 8 However, after the age of 85, significantly more women suffer from ischaemic strokes compared to men,7 and this is often attributed to their longer life expectancy. Studies on ischaemic subtypes have consistently shown that atrial fibrillation-related cardioembolic strokes are more common among women, notably in the elderly population, while large and small vessel strokes are more common in men.9 , 10 In the USA, ∼58% of stroke-related deaths in 2017 occurred in women (84 738 deaths in women, 61 645 deaths in men), where a large majority occurred particularly in the ≥85 age group.11
The influence of sex and gender on stroke awareness
Stroke awareness is a critical factor in predicting stroke survival. The faster the signs of stroke are recognized, the earlier treatment is administered and the greater the chances of good recovery. Few studies have assessed differences in knowledge of stroke signs and symptoms between men and women. Most of this limited evidence has identified women as being more likely to recognize traditional warning signs of stroke compared to men.12 , 13 Particularly, one survey of >130 000 US residents reported female gender to be associated with better knowledge of traditional stroke warning signs than male gender, suggesting the need for gender-targeted stroke education.12 These disparities in stroke symptom awareness may be partly explained by differences in patterns of health information behaviour across genders. Female gender has been associated with increased interest in obtaining health information and increased health-seeking behaviour, while male gender has been associated with decreased engagement, attentiveness, and receptiveness to health-related information.14 , 15
Unfortunately, even if women are able to recognize traditional warning signs of stroke, many studies have reported that female sex is associated with ‘atypical’ stroke symptoms (change in mental status, pain, general weakness or fatigue, headache, and other unclassifiable symptoms) compared to male sex, although the differences reported were rather small.16–18 A study performed by the American Heart Association demonstrated that a low proportion of women in the US associated ‘atypical’ stroke symptoms with warning signs of an ischaemic event [severe headache (23%), unexplained dizziness (20%), loss of vision (18%)].19 In many cases, the presence of atypical symptoms is often associated with missed diagnosis of stroke20–23 (i.e. more likely to receive a diagnosis of stroke mimic).
The influence of sex and gender on acute tissue plasminogen activator intervention
The failure to recognize an ischaemic stroke inevitably leads to missed opportunity for acute intervention. During a stroke, up to 2.03 million neurons die per minute.24 Therefore, the earlier blood flow is restored, the greater the likelihood of a good outcome. Tissue plasminogen activator (tPA) is the only Food and Drug Administration-approved therapy for acute ischaemic stroke. Unfortunately, this agent has an extremely short therapeutic window (3–4.5 h after symptom onset), and even the shortest delays in hospital arrival can make patients ineligible to receive tPA.
Differences in tPA administration have been reported between the sexes, with men receiving treatment more often than women.25–28 According to a meta-analysis (n = 16 studies) that reported sex-specific rates of tPA administration for acute ischaemic stroke, the odds of receiving tPA were 30% lower in women compared to men [odds ratio (OR), 0.70; 95% confidence interval (CI), 0.55–0.88].25 This association remained significant even after excluding, in a sensitivity analysis, the study that was the primary source of significant between-study variation (OR, 0.75; 95% CI, 0.69–0.82).25 The most common tPA exclusion criterion is delayed hospital arrival, and the lower proportion of women receiving tPA than men was partly attributed to their later arrival.28 Specifically, fewer women than men presented themselves at the hospital within 4 h from ischaemic stroke onset (27% vs. 33%, OR, 0.80; 95% CI, 0.70–0.90); a ∼27-min longer ‘symptom onset-to-door’ time was noted in women compared to men.28 Yet, another cohort study demonstrated women to still be independently (although weakly) associated with failure to receive tPA treatment (OR, 1.08; 95% CI, 1.02–1.15; P = 0.008), despite arriving within the therapeutic window of tPA.27
Reported delays among women vs. men in receiving tPA treatment may be associated with both sex- and gender-related factors. As mentioned above, women are more prone to present with atypical symptomatology than men and are more likely to be older at the time of stroke presentation, both contributing to delays in hospital arrival and delays in diagnosis. Gender differences in caregiver roles and social support are also important to take into consideration; it is commonly believed that women are more likely to down-play their symptoms when they first appear, continue with their usual responsibilities, and delay seeking medical help.4
The influence of sex and gender on stroke outcomes
Since women tend to have strokes at a much older age compared to men, recovery post-stroke is more challenging. In a pooled analysis of 19 652 patients from 5 acute stroke randomized controlled trials (RCTs), women were observed to have greater disability (modified Rankin Scale score 3–6) (OR, 1.20; 95% CI, 1.06–1.36) after ischaemic stroke than men, and experienced poorer scores across all health-related quality of life outcomes (mobility, self-care, pain/discomfort, and anxiety/depression).29 Likewise, a meta-analysis found that most studies generally reported women stroke survivors having worse functional recovery and greater long-term disability and handicap compared to men.30 However, differences between sexes were greatly attenuated when confounding factors were taken into account, particularly age and stroke severity.30
