-
PDF
- Split View
-
Views
-
Cite
Cite
Magalie Ladouceur, Alexander Van De Bruaene, Robert Kauling, Werner Budts, Jolien Roos-Hesselink, Sandra Villagrá Albert, Inmaculada Sanchez Perez, Berardo Sarubbi, Flavia Fusco, Pastora Gallego, Maria Jose Rodriguez-Puras, Judith Bouchardy, Coralie Blanche, Tobias Rutz, Katja Prokselj, Fabien Labombarda, Laurence Iserin, Tom Wong, Michael A Gatzoulis, A new score for life-threatening ventricular arrhythmias and sudden cardiac death in adults with transposition of the great arteries and a systemic right ventricle, European Heart Journal, Volume 43, Issue 28, 21 July 2022, Pages 2685–2694, https://doi.org/10.1093/eurheartj/ehac288
Close - Share Icon Share
Abstract
To investigate the incidence of major adverse ventricular arrhythmias and related events (MAREs) and to develop a stratification tool predicting MAREs in adults with a systemic right ventricle (sRV).
In a multicentre approach, all adults (≥16 years old) with a sRV undergoing follow-up between 2000 and 2018 were identified. The incidence of MAREs, defined as sudden cardiac death, sustained ventricular tachycardia, and appropriate implantable cardioverter-defibrillator (ICD) therapy, was analysed. The association of MAREs with clinical, electrical, and echocardiographic parameters was evaluated. A total of 1184 patients (median age 27.1 years; interquartile range 19.9–34.9 years; 59% male; 70% with atrial switch repair for D-transposition of the great arteries) were included. The incidence of MAREs was 6.3 per 1000 patient-years. On multivariate analysis, age, history of heart failure, syncope, QRS duration, severe sRV dysfunction and at least moderate left ventricular outflow tract obstruction were retained in the final model with a C-index of 0.78 [95% confidence interval (CI) 0.72–0.83] and a calibration slope of 0.93 (95% CI 0.64–1.21). For every five ICDs implanted in patients with a 5-year MARE risk >10%, one patient may potentially be spared from a MARE.
Sudden cardiac death remains a devastating cause of death in a contemporary adult cohort with a sRV. A prediction model based on clinical, electrocardiographic, and echocardiographic parameters was devised to estimate MARE risk and to identify high-risk patients who may benefit from primary prevention ICD implantation.
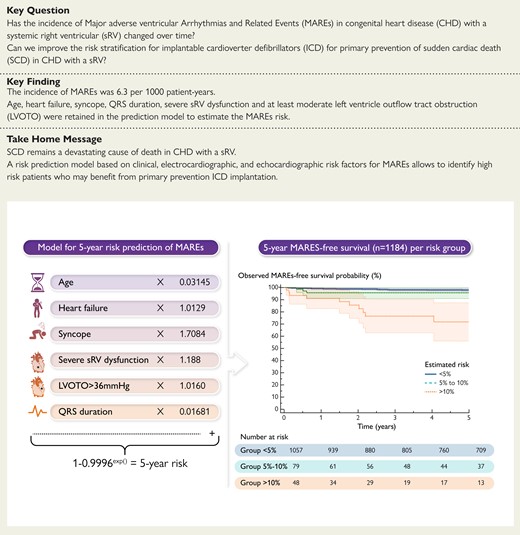
Prediction of malignant ventricular arrhythmia and/or sudden cardiac death in the systemic right ventricle (sRV). Major adverse ventricular arrhythmias and related event (MARE) risk prediction model and Kaplan–Meier survival curves for freedom from MAREs plotted and compared according to the risk groups. Shaded areas correspond to 95% confidence intervals. LVOTO, left ventricular outflow tract obstruction; MAREs, major adverse ventricular arrhythmias and related events.
See the editorial comment for this article ‘Sudden cardiac death in adults with transposition of the great arteries and systemic right ventricles: preventing (night)MAREs’, by Paul Khairy, https://doi.org/10.1093/eurheartj/ehac228.
Introduction
Sudden cardiac death (SCD) after repair of congenital heart disease (CHD) remains devastating, concerning an increasing number of late survivors who have benefited from improved medical and surgical care.1,2 The risk of SCD has been extensively studied in patients with tetralogy of Fallot, where the right ventricle is typically subjected to volume and/or pressure overload supporting the pulmonary circulation. In contrast, in patients with a right ventricle in the systemic position, subjected to systemic pressures while supporting the systemic circulation, as in complete transposition of the great arteries (D-TGA) with previous atrial switch repair (Mustard or Senning) and in congenitally corrected transposition (L-TGA), SCD has been much less studied.3 Late complications in TGA include systemic right ventricular (sRV) failure, systemic (tricuspid) atrioventricular valve regurgitation, rhythm disturbances, and SCD, the latter being one of the leading causes of death in this setting.4–7 A population-based study of D-TGA in the late 1990s reported a SCD incidence of 4.9 per 1000 patient-years, second only to aortic stenosis and more than three-fold greater compared to tetralogy of Fallot.1 With regard to L-TGA, SCD due to documented ventricular tachycardia has been reported in 5.4% of patients with reduced sRV ejection fraction (<35%) during a mean follow-up of 7.2 years.8
To date, the identified risk factors for SCD in patients with sRV are mainly confined to atrial tachyarrhythmias, and heart failure.4,8,9 Risk stratification for implantable cardioverter-defibrillators (ICDs) for primary prevention of SCD remains elusive, even in patients with a clinical profile deemed high risk in the absence of a near-fatal event.10,11 As a result, international recommendations for ICD implantation for primary prevention in these patients are supported by weak evidence and thus not regularly applied. The small sample size of previous studies on the sRV was an important limitation to robustly determine predictors of SCD.4,8,9,12,13 Moreover, over the last decade the population of patients with sRV has grown older and consequently the incidence of such events may have changed. Therefore, we sought to determine the incidence and risk factors of SCD and important ventricular arrhythmic events [major adverse ventricular arrhythmias and related events (MAREs)] in a large, contemporary, multicentre cohort of adults with a sRV, and to develop a risk stratification model to identify high-risk patients who could benefit from ICD implantation for primary prevention.
Methods
We conducted a retrospective, observational study involving 11 European centres with dedicated adult CHD (ACHD) care (Hôpital Européen Georges Pompidou, Paris—Royal Brompton Hospital, London—Erasmus Medical Center, Rotterdam—Leuven University Hospital, Leuven—Monaldi Hospital, Naples—Hospital Virgen del Rocio, Seville—Ramon y Cajal Hospital, Madrid—Centre Hospitalier Universitaire Vaudois, Lausanne—Hôpitaux Universitaires de Genève, Geneva—University Medical Center Ljubljana, Ljubljana—Centre Hospitalo-Universitaire de Caen, Caen). The study complies with the Declaration of Helsinki and ethical or research committee approval was obtained in each contributing centre.
Study population
We included all patients who (i) were ≥16 years of age with D-TGA or L-TGA and a sRV, (ii) with available data from clinical assessment at their first visit at the ACHD centre between January 2000 and December 2018 and (iii) a follow-up of 2 years or longer. Patients presenting with a history of MAREs at or before their index clinical assessment, and patients with univentricular heart physiology were excluded.
Study outcome
The main outcome was MAREs defined as SCD, resuscitated SCD, sustained ventricular tachycardia with or without hemodynamic compromise, appropriate therapy (shock or anti-tachycardia pacing) from ICD implanted for primary prevention.
