-
PDF
- Split View
-
Views
-
Cite
Cite
Filippo Crea, Screening, diagnosis, and treatment of familial hypercholesterolaemia: a call to action, European Heart Journal, Volume 43, Issue 34, 7 September 2022, Pages 3185–3188, https://doi.org/10.1093/eurheartj/ehac479
Close - Share Icon Share
With thanks to Amelia Meier-Batschelet, Johanna Huggler, and Martin Meyer for help with compilation of this article.
 For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
This Focus Issue on dyslipidaemias contains the Special Article ‘The dawn of a new era of targeted lipid-lowering therapies’ by Lale Tokgözoğlu from the Hacettepe University Faculty of Medicine in Ankara, Turkey and Peter Libby from Harvard Medical School in Boston, MA, USA.1 The authors note that lipid risk factors for cardiovascular disease (CVD) depend in part on lifestyle, but optimum control of lipids often demands additional measures.2,3 LDL doubtless contributes causally to atherosclerosis.4,5 Recent human genetic findings have substantiated a number of novel targets for lipid-lowering therapy including apolipoprotein C-III, angiopoietin-like protein 3 and 4, apolipoprotein V, and ATP citrate lyase. These discoveries coupled with advances in biotechnology development afford new avenues for management of LDL and other aspects of lipid risk. Beyond LDL, new treatments targeting triglyceride-rich lipoproteins and lipoprotein(a) have become available and have entered clinical development. Biological and RNA-directed agents have joined traditional small-molecule approaches, which themselves have undergone considerable refinement. Innovative targeting strategies have increased the efficacy of some of these novel interventions and markedly improved their tolerability. Gene editing approaches have appeared on the horizon of lipid management. This article reviews this progress, offering insight into novel biological and therapeutic discoveries, and places them into a practical patient care perspective.
Familial hypercholesterolaemia (FH) is the most common inherited life-threatening metabolic disorder, affecting 1:300 individuals.6,7 In a Viewpoint article entitled ‘Screening in children for familial hypercholesterolaemia: start now’, Urh Groselj from the University Medical Centre Ljubljana in Slovenia, and colleagues note that with a 50% chance of inheriting the condition, every individual with an FH-causing variant also has at least one parent, and often siblings, with the same variant, presenting a health burden for affected families.8 In Europe, there are >500 000 children and 2 000 000 adults with FH. However, <5% of these children are identified and only a small fraction of all affected individuals receive life-saving treatment. Elevated LDL-cholesterol (LDL-C) levels in individuals with FH and consequent lifelong LDL-C exposure accelerate the process of atherosclerosis and lead to a 10 times excess risk of premature CVD morbidity and mortality. About 50% of untreated men with FH will have heart attacks by age 50 years and ∼30% women by age 60. Identifying individuals with FH early in life leads to early introduction of lipid-lowering therapy and improved outcomes, but too often this does not happen. The Familial Hypercholesterolemia Studies Collaboration (EAS FHSC) global registry analysis, including 42 000 adults from 56 countries, showed that FH is diagnosed late in life (at a median of 44 years) and only 2.1% of the cohort was diagnosed in childhood. Thus, the authors propose the following suggestions: (i) every country should have an FH screening programme; (ii) combined (universal, cascade, and opportunistic) screening strategies should be implemented to best fit the individual country's healthcare system; (iii) governments should provide financial support for these programmes and related care; and (iv) further research to optimize care and implementations should be conducted.
