-
PDF
- Split View
-
Views
-
Cite
Cite
Deepthi Rajan, Rodrigue Garcia, Jesper Svane, Jacob Tfelt-Hansen, Risk of sports-related sudden cardiac death in women, European Heart Journal, Volume 43, Issue 12, 21 March 2022, Pages 1198–1206, https://doi.org/10.1093/eurheartj/ehab833
Close - Share Icon Share
Abstract
Sudden cardiac death (SCD) is a tragic incident accountable for up to 50% of deaths from cardiovascular disease. Sports-related SCD (SrSCD) is a phenomenon which has previously been associated with both competitive and recreational sport activities. SrSCD has been found to occur 5–33-fold less frequently in women than in men, and the sex difference persists despite a rapid increase in female participation in sports. Establishing the reasons behind this difference could pinpoint targets for improved prevention of SrSCD. Therefore, this review summarizes existing knowledge on epidemiology, characteristics, and causes of SrSCD in females, and elaborates on proposed mechanisms behind the sex differences. Although literature concerning the aetiology of SrSCD in females is limited, proposed mechanisms include sex-specific variations in hormones, blood pressure, autonomic tone, and the presentation of acute coronary syndromes. Consequently, these biological differences impact the degree of cardiac hypertrophy, dilation, right ventricular remodelling, myocardial fibrosis, and coronary atherosclerosis, and thereby the occurrence of ventricular arrhythmias in male and female athletes associated with short- and long-term exercise. Finally, cardiac examinations such as electrocardiograms and echocardiography are useful tools allowing easy differentiation between physiological and pathological cardiac adaptations following exercise in women. However, as a significant proportion of SrSCD causes in women are non-structural or unexplained after autopsy, channelopathies may play an important role, encouraging attention to prodromal symptoms and family history. These findings will aid in the identification of females at high risk of SrSCD and development of targeted prevention for female sport participants.
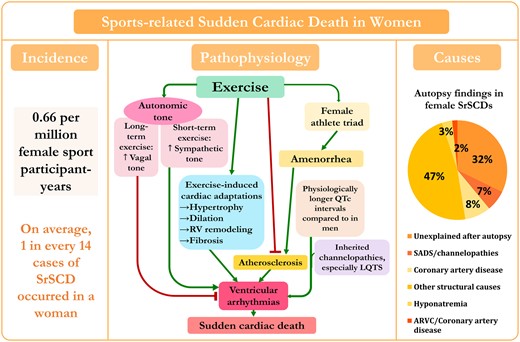
Diagram of incidence, pathophysiology, and causes of sports-related sudden cardiac death in women. The pie chart shows causes of 59 sports-related sudden cardiac deaths in women based on reported data from different studies.7–9 , 23 , 24 , 26 , 27 , 29 , 38 SADS, sudden arrhythmic death syndrome; RV, right ventricular; LQTS, long QT syndrome; ARVC, arrhythmogenic right ventricular cardiomyopathy. ‡The primary autopsy finding and stated cause of death in one of the hyponatraemia cases was hyponatraemia, yet additional clinical and autopsy data also showed the presence of myxomatous polyvalvular (mitral, tricuspid, aortic) heart disease.
Introduction
Sudden cardiac death (SCD) is a major health issue and responsible for up to 13–20% of all deaths in Western societies, a proportion of which are related to sports activities.1–3 Sports are defined, according to the Council of Europe, as all forms of physical activity which, through casual or organized participation, aim at expressing or improving physical fitness and mental well-being, forming social relationships, or obtaining results in competition at all levels.4 Sports-related SCD (SrSCD) is the subset of SCD occurring in temporal relation to physical exercise. While some definitions of SrSCD allow a time interval between sports participation and the occurrence of SCD of 1 h, others allow up to 96 h. However, the most common definition of SrSCD is a non-traumatic SCD occurring during or within 1 h of moderate- to high-intensity exercise.5–9
Sports were previously viewed as beneficial in preventing leading causes of SCD such as coronary artery disease (CAD) and diabetes. CAD is the most common cause of SCD3 and diabetes is a risk factor for SCD.10
SrSCD has been found to occur significantly more often in males than females.1 While both short- and long-term exercise are known to be associated with a multitude of cardiac structural and functional changes to accommodate increased demand for cardiac output,11–13 biological differences between males and females may result in distinct cardiac adaptations following exercise, leading to a disproportion in SrSCD incidence between the sexes.14 Furthermore, sex differences in catecholamine release during exercise may be consequential.
Uncovering the role of female sex in lower SrSCD incidences could reveal underlying mechanisms and consequently how SrSCD can be prevented. Therefore, in this review, we focus on the epidemiology of SrSCD, characteristics and causes of SrSCD in females, and elucidate how and why cardiac adaptations to exercise vary between males and females (Graphical Abstract).