Sustained ventricular tachycardia was defined as >30 s with a rate >100 b.p.m. Death was classified as sudden if it occurred unexpectedly (i) within 1 h of onset of cardiac manifestations in the absence of preceding hemodynamic deterioration, (ii) during sleep or (iii) within 24 h after the patient was last seen alive and apparently clinically stable.14
Baseline measurements and follow-up
Data were independently collected at each participating centre using uniform methodology. The patients’ medical records were reviewed for demographics, medical, and surgical details. Parameters obtained were chosen on the basis of a literature review on univariate predictors of outcome in patients with a sRV.8,9,12,13,15 Complex TGA was defined as TGA associated with ventricular septal defect (VSD), left ventricular outflow tract (LVOT) obstruction, and/or aortic coarctation. Transposition of the great arteries associated with isolated atrial septal defect or persistent ductus arteriosus was not considered complex. Associated pulmonary arterial hypertension was noted in the presence of Eisenmenger syndrome or when pre-capillary pulmonary hypertension was invasively confirmed according to the ESC guidelines.16
Heart failure was defined as hospitalization for decompensated heart failure or initiation of diuretic therapy for clinical signs and symptoms of heart failure. Atrial arrhythmia encompassed all the types of supraventricular arrhythmia including ectopic atrial tachycardia, atrioventricular nodal re-entrant tachycardia, atrioventricular reciprocating tachycardia, intra-atrial re-entry tachycardia, atrial flutter, and atrial fibrillation.
Baseline was determined as the first visit at the ACHD centre during the study period including a clinical examination and a 12-lead electrocardiography. History of syncope, palpitations, and New York Heart Association (NYHA) class reported during this first visit were collected. Syncope was defined as a transient loss of consciousness with no identifiable cause at or before first evaluation. Echocardiography was considered if it was performed within the year before or after the baseline visit by an experienced operator. Systemic right ventricular function was assessed with the use of a 4-point semiquantitative scale (normal, mildly, moderately, or severely impaired) because of inherent difficulties in applying quantitative measures.17 Tricuspid regurgitation was graded as none, mild, moderate, or severe.17 Left ventricular outflow tract left ventricular outflow tract was based on Doppler gradients considered at least moderate, when maximum LVOT gradient >36 mmHg or Doppler maximum velocity >3 m/s.18 Brain natriuretic peptide (BNP), cardiac magnetic resonance (CMR), and imaging and cardiopulmonary exercise testing (CPET) were considered when carried out within the 3 years preceding or following the date of inclusion. Holter and pacemaker/ICD monitoring, and electrophysiological studies were obtained from medical records just before or at baseline. Rhythm abnormalities recorded by Holter or pacemaker/ICD monitoring were classified as sustained atrial arrhythmia (≥30 s), non-sustained ventricular tachycardia (<30 s), and conduction abnormalities with sinus node dysfunction and complete heart block.
The first episode of heart failure or atrial arrhythmia and the first pacemaker implantation were longitudinally recorded from birth in patients with L-TGA and from the atrial switch in patients with D-TGA to the end of the study. All events occurring after patient inclusion were managed as time-dependent variables. The latest follow-up data were obtained by reviewing clinical medical records or by telephone contact and/or consultation with the patient.
Statistical analysis
Data were expressed as mean ± standard deviation (SD) for normally distributed variables and as median with interquartile range (IQR) for non-normal data. The normality of quantitative variables was determined using the Shapiro–Wilk test. Between-group comparison was performed using Student’s t-test or Mann–Whitney U test for normally and non-normally distributed data, respectively. Between-group comparison of categorical data was done using the Pearson χ2 test or the Fisher’s exact test, as appropriate.
Follow-up duration for all patients was calculated from the date of their first, index evaluation as an adult at the tertiary centre to the date of reaching the study endpoint, death from another cause, heart transplantation, or the date of their latest evaluation within the study period. Patient-years were accrued from time of entry until the occurrence of MAREs or the study end. Freedom from MAREs was plotted with the Kaplan–Meier method.
Missing data were assumed to be missing at random and imputed using multiple imputation with chained equations.19 The imputation model included all predictors of missingness, the outcome, all predictors of the risk model, and the estimate of the cumulative hazard function.20 A total of 17 imputed data sets were generated, and estimates obtained from the imputed data sets were combined using the Rubin rule.21 Finally, a complete case analysis was conducted as a sensitivity analysis.
The association of predictors with survival free from MAREs was assessed using Cox proportional hazards method. First episodes of atrial arrhythmia, pacemaker implantation, and heart failure occurring during follow-up were considered as time-dependent covariates. Proportionality of hazards was confirmed in each case by assessing the correlation between the scaled Schoenfeld residuals and time.
All predictors with a univariate value <0.05 were included into a multivariate model, after which stepwise backward selection based on Akaike information criterion (AIC) allowed to determine the best-fit model.
Bootstrapping was used to evaluate the performance of the score model. For this purpose, 200 bootstrap samples were generated. The calibration slope was used to assess the degree of agreement between the observed and predicted hazards of MAREs. The C-index was used to measure how well the model discriminated between high- and low-risk patients, using the prediction score on a continuous scale. The C-index and calibration results presented are an average of the bootstrapped samples. Graphic comparisons of the observed and predicted risk of MAREs at 5 years by clinical risk group (0% to <5%, 5% to ≤10%, and >10%) are provided. A P-value < 0.05 was considered statistically significant. Analyses were performed using SAS statistical software (Version 9.4, SAS Institute Inc., Cary, NC, USA) and MedCalc (MedCalc Software, Mariakerke, Belgium).
Results
Study population
Overall, 1240 patients were considered, with 1184 fulfilling entry criteria and constituting our study population (Figure 1). Their characteristics are shown in Table 1: median age at baseline was 27.1 years (19.9–34.9), 700 patients (59%) were male. Seventy per cent of patients had D-TGA palliated with an atrial switch procedure (64.2% Mustard, 35.8% Senning). A quarter of patients had associated cardiac defects: 292 (25%) a VSD, 190 (16%) pulmonary stenosis or atresia, and 22 (2%) aortic coarctation. Tricuspid valve surgery was performed in 84 (7%; 82% in L-TGA), a left ventricle to pulmonary artery conduit operation in 47 (4%), and a pulmonary valvulotomy or a subpulmonary resection in 13 (1%).
![Flowchart of the study population. The 41 patients with inadequate data were considered as lost to follow-up. They were 28 patients with complete transposition of the great arteries and 13 with congenitally corrected transposition of the great arteries [41% men, median age 30 (25–34) years]. Their characteristics are shown in Supplementary material online, Table S1. D-TGA, complete transposition of the great arteries; L-TGA, congenitally corrected transposition of the great arteries; sRV, systemic right ventricle.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/eurheartj/43/28/10.1093_eurheartj_ehac288/1/m_ehac288f1.jpeg?Expires=1750185065&Signature=vJzPZrIOBeRNq4N5IEXPRg7W5og9VIJqn84N1lENRhXU-~C-cHH71lzdwbbtffXCija4DITb3C9owAB7yzL-dAPnQxl5nNNyWQR7vS-upuGwQB51QvPR00Bnqdt6rJZKksDjrkhT~zOIDpKvjFkhVH4CjpGie2NC38cEcbWjz~qHmjYvNIjNvNmOy69ld1ByQJDXXC8H~Rm5L0VOPTNefv9bPOmyTcSpV8517KXpYl3ZFv9HHdWngzx5EKp8TByTAy4QCBNdIaMxYZL8LdsFPjB9hRTiglqO50f1r-QADkKUzqQcFcpj1YNKQXXcVcmVKnbyi0pfDeMLIrdur8QstQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Flowchart of the study population. The 41 patients with inadequate data were considered as lost to follow-up. They were 28 patients with complete transposition of the great arteries and 13 with congenitally corrected transposition of the great arteries [41% men, median age 30 (25–34) years]. Their characteristics are shown in Supplementary material online, Table S1. D-TGA, complete transposition of the great arteries; L-TGA, congenitally corrected transposition of the great arteries; sRV, systemic right ventricle.