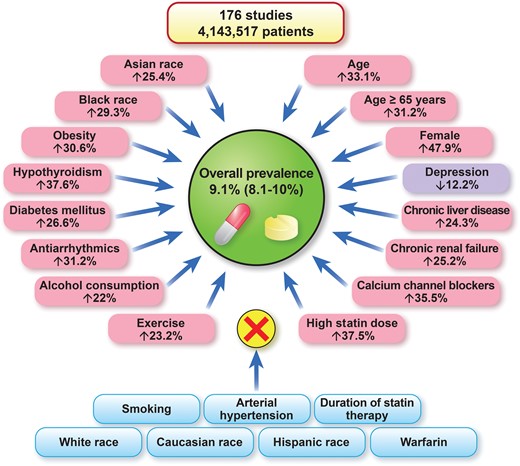
The worldwide prevalence of statin intolerance and risk factors/conditions that affect or do not affect the risk of statin intolerance.12
Statins play a key role in the management of patients with cardiovascular disease.9–11 Thus, statin intolerance (SI) represents a significant public health problem for which precise estimates of prevalence are needed as it is associated with an increased risk of cardiovascular events. In a Meta-Analysis article entitled ‘Prevalence of statin intolerance: a meta-analysis’, Ibadete Bytyçi from Umeå University in Sweden, and colleagues estimate the prevalence of SI according to different diagnostic criteria and in different disease settings, and identify possible risk factors/conditions that might increase its risk.12 The authors searched several databases up to 31 May 2021 for studies that reported the prevalence of SI. The primary endpoint was overall prevalence and prevalence according to a range of diagnostic criteria [National Lipid Association (NLA), International Lipid Expert Panel (ILEP), and European Atherosclerosis Society (EAS)] and in different disease settings. The secondary endpoint was to identify possible risk factors for SI. A random-effects model was applied to estimate the overall pooled prevalence. A total of 176 studies [112 randomized controlled trials (RCTs) and 64 cohort studies] with 4 143 517 patients were ultimately included in the analysis. The prevalence was similar when defined using NLA, ILEP, and EAS criteria (7.0, 6.7, and 5.9%, respectively). The prevalence of SI in RCTs was significantly lower compared with cohort studies (4.9% vs. 17%). The prevalence of SI in studies including both primary and secondary prevention patients was much higher than when primary or secondary prevention patients were analysed separately (18, 8.2, and 9.1%, respectively]. Statin lipid solubility did not affect the prevalence of SI (4.0% vs. 5.0%). Age [odds ratio (OR) 1.33, P = 0.04], female gender (OR 1.47, P = 0.007), Asian and Black race (P < 0.05 for both), obesity (OR 1.30, P = 0.02), diabetes mellitus (OR 1.26, P = 0.02), hypothyroidism (OR 1.37, P = 0.01), chronic liver, and renal failure (P < 0.05 for both) were significantly associated with SI in the meta-regression model. Antiarrhythmic agents, calcium channel blockers, alcohol use, and higher statin doses were also associated with a higher risk of SI (Figure 1).
The authors conclude that based on the present analysis of >4 million patients, the prevalence of SI is low when diagnosed according to international definitions. These results support the concept that the prevalence of SI might often be overestimated and highlight the need for the careful assessment of patients with potential symptoms related to SI. The contribution is accompanied by an Editorial by Christopher P. Cannon from the Brigham & Women's Hospital in Boston, MA, USA.13 Cannon notes that statin therapy is perhaps the most beneficial therapy in all of cardiology. Yet, on the internet and in public perception, statins are perhaps best known for their side effects and often viewed with great scepticism. Whenever the topic comes up, patients will report themselves as having had or knowing others who had side effects and ‘couldn't take’ statins. This has become known broadly as ‘statin intolerance’, Cannon acknowledges the reassuring findings of the study by Bytyçi et al. and describes his clinical approach in patients on statin treatment.