A biological explanation for the disparities in ischaemic stroke outcomes between sexes may be related to sex-specific brain lesion patterns post-stroke.31 Brain lesions in regions underlying motor and language functions were generally more widespread and pronounced in female vs. male patients.31 Moreover, additional lesions in the posterior circulation of the left hemisphere were associated with higher stroke severity exclusively in women.31 Other factors (i.e. comorbidities, hormonal and reproductive factors, gender-related socio-demographic factors) may also impact stroke outcomes in women and in men. Social support is an example of a gender-related factor linked to stroke recovery. Women are more likely to live alone and lack a support system at the time of stroke presentation compared to men (who are more likely to have a caregiver, such as a spouse, at the time of stroke), impacting their recovery and rehabilitation post-stroke.4 , 32 This may contribute to the higher rates of depression reported among women post-stroke.33 , 34
Carotid atherosclerotic plaque prevalence, morphology and composition, and gene connectivity
Sex differences in plaque prevalence
The Tromsø Study was among the first to investigate sex differences in the prevalence of carotid atherosclerosis.35 In the majority of age groups, plaque prevalence was greater among men than women, until the age of 75, where more women (81.2%) than men (76.5%) were observed to have carotid atherosclerosis.35 These trends mirror the lifetime risk for ischaemic stroke among both sexes.7
Sex differences in plaque morphology and composition
Differences in plaque morphology and composition between sexes have been observed by histology or non-invasive imaging (Table 1).35–41 While data revealed that carotid artery stenosis and carotid plaque area increase with age in both sexes, women have greater stenosis than men while men have greater plaque area compared to women.41
Studies highlighting sex differences in carotid atherosclerotic plaque morphology and composition as assessed via histology or non-invasive imaging
| Histology . | ||||
|---|---|---|---|---|
| Author, year . | Population description . | Total # of patients, % Symptomatic . | Plaque classification . | Plaque description . |
| Hellings et al.,36 2007 | Consecutive patients who underwent CEA in two participating Dutch hospitals |
|
|
|
| Sangiorgi et al.,37 2013 | Patients who underwent CEA in Rome, Italy |
|
|
|
| Wendorff et al.,40 2015 | Patients who underwent CEA between 2004 and 2013 |
|
|
|
| Histology . | ||||
|---|---|---|---|---|
| Author, year . | Population description . | Total # of patients, % Symptomatic . | Plaque classification . | Plaque description . |
| Hellings et al.,36 2007 | Consecutive patients who underwent CEA in two participating Dutch hospitals |
|
|
|
| Sangiorgi et al.,37 2013 | Patients who underwent CEA in Rome, Italy |
|
|
|
| Wendorff et al.,40 2015 | Patients who underwent CEA between 2004 and 2013 |
|
|
|
| Non-invasive imaging . | ||||
|---|---|---|---|---|
| Author, year . | Population description . | Total # of patients . | Type of imaging . | Plaque description . |
| Joakimsen et al.,35 1999 | Single-centre study of inhabitants aged 25–84 years in tde municipality of Tromsø, Norway |
| High-resolution B-mode ultrasound |
|
| Ota et al.,38 2010 | Patients with ≥50% asymptomatic carotid stenosis |
| 3.0-T multi-contrast MRI |
|
| Ota et al.,39 2013 | Patients with <50% asymptomatic carotid stenosis |
| 3.0-T multi-contrast MRI |
|
| Non-invasive imaging . | ||||
|---|---|---|---|---|
| Author, year . | Population description . | Total # of patients . | Type of imaging . | Plaque description . |
| Joakimsen et al.,35 1999 | Single-centre study of inhabitants aged 25–84 years in tde municipality of Tromsø, Norway |
| High-resolution B-mode ultrasound |
|
| Ota et al.,38 2010 | Patients with ≥50% asymptomatic carotid stenosis |
| 3.0-T multi-contrast MRI |
|
| Ota et al.,39 2013 | Patients with <50% asymptomatic carotid stenosis |
| 3.0-T multi-contrast MRI |
|
AHA, American Heart Association; CEA, carotid endarterectomy; CI, confidence interval; MRI, magnetic resonance imaging; OR, odds ratio.
Studies highlighting sex differences in carotid atherosclerotic plaque morphology and composition as assessed via histology or non-invasive imaging
| Histology . | ||||
|---|---|---|---|---|
| Author, year . | Population description . | Total # of patients, % Symptomatic . | Plaque classification . | Plaque description . |
| Hellings et al.,36 2007 | Consecutive patients who underwent CEA in two participating Dutch hospitals |
|
|
|
| Sangiorgi et al.,37 2013 | Patients who underwent CEA in Rome, Italy |
|
|
|
| Wendorff et al.,40 2015 | Patients who underwent CEA between 2004 and 2013 |
|
|
|
| Histology . | ||||
|---|---|---|---|---|
| Author, year . | Population description . | Total # of patients, % Symptomatic . | Plaque classification . | Plaque description . |
| Hellings et al.,36 2007 | Consecutive patients who underwent CEA in two participating Dutch hospitals |
|
|
|
| Sangiorgi et al.,37 2013 | Patients who underwent CEA in Rome, Italy |
|
|
|
| Wendorff et al.,40 2015 | Patients who underwent CEA between 2004 and 2013 |
|
|
|
| Non-invasive imaging . | ||||
|---|---|---|---|---|
| Author, year . | Population description . | Total # of patients . | Type of imaging . | Plaque description . |
| Joakimsen et al.,35 1999 | Single-centre study of inhabitants aged 25–84 years in tde municipality of Tromsø, Norway |
| High-resolution B-mode ultrasound |
|
| Ota et al.,38 2010 | Patients with ≥50% asymptomatic carotid stenosis |
| 3.0-T multi-contrast MRI |
|
| Ota et al.,39 2013 | Patients with <50% asymptomatic carotid stenosis |
| 3.0-T multi-contrast MRI |
|
| Non-invasive imaging . | ||||
|---|---|---|---|---|
| Author, year . | Population description . | Total # of patients . | Type of imaging . | Plaque description . |
| Joakimsen et al.,35 1999 | Single-centre study of inhabitants aged 25–84 years in tde municipality of Tromsø, Norway |
| High-resolution B-mode ultrasound |
|
| Ota et al.,38 2010 | Patients with ≥50% asymptomatic carotid stenosis |
| 3.0-T multi-contrast MRI |
|
| Ota et al.,39 2013 | Patients with <50% asymptomatic carotid stenosis |
| 3.0-T multi-contrast MRI |
|
AHA, American Heart Association; CEA, carotid endarterectomy; CI, confidence interval; MRI, magnetic resonance imaging; OR, odds ratio.
