-
PDF
- Split View
-
Views
-
Cite
Cite
Pietro Enea Lazzerini, Mohamed Boutjdir, Pier Leopoldo Capecchi, Anti-Ro/SSA-antibodies and heart rhythm disturbances in the general population: the ‘dark side of the immune’, European Heart Journal, Volume 43, Issue 47, 14 December 2022, Pages 4920–4922, https://doi.org/10.1093/eurheartj/ehac575
Close - Share Icon Share
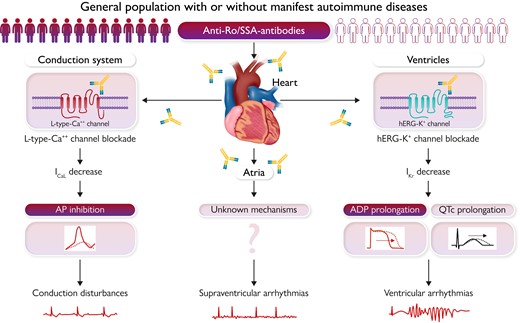
Impact of anti-Ro/SSA-antibodies on cardiac arrhythmia risk in the general population and putative underlying mechanisms. QTc, corrected QT interval; ICaL, L-type calcium current; AP, action potential; hERG-K+, human ether-a-go-go-related gene potassium channel; IKr, rapid component of the delayed rectifier potassium current; APD, action potential duration.
This editorial refers to ‘Association of anti-Ro seropositivity with cardiac rhythm and conduction disturbances’, by A. Akuka et al., https://doi.org/10.1093/eurheartj/ehac516.
Cardiac arrhythmias, including tachyarrhythmias and conduction defects/bradyarrhythmias, are one of the most important contributors to morbidity and mortality worldwide, at least in part due to incomplete understanding of underlying mechanisms limiting the effectiveness of therapeutic interventions. Specifically, malignant tachyarrhythmias and bradyarrhythmias are responsible for most cases of sudden cardiac death (SCD), representing one of the leading causes of death in Western countries.1 Whereas coronary artery disease and heart failure are the prevalent underlying substrates, cardiac structural alterations are not usually identified at post-mortem examination in up to 15% of patients, increasing to 30% in subjects younger than 40.1 It is well recognized that a percentage of the unexplained cases is due to inherited cardiac channelopathies, a group of genetically-mediated arrhythmogenic diseases caused by the dysfunction of specific ion channels, resulting in disruption of the cardiac action potential. However, post-mortem genetic testing using DNA extracted from tissue samples (molecular autopsy) failed to identify a genetic cause of death in a ∼70% of cases of unexplained SCD.1
In this scenario, accumulating recent evidence demonstrates that factors other than genetic mutations can promote arrhythmias by inducing a selective cardiac ion channel dysfunction in the absence of any structural heart defect. In particular, several arrhythmogenic autoantibodies targeting specific peptide sequences of the calcium, potassium, or sodium channels in the heart have been identified, and the term ‘autoimmune cardiac channelopathies’ has been recently coined.2
The anti-ion channel autoantibodies whose role has been most established are anti-Ro/SSA (Sjögren’s syndrome-related antigen A)-antibodies, critically involved in the pathogenesis of the autoimmune congenital heart block by cross-reacting with and inhibiting the L-type and T- type calcium channels in fetal nodal cardiomyocytes.2 Anti-Ro/SSA- and anti-La/SSB(Sjögren’s syndrome-related antigen B)-antibodies originate from an autoimmune reaction primarily directed against the intracellular Ro/La ribonucleoprotein complex, an heterogeneous antigenic structure constituted by three protein subunits (Ro-52kD, Ro-60 kD, and La-48kD). Anti-La/SSB-antibodies are mostly associated with Sjögren’s syndrome, and anti-Ro/SSA-antibodies, including the anti-Ro/SSA-52kD, and anti-Ro/SSA-60kD subtypes, are commonly found in many connective tissue diseases (CTD) and other autoimmune diseases (ADs),2 but also in a significant proportion of the adult general population (up to 3–8%), in 50–60% of cases without a manifest AD.2–4 Thus, it can be estimated that, in only Europe, up to ∼5–20 million people may present an undiagnosed anti-Ro/SSA-positivity.