| Characteristics . | All patients (n = 1184) . | MAREs (n = 59) . | Remainder (n = 1125) . | P-value . |
|---|---|---|---|---|
| Age at baseline (years) | 27.1 (19.9–34.9) | 32.8 (24.2–42.0) | 26.9 (19.7–34.2) | <0.001 |
| Male sex | 700 (59.1) | 40 (67.8) | 660 (58.7) | 0.165 |
| Type of TGA | ||||
| D-TGA | 834 (70.4) | 37 (62.7) | 797 (70.8) | 0.182 |
| L-TGA | 350 (29.6) | 22 (37.2) | 328 (29.2) | |
| Complex form | 299 (25.3) | 20 (33.9) | 279 (24.8) | <0.117 |
| PAH | 91 (7.7) | 12 (20.3) | 79 (7.0) | <0.001 |
| Medical history before inclusion | ||||
| Atrial arrhythmia | 286 (24.2) | 30 (50.8) | 256 (22.8) | <0.001 |
| Heart failure | 107 (9.0) | 21 (35.6) | 86 (7.6) | <0.001 |
| Pacemaker | 221 (18.7) | 22 (37.3) | 199 (17.7) | <0.001 |
| Holter (n = 949) | ||||
| NSVT | 116 (13.9) | 12 (30.0) | 104 (13.0) | 0.001 |
| Atrial arrhythmia (>30 s) | 214 (25.6) | 24 (60.0) | 190 (23.8) | <0.001 |
| Conduction abnormalitiesa | 112 (13.4) | 4 (10.0) | 108 (13.5) | 0.841 |
| Baseline (n, available data) | ||||
| Symptoms (n = 1184) | ||||
| NYHA class > II | 81 (6.98) | 9 (15.3) | 72 (6.4) | 0.009 |
| Palpitations | 234 (19.8) | 19 (32.2) | 215 (19.1) | 0.014 |
| Syncope | 46 (3.9) | 7 (11.9) | 39 (3.5) | <0.001 |
| Cardiac medication (n = 1184) | ||||
| Beta-blockers | 227 (19.2) | 14 (23.7) | 213 (18.9) | 0.362 |
| ACEi/ARB | 352 (29.7) | 21 (35.6) | 331 (29.4) | 0.312 |
| Diuretics | 140 (11.8) | 17 (28.8) | 123 (10.9) | <0.001 |
| ECG (n = 972) | ||||
| Loss of sinus rhythmb (n = 1084) | 238 (24.5) | 20 (33.9) | 218 (21.3) | 0.083 |
| QRS durationc (ms) (n = 970) | 106 (94–121) | 122 (108.5–158.5) | 106 (93–120) | <0.001 |
| QT duration (ms) (n = 557) | 402 (372–435) | 405 (374–450) | 402 (372–434) | 0.316 |
| Echocardiography (n = 1184) | ||||
| sRV function | <0.001 | |||
| Normal | 382 (32.3) | 11 (18.6) | 371 (33.0) | |
| Mildly impaired | 394 (33.3) | 14 (23.7) | 380 (33.8) | |
| Moderately impaired | 272 (23.0) | 15 (25.4) | 257 (22.8) | |
| Severely impaired | 136 (11.5) | 19 (32.2) | 117(10.4) | |
| TR grade (moderate to severe) | 374 (31.6) | 29 (49.2) | 345 (30.7) | 0.003 |
| LVOT obstruction (>36 mmHg) | 198 (16.7) | 20 (33.9) | 178 (15.8) | <0.001 |
| Characteristics . | All patients (n = 1184) . | MAREs (n = 59) . | Remainder (n = 1125) . | P-value . |
|---|---|---|---|---|
| Age at baseline (years) | 27.1 (19.9–34.9) | 32.8 (24.2–42.0) | 26.9 (19.7–34.2) | <0.001 |
| Male sex | 700 (59.1) | 40 (67.8) | 660 (58.7) | 0.165 |
| Type of TGA | ||||
| D-TGA | 834 (70.4) | 37 (62.7) | 797 (70.8) | 0.182 |
| L-TGA | 350 (29.6) | 22 (37.2) | 328 (29.2) | |
| Complex form | 299 (25.3) | 20 (33.9) | 279 (24.8) | <0.117 |
| PAH | 91 (7.7) | 12 (20.3) | 79 (7.0) | <0.001 |
| Medical history before inclusion | ||||
| Atrial arrhythmia | 286 (24.2) | 30 (50.8) | 256 (22.8) | <0.001 |
| Heart failure | 107 (9.0) | 21 (35.6) | 86 (7.6) | <0.001 |
| Pacemaker | 221 (18.7) | 22 (37.3) | 199 (17.7) | <0.001 |
| Holter (n = 949) | ||||
| NSVT | 116 (13.9) | 12 (30.0) | 104 (13.0) | 0.001 |
| Atrial arrhythmia (>30 s) | 214 (25.6) | 24 (60.0) | 190 (23.8) | <0.001 |
| Conduction abnormalitiesa | 112 (13.4) | 4 (10.0) | 108 (13.5) | 0.841 |
| Baseline (n, available data) | ||||
| Symptoms (n = 1184) | ||||
| NYHA class > II | 81 (6.98) | 9 (15.3) | 72 (6.4) | 0.009 |
| Palpitations | 234 (19.8) | 19 (32.2) | 215 (19.1) | 0.014 |
| Syncope | 46 (3.9) | 7 (11.9) | 39 (3.5) | <0.001 |
| Cardiac medication (n = 1184) | ||||
| Beta-blockers | 227 (19.2) | 14 (23.7) | 213 (18.9) | 0.362 |
| ACEi/ARB | 352 (29.7) | 21 (35.6) | 331 (29.4) | 0.312 |
| Diuretics | 140 (11.8) | 17 (28.8) | 123 (10.9) | <0.001 |
| ECG (n = 972) | ||||
| Loss of sinus rhythmb (n = 1084) | 238 (24.5) | 20 (33.9) | 218 (21.3) | 0.083 |
| QRS durationc (ms) (n = 970) | 106 (94–121) | 122 (108.5–158.5) | 106 (93–120) | <0.001 |
| QT duration (ms) (n = 557) | 402 (372–435) | 405 (374–450) | 402 (372–434) | 0.316 |
| Echocardiography (n = 1184) | ||||
| sRV function | <0.001 | |||
| Normal | 382 (32.3) | 11 (18.6) | 371 (33.0) | |
| Mildly impaired | 394 (33.3) | 14 (23.7) | 380 (33.8) | |
| Moderately impaired | 272 (23.0) | 15 (25.4) | 257 (22.8) | |
| Severely impaired | 136 (11.5) | 19 (32.2) | 117(10.4) | |
| TR grade (moderate to severe) | 374 (31.6) | 29 (49.2) | 345 (30.7) | 0.003 |
| LVOT obstruction (>36 mmHg) | 198 (16.7) | 20 (33.9) | 178 (15.8) | <0.001 |
Continuous variables are reported as median and interquartile range and quantitative variables as crude value and percentage (%). LVOT, left ventricle outflow tract; MARES, major adverse ventricular arrhythmias, and related events; NSVT, non-sustained ventricular tachycardia; PAH, pulmonary arterial hypertension; sRV, systemic right ventricle; VT, ventricular tachycardia.
Conduction abnormalities included sinus node dysfunction and complete heart block.
Loss of sinus rhythm corresponded to atrial arrhythmia, sinus node dysfunction, atrial pacing, ventricular pacing, complete heart block, and atrial/ventricular premature beats on ECG at baseline.