Moreover, men tend to have a higher prevalence of unstable plaques than women, with high-risk features (large intraplaque haemorrhage, thin fibrous cap, large lipid core, more inflammatory cells; Figure 1A and B).35–40 Using high-resolution B-mode ultrasonography, a greater proportion of echolucent plaques (i.e. lipid-rich plaques) were observed in men than in women, among all age groups (25–84 years).35 Similarly, using in vivo magnetic resonance imaging in asymptomatic individuals regardless of degree of artery stenosis, the presence of high-risk plaque features (thin/ruptured fibrous cap; lipid-rich/necrotic core) were more common among men than women.38 , 39
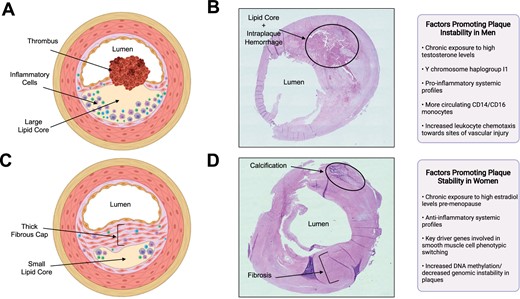
Differences in plaque morphology and composition between male vs. female carotid atherosclerotic plaque specimens and associated factors contributing to the pathologies. (A). A drawing illustrating a typical plaque isolated from men (unstable plaque), which includes a large lipid core, a thin ruptured fibrous cap, abundant inflammatory cells, and thrombus formation. Created with BioRender.com. (B) Representative photomicrograph of haematoxylin and eosin-stained carotid plaque obtained from a symptomatic man who underwent a carotid endarterectomy at the McGill University Health Centre, Montreal, Canada. The plaque exhibits unstable features; a large lipid core, intraplaque haemorrhage, and a thin fibrous cap. Image photographed at magnification ×20. (C) A drawing illustrating a typical plaque isolated from women (stable plaque), which includes a smaller lipid core, and thick intact fibrous cap. Created with BioRender.com. (D) Representative photomicrograph of haematoxylin and eosin-stained carotid plaque obtained from a symptomatic woman who underwent a carotid endarterectomy at the McGill University Health Centre, Montreal, Canada. The plaque exhibits stable features; composed mainly of fibrous tissue and calcification and has no apparent lipid core. Image photographed at magnification ×20.
Histological analyses of carotid plaque specimens reported that male sex was associated with greater cellularity (OR, 1.56; 95% CI, 1.17–2.10), more inflammation (OR, 1.75; 95% CI, 1.31–2.34), and more neovascularization (OR, 1.47; 95% CI, 1.10–1.97), independent of smoking status, medication use, and medical history.40 Notably, plaques from men were observed to have more macrophages, as well as higher levels of CD86 (a costimulatory molecule that facilitates communication with T cells) compared to plaques from women.42 Conversely, women had higher rates of stable fibrous/fibrocalcific plaques than men independent of cerebrovascular symptom status (Figure 1C and D).36 , 40 The inflammatory accumulation observed in plaques in men compared to women is hypothesized to be related to differences in systemic inflammatory profiles.43 Men have been reported to have more circulating CD14 and CD16 monocytes compared to women.44 Particularly, CD16 monocytes have been associated with endothelial dysfunction, carotid intima-media thickness, and atherosclerosis development.43 , 45 Moreover, men tend to have increased CCR2 expression on monocytes, increased toll-like receptor-4 expression on macrophages and neutrophils, and elevated inflammatory cytokine and chemokine production (i.e. interleukin-6, interleukin-1ß, tumour necrosis factor-alpha) compared to women.46–48 These sex differences in systemic inflammatory profiles may contribute to increased chemotaxis of leukocytes towards the site of vascular injury in men. As a result of differences in plaque phenotype and inflammation, thrombosis can occur via diverse mechanisms in either sex. Women’s plaques are more prone to surface erosion as opposed to typical rupture, which is frequently observed in men’s plaques.49
Sex differences in plaque gene expression and network connectivity
Biological sex can also influence gene expression and network connectivity within atherosclerotic plaques.50 , 51 Studies have demonstrated plaques from men to be enriched for genes mainly associated with the myeloid, immune, and haematological system, while genes most active in plaques from women were associated with endothelial and mesenchymal cells.50 , 51 These findings coincide with the above-mentioned histological and imaging studies. Through generation of sex-stratified gene regulatory networks, vascular smooth muscle cell (VSMC) biology appeared to be a key driver of pathophysiological differences in atherosclerosis between sexes, with the female key driver genes mainly involved in smooth muscle cell phenotypic switching.50 Large differences in DNA methylation between women and men may also lead to sex differences in gene regulation that influence atherosclerosis development. Methylation was observed to be greater in female plaques compared to male plaques, which suggests that women may be less prone to genomic instability within their plaque cells compared to men.51
Limitations of preclinical animal studies
Limited data exist in the preclinical literature to address mechanisms underlying sex differences in atherosclerosis. An analysis of all preclinical studies of atherosclerosis and other vascular diseases published between the years 2006 and 2018 in leading American Heart Association journals found that 18.8% of the studies did not report the sex of the animals.52 Among those that did, only 24.1% studied both sexes. They also noted that the proportion of studies using exclusively male animals had increased over time (P < 0.0001).52 Man et al. noted that among the ∼25% of studies that included both male and female animal models, <50% used appropriate statistical tests to compare the two sexes.43 When sexes were compared, plaque size was the most common measurement.43 However, plaque size was found to be generally larger in females vs. males in most animal models of atherosclerosis, which is not reflective of human findings.43 Instead, plaque inflammation may serve as a more useful surrogate endpoint to study plaque vulnerability in animal models.43 Unfortunately, the number of preclinical studies directly comparing plaque inflammation between sexes remain scarce.