Although traditionally considered invulnerable, accumulating data from basic and clinical studies indicate that anti-Ro/SSA-antibodies can be pro-arrhythmic also for the adult heart, as a result of direct and potentially reversible effects on cardiac electrophysiology.2 In fact, several recent studies demonstrated that in adults, regardless of the presence or absence of ADs, anti-Ro/SSA-positivity (specifically anti-Ro/SSA-52kD) is associated with an increased prevalence of heart rate-corrected QT-interval (QTc) prolongation and complex ventricular arrhythmias (VAs), including Torsades de Pointes ventricular tachycardia (TdP),4–9 due to an inhibitory cross-reaction with the human ether-a-go-go-related gene (hERG) potassium channel.8–11 More particularly, it has been proven that anti-Ro/SSA-52kD-antibodies can directly target the extracellular pore region of the channel, and that this interaction inhibits the hERG-potassium current, both acutely impairing the pore gating properties9,10 and chronically reducing the expression of the channel on the cell surface via facilitated endocytic degradation.8,11 Moreover, increasing evidence suggests that anti-Ro/SSA-antibodies can be also pathogenetically involved in a significant proportion of severe atrioventricular blocks(AVBs) of unknown origin in the adult,12,13 possibly as a result of a direct and reversible block of the L-type calcium channels.14
The data above point to anti-Ro/SSA-antibodies as a novel, and to date largely overlooked, risk factor for VAs and conduction disturbances in adults, but current evidence stems only from mechanistic experimental studies and clinical investigations in most cases involving rather small samples of selected patients. Thus, until now it was unknown how much these findings could be translated in a larger scale, i.e. whether circulating anti-Ro/SSA-antibodies actually impact on the risk of developing arrhythmic events in the adult general population.
An important contribution to fill this gap of knowledge now comes from Akuka and co-workers who reported, in this issue of the European Heart Journal, the results of a large (>100 000 subjects), nationwide, population-based, cross-sectional study conducted in Israel, aimed at investigating the association between anti-Ro/SSA- (and anti-La/SSB(Sjögren’s syndrome-related antigen B)-) antibodies and cardiac arrhythmias in the general population. Overall, this paper provides convincing evidence that anti-Ro/SSA seropositivity is independently associated with an increased prevalence of conduction disturbances and tachyarrhythmias in adults, regardless of the presence or not of a manifest AD.
By comparing 17 231 anti-Ro/La-seropositive subjects and 84 368 controls, the authors found that anti-Ro/SSA-positive subjects had significantly higher rates of both conduction disturbances [odds raio (OR) = 1.70, 95% confidence interval (CI) (1.50–1.91), P < .001] and tachyarrhythmias [OR = 1.47, (1.37–1.57), P < .001]. Specifically, anti-Ro/SSA-positive subjects showed a significantly higher prevalence of intraventricular conduction disturbances, i.e. right bundle branch block [OR = 1.80, (1.47–2.21), P < 0.001], left bundle branch block [OR = 1.56, (1.16–2.10), P = 0.003], left bundle hemiblock [OR = 1.40, (1.07–1.83), P = 0.012], and even more of AVBs, complete [(OR = 1.91, (1.40–2.60), P < 0.001] and incomplete [OR = 1.84, (1.46–2.32), P < 0.001], which was twice the control group. Regarding tachyarrhythmias, the presence of anti-Ro/SSA-antibodies was associated with increased rates of both VAs, i.e. paroxysmal ventricular tachycardia [OR = 1.49, (1.10–2.01), P = 0.009], and supraventricular arrhythmias, including atrial fibrillation/atrial flutter (AF/AFl) [OR = 1.44, (1.33–1.56), P < 0.001] and paroxysmal supraventricular tachycardia [OR = 1.35 (1.12–1.62), P < 0.001]. Conversely, no association was demonstrated between anti-La/SSB-positivity and any of these rhythm disorders.