Homozygous FH (HoFH) is an orphan disease defined by extreme elevations in LDL-C, cutaneous xanthomas, and premature atherosclerotic CVD. In a Clinical Research article entitled ‘Aortic stenosis in homozygous familial hypercholesterolaemia: a paradigm shift over a century’, Alexandre Bélanger from the Research Institute of the McGill University Health Centre in Montréal, Canada, and colleagues note that survival has more than doubled over the past three decades. Aortic stenosis (AS) [supravalvular aortic stenosis (SVAS) or valvular aortic stenosis (VAS)] is commonly encountered.14 A systematic review was performed to summarize the current evidence on AS in HoFH and to determine whether pharmacological treatments (statins) have had an impact on clinical presentation, phenotype, and clinical course over the past nine decades. MEDLINE, Embase Classic + Embase, Cochrane Central Register of Controlled Trials, PubMed, AfricaWide, and Scopus were searched from inception to 10 November 2021. Searches identified 381 publications, of which 19 were retained; they were cross-sectional or retrospective studies. Separately, 108 individual case reports were described. Within the 424 HoFH cases, AS was identified in 57% of patients in the pre-statin era vs. 35% in patients reported more recently. With an increase in longevity due to statins and lipoprotein apheresis, a change in the proportion of patients with SVAS and VAS with a SVAS:VAS ratio of 47:53 and 10:90 for HoFH patients not on statin and on long-term statin, respectively, was noted.
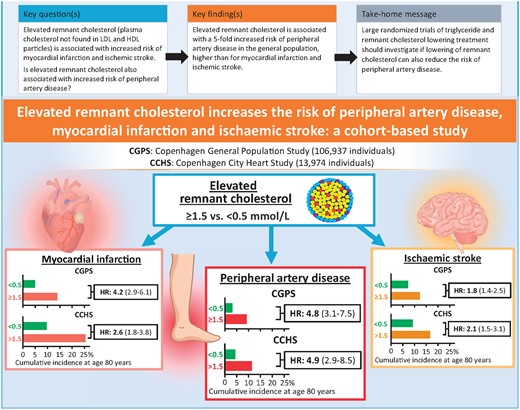
Comparison of risk of peripheral artery disease, myocardial infarction, and ischaemic stroke as a function of elevated remnant cholesterol in two studies of the Danish general population. HR, hazard ratio (95% confidence interval) after multivariable adjustment.23
The authors conclude that these data suggest that SVAS and VAS are frequent in HoFH and that the phenotype has shifted towards calcific VAS as statins and lipoprotein apheresis improve survival in these patients. This manuscript is accompanied by an Editorial by Archna Bajaj and Marina Cuchel from the Perelman School of Medicine at the University of Pennsylvania, USA.15 Bajaj and Cuchel note that the study by Belanger et al. highlights an important and relatively understudied aspect of HoFH. The extensive summary of the published data shows the significant impact that AS has in this population. Screening for AS should be done for all patients with HoFH at the time of diagnosis, and regularly after that. Until a treatment to alter the progression of AS becomes available, the data support the initiation of aggressive lipid-lowering in order to prevent the onset of disease, starting at the time of diagnosis of HoFH. There remain many unanswered questions regarding the mechanisms underlying the development of VAS, and future work in this area is desperately needed in order to develop treatments that will be likely to have a larger impact beyond the HoFH population. Access to detailed patient-level information including laboratory tests, treatment, and imaging studies over time would be essential for achieving this goal.
In a Clinical Research article entitled ‘Population genomic screening of young adults for familial hypercholesterolaemia: a cost–effectiveness analysis’, Clara Marquina from the Monash University in Melbourne, Australia, and colleagues assess the impact and cost–effectiveness of offering population genomic screening to all young adults in Australia to detect heterozygous FH.6 The authors designed a decision analytic Markov model to compare the current standard of care for heterozygous FH diagnosis in Australia (opportunistic cholesterol screening and genetic cascade testing) with the alternative strategy of population genomic screening of adults aged 18–40 years to detect pathogenic variants in the LDLR/APOB/PCSK9 genes. They used a validated cost–adaptation method to adapt findings to eight high-income countries. The model captured coronary heart disease (CHD) morbidity/mortality over a lifetime horizon, from healthcare and societal perspectives. Risk of CHD, treatment effects, prevalence, and healthcare costs were estimated from published studies. Outcomes included quality-adjusted life years (QALYs), costs, and the incremental cost–effectiveness ratio (ICER), discounted 5% annually. Sensitivity analyses were undertaken to explore the impact of key input parameters on the robustness of the model. Over the lifetime of the population (4 167 768 men and 4 129 961 women), the model estimated a gain of 33 488 years of life lived and 51 790 QALYs due to CHD prevention. Population genomic screening for FH would be cost-effective from a healthcare perspective if the per test cost was ≤AU$250, yielding an ICER of <AU$28 000 per QALY gained. From a societal perspective, population genomic screening would be cost-saving. ICERs from a societal perspective remained cost-saving after adaptation to other countries.