The role of sex hormones and sex chromosomes in atherosclerosis development and ischaemic stroke risk
Sex hormones
Sex hormones impact the vasculature in men and women; they affect vascular reactivity, inflammation, and lipoprotein metabolism, which all contribute to atherosclerosis development, plaque instability, and stroke risk.53
Oestradiol
Oestradiol exerts atheroprotective effects at the vascular endothelium; by preventing circulating monocytes from adhering to the vasculature, oestradiol inhibits the recruitment of inflammatory cells to the lesion site.54 Oestradiol also inhibits macrophage low-density lipoprotein (LDL) uptake and metabolism,54 thereby reducing the formation of macrophage-derived foam cells within the plaque. Moreover, oestradiol signalling has been shown to trigger the resolution of inflammation in macrophages.55 Similar to its effects on macrophages, oestradiol can promote cholesterol efflux from VSMCs and reduce VSMC-derived foam cell formation.56 While oestradiol exerts a variety of atheroprotective effects, it also has prothrombotic actions affecting many haemostatic pathways to promote platelet aggregation.57 , 58
It is widely accepted that oestradiol protects premenopausal women against stroke. This protection diminishes with age after menopause, paralleled by the significant decrease in circulating oestradiol in postmenopausal women. The effects of oestradiol on the vasculature have led to many studies examining the relationship between hormone therapy (HT) and stroke. Key RCTs are highlighted below and presented in Table 2. Most trials examining the effectiveness of either oestrogen, oestrogen + progestin, or 17β-oestradiol in reducing stroke risk, stroke recurrence, or reducing carotid intima-media thickness/atherosclerosis deemed HT ineffective (i.e. HERS, HERS-II, Estrogen Replacement and Atherosclerosis Trial, WEST) and very few found HT to exert beneficial effects (i.e. EPAT).59–64 Conversely, both the oestrogen and the oestrogen + progestin arms of the WHI trial found that HT increased the risk of stroke, and the trials were terminated prematurely.65–67
| Study . | Design . | Cohort . | Agea . | Dose . | Mode of administration . | Mean follow-up . | Relevant findings . |
|---|---|---|---|---|---|---|---|
| Heart and Estrogen/ progestin Replacement Study (HERS)59 | Randomized, double-blinded, placebo-controlled secondary prevention trial | 2763 postmenopausal women with established CHD | 67, 44–79 | 0.625mg/day CEE + 2.5 mg/day MPA | Oral tablets | 4.1 years | HT was not associated with risk of nonfatal stroke, fatal stroke, or TIA |
| Unblinded follow-up | 2321 women from the HERS study | 67, 44–79 | 0.625mg/day CEE + 2.5 mg/day MPA | Oral tablets | 2.7 years | HT was not associated with risk of nonfatal stroke, fatal stroke, or TIA |
| Estrogen Replacement and Atherosclerosis Trial64 | Randomized, double-blind, placebo-controlled trial | 309 postmenopausal women aged with coronary artery disease | 68.5, 42–80 | 0.625 mg/day CEE or 0.625 mg/day CEE + 2.5 mg/day MPA | Oral tablets | 3.2 years |
|
| Estrogen in the Prevention of Atherosclerosis Trial (EPAT)61 | Randomized, double-blinded, placebo-controlled trial | 222 healthy postmenopausal women | 62.2, 46–80 | 1 mg/day 17β-oestradiol | Oral tablets | 2 years | HRT significantly slowed the progression of subclinical atherosclerosis, as measured by CIMT, compared to placebo |
| Women’s Estrogen for Stroke Trial (WEST)60 | Randomized, double-blind, placebo-controlled trial | 664 postmenopausal women recently having had an ischaemic stroke or TIA | 71, 46–91 | 1 mg/day 17β-oestradiol | Oral tablets | 2.8 years | No significant differences in stroke recurrence between HT and placebo groups |
| Women’s Health Initiative (WHI)65–67 | Two parallel, randomized, double-blind, placebo-controlled trials |
|
|
| Oral tablets |
|
|
| Early vs. Late Intervention Trial with Estradiol (ELITE)68 | Randomized, double-blind, placebo-controlled trial | 643 healthy postmenopausal women separated based on time since menopause |
| 1 mg/day 17β-oestradiol + 45 mg progesterone | Oral 17β-oestradiol + vaginal progesterone gel | 5 years |
|
| Study . | Design . | Cohort . | Agea . | Dose . | Mode of administration . | Mean follow-up . | Relevant findings . |
|---|---|---|---|---|---|---|---|
| Heart and Estrogen/ progestin Replacement Study (HERS)59 | Randomized, double-blinded, placebo-controlled secondary prevention trial | 2763 postmenopausal women with established CHD | 67, 44–79 | 0.625mg/day CEE + 2.5 mg/day MPA | Oral tablets | 4.1 years | HT was not associated with risk of nonfatal stroke, fatal stroke, or TIA |
| Unblinded follow-up | 2321 women from the HERS study | 67, 44–79 | 0.625mg/day CEE + 2.5 mg/day MPA | Oral tablets | 2.7 years | HT was not associated with risk of nonfatal stroke, fatal stroke, or TIA |
| Estrogen Replacement and Atherosclerosis Trial64 | Randomized, double-blind, placebo-controlled trial | 309 postmenopausal women aged with coronary artery disease | 68.5, 42–80 | 0.625 mg/day CEE or 0.625 mg/day CEE + 2.5 mg/day MPA | Oral tablets | 3.2 years |
|
| Estrogen in the Prevention of Atherosclerosis Trial (EPAT)61 | Randomized, double-blinded, placebo-controlled trial | 222 healthy postmenopausal women | 62.2, 46–80 | 1 mg/day 17β-oestradiol | Oral tablets | 2 years | HRT significantly slowed the progression of subclinical atherosclerosis, as measured by CIMT, compared to placebo |
| Women’s Estrogen for Stroke Trial (WEST)60 | Randomized, double-blind, placebo-controlled trial | 664 postmenopausal women recently having had an ischaemic stroke or TIA | 71, 46–91 | 1 mg/day 17β-oestradiol | Oral tablets | 2.8 years | No significant differences in stroke recurrence between HT and placebo groups |
| Women’s Health Initiative (WHI)65–67 | Two parallel, randomized, double-blind, placebo-controlled trials |
|
|
| Oral tablets |
|
|
| Early vs. Late Intervention Trial with Estradiol (ELITE)68 | Randomized, double-blind, placebo-controlled trial | 643 healthy postmenopausal women separated based on time since menopause |
| 1 mg/day 17β-oestradiol + 45 mg progesterone | Oral 17β-oestradiol + vaginal progesterone gel | 5 years |
|
CEE, conjugated equine oestrogen; CHD, coronary heart disease; CIMT, carotid intima-media thickness; HT, hormone replacement therapy; MPA, medroxyprogesterone acetate; TIA, transient ischaemic attack.