Excluding patients with ventricular pacing on ECG at baseline
| Characteristics . | All patients (n = 1184) . | MAREs (n = 59) . | Remainder (n = 1125) . | P-value . |
|---|---|---|---|---|
| Age at baseline (years) | 27.1 (19.9–34.9) | 32.8 (24.2–42.0) | 26.9 (19.7–34.2) | <0.001 |
| Male sex | 700 (59.1) | 40 (67.8) | 660 (58.7) | 0.165 |
| Type of TGA | ||||
| D-TGA | 834 (70.4) | 37 (62.7) | 797 (70.8) | 0.182 |
| L-TGA | 350 (29.6) | 22 (37.2) | 328 (29.2) | |
| Complex form | 299 (25.3) | 20 (33.9) | 279 (24.8) | <0.117 |
| PAH | 91 (7.7) | 12 (20.3) | 79 (7.0) | <0.001 |
| Medical history before inclusion | ||||
| Atrial arrhythmia | 286 (24.2) | 30 (50.8) | 256 (22.8) | <0.001 |
| Heart failure | 107 (9.0) | 21 (35.6) | 86 (7.6) | <0.001 |
| Pacemaker | 221 (18.7) | 22 (37.3) | 199 (17.7) | <0.001 |
| Holter (n = 949) | ||||
| NSVT | 116 (13.9) | 12 (30.0) | 104 (13.0) | 0.001 |
| Atrial arrhythmia (>30 s) | 214 (25.6) | 24 (60.0) | 190 (23.8) | <0.001 |
| Conduction abnormalitiesa | 112 (13.4) | 4 (10.0) | 108 (13.5) | 0.841 |
| Baseline (n, available data) | ||||
| Symptoms (n = 1184) | ||||
| NYHA class > II | 81 (6.98) | 9 (15.3) | 72 (6.4) | 0.009 |
| Palpitations | 234 (19.8) | 19 (32.2) | 215 (19.1) | 0.014 |
| Syncope | 46 (3.9) | 7 (11.9) | 39 (3.5) | <0.001 |
| Cardiac medication (n = 1184) | ||||
| Beta-blockers | 227 (19.2) | 14 (23.7) | 213 (18.9) | 0.362 |
| ACEi/ARB | 352 (29.7) | 21 (35.6) | 331 (29.4) | 0.312 |
| Diuretics | 140 (11.8) | 17 (28.8) | 123 (10.9) | <0.001 |
| ECG (n = 972) | ||||
| Loss of sinus rhythmb (n = 1084) | 238 (24.5) | 20 (33.9) | 218 (21.3) | 0.083 |
| QRS durationc (ms) (n = 970) | 106 (94–121) | 122 (108.5–158.5) | 106 (93–120) | <0.001 |
| QT duration (ms) (n = 557) | 402 (372–435) | 405 (374–450) | 402 (372–434) | 0.316 |
| Echocardiography (n = 1184) | ||||
| sRV function | <0.001 | |||
| Normal | 382 (32.3) | 11 (18.6) | 371 (33.0) | |
| Mildly impaired | 394 (33.3) | 14 (23.7) | 380 (33.8) | |
| Moderately impaired | 272 (23.0) | 15 (25.4) | 257 (22.8) | |
| Severely impaired | 136 (11.5) | 19 (32.2) | 117(10.4) | |
| TR grade (moderate to severe) | 374 (31.6) | 29 (49.2) | 345 (30.7) | 0.003 |
| LVOT obstruction (>36 mmHg) | 198 (16.7) | 20 (33.9) | 178 (15.8) | <0.001 |
| Characteristics . | All patients (n = 1184) . | MAREs (n = 59) . | Remainder (n = 1125) . | P-value . |
|---|---|---|---|---|
| Age at baseline (years) | 27.1 (19.9–34.9) | 32.8 (24.2–42.0) | 26.9 (19.7–34.2) | <0.001 |
| Male sex | 700 (59.1) | 40 (67.8) | 660 (58.7) | 0.165 |
| Type of TGA | ||||
| D-TGA | 834 (70.4) | 37 (62.7) | 797 (70.8) | 0.182 |
| L-TGA | 350 (29.6) | 22 (37.2) | 328 (29.2) | |
| Complex form | 299 (25.3) | 20 (33.9) | 279 (24.8) | <0.117 |
| PAH | 91 (7.7) | 12 (20.3) | 79 (7.0) | <0.001 |
| Medical history before inclusion | ||||
| Atrial arrhythmia | 286 (24.2) | 30 (50.8) | 256 (22.8) | <0.001 |
| Heart failure | 107 (9.0) | 21 (35.6) | 86 (7.6) | <0.001 |
| Pacemaker | 221 (18.7) | 22 (37.3) | 199 (17.7) | <0.001 |
| Holter (n = 949) | ||||
| NSVT | 116 (13.9) | 12 (30.0) | 104 (13.0) | 0.001 |
| Atrial arrhythmia (>30 s) | 214 (25.6) | 24 (60.0) | 190 (23.8) | <0.001 |
| Conduction abnormalitiesa | 112 (13.4) | 4 (10.0) | 108 (13.5) | 0.841 |
| Baseline (n, available data) | ||||
| Symptoms (n = 1184) | ||||
| NYHA class > II | 81 (6.98) | 9 (15.3) | 72 (6.4) | 0.009 |
| Palpitations | 234 (19.8) | 19 (32.2) | 215 (19.1) | 0.014 |
| Syncope | 46 (3.9) | 7 (11.9) | 39 (3.5) | <0.001 |
| Cardiac medication (n = 1184) | ||||
| Beta-blockers | 227 (19.2) | 14 (23.7) | 213 (18.9) | 0.362 |
| ACEi/ARB | 352 (29.7) | 21 (35.6) | 331 (29.4) | 0.312 |
| Diuretics | 140 (11.8) | 17 (28.8) | 123 (10.9) | <0.001 |
| ECG (n = 972) | ||||
| Loss of sinus rhythmb (n = 1084) | 238 (24.5) | 20 (33.9) | 218 (21.3) | 0.083 |
| QRS durationc (ms) (n = 970) | 106 (94–121) | 122 (108.5–158.5) | 106 (93–120) | <0.001 |
| QT duration (ms) (n = 557) | 402 (372–435) | 405 (374–450) | 402 (372–434) | 0.316 |
| Echocardiography (n = 1184) | ||||
| sRV function | <0.001 | |||
| Normal | 382 (32.3) | 11 (18.6) | 371 (33.0) | |
| Mildly impaired | 394 (33.3) | 14 (23.7) | 380 (33.8) | |
| Moderately impaired | 272 (23.0) | 15 (25.4) | 257 (22.8) | |
| Severely impaired | 136 (11.5) | 19 (32.2) | 117(10.4) | |
| TR grade (moderate to severe) | 374 (31.6) | 29 (49.2) | 345 (30.7) | 0.003 |
| LVOT obstruction (>36 mmHg) | 198 (16.7) | 20 (33.9) | 178 (15.8) | <0.001 |
Continuous variables are reported as median and interquartile range and quantitative variables as crude value and percentage (%). LVOT, left ventricle outflow tract; MARES, major adverse ventricular arrhythmias, and related events; NSVT, non-sustained ventricular tachycardia; PAH, pulmonary arterial hypertension; sRV, systemic right ventricle; VT, ventricular tachycardia.
Conduction abnormalities included sinus node dysfunction and complete heart block.
Loss of sinus rhythm corresponded to atrial arrhythmia, sinus node dysfunction, atrial pacing, ventricular pacing, complete heart block, and atrial/ventricular premature beats on ECG at baseline.
Excluding patients with ventricular pacing on ECG at baseline
At baseline, most patients (n = 1077, 91.0%) were free of a history of heart failure hospitalization albeit 34.5% had moderate to severe sRV dysfunction, 31% moderate to severe tricuspid regurgitation, and 32% were receiving cardiac medication: angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers (29.7%) and/or diuretics (11.8%). Nineteen per cent of patients were treated with beta-blockers. Two hundred eighty-six (24.2%) had already experienced episodes of atrial arrhythmia, whereas 221 (18.7%) had a pacemaker, and 24 (2.0%) had received an ICD for primary prevention. Brain natriuretic peptide, CPET, and CMR values were available in <57% of patients (values are shown in Supplementary material online, Table S2).
Seventy-nine patients (6.7%) died during a median follow-up of 9.4 (4.9–12.9) years, 37 from heart failure (46.8%), 13 from MAREs (16.4%), 8 from non-cardiac causes (10.1%), whereas in 21 cases the cause of death was not specified. Twenty-nine (2.4%) patients received a heart transplant during the study. The overall actuarial survival rate, with censure of patients transplanted, was 92.5%, 89.3%, and 86.1% at 10, 15, and 20 years, respectively, corresponding to an averaged actuarial mortality rate of 0.43% per annum.
During the study period, 279 (23%) patients experienced new cardiovascular events including atrial arrhythmia in 146 (12%), heart failure in 117 (10%), and pacemaker insertion in 105 (9%). Furthermore, 37 (3%) patients underwent ICD implantation for primary prevention during the study.
