-
PDF
- Split View
-
Views
-
Cite
Cite
Filippo Crea, Cardiac magnetic resonance: challenges, opportunities, and developments, European Heart Journal, Volume 43, Issue 26, 7 July 2022, Pages 2427–2431, https://doi.org/10.1093/eurheartj/ehac355
Close - Share Icon Share
With thanks to Amelia Meier-Batschelet, Johanna Huggler, and Martin Meyer for help with compilation of this article.
 For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
This Issue opens with the Special Article entitled ‘Integrated care for optimizing the management of stroke and associated heart disease: a position paper of the European Society of Cardiology Council on Stroke’ by Gregory Y.H. Lip from the University of Liverpool and Liverpool Heart & Chest Hospital in the UK, and colleagues.1 The authors note that the management of patients with stroke is often multidisciplinary, involving various specialties and healthcare professionals. Given the common shared risk factors for stroke and cardiovascular disease, input may also be required from the cardiovascular teams, as well as patient caregivers and next of kin.2–7 Ultimately, the patient is central to all this, requiring a coordinated and uniform approach to the priorities of post-stroke management, which can be consistently implemented by different multidisciplinary healthcare professionals, as part of the patient ‘journey’ or ‘patient pathway’, supported by appropriate education and tele-medicine approaches. All these aspects would ultimately aid delivery of care and improve patient (and caregiver) engagement and empowerment. Given the need to address the multidisciplinary approach to holistic or integrated care of patients with heart disease and stroke, the European Society of Cardiology Council on Stroke convened a Task Force, with the remit to propose a consensus on integrated care management for optimizing the management of stroke and associated heart disease. The present position paper summarizes the available evidence and proposes consensus statements that may help to define evidence gaps and simple practical approaches to assist in everyday clinical practice. A post-stroke ABC pathway is proposed, as a more holistic approach to integrated stroke care, which would include three pillars of management, as follows.
A. Appropriate antithrombotic therapy.
B. Better functional and psychological status.
C. Cardiovascular risk factors and comorbidity optimization (including lifestyle changes).
The issue continues with a focus on Imaging. In a State of the Art Review article entitled ‘Autopsy in the era of advanced cardiovascular imaging’, Cristina Basso from the University of Padua in Italy and James R. Stone from the Massachusetts General Hospital and Harvard Medical School in Boston, MA, USA note that historically, autopsy contributed to our current knowledge of cardiovascular anatomy, physiology, and pathology.8 Major advances in the understanding of cardiovascular diseases, including atherosclerosis and coronary artery disease, congenital heart diseases, and cardiomyopathies, were possible through autopsy investigations and clinicopathological correlations. This review addresses the importance of performing clinical autopsies in people dying from cardiovascular disease, even in the era of advanced cardiovascular imaging. Autopsies are most helpful in the setting of sudden unexpected deaths, particularly when advanced cardiovascular imaging has not been performed. In this setting, the autopsy is often the only chance to make the correct diagnosis. In previously symptomatic patients who had undergone advanced cardiovascular imaging, autopsies still play many roles. Post-mortem examinations are important for furthering the understanding of key issues related to the underlying diseases. Autopsy can help to increase the knowledge of the sensitivity and specificity of advanced cardiovascular imaging modalities. Autopsies are particularly important to gain insights into both the natural history of cardiovascular diseases and less common presentations and therapeutic complications. Finally, autopsies are a key tool to quickly understand the cardiac pathology of new disorders, as emphasized during the recent coronavirus disease 2019 pandemic.