Mean or median, range.
| Study . | Design . | Cohort . | Agea . | Dose . | Mode of administration . | Mean follow-up . | Relevant findings . |
|---|---|---|---|---|---|---|---|
| Heart and Estrogen/ progestin Replacement Study (HERS)59 | Randomized, double-blinded, placebo-controlled secondary prevention trial | 2763 postmenopausal women with established CHD | 67, 44–79 | 0.625mg/day CEE + 2.5 mg/day MPA | Oral tablets | 4.1 years | HT was not associated with risk of nonfatal stroke, fatal stroke, or TIA |
| Unblinded follow-up | 2321 women from the HERS study | 67, 44–79 | 0.625mg/day CEE + 2.5 mg/day MPA | Oral tablets | 2.7 years | HT was not associated with risk of nonfatal stroke, fatal stroke, or TIA |
| Estrogen Replacement and Atherosclerosis Trial64 | Randomized, double-blind, placebo-controlled trial | 309 postmenopausal women aged with coronary artery disease | 68.5, 42–80 | 0.625 mg/day CEE or 0.625 mg/day CEE + 2.5 mg/day MPA | Oral tablets | 3.2 years |
|
| Estrogen in the Prevention of Atherosclerosis Trial (EPAT)61 | Randomized, double-blinded, placebo-controlled trial | 222 healthy postmenopausal women | 62.2, 46–80 | 1 mg/day 17β-oestradiol | Oral tablets | 2 years | HRT significantly slowed the progression of subclinical atherosclerosis, as measured by CIMT, compared to placebo |
| Women’s Estrogen for Stroke Trial (WEST)60 | Randomized, double-blind, placebo-controlled trial | 664 postmenopausal women recently having had an ischaemic stroke or TIA | 71, 46–91 | 1 mg/day 17β-oestradiol | Oral tablets | 2.8 years | No significant differences in stroke recurrence between HT and placebo groups |
| Women’s Health Initiative (WHI)65–67 | Two parallel, randomized, double-blind, placebo-controlled trials |
|
|
| Oral tablets |
|
|
| Early vs. Late Intervention Trial with Estradiol (ELITE)68 | Randomized, double-blind, placebo-controlled trial | 643 healthy postmenopausal women separated based on time since menopause |
| 1 mg/day 17β-oestradiol + 45 mg progesterone | Oral 17β-oestradiol + vaginal progesterone gel | 5 years |
|
| Study . | Design . | Cohort . | Agea . | Dose . | Mode of administration . | Mean follow-up . | Relevant findings . |
|---|---|---|---|---|---|---|---|
| Heart and Estrogen/ progestin Replacement Study (HERS)59 | Randomized, double-blinded, placebo-controlled secondary prevention trial | 2763 postmenopausal women with established CHD | 67, 44–79 | 0.625mg/day CEE + 2.5 mg/day MPA | Oral tablets | 4.1 years | HT was not associated with risk of nonfatal stroke, fatal stroke, or TIA |
| Unblinded follow-up | 2321 women from the HERS study | 67, 44–79 | 0.625mg/day CEE + 2.5 mg/day MPA | Oral tablets | 2.7 years | HT was not associated with risk of nonfatal stroke, fatal stroke, or TIA |
| Estrogen Replacement and Atherosclerosis Trial64 | Randomized, double-blind, placebo-controlled trial | 309 postmenopausal women aged with coronary artery disease | 68.5, 42–80 | 0.625 mg/day CEE or 0.625 mg/day CEE + 2.5 mg/day MPA | Oral tablets | 3.2 years |
|
| Estrogen in the Prevention of Atherosclerosis Trial (EPAT)61 | Randomized, double-blinded, placebo-controlled trial | 222 healthy postmenopausal women | 62.2, 46–80 | 1 mg/day 17β-oestradiol | Oral tablets | 2 years | HRT significantly slowed the progression of subclinical atherosclerosis, as measured by CIMT, compared to placebo |
| Women’s Estrogen for Stroke Trial (WEST)60 | Randomized, double-blind, placebo-controlled trial | 664 postmenopausal women recently having had an ischaemic stroke or TIA | 71, 46–91 | 1 mg/day 17β-oestradiol | Oral tablets | 2.8 years | No significant differences in stroke recurrence between HT and placebo groups |
| Women’s Health Initiative (WHI)65–67 | Two parallel, randomized, double-blind, placebo-controlled trials |
|
|
| Oral tablets |
|
|
| Early vs. Late Intervention Trial with Estradiol (ELITE)68 | Randomized, double-blind, placebo-controlled trial | 643 healthy postmenopausal women separated based on time since menopause |
| 1 mg/day 17β-oestradiol + 45 mg progesterone | Oral 17β-oestradiol + vaginal progesterone gel | 5 years |
|
CEE, conjugated equine oestrogen; CHD, coronary heart disease; CIMT, carotid intima-media thickness; HT, hormone replacement therapy; MPA, medroxyprogesterone acetate; TIA, transient ischaemic attack.
Mean or median, range.
The discrepancies between trials have led to the hypothesis that HT may exert differential effects depending on the timing of hormone initiation relative to menopause onset. The effects of oestradiol on atherosclerosis are related to the health of the underlying vasculature: oestradiol is believed to slow the progression of early atherosclerosis if HT is initiated soon after menopause when the endothelium is relatively healthy.2 If commenced several years after menopause when the vessel wall is diseased, oestradiol may instead exert pro-atherogenic effects.2 The ELITE trial confirmed that oestradiol HT was significantly associated with decreased progression of atherosclerosis only when initiated within 6 years of menopause onset.68 A variety of other factors, such as type of hormone administered (including progesterone component), route of delivery (oral or transdermal), dosage (high vs. low), decrease in oestrogen receptors, and adherence to treatment regimen may also impact the effects of HT on the vascular system, highlighting the complex associations and further explaining the controversial evidence in the literature.
Testosterone
Androgens also exert beneficial effects on vascular cells. Specifically, testosterone can prevent endothelial dysfunction by increasing endothelial cell production of nitric oxide, increasing endothelial cell motility and proliferation,69 and promoting vasodilation. Testosterone also exerts effects on macrophages and foam cells by reducing inflammatory cell activation.69 A study performed on a rabbit model of atherosclerosis demonstrated that administration of dihydrotestosterone (DHT), the active form of testosterone, reduced foam cell formation within plaques and suppressed inflammatory cytokine production.70 Moreover, castration in a rabbit model of atherosclerosis led to increased plaque area and foam cell formation.71 Although testosterone is largely anti-atherogenic and immunosuppressive, some studies have also demonstrated high levels of testosterone as well as chronic exposure to testosterone to have the opposite effects.72 , 73
The relationship between testosterone and stroke also presents with conflicting data. While lower circulating testosterone has been associated with higher incidence of atherosclerosis,74 testosterone replacement therapy significantly increased the risk of cardiovascular adverse events in men aged ≥65 years.75 Alternatively, a more recent study did not establish any associations between endogenous levels of total testosterone and plaque composition or stroke risk among men (≥45 years of age) or post-menopausal women with atherosclerosis.76 We cannot discern with certainty the relationship between androgens and stroke as testosterone can be aromatized to oestradiol or converted to DHT at the level of the tissue. Therefore, it is difficult to determine whether the effects of testosterone are ultimately androgen or oestrogen derived.