Major adverse ventricular arrhythmia and related events
Fifty-nine patients (5.0%; 37 with D-TGA and 22 with L-TGA) experienced MAREs during the follow-up [median 9.4 (5.5–12.9) years]: SCD (n = 12), resuscitated SCD (n = 7), sustained VT with hemodynamic compromise (n = 21), and without (n = 8), appropriate anti-tachycardia pacing (n = 3), or ICD shocks (n = 8). The overall incidence of the primary endpoint was 6.3 per 1000 patient-years (Figure 2A). Patients reaching the combined endpoint were older and had more cardiovascular comorbidities including atrial arrhythmia (51% vs. 23%, P < 0.001), heart failure (35% vs. 7%, P < 0.001), pacemaker (37% vs. 18%, P < 0.001), and/or pulmonary arterial hypertension (20% vs. 7%, P < 0.001). Major adverse ventricular arrhythmias and related events were also more common amongst patients with moderate to severe LVOT obstruction (34% vs. 16% in the remainder, P < 0.001).
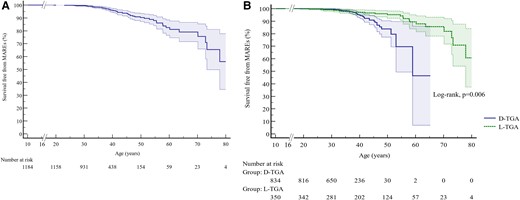
Survival free from major adverse ventricular arrhythmias and related events in the entire population of patients with a systemic right ventricle (A), and in patients with transposition of the great arteries palliated by atrial switch and congenitally corrected transposition of the great arteries (B). Entry time was defined as age at the beginning of the follow-up, exit time was defined as age at death, age at major adverse ventricular arrhythmias and related events, or age at the end of follow-up. Shaded areas correspond to 95% confidence intervals.
Holter electrocardiogram (ECG) or pacemaker/ICD monitoring at follow-up was available in 949 patients (80%). Patients who experienced a cardiac arrest or a life-threatening ventricular arrhythmia had more frequent episodes of atrial arrhythmia or non-sustained ventricular tachycardia prior to the event (Table 1, P ≤ 0.001). Sinus node dysfunction or complete heart block was not associated with MAREs. A programmed ventricular stimulation was performed in only 31 patients (Supplementary material online).
Considering symptoms at baseline, NYHA functional class >II, palpitations and syncope were more frequently reported amongst patients who subsequently met the primary endpoint (Table 1, P < 0.05). Baseline electrocardiographic abnormalities showed a longer QRS duration in patients who subsequently experienced MAREs [122 ms (95% CI 108.5–158.5) vs. 106 ms (95% CI 93.0–120.0); P < 0.001].
Predictors and the risk prediction score of major adverse ventricular arrhythmias and related events
Table 2 presents the results of univariate Cox regression analysis for MAREs. Each predictor with a significant (P < 0.05) univariate linear relationship with the primary outcome was fitted into a multivariate model, after which stepwise backward selection was performed leading to the selection of six variables: age, history of heart failure, syncope, QRS duration, severe sRV dysfunction, and at least moderate LVOT obstruction. Supplementary material online, Table S3 illustrates the process of models’ selection based on AIC value. Models including the history of atrial arrhythmia or non-sustained ventricular tachycardia showed a difference in AIC relative to AICmin (final model) >2 and were not retained. The estimates of hazard ratios for the final model are reported in Table 3. Complete data were available for 817 patients (69.0%). Among predictors, QRS duration including paced QRS was missing in 18.1% and atrial arrhythmia and or non-sustained ventricular tachycardia on Holter ECG or pacemaker/ICD monitoring in 19.8%. As a sensitivity analysis, we repeated the process of developing the risk prediction model for patients with complete data. As can be appreciated from Supplementary material online, Table S4, this resulted in the inclusion of the same predictor variables with only small changes to the coefficients in the resulting model.
| . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| Age at baseline (per year increase) | 1.05 | 1.03–1.06 | <0.001 |
| Female sex | 1.46 | 0.85–2.53 | 0.171 |
| Type of TGA (L-TGA) | 1.74 | 1.02–2.98 | 0.042 |
| Complex form | 1.54 | 0.90–2.45 | 0.116 |
| PAH | 3.24 | 1.71–6.15 | <0.001 |
| Medical historya | |||
| Atrial arrhythmia | 2.67 | 1.60–4.47 | <0.001 |
| Heart failure | 5.55 | 3.19–9.64 | <0.001 |
| Pacemaker | 2.13 | 1.26–3.60 | 0.005 |
| Holter | |||
| NSVT | 3.46 | 1.97–6.12 | <0.001 |
| Atrial arrhythmia (>30 s) | 5.33 | 2.94–9.67 | <0.001 |
| Conduction abnormalities | 0.57 | 0.21–1.60 | 0.287 |
| Baseline | |||
| Symptoms | |||
| NYHA class > II | 3.89 | 1.90–7.98 | <0.001 |
| Palpitations | 2.11 | 1.22–3.65 | 0.008 |
| Syncope | 4.36 | 1.97–9.65 | <0.001 |
| Cardiac medication | |||
| Beta-blockers | 1.85 | 1.00–3.41 | 0.049 |
| ACEi/ARB | 1.70 | 0.99–2.94 | 0.055 |
| Diuretics | 4.56 | 2.57–8.01 | <0.001 |
| ECG | |||
| Loss of sinus rhythm | 1.02 | 0.60–1.73 | 0.942 |
| QRS duration, ms | 1.02 | 1.01–1.03 | <0.001 |
| Echocardiography | |||
| sRV dysfunction | |||
| Normal | 0.42 | 0.22–0.81 | 0.010 |
| Mildly impaired | 0.69 | 0.38–1.24 | 0.210 |
| Moderately impaired | 1.17 | 0.65–2.09 | 0.598 |
| Severely impaired | 3.54 | 1.99–6.30 | <0.001 |
| TR grade (moderate to severe) | 2.34 | 1.40–3.90 | 0.001 |
| LVOT obstruction (>36 mmHg) | 2.44 | 1.42–4.19 | 0.001 |
| . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| Age at baseline (per year increase) | 1.05 | 1.03–1.06 | <0.001 |
| Female sex | 1.46 | 0.85–2.53 | 0.171 |
| Type of TGA (L-TGA) | 1.74 | 1.02–2.98 | 0.042 |
| Complex form | 1.54 | 0.90–2.45 | 0.116 |
| PAH | 3.24 | 1.71–6.15 | <0.001 |
| Medical historya | |||
| Atrial arrhythmia | 2.67 | 1.60–4.47 | <0.001 |
| Heart failure | 5.55 | 3.19–9.64 | <0.001 |
| Pacemaker | 2.13 | 1.26–3.60 | 0.005 |
| Holter | |||
| NSVT | 3.46 | 1.97–6.12 | <0.001 |
| Atrial arrhythmia (>30 s) | 5.33 | 2.94–9.67 | <0.001 |
| Conduction abnormalities | 0.57 | 0.21–1.60 | 0.287 |
| Baseline | |||
| Symptoms | |||
| NYHA class > II | 3.89 | 1.90–7.98 | <0.001 |
| Palpitations | 2.11 | 1.22–3.65 | 0.008 |
| Syncope | 4.36 | 1.97–9.65 | <0.001 |
| Cardiac medication | |||
| Beta-blockers | 1.85 | 1.00–3.41 | 0.049 |
| ACEi/ARB | 1.70 | 0.99–2.94 | 0.055 |
| Diuretics | 4.56 | 2.57–8.01 | <0.001 |
| ECG | |||
| Loss of sinus rhythm | 1.02 | 0.60–1.73 | 0.942 |
| QRS duration, ms | 1.02 | 1.01–1.03 | <0.001 |
| Echocardiography | |||
| sRV dysfunction | |||
| Normal | 0.42 | 0.22–0.81 | 0.010 |
| Mildly impaired | 0.69 | 0.38–1.24 | 0.210 |
| Moderately impaired | 1.17 | 0.65–2.09 | 0.598 |
| Severely impaired | 3.54 | 1.99–6.30 | <0.001 |
| TR grade (moderate to severe) | 2.34 | 1.40–3.90 | 0.001 |
| LVOT obstruction (>36 mmHg) | 2.44 | 1.42–4.19 | 0.001 |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker, CI, confidence interval; HR, hazard ratio; LVOT, left ventricular outflow tract; NSVT, non-sustained ventricular tachycardia; PAH, pulmonary arterial hypertension; sRV, systemic right ventricle; VT, ventricular tachycardia.