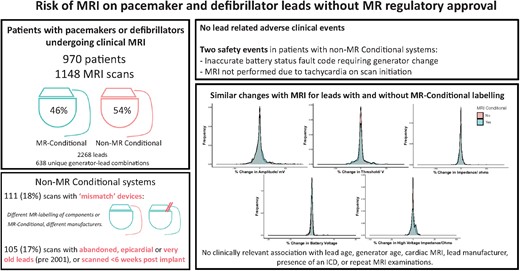
Clinicians can safely perform magnetic resonance imaging in non-specialist centres for patients with pacemakers and defibrillators if the generator is MR-conditional, irrespective of the magnetic resonance safety labelling of the attached leads, by following standardized protocols adapted from fully MR-conditional systems and excluding other high-risk scenarios.9
Many cardiac pacemakers and defibrillators are not approved by regulators for magnetic resonance imaging (MRI). Even following generator exchange to an approved MR-conditional model, many systems remain classified ‘non-MR conditional’ due to the leads. In a Clinical Research article entitled ‘Evidence to support magnetic resonance conditional labelling of all pacemaker and defibrillator leads in patients with cardiac implantable electronic devices’, Anish N. Bhuva from the Barts Heart Centre, Barts Health NHS Trust in the UK, and colleagues note that this classification makes patient access to MRI challenging, while the evidence of increased clinical risk appears to be unsubstantiated. The authors compared the effect of MRI on non-MR conditional and MR-conditional pacemaker and defibrillator leads.9 Patients undergoing clinical 1.5 T MRI with pacemakers and defibrillators in three centres over 5 years were included. MRI protocols were similar for MR-conditional and non-MR conditional systems. Devices were interrogated pre- and immediately post-scan, and at follow-up, and adverse clinical events were recorded. Lead parameter changes peri-scan were stratified by MR-conditional labelling. A total of 1148 MRI examinations were performed in 970 patients (54% non-MR conditional systems, 39% defibrillators, 15% pacing-dependent) with 2268 leads. There were no lead-related adverse clinical events, and no clinically significant immediate or late lead parameter changes following MRI in either MR-conditional or non-MR conditional leads. Small reductions in atrial and right ventricular sensed amplitudes and impedances were similar between groups, with no difference in the proportion of leads with parameter changes greater than pre-defined thresholds (7.1%, 95% confidence interval 6.1–8.3) (Figure 1).
The authors conclude that there was no increased risk of MRI in patients with non-MR conditional pacemaker or defibrillator leads when following recommended protocols. Standardizing MR conditions for all leads would significantly improve access to MRI by enabling patients to be scanned in non-specialist centres, with no discernible incremental risk. The contribution is accompanied by an Editorial by Chiara Bucciarelli-Ducci from King's College London in the UK and Panos Vardas from the Hygeia Group Hospitals in Athens, Greece.10 The authors conclude that the clinical impact of the study of Bhuva et al. is likely to be substantial and practice-changing. The 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy stated that in patients with MR-conditional devices, MRI at 1.5 T can be done safely following the manufacturer's instructions (class of recommendation IIa, level of evidence B), whilst in patients with conventional (legacy) cardiac devices, MRI at 1.5 T can be performed with a low risk of complications if appropriate precautions are taken (class of recommendation IIb, level of evidence B). This latter indication is likely to change in future guidelines given the new clinical evidence provided in recent papers, including this study.