Sex chromosomes
The effects of chromosomal sex on atherosclerosis and stroke have been largely under-studied, as it is difficult to dissociate effects of sex chromosomes from those of sex hormones. However, the Four Core Genotypes mouse model is frequently used to circumvent this limitation.77 This mouse model involves the translocation of the SRY testes-determining gene from the Y chromosome to chromosome 3, allowing gonadal sex to be segregated from chromosomal sex and producing XX and XY mice with ovaries and XX and XY mice with testes.77 Studies using this model found the XX complement to increase circulating HDL levels, while others found it to also increase circulating LDL, both of which influence the atherosclerotic process.77 , 78 Moreover, XX gonadectomized Ldlr− / − mice (male or female) had increased atherosclerosis compared to XY gonadectomized male or female Ldlr− / − mice, providing evidence of a direct chromosomal contribution to atherosclerosis, irrespective of sex hormones.78 The sex chromosome complement can also contribute to ischaemic stroke sensitivity; aged animals with a XX chromosome complement had larger infarcts, higher neurological-deficit scores, and greater immune cell infiltration and activation in the plaque compared to animals with an XY complement.79 Yet, in humans, a genetic association study from the UK Biobank showed that the Y chromosome haplogroup I1 was associated with changes in pathways involved in atherosclerosis—namely immunity, lipid metabolism, and coagulation—compared to men without this haplogroup, suggesting that the inheritance of this Y chromosome haplotype increases cardiovascular risk.80 Much work remains to be performed in both basic and clinical research to fully elucidate the effects of sex chromosomes on the development of atherosclerosis and risk for stroke.
Carotid disease management
Sex differences in surgical management
Surgical management for carotid atherosclerosis includes either carotid endarterectomy (CEA) or carotid artery stenting (CAS). CEA is the gold standard method for carotid revascularization, as CAS is associated with a higher risk of periprocedural stroke.81–83 Current guidelines recommend CEA or CAS (plus medical treatment) for stroke prevention in both symptomatic and asymptomatic patients with carotid plaques, when carotid artery stenosis is ≥50% or ≥60%, respectively.84 , 85 However, the strength of the conclusions upon which these guidelines are based has been debated, particularly in the asymptomatic population. The trials on surgical therapy are outdated, taking place before the widespread use of statins and other contemporary medications, which has since redefined the current management of carotid disease.86 , 87 Moreover, these guidelines are based on trials where women were considerably under-represented (≤30%). Sex-specific post-hoc analyses of these trials demonstrated important sex differences in surgical benefit.88–91 However, these analyses were under-powered. Therefore, new trials designed to study the benefit of CEA or CAS for stroke prevention specifically in women and in men are essential.
Carotid endarterectomy
Overall, post-hoc analyses suggest that women benefit less from surgical intervention vs. medical management than men, particularly those with asymptomatic carotid disease.88 , 89 Data combined from NASCET and the ASA and Carotid Endarterectomy Trial reported that women with symptomatic carotid stenosis ≥70% had a similar 5-year absolute risk reduction (ARR) in ischaemic stroke from CEA (ARR, 15.1%; P = 0.007) compared to men (ARR, 17.3%; P < 0.001).88 However, when stenosis was between 50% and 69%, CEA was not beneficial in symptomatic women (ARR, 3.0%, P = 0.94), while it was in men (ARR, 10.0%, P = 0.02).88 In women with asymptomatic carotid stenosis, the stroke relative risk reduction from CEA was significantly lower compared to men, only 4% in women compared to 51% in men (P = 0.008).89 Post-hoc analyses also suggest an increased surgical risk among women. Women with symptomatic carotid stenosis had a higher perioperative risk of stroke or death 30 days post- CEA compared to men (OR, 1.50; 95% CI, 1.14–1.97; P = 0.004).91 Similarly, asymptomatic women were also reported to have increased perioperative stroke and death rate (3.6%) associated with CEA compared to asymptomatic men (1.7%).90
Carotid artery stenting
In contrast to CEA, less evidence exists regarding the comparison of CAS outcomes between men and women. A retrospective analysis demonstrated no significant differences in 30-day periprocedural stroke rate (2.1% in women vs. 4.2% in men, P = 0.48) or death rate (0% in women vs. 0.70% in men, P = 0.99) between men and women following CAS.92 In contrast, in the CREST study, higher rates of combined periprocedural endpoints (stroke + death + myocardial infarction) after CAS were trending among women (6.8%) compared to men (4.3%; P = 0.064).93 Moreover, women assigned to CAS had higher rates of periprocedural stroke events compared to women who underwent CEA [hazard ratio (HR), 2.63, 95% CI, 1.23–5.65; P = 0.013], while for men there was no difference in risk between surgical groups.93 However, a more recent study evaluating data from the Carotid Stenosis Trialists’ Collaboration reported major inconsistencies across trials regarding sex differences in the CAS-to-CEA risk.94 Thus, results must be interpreted with caution. The CREST-2 (ClinicalTrials.gov identifier: NCT02089217) trial is actively enrolling a more representative percentage of women (>40%) to ensure adequate information is recorded on the risk and benefit ratio of CEA or CAS plus contemporary medical management.95
Timing of intervention
The timing of carotid revascularization is essential in the prevention of secondary stroke. Ideally, surgery should be performed within 14 days of ischaemic event onset.91 In actuality, timing of surgery is more critical in women than in men, where women were observed to benefit most when CEA was performed within 2 weeks of their last ischaemic event, irrespective of stenosis severity.96 For instance, in women the ARR in stroke dropped from 41.7% when CEA was conducted within 2 weeks to only 6.6% when CEA was conducted between 2 and 4 weeks following the event.96 After 4 weeks, surgery was found to be harmful, with an ARR of −2.2%.96 This is in contrast to men, where surgery remained beneficial even >12 weeks after their last symptomatic event (ARR for CEA <2 weeks from event, 23.5%; ARR for CEA >12 weeks from event, 20.4%).96 However, timely CEAs for symptomatic women are not often achieved in practice. For example, a study compiling data from 19 emergency departments in California identified that time to CEA was significantly delayed in women with a transient ischaemic attack (TIA) diagnosis compared with men (median time to CEA: 35 vs. 18 days; P = 0.03), despite adjusting for patient-related covariates, clinical presentation, and degree of carotid stenosis.97 Another study of consecutive patients with acute stroke or TIA admitted to 11 Ontario stroke centres reported that women were half as likely to undergo carotid revascularization within 6 months of the event (2.2% vs. 4.3%; OR, 0.51; 95% CI, 0.37–0.70).98 Moreover, multiple studies have also pointed out that women experience longer delays in door-to-imaging compared to men following an acute ischaemic stroke.98–100
Sex differences in medical management
Optimal medical management is important for the treatment of all patients with carotid atherosclerosis, regardless of stenosis and whether surgical intervention is planned. Management includes lifestyle modifications (smoking cessation, healthy diet, and physical activity) and treatment of traditional vascular risk factors.84 , 85 , 101 Large carotid stenosis trials showed that women exhibited greater stroke risk reduction when offered medical management compared to men; medically treated women with 50–69% carotid artery stenosis had a lower 5-year risk of stroke compared to similarly treated men (16.1% vs. 25.3%; P = 0.03).88 Unfortunately, women are less likely than men to receive medical therapy (i.e. statins, anti-hypertensive medication, anti-platelets) for primary and secondary prevention of atherosclerotic disease2 , 102 (Figure 2).