History of heart failure, atrial arrhythmia, and pacemaker implantation are time-dependant covariates.
| . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| Age at baseline (per year increase) | 1.05 | 1.03–1.06 | <0.001 |
| Female sex | 1.46 | 0.85–2.53 | 0.171 |
| Type of TGA (L-TGA) | 1.74 | 1.02–2.98 | 0.042 |
| Complex form | 1.54 | 0.90–2.45 | 0.116 |
| PAH | 3.24 | 1.71–6.15 | <0.001 |
| Medical historya | |||
| Atrial arrhythmia | 2.67 | 1.60–4.47 | <0.001 |
| Heart failure | 5.55 | 3.19–9.64 | <0.001 |
| Pacemaker | 2.13 | 1.26–3.60 | 0.005 |
| Holter | |||
| NSVT | 3.46 | 1.97–6.12 | <0.001 |
| Atrial arrhythmia (>30 s) | 5.33 | 2.94–9.67 | <0.001 |
| Conduction abnormalities | 0.57 | 0.21–1.60 | 0.287 |
| Baseline | |||
| Symptoms | |||
| NYHA class > II | 3.89 | 1.90–7.98 | <0.001 |
| Palpitations | 2.11 | 1.22–3.65 | 0.008 |
| Syncope | 4.36 | 1.97–9.65 | <0.001 |
| Cardiac medication | |||
| Beta-blockers | 1.85 | 1.00–3.41 | 0.049 |
| ACEi/ARB | 1.70 | 0.99–2.94 | 0.055 |
| Diuretics | 4.56 | 2.57–8.01 | <0.001 |
| ECG | |||
| Loss of sinus rhythm | 1.02 | 0.60–1.73 | 0.942 |
| QRS duration, ms | 1.02 | 1.01–1.03 | <0.001 |
| Echocardiography | |||
| sRV dysfunction | |||
| Normal | 0.42 | 0.22–0.81 | 0.010 |
| Mildly impaired | 0.69 | 0.38–1.24 | 0.210 |
| Moderately impaired | 1.17 | 0.65–2.09 | 0.598 |
| Severely impaired | 3.54 | 1.99–6.30 | <0.001 |
| TR grade (moderate to severe) | 2.34 | 1.40–3.90 | 0.001 |
| LVOT obstruction (>36 mmHg) | 2.44 | 1.42–4.19 | 0.001 |
| . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| Age at baseline (per year increase) | 1.05 | 1.03–1.06 | <0.001 |
| Female sex | 1.46 | 0.85–2.53 | 0.171 |
| Type of TGA (L-TGA) | 1.74 | 1.02–2.98 | 0.042 |
| Complex form | 1.54 | 0.90–2.45 | 0.116 |
| PAH | 3.24 | 1.71–6.15 | <0.001 |
| Medical historya | |||
| Atrial arrhythmia | 2.67 | 1.60–4.47 | <0.001 |
| Heart failure | 5.55 | 3.19–9.64 | <0.001 |
| Pacemaker | 2.13 | 1.26–3.60 | 0.005 |
| Holter | |||
| NSVT | 3.46 | 1.97–6.12 | <0.001 |
| Atrial arrhythmia (>30 s) | 5.33 | 2.94–9.67 | <0.001 |
| Conduction abnormalities | 0.57 | 0.21–1.60 | 0.287 |
| Baseline | |||
| Symptoms | |||
| NYHA class > II | 3.89 | 1.90–7.98 | <0.001 |
| Palpitations | 2.11 | 1.22–3.65 | 0.008 |
| Syncope | 4.36 | 1.97–9.65 | <0.001 |
| Cardiac medication | |||
| Beta-blockers | 1.85 | 1.00–3.41 | 0.049 |
| ACEi/ARB | 1.70 | 0.99–2.94 | 0.055 |
| Diuretics | 4.56 | 2.57–8.01 | <0.001 |
| ECG | |||
| Loss of sinus rhythm | 1.02 | 0.60–1.73 | 0.942 |
| QRS duration, ms | 1.02 | 1.01–1.03 | <0.001 |
| Echocardiography | |||
| sRV dysfunction | |||
| Normal | 0.42 | 0.22–0.81 | 0.010 |
| Mildly impaired | 0.69 | 0.38–1.24 | 0.210 |
| Moderately impaired | 1.17 | 0.65–2.09 | 0.598 |
| Severely impaired | 3.54 | 1.99–6.30 | <0.001 |
| TR grade (moderate to severe) | 2.34 | 1.40–3.90 | 0.001 |
| LVOT obstruction (>36 mmHg) | 2.44 | 1.42–4.19 | 0.001 |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker, CI, confidence interval; HR, hazard ratio; LVOT, left ventricular outflow tract; NSVT, non-sustained ventricular tachycardia; PAH, pulmonary arterial hypertension; sRV, systemic right ventricle; VT, ventricular tachycardia.
History of heart failure, atrial arrhythmia, and pacemaker implantation are time-dependant covariates.
| Covariate . | β . | HR . | 95% CI . | P-value . |
|---|---|---|---|---|
| Age at baseline (per year increase) | 0.03145 | 1.03 | 1.01–1.05 | 0.003 |
| History of heart failurea | 1.0129 | 2.75 | 1.47–5.16 | 0.002 |
| Syncope | 1.7084 | 5.52 | 2.47–12.34 | <0.001 |
| QRS duration (per ms increase) | 0.01681 | 1.02 | 1.01–1.03 | <0.001 |
| Severe sRV dysfunction | 1.1188 | 3.06 | 1.62–5.77 | <0.001 |
| LVOT obstruction (>36 mmHg) | 1.0160 | 2.76 | 1.56–4.90 | <0.001 |
| Covariate . | β . | HR . | 95% CI . | P-value . |
|---|---|---|---|---|
| Age at baseline (per year increase) | 0.03145 | 1.03 | 1.01–1.05 | 0.003 |
| History of heart failurea | 1.0129 | 2.75 | 1.47–5.16 | 0.002 |
| Syncope | 1.7084 | 5.52 | 2.47–12.34 | <0.001 |
| QRS duration (per ms increase) | 0.01681 | 1.02 | 1.01–1.03 | <0.001 |
| Severe sRV dysfunction | 1.1188 | 3.06 | 1.62–5.77 | <0.001 |
| LVOT obstruction (>36 mmHg) | 1.0160 | 2.76 | 1.56–4.90 | <0.001 |
CI, confidence interval; HR, hazard ratio; LVOT, left ventricular outflow tract; sRV, systemic right ventricle.
History of heart failure was handled as a time-dependant covariate.
| Covariate . | β . | HR . | 95% CI . | P-value . |
|---|---|---|---|---|
| Age at baseline (per year increase) | 0.03145 | 1.03 | 1.01–1.05 | 0.003 |
| History of heart failurea | 1.0129 | 2.75 | 1.47–5.16 | 0.002 |
| Syncope | 1.7084 | 5.52 | 2.47–12.34 | <0.001 |
| QRS duration (per ms increase) | 0.01681 | 1.02 | 1.01–1.03 | <0.001 |
| Severe sRV dysfunction | 1.1188 | 3.06 | 1.62–5.77 | <0.001 |
| LVOT obstruction (>36 mmHg) | 1.0160 | 2.76 | 1.56–4.90 | <0.001 |
| Covariate . | β . | HR . | 95% CI . | P-value . |
|---|---|---|---|---|
| Age at baseline (per year increase) | 0.03145 | 1.03 | 1.01–1.05 | 0.003 |
| History of heart failurea | 1.0129 | 2.75 | 1.47–5.16 | 0.002 |
| Syncope | 1.7084 | 5.52 | 2.47–12.34 | <0.001 |
| QRS duration (per ms increase) | 0.01681 | 1.02 | 1.01–1.03 | <0.001 |
| Severe sRV dysfunction | 1.1188 | 3.06 | 1.62–5.77 | <0.001 |
| LVOT obstruction (>36 mmHg) | 1.0160 | 2.76 | 1.56–4.90 | <0.001 |
CI, confidence interval; HR, hazard ratio; LVOT, left ventricular outflow tract; sRV, systemic right ventricle.