The significance of these findings was confirmed and strengthened by a wide multivariate logistic-regression-analysis [adjusted for age, sex, smoking, presence of ischaemic heart disease, obesity, diabetes, hypertension, cardiomyopathy, chronic renal failure, hyper/hypothyroidism, and manifest ADs (systemic lupus erythematosus, systemic sclerosis, Sjogren’s syndrome, rheumatoid arthritis)] which demonstrated that anti-Ro/SSA seropositivity was independently and still significantly associated with both conduction disturbances [OR = 1.44, (1.25–1.66), P < 0.001] and tachyarrhythmias [OR = 1.21 (1.11–1.31), P < 0.001]. Moreover, the authors conducted several sensitivity analyses refining the definition of anti-Ro/SSA seronegativity, matching between anti-Ro/SSA-positive subjects and controls (propensity score matching), or by focusing on specific subgroups of subjects, in all cases confirming the results of the primary analysis. In this regard, it is particularly important to note, as in one of the subgroup analyses where only patients without a diagnosis of AD were considered, adjusted odds ratios between anti-Ro/SSA-positive subjects (representing 44% of the total positive cohort) and controls overlapped those found in the entire population [conduction disturbances:1.47 (1.27–1.71), P < 0.001; tachyarrhythmias:1.22 (1.12–1.37), P < 0.001], thereby providing further support to the hypothesis that anti-Ro/SSA-antibodies are per se able to promote cardiac arrhythmias by inducing direct effects on cardiac electrophysiology. Moreover, whereas the increased incidence of conduction disturbances and ventricular tachyarrhythmias in anti-Ro/SSA-positive subjects was in some way anticipated by previous studies, the higher rates of supraventricular tachyarrhythmias, particularly AF/AFl, are a completely novel finding warranting specific investigations to dissect underlying mechanisms.
This study has important strengths, primarily the large size of the investigated population and the accurate handling of potential confounding factors, but also limitations. First, the cross-sectional nature does not permit ascertaining causal and temporal relationships, although a number of previous clinical and mechanistic studies make highly conceivable the existence of both these associations. Moreover, no information is available regarding plasma levels and subtype specificities in anti-Ro/SSA-positive subjects. This point is potentially relevant, given that accumulating evidence suggests that the arrhythmogenic activity of anti-Ro/SSA-antibodies is restricted to the anti-Ro/SSA-52kD subtype, and that relatively high antibody levels are necessary to induce clinically significant electric changes.2 Indeed, it is known that among anti-Ro/SSA-positive subjects, a significant percentage does not present circulating anti-Ro/SSA-52kD-antibodies or at low levels only.4 That said, the evidence that, despite such a likely overestimation of the number of anti-Ro/SSA-positive subjects included in the study who actually carried pathogenic autoantibodies, an independent association with cardiac arrhythmias was nevertheless found, further supports the conclusion that anti-Ro/SSA-antibodies have a clinically relevant impact on the arrhythmic risk in the general population.
The key novel message of this study which translates in daily clinical practice is that anti-Ro/SSA positivity represents a significant risk factor independently contributing to conduction disturbances and tachyarrhythmias onset in the general population, regardless of the presence or not of a manifest AD (see Graphical Abstract). These findings, in association with the results of previous clinical and experimental studies demonstrating the arrhythmogenic potential of anti-Ro/SSA-antibodies, provide strong additional evidence supporting the hypothesis that these autoantibodies not only can promote the development of cardiac arrhythmias in patients with CTD or other ADs, but also are silently involved in a number of unexplained/not fully explained arrhythmic events or SCD in the general population. This is a very important point because to date, in the management of patients with cardiac arrhythmias, most physicians think about the possibility of an autoimmune mechanism only if the ‘red flag’ of a manifest AD is present. This study confirms that it is time to revisit this preconception and consider specific anti-Ro/SSA testing also in patients asymptomatic for ADs, as in some of these cases the arrhythmia itself may be ‘the autoimmune disease’! The concept is not trivial because, as detailed above, it is estimated that millions of subjects in the worldwide general population are potentially exposed to this silent risk factor. Thus, identifying circulating anti-Ro/SSA-antibodies in patients with ‘idiopathic’ rhythm disturbances (or even with structural heart disease or inherited channelopathies not responding to conventional therapeutic approaches) could lead to innovative treatment and prophylactic opportunities (including immunomodulatory interventions, decoy peptide- and peptide/antibody-based antiarrhythmic therapies)2,15 with unanticipated epidemiological implications. Large prospective studies and interventional trials are warranted to further confirm the actual clinical impact of anti-Ro/SSA-antibodies on arrhythmic events in the general population.
Funding
This work was supported by: Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR), Progetti di Rilevante Interesse Nazionale (PRIN), Bando 2017, protocollo 2017XZMBYX (to P.E.L. and P.L.C.); National Heart, Lung, and Blood Institute 1R01HL164415-01 (to MB); U.S. Department of Defense award number W81XWH-21-1-0424 (to M.B.); and a Merit Review grant I01 BX002137 from Biomedical Laboratory Research & Service of Veterans Affairs Office of Research and Development (to M.B).
References
Author notes
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
Mohamed Boutjdir and Pier Leopoldo Capecchi contributed equally to this work.
Conflict of interest: Dr. Pietro Enea Lazzerini received a grant from Roche Italia S.p.A. outside the submitted work, in 2018.