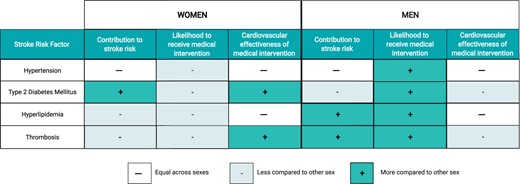
Differences in medical management for stroke risk factors between men and women. Summary of differences between men and women in (i) the relative contribution of hypertension, type 2 diabetes mellitus, hyperlipidaemia, and thrombosis to stroke risk, (ii) the likelihood to receive medical intervention, and (iii) the cardiovascular effectiveness of medical intervention. Created with BioRender.com.
Hypertension
Hypertension remains the most prevalent modifiable risk factor for ischaemic stroke globally among both sexes. Hypertension influences stroke risk similarly in men and women regardless of age103 and both sexes show similar benefit in stroke reduction from anti-hypertensive medications, across all drug classes.104 Despite these similarities, postmenopausal women are less likely to have their blood pressure controlled compared to their male counterparts.105–109 Hence, understanding sex- and gender-specific factors related to hypertension control remains important.
It is worth noting that blood pressure values in healthy women are typically lower compared to men.110 This results in increased risk of stroke in women with blood pressures exceeding their normal range yet falling within the normal range of blood pressures in men. A recent study reported that women with systolic blood pressure (SBP) 120–129 mmHg had a significant risk for stroke (HR, 1.53; 95% CI, 1.07–2.21), which was comparable to stroke risk among men with SBP 140–149 mmHg.110 Therefore, blood pressure treatment targets could benefit from careful reconsideration according to sex.
Type 2 diabetes mellitus
Although type 2 diabetes mellitus (T2DM) is an important risk factor for ischaemic stroke in both sexes, contributing an overall two-fold increased risk,111 , 112 it has been classified as a stronger risk factor in women than in men.113 , 114 A meta-analysis of 64 cohort studies reported a 27% higher stroke risk among women with T2DM than men (relative risk, 1.27; 95% CI, 1.10–1.46), independent of other cardiovascular risk factors.115 Interestingly, the prevalence of atherosclerosis was also observed to be higher in women with newly diagnosed T2DM compared to women without T2DM, as opposed to men with and without T2DM.116 This suggests that diabetes, even at an early stage, can increase the risk for CVD and stroke in women to a greater extent than in men.116 Despite these known sex differences, women are generally less likely than men to have their glycated haemoglobin levels controlled.117
Hyperlipidaemia
Hyperlipidaemia is a stronger risk factor for stroke in men than women.118 Generally, men have a worse overall pattern of cholesterol levels than women, with higher levels of LDL and lower levels of HDL. Despite the effectiveness of statins in lowering stroke risk being similar between women and men,119 women are less likely than men to receive statins for hyperlipidaemia or atherosclerotic disease, or to reach recommended LDL goals.102 , 120 , 121
Thrombosis
Since ischaemic stroke is an atherothrombotic complication, antiplatelet agents are highly recommended for primary and secondary prevention.84 , 85 , 101 Interestingly, aspirin has been found to reduce ischaemic stroke risk in women but have minimal benefit in men; in a sex-specific meta-analysis of six RCTs, women had a 24% reduced risk of ischaemic stroke when taking aspirin for primary prevention of CVD (OR, 0.76; 95% CI, 0.63–0.93), while no stroke preventative benefit was observed in men (OR, 1.00; 95% CI, 0.72–1.41).122 Ironically, a US-wide healthcare system-based study of individuals with premature ischaemic cerebrovascular disease found that women were less likely than men to be taking antiplatelet medications (64.5% vs. 77.9%; OR: 0.76, 95% CI: 0.70–0.82).102
Multi-morbidity
The presence of multiple comorbidities contributes to greater stroke severity, lower baseline functional status, and higher risk of disability and mortality post-stroke.123 It may also interfere with recommended treatments by enhancing drug-drug adverse interactions and risk of non-adherence.