History of heart failure was handled as a time-dependant covariate.
Brain natriuretic peptide, CMR, and CPET data were missing for more than 50% of patients at baseline. An analysis of the subgroup of patients with available BNP, CMR, and CPET data failed to demonstrate an effect on the final model (see Supplementary material online, Table S5). Incidence of MAREs was significantly higher in D-TGA compared to L-TGA when adjusted for age (Figure 2B, log-rank test, P = 0.006). A separate analysis of predictors of MAREs in L-TGA and D-TGA was, therefore, realised and did not allow to obtain better prediction models (see Supplementary material online, Tables S6 and S7).
The C-statistic was 0.78 (95% CI 0.72–0.84) and the calibration slope was 0.93 (95% CI 0.64–1.21). Patients were categorized into low, intermediate, and high risk, with a 5-year predicted risk of MAREs of <5%, 5–10%, and >10%, respectively. Figure 3 shows the comparison between the observed and the predicted 5-year risk of MAREs in the whole population. The model’s performance in predicting the risk of MAREs at 5 years was validated in the group of patients with L-TGA and D-TGA. However, in L-TGA, discriminatory ability (C-index 0.67; 95% CI 0.51–0.82) and agreement between observed and predicted outcomes [calibration slope 0.81 (95% CI 0.26–1.35) were modest, with an underestimation of the risk of MAREs in the high-risk group (see Supplementary material online, Figure S1]. In D-TGA, C-index was 0.85 (95% CI 0.78–0.91) and the calibration slope was 1.05 (95% CI 0.52–1.59) (see Supplementary material online, Figure S2). Comparison by the centre of predictions and Kaplan–Meier estimations at 5-year follow-up is shown in Supplemental material online, Table S9.
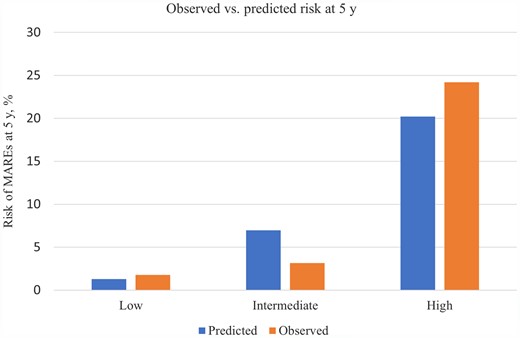
Comparison of observed and predicted risk by risk group of the major adverse ventricular arrhythmias and related event proposed risk model. Vertical bars represent observed (the left column for each risk group) and model-based predicted (the right column for each risk group) probability of major adverse ventricular arrhythmias and related events at 5 years. The low-risk group corresponds to a predicted 5-year risk of major adverse ventricular arrhythmias and related events <5%, the intermediate-risk group to a predicted risk of 5% to ≤10%, and the high-risk group to a predicted risk >10%.
Patients with an implantable cardioverter-defibrillator for primary prevention
Implantable cardioverter-defibrillator for primary prevention was implanted in 121 (10.2%) patients including 97 at follow-up. Patients receiving an ICD for primary prevention were older [43.5 years (39.0–47.5) vs. 36.7 (36.0–37.5), P < 0.001] and more likely to have L-TGA (16.6% of L-TGA patients vs. 7.6% D-TGA patients, P < 0.001) compared to the remainder. Interestingly, according to our prediction model, 71 (59%) patients with ICD were in the low-risk category (5-year MARE risk <5%). Inappropriate shocks occurred in 14 (11.6%) patients, 11 due to atrial arrhythmia and 3 due to lead dysfunction. Appropriate shocks occurred in 8 (6.6%) patients with an incidence of 1.6% patient-years during the study period: five of these eight patients were in the intermediate-risk category, whereas two in the high-risk category.
The clinical implications of the model were examined within the first 5 years of follow-up. During this period, the MARE endpoint was reached by 19 patients (1.8%) with a predicted risk lower than 5% (n = 1057), 3 patients (3.8%) with a predicted risk of 5% to <10% (n = 79), and 10 patients (20.8%) with a predicted risk of 10% or greater (n = 48) (Structured Graphical abstract). Using a 5-year MARE risk of 5% or greater to consider primary prevention with ICD implantation would identify 13 of 32 MARE endpoints (40.6%), with ICD implantation in 114 of 1152 patients (9.9%) not reaching MARE endpoints within 5 years. Only 27 out of the 127 patients with a 5-year MARE risk of 5% or greater had an ICD during this period, of whom 5 (18.5%) received appropriate ICD therapy. Using a 5-year MARE risk >10% to consider primary prevention ICD implantation would identify 10 of 32 MARE endpoints (31.3%), with ICD implantation in 38 of 1152 patients (3.3%) not reaching MARE endpoints within 5 years. Eleven out of the 48 patients with a 5-year MARE risk of 10% or greater had an ICD, of whom 4 (36.3%) received appropriate ICD therapy. One patient, thus, may potentially be saved from MAREs at 5 years, for every 10 ICDs implanted in patients with a 5-year MARE risk >5% or more, and for every 5 ICDs implanted in patients with a 5-year MARE risk >10%.
Discussion
To the best of our knowledge, we conducted the largest, contemporary multicentre study on MAREs in patients with a sRV. We observed a slight increase in the incidence of ventricular arrhythmias and SCD compared to previous reports,1 albeit our patients were older. Major adverse ventricular arrhythmias and related events occurred in 5% of patients at a median follow-up of 9 years with an incidence of 6.3 per 1000 patient-years. Sudden cardiac death in our cohort was the second most common cause of death after heart failure, with the confluence of factors leading to either. The risk factors for MAREs were age, history of heart failure, syncope, QRS duration, severe sRV dysfunction, and at least moderate LVOT obstruction. We present herewith a prediction model for MAREs based on simple, non-invasive parameters to aid the management of adult patients with a sRV and refine the selection of candidates for ICD for primary prevention.
Our findings suggest that ventricular arrhythmias are more common in patients with sRV failure. This finding is consistent with several other studies.8,12,13 Intra-myocardial fibrosis putatively develops as a maladaptive response in the failing ventricle22 and serves to delay electrical activation within the heart that may increase the risk of SCD. This process in the ventricles can frequently be inscribed on the surface electrocardiogram as QRS prolongation, QRS notching, and QRS fractionation/fragmentation. In our large patient cohort, there was a significant correlation between prolonged QRS duration and risk of ventricular arrhythmia and/or sudden arrhythmic death, which has already been observed in adults with a sRV.8,12 However, QRS duration itself does not seem to be as prolonged in this group as in those with tetralogy of Fallot and this may be related to the lack of surgical scar involving the right ventricular outflow tract and/or VSD closure as in the latter. History of heart failure and severe sRV dysfunction were strong risk factors of MAREs. Myocardial microcirculation impairment and ischaemia seem to be associated with sRV dysfunction23,24 and have been suggested as a cause of SCD. Indeed, autopsy studies in patients who died suddenly revealed acute massive myocardial infarction of the sRV in the setting of chronic subendocardial ischaemia and in the absence of conventional coronary atherosclerosis.25
A novel finding from our study is that when both ventricles are pressure overloaded (i.e. the sRV with systemic load and subpulmonary LVOT obstruction), the risk of ventricular arrhythmia and SCD was significantly increased. Although pulmonary outflow obstruction in L-TGA was found to be associated with a lower risk of developing heart failure,26 the resulting left ventricular hypertrophy may contribute to the ventricular arrhythmia substrate. Indeed, a recent study demonstrated that adverse remodelling of the subpulmonary left ventricle was associated with a worse clinical outcome.27 Consequently, the subpulmonary left ventricle, which can be the ‘forgotten chamber’ in patients with a sRV, should be carefully surveyed.28
Not surprisingly, the risk of ventricular arrhythmias increased with age. Progress in ACHD care has considerably improved the long-term survival of these patients, including those with complex disease such as patients with a sRV, albeit this progress comes with new challenges and opportunities. Consequently, the age at which heart failure and ventricular arrhythmias ensue may have increased, and this is likely a confounder when our study is compared with the study by Silka et al.1
Among rhythm parameters, we observed that non-sustained ventricular tachycardia and atrial arrythmias were significantly associated with MAREs. Several studies have identified atrial arrhythmia and non-sustained ventricular tachycardia as risk factors, mainly in D-TGA.4,9,12,13 In our study, these covariates were significantly associated with MAREs on univariate Cox analysis. However, they were not selected in the final model probably due to the limited number of events. Indeed, from an inspection of the backward stepwise selection, the difference in the AIC values between the best model (final model) and the models that included non-sustained ventricular tachycardia and atrial arrhythmia was quite small. Moreover, data obtained from Holter ECG and pacemaker/ICD monitoring were missing in 20%, which may constitute a potential selection bias.