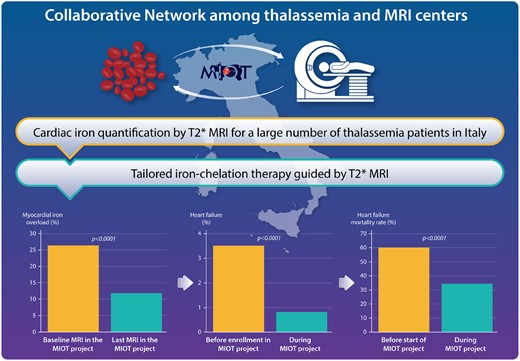
A national clinical and imaging networking for the continuous and standardized monitoring of cardiac iron levels was effective in improving the care and in reducing the cardiac complications and mortality in thalassaemia major patients.11
A tailored chelation therapy-guided MRI is a strategy to improve the prognosis in iron-loaded patients, in many cases still hampered by limited MRI availability. In a Clinical Research article entitled ‘National networking in rare diseases and reduction of cardiac burden in thalassaemia major’, Alessia Pepe from the Fondazione G. Monasterio CNR-Regione Toscana in Pisa, Italy, and colleagues note that the Myocardial Iron Overload in Thalassemia (MIOT) network was established in Italy to address this issue. The authors aimed to describe the impact of 10-year activity of this network on cardiac burden in thalassaemia major (TM).11 Within the MIOT network, 1746 TM patients (911 females; mean age 31 years) were consecutively enrolled and prospectively followed by 70 thalassaemia and 10 MRI centres. Patients were scanned using a multiparametric approach for assessing myocardial iron overload (MIO), biventricular function, and myocardial fibrosis. At the last MRI scan, a significant increase in global heart T2* values and a significantly higher frequency of patients with no MIO (all segmental T2* ≥20 ms) were detected, with a concordant improvement in biventricular function, particularly in patients with baseline global heart T2* <20 ms. Forty-seven per cent of patients changed their chelation regimen based on MRI. The frequency of heart failure (HF) significantly decreased after baseline MRI from 3.5% to 0.8% (P <0.0001). Forty-six patients died during the study, and HF accounted for 35% of deaths (Figure 2).
Pepe and colleagues conclude that over 10 years, continuous monitoring of cardiac iron and a tailored chelation therapy allowed MIO reduction, with consequent improvement of cardiac function and reduction of cardiac complications and mortality from MIO-related HF. A national networking for rare diseases therefore is effective in improving the care and reducing cardiac outcomes of TM patients. This manuscript is accompanied by an Editorial by Mark Westwood and Dudley Pennell from the Royal Brompton Hospital in London, UK.12 The authors conclude that overall, the study by Pepe et al. has shown that the use of integrated care at a regional and national level can drive and improve outcomes and has yet again confirmed the simplicity and validity of the T2* technique. Even though a more complex analysis technique has been used, the results confirm the enormous power of myocardial T2*, how revolutionary it has been in the management of cardiac iron loading, and that, using this very simple strategy, how many young lives have and continue to be saved.
In a Clinical Research article entitled ‘Improving cardiovascular magnetic resonance access in low- and middle-income countries for cardiomyopathy assessment: rapid cardiovascular magnetic resonance’, Katia Devorha Menacho from the University College London in the UK, and colleagues note that cardiovascular magnetic resonance (CMR) complements echocardiography and is recommended in multiple international guidelines, including the majority from the ESC (14 guidelines, 39 Class I, and 22 Class II recommendations). It is the gold standard for the evaluation of ventricular function, scar imaging using late gadolinium enhancement, and iron quantification (CMR-T2*).13–15 The authors evaluated the impact of a simplified, rapid cardiovascular CMR protocol embedded in care and supported by a partner education programme on the management of cardiomyopathy in low- and middle-income countries (LMICs).16 Rapid CMR focused particularly on cardiomyopathy was implemented in 11 centres, 7 cities, 5 countries, and 3 continents linked to training courses for local professionals. Patients were followed up for 24 months to assess the impact. The rate of subsequent adoption was tracked. Five CMR conferences were delivered (920 attendees—potential referrers, radiographers, reporting cardiologists, or radiologists) and five new centres started CMR. A total of 601 patients were scanned. CMR indications were 24% non-contrast T2* scans (MIO) and 72% suspected/known cardiomyopathies (including ischaemic and viability). Ninety-eight per cent of studies were of diagnostic quality. The average scan time was 22 ± 6 min (contrast) and 12 ± 4 min (non-contrast), a potential cost/throughput reduction of between 30% and 60%. CMR findings impacted management in 62%, including a new diagnosis in 22% and MIO detected in 30% of non-contrast scans.