There has been limited comparison of comorbidity prevalence between men and women with ischaemic stroke. One cohort study (n = 8751) reported women with stroke to have a significantly higher number of comorbidities (≥4) than their male counterparts.124 Interestingly, women without comorbidities have a lower 10-year stroke rate than men (1.1% vs. 2.6%); however, this rate is greater among women than men with presence of four or more comorbidities (19.1% vs. 14.8%).125
Aside from traditional risk factors, there are sex-specific factors that may increase stroke risk and impact stroke outcomes. In women, these factors include history of adverse pregnancy outcomes (i.e. preeclampsia and gestational hypertension, gestational diabetes, preterm delivery), early-onset menopause, polycystic ovarian syndrome, breast or ovarian cancer, and inflammatory disorders (i.e. rheumatoid arthritis, psoriasis, systemic erythematous lupus).126 , 127 Male-specific risk factors include androgen deprivation therapy, orchiectomy, and erectile dysfunction.127 These sex-specific risk factors should be implemented into current risk stratification estimations to improve stroke care.
Sex- and gender-related factors influencing carotid disease management
Biological sex- and gender-related attitudes/roles have been suggested to influence post-surgical outcomes among men and women, as well as promote delays in diagnosis and decision-making regarding surgical and/or medical management.128–130
Sex-related factors
The increased post-operative risk reported among women is speculated to be due to their carotid artery mean diameters being significantly smaller than those of men—independent of body size, neck size, age, and blood pressure—making CEA more technically difficult.131 Differences in plaque morphology and composition (women having more stable plaques) may also partly explain why women benefit less from carotid revascularization than men.129 Thus, assessment of plaque vulnerability during the preoperative phase may help predict which patients/women would most benefit from surgical and/or medical intervention.
Gender-related factors
Personality traits and social roles traditionally ascribed to women may help explain longer delays before diagnosis and treatment, and differences in treatment rates.128 , 130 Gender role expectations of women as caregivers may contribute to the observed delays in diagnosis and treatment, as women may prioritize the health of others before their own and delay immediate action upon symptom presentation.128 , 130 , 132 , 133 Furthermore, women are generally more concerned with the risk associated with interventions and tend to seek additional information to guide them in decision-making.14 , 15 , 133 Women may therefore be less likely to accept or adhere to treatment (whether surgical or medical) when offered.133 Differences in stroke care between women and men may also be due to clinician awareness and bias: the misperception that women are less affected by stroke or its risk factors and that they respond to stroke treatment similarly to men still exists within the medical community.128 , 130 To reduce these disparities, future studies are needed to better understand the influence of these gender-related factors on carotid disease management, and measures should be taken to better educate the medical community and general public about their impact. Once proven in future studies, these factors may help refine the guidelines for carotid disease management and stroke prevention.
Conclusion
Clinical stroke research has historically included mostly men and studies were not properly designed to perform sex- and gender-based analyses, leading to the under-appreciation of differences between men and women in stroke presentation and outcomes, and in their response to treatment for carotid disease. The reasons for these differences are likely multifactorial; some differences are due to socioeconomic and gender-related factors, such as decreased social support and lack of stroke awareness, yet others result from clear biological differences between males and females (Figure 3). Given that these sexes exhibit different plaque phenotypes, which can contribute to their individual stroke risk, understanding the sex-specific mechanisms that underlie atherosclerosis is a research priority. Animal studies are used to investigate these biological processes; however, most are not conducted in both sexes and are not translationally relevant. Thus, a paradigm shift in scientific research must occur with sex- and gender-based analyses being a necessity (Figure 4). Experimental research should be conducted on animals and cells of both sexes, and clinical research should have equal representation of both men and women and provide appropriately designed analyses for the examination of potential sex and gender differences. Moreover, the terms ‘sex’ and ‘gender’ should be appropriately distinguished and utilized in scientific literature, and clearly defined in the methodology, which is not the current reality. The implementation of these recommendations will lead to more accurate patient risk stratification and pave the way for the identification of novel therapeutic targets aiming to reduce stroke risk specifically in women and in men.
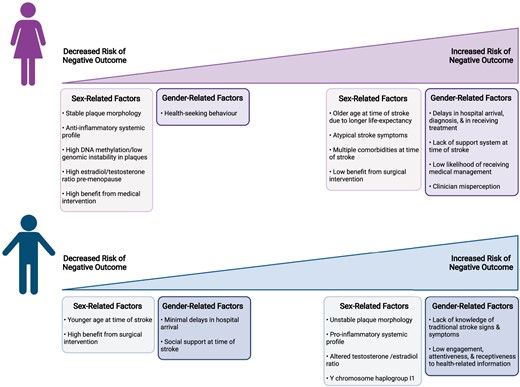
Summary of sex and gender-related factors contributing to increased or decreased risk of negative outcomes post-stroke within each sex. Created with BioRender.com.
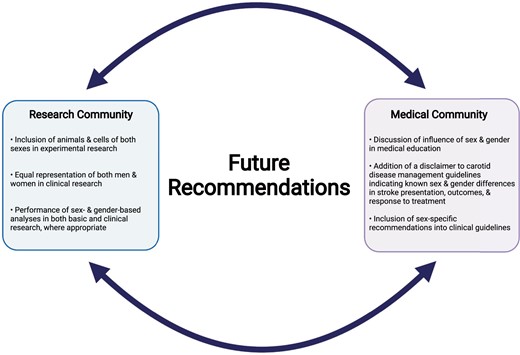
Future recommendations for the consideration of sex and gender within the research and medical communities. Created with BioRender.com.
Importantly, this review highlights that a ‘one-size fits all’ approach should not be applied to carotid disease management and stroke prevention. Instead, the influence of sex and gender should be implemented into medical education. Unfortunately, the current guidelines for carotid disease management are lacking sex- and gender-specific orientation.134 At present, a disclaimer could be added in the guidelines indicating that men and women differ in stroke presentation and outcomes, and in their response to surgical/medical intervention for carotid disease management. This may promote increased awareness among healthcare providers and may lead to the use of more appropriate treatment options. Furthermore, female- and male-specific risk factors should be considered in clinical recommendations, as they may help improve the accuracy and timeliness of stroke risk assessment. As properly designed studies are conducted with a sex and gender lens, these results should form the basis of updated clinical guidelines with recommendations that are specific for women and for men.
Funding
This work was supported by the Canadian Institutes of Health Research (PJT-148966, FRN 145589) and the Heart & Stroke Foundation of Canada (G-17-0018755). S.S.D. is a Senior Chercheur-Boursier Clinicien supported by the Fonds de recherche du Québec—Santé.
Conflict of interest: none declared.
Data availability
There are no new data associated with this article.
References