Marquina et al. conclude that based on their model, offering population genomic screening to all young adults for FH could be cost-effective, at testing costs that are feasible. The contribution is accompanied by an Editorial by Corey Bradley from the Columbia University Irving Medical Center in New York, NY, USA and Amit Khera and Ann Marie Navar from the University of Texas Southwestern Medical Center in Dallas, TX, USA.16 The authors conclude that healthcare systems around the world are failing people with FH by underscreening, underdiagnosing, and undertreating most patients with FH, leading to unnecessary morbidity and mortality from atherosclerotic disease. Novel approaches are urgently needed to identify those with FH and initiate therapy. Multiple interventions have been proposed as alternatives to the opportunistic or cascade screening programmes in place today, many of which may be cost-effective but have yet to be tested broadly. Regardless of the strategy used, any screening programmes for FH should be coupled with aggressive interventions to treat persons with FH before the onset of disease and systems of care that encourage long-term adherence.
The atherogenic potential of cholesterol in triglyceride-rich lipoproteins, also called remnant cholesterol, is being increasingly acknowledged.17–22 In a Clinical Research article entitled ‘Elevated remnant cholesterol increases the risk of peripheral artery disease, myocardial infarction, and ischaemic stroke: a cohort-based study’, Benjamin Nilsson Wadström from the Copenhagen University Hospital in Denmark, and colleagues tested the hypothesis that elevated remnant cholesterol is also associated with increased risk of peripheral artery disease (PAD).23 The authors studied 106 937 individuals from the Copenhagen General Population Study recruited in 2003–2015. During up to 15 years of follow-up, 1586 were diagnosed with PAD, 2570 with myocardial infarction, and 2762 with ischaemic stroke. They also studied 13 974 individuals from the Copenhagen City Heart Study recruited in 1976–1978. During up to 43 years of follow-up, 1033 were diagnosed with PAD, 2236 with myocardial infarction, and 1976 with ischaemic stroke. Remnant cholesterol was calculated from a standard lipid profile. Diagnoses were from Danish nationwide health registries. In the Copenhagen General Population Study, elevated remnant cholesterol levels were significantly associated with higher risk of PAD, up to a multivariable adjusted hazard ratio (HR) of 4.8 for individuals with levels ≥1.5 mmol/L (58 mg/dL) vs. <0.5 mmol/L (19 mg/dL). Corresponding results were 4.2 for myocardial infarction and 1.8 for ischaemic stroke. In the Copenhagen City Heart Study, corresponding HRs were 4.9 for PAD, 2.6 for myocardial infarction, and 2.1 for ischaemic stroke (Figure 2).
Wadström and colleagues conclude that elevated remnant cholesterol is associated with a five-fold increased risk of PAD in the general population, higher than for myocardial infarction and ischaemic stroke. The manuscript is accompanied by an Editorial by Connie Hess and Marc Bonaca from University of Colorado School of Medicine in Aurora, CO, USA.17 The authors note that there have been important gains in therapeutic development to reduce risk in patients with atherosclerotic cardiovascular disease (ASCVD). In addition, the advent of lower cost, lower frequency therapies such as inclisiran creates opportunities for earlier intervention for primary or even primordial prevention. As options increase, clinicians and patients will need more information to better personalize preventive strategies. Remnant cholesterol holds promise as an additional risk factor that can be measured and potentially modified to better understand and reduce risk for ASCVD and specifically PAD.
The editors hope that this issue of the European Heart Journal will be of interest to its readers.
Dr. Crea reports speaker fees from Amgen, Astra Zeneca, Servier, BMS, other from GlyCardial Diagnostics, outside the submitted work.