Even if the type of TGA was not selected in the final model, the incidence of MAREs was higher in D-TGA than in L-TGA (Figure 2B). Pathophysiological processes of SCD may be different between L-TGA and D-TGA. Indeed, abnormal cardiac conduction, part of the L-TGA phenotype, may increase the risk of sudden death. Daliento et al.29 described fibrosis and disruption of the proximal non-bifurcating His bundle, which can constitute an underlying arrhythmogenic myocardial substrate. This conduction anomaly specific to L-TGA may explain the modest performance of the MARE prediction model in this subgroup, whereas myocardial ischaemia and consequently sRV dysfunction seem to be prominent causes of ventricular arrhythmias in D-TGA.25
Possible prevention of major adverse ventricular arrhythmias and related events: considerations for primary prevention implantable cardioverter-defibrillator implantation
Our study has several important implications for the management of patients with a sRV. To our knowledge, there is no model for ventricular arrhythmia risk prediction to date in sRV and current recommendations for primary prevention ICD implantation in patients with a sRV are only IIb due to the sparsity of data.15,16 By including patients from many large centres and integrating multiple associated risk factors, our model improves the identification of patients at risk of MAREs who could benefit from ICD implantation for primary prevention. Surprisingly, most of ICD implants for primary prevention concerned patients in the low-risk group (predicted 5-year risk of MAREs <5%). Current recommendations for ICD in ACHD are known to have a low discriminative power to identify patients at risk, resulting in underimplantation in patients who are truly at risk of SCD and overimplantation in low SCD risk subjects.30 By using the MARE risk prediction model developed herewith, the selection of candidates with a sRV for primary prevention ICD implantation may be improved. Furthermore, in this relatively young population, the risk of supraventricular arrhythmias causing inappropriate shocks is clinically relevant9 with a potentially greater psychological impact compared to older populations31 underscoring the need for improving the selection of candidates for ICD. Moreover, hemodynamic prognosis in patients with a sRV seems to be more prominent than arrhythmic prognosis, with several overlapping risk factors for heart failure and MAREs. However, the higher incidence of ventricular tachyarrhythmia in patients with high scores supports the potential role of ICDs in this subgroup. Ideally, a multicentre, double-blind, randomized controlled study with longer follow-up, exploring the clinical impact of ICD implantation for primary prevention in sRVs, including survival and quality of life, would provide more evidence on ICD indication in patients with a sRV; however, such a trial remains challenging.
Limitations
As CHD with a sRV is an uncommon condition and SCD is a relatively uncommon, albeit devastating complication in this setting, a multicentre, retrospective, longitudinal design was necessary to develop a specific model. This study is, therefore, limited by inherent problems of retrospective studies, notably, missing data. This specifically concerns BNP, CMR, measurements which may improve risk stratification of MAREs in this population. Indeed, even if we observed a significant increase in BNP level, an increase in CMR sRV volumes and a decrease in CMR sRV ejection fraction in patients who met the primary outcome, these variables were not retained in the final model when we performed an analysis of the subgroup with available data. Because these measurements were missing in more than half of our cohort, we cannot definitively conclude on how they may affect our risk model. Nevertheless, (serial) measurements of natriuretic peptides play a key role in both monitoring and risk stratifying adult patients with CHD and patients with a sRV should not be an exemption.32 Similarly, myocardial scar on CMR is recognized as a risk marker for SCD notably in non-ischaemic cardiomyopathy.33 Clearly, such types of biomarkers should be validated and potentially incorporated in any future risk prediction model. Moreover, CMR offers highly reproducible RV volume and ejection fraction measurements in CHD,34 while qualitative assessment of sRV systolic function by echocardiography is known to have a poor interobserver reproducibility.35 However, we observed that only severe sRV dysfunction was significantly associated with MAREs. We presumed diagnosis was more discriminatory between a severely impaired and a normal to moderately impaired sRV function. Among quantitative echocardiographic measurements of sRV function, only the sRV global longitudinal strain was demonstrated to be associated with ventricular arrhythmias in patients with D-TGA.36 Because these data were rarely reported, it could not be selected as a predictor of MAREs. Subclinical impairment of sRV ejection fraction and syncope were not assessed as time-varying covariates in our risk prediction model. This may induce some bias in our results by underestimating their association with the primary outcome. Indeed, clinical heart failure is usually preceded by a decline in sRV function. However, alteration of sRV function and syncope were difficult to longitudinally capture due to the retrospective design of our study unlike events marked by hospitalization and/or a change in treatment. With 59 events and 6 selected predictors, the rule of thumb that Cox model should be used with a minimum of 10 events per predictor variable was partially reached. This limitation may have led to overestimate the risk score performance, which may be poor on new data. However, some authors have shown that this rule can be relaxed to 5–9 events per variables with acceptable coverage and bias.37 An external validation of our point-based predictive model is required prior to a prospective validation, which would require an even longer period of observation. The alternative would be to validate our model on another large registry or by extracting data from ICDs implanted for the prevention of SCD in patients with a sRV in again a multicentre operation. Because the cohort was recruited longitudinally, the length of follow-up for individual patients varied. However, the medical management of patients with a sRV has not changed significantly over the study period.
Seventy per cent of our population included patients with a D-TGA palliated by atrial switch operation which was routinely performed in the 1970s. Since the late 1980s, the arterial switch operation has superseded the Mustard and Senning operations and appears to provide superior long-term outcomes, albeit with shorter period of observation.
Conclusions
Sudden cardiac death remains a devastating cause of death in a large contemporary cohort of patients with a sRV. Older age, history of heart failure, syncope, QRS duration, severe sRV dysfunction, and at least moderate LVOT obstruction were predictive of MAREs and formed the base of a proposed risk prediction model for consideration of primary prevention ICD implantation. Further studies validating our data and incorporating other biomarkers in prospective models are clearly warranted.
Supplementary material
Supplementary material is available at European Heart Journal online.
Acknowledgements
We thank Mrs Anissa Boubrit, a French research technician from the reference centre of complex congenital heart disease M3C, who helped with the recording of data from the Paris, Lausanne and Geneve Centres. We also thank Dr Matthew O'Connor, Consultant Electrophysiologist from the Royal Brompton, London, UK for his critique and feedback on our paper before submission.
Funding
This work was supported by the Fédération Française de Cardiologie and the Assistance Publique des Hôpitaux de Paris.
Conflict of interest: None declared.
References
- primary prevention
- left ventricular outflow obstruction
- syncope
- echocardiography
- sudden cardiac death
- transposition of great vessels
- atrium
- implantable defibrillators
- heart failure
- right ventricle
- adult
- cause of death
- follow-up
- ventricular arrhythmia
- survivin
- implantable defibrillator insertion
- qrs complex duration
- d-transposition of the great vessels