The authors conclude that rapid CMR of diagnostic quality can be delivered using available technology in LMICs. When embedded in care and a training programme, costs are lower, care is improved, and services can be sustained over time. This contribution is accompanied by an Editorial by Subha Raman from the Indiana University School of Medicine/IU Health Cardiovascular Institute in Indianapolis, IN, USA.17 Raman states that ideally, all stakeholders—healthcare recipients, healthcare providers, and healthcare payors—should work together to address patients’ unmet needs and deliver innovative solutions. The author concludes that together with local, national, multinational, and societal partners, we should accelerate our shared pursuit of equitable, population-scale access to CMR as a key component of high-value cardiovascular care.
In a Clinical Research article entitled ‘Cardiac magnetic resonance identifies raised left ventricular filling pressure: prognostic implications’, Pankaj Garg from the University of Sheffield in the UK, and colleagues18 note that HF presents a significant social and economic burden, and it is on the rise.19 The underlying pathophysiology of HF is raised intracardiac filling pressures.20–24 Identification of raised left ventricular filling pressure (LVFP) is the cornerstone of HF diagnosis. CMR is emerging as an important imaging tool for subphenotyping HF. However, currently, LVFP cannot be estimated from CMR. This study sought to investigate (i) if CMR can estimate LVFP in patients with suspected HF and (ii) if CMR-modelled LVFP has prognostic power. Suspected HF patients underwent right heart catheterization (RHC), CMR, and transthoracic echocardiography (TTE) (validation cohort only) within 24 h of each other. Right heart catheterization-measured pulmonary capillary wedge pressure (PCWP) was used as a reference for LVFP. At follow-up, death was considered as the primary endpoint. The authors enrolled 835 patients (mean age: 65 years, 40% male). In the derivation cohort (n = 708), two CMR metrics were associated with RHC PCWP: LV mass and left atrial volume. When applied to the validation cohort (n = 127), the correlation coefficient between RHC PCWP and CMR-modelled PCWP was 0.55 (P < 0.0001). CMR-modelled PCWP was superior to TTE in classifying patients as having normal or raised filling pressures. A raised CMR-modelled PCWP was associated with an increased risk of death (hazard ratio: 1.77, P < 0.001). At Kaplan–Meier analysis, A raised CMR-modelled PCWP was comparable with RHC PCWP (≥15 mmHg) to predict survival at 7-year follow-up (35% vs. 37%, P = 0.52).
Garg et al. conclude that a physiological CMR model can estimate LVFP in patients with suspected HF. In addition, CMR-modelled LVFP predicts the outcome. The manuscript is accompanied by an Editorial by Anna Baritussio from the University of Padua in Italy and Vivek Muthurangu from the University College London in the UK.25 Baritussio and Muthurangu highlight that the study by Garg et al. further expands the role of CMR as a one-stop-shop technique by providing data on HF aetiology, accurate and highly reproducible assessment of biventricular volumes and function, and an accurate estimation of LVFP. Given the good specificity and negative predictive value, CMR could be used as a tool to stratify patients for further invasive LVFP assessment. Finally, CMR can be proposed, based on the findings from Garg et al., not only as a diagnostic and prognostic tool in HF assessment, but also as a non-invasive test to monitor response to HF treatment.
The issue is also complemented by two Discussion Forum contributions. In a commentary entitled ‘Dual antiplatelet therapy analysis inconclusive in DISCO registry for spontaneous coronary artery dissection’, Cameron McAlister and Jacqueline Saw from the University of British Columbia in Canada and colleagues comment on the recent publication ‘Antiplatelet therapy in patients with conservatively managed spontaneous coronary artery dissection from the multicentre DISCO registry’ by Enrico Cerrato from San Luigi Gonzaga University Hospital in Turin, Italy, and colleagues.26,27 Cerrato et al. respond in a separate comment.28
Dr. Crea reports speaker fees from Amgen, Astra Zeneca, Servier, BMS, other from GlyCardial Diagnostics, outside the submitted work.
The editors hope that this issue of the European Heart Journal will be of interest to its readers.