Women in sports
Traditionally, female participation in sports activities has to a great extent trailed behind that of men. Notions of sports being harmful to female reproductive organs prevailed for a long time and women were not allowed to participate in the first modern Olympics in 1896. Thus, the implementation of Title IX, a federal civil rights law, in the USA in 1972 advocating for equal access to sports participation for both sexes at higher education institutions was monumental in compelling more women to engage in sports.15 As a consequence, female participation in sports increased by more than 1000% over the last 50 years, with jogging and cycling being the most popular activities among women.16 , 17 This coincided with an upsurge in women engaging in professional elite sports, with females representing 10% of participants in the Olympic games in 1948 in London, yet 45% by the 2016 Olympics in Rio.18
Football is the sport with most SrSCDs in Europe,19 and Fédération Internationale de Football Association (FIFA) implemented numerous initiatives to prevent SrSCD in football players. Previously, football was dominated by males, yet FIFA aims to increase global female participation to 60 million by 2026.20
Hitherto, the lower participation of females in sports activities offered a possible explanation for the lower occurrence of SrSCD encountered in women. However, considering the continuously rising participation of women in sports,21 it is paramount to determine whether this explanation still upholds.
Epidemiology of sports-related sudden cardiac death in women
Numerous studies from different countries provide estimates for the incidence of SrSCD in women (see Supplementary material online, Data S1 for literature search strategy). Nevertheless, comparison of these incidences is met by a number of challenges. There are inconsistencies in SrSCD definitions, methods used to record SrSCD cases (some more exhaustive, i.e. construction of systematic nationwide SrSCD databases compared with review of autopsy reports, death certificates, media searches, and insurance claims), prospective22 vs. retrospective5 study designs, and the population investigated (nationwide population vs. athletes, competitive vs. non-competitive athletes, and age groups included). In addition, there are potential errors in incidences reported, depending on the population selected as the denominator, and referral bias to autopsy. Limited autopsies are performed and levels of expertise in cardiovascular autopsy vary between centres.
In spite of these issues, the occurrence of SrSCD in women is found to be considerably lower than in men across various studies. In a prospective, community-based registry study from France, only 5.2% of 820 SrSCDs occurred in women, corresponding to a male:female ratio of 18:1.23 The male:female ratio in SrSCDs varied from 7:1 to 32:1 according to different studies.6 , 7 Overall, previous studies from different countries (Figure 1) demonstrated that females constitute between 0 and 17% of SrSCDs and appear to represent a higher proportion in later years.5–9 , 24–30 The sex difference persists among different ethnicities, as female athletes had lower incidence of SrSCD compared with male athletes of African-American/other minorities.31
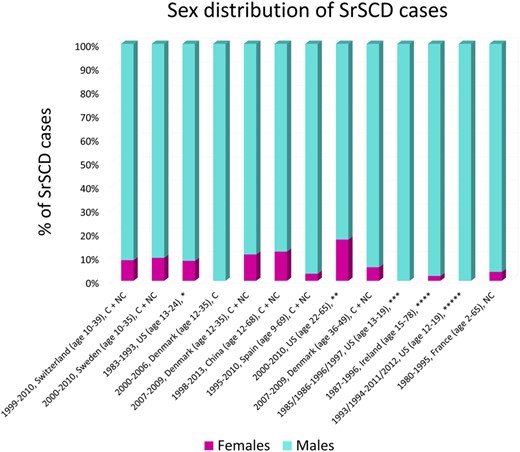
Bar chart showing percentage of sports-related sudden cardiac deaths occurring in women as found in studies from different countries with calendar period, country, and age of population reported. C, competitive athletes; NC, non-competitive athletes; SrSCD, sports-related sudden cardiac death. Competitive and non-competitive athletes both participate in moderate-to high-intensity sports on a regular level in the months prior to the SrSCD; however, competitive athletes additionally participate in competitions.24 *, high school and college athletes; **, full and half marathoners; ***, competitive high school athletes; ****, type of athlete not reported; *****, high school athletes, screened every 3 years with a standardized pre-participation health screening examination form including all components of the 2007 American Heart Association cardiovascular pre-participation health screening examination recommendations.
One could presume the sex difference in SrSCD occurrence is attributed to a lower participation of females in sports. On the other hand, this is countered by studies from the USA (1983–93), France (2005–10), and Germany (2012–14) reporting the incidence of SrSCD in females per million female sports participants and finding lower incidences compared with males. For example, the SrSCD incidence in females was 0.66 per million female athlete-years vs. 5.01 in males per million male athlete-years in the USA.9 Including resuscitated cases, the mean SrSCD incidence in France was 0.59–2.17 per million female sports participant-years and 11.24–33.84 per million male sports participant-years.23 The German study also included resuscitated cases and found a SrSCD incidence of 0.2 per million female sports participant-years vs. 3.60 per million male sports participant-years.32
SCD is twice as common in males than females, and the most common causes are CAD and secondly cardiomyopathy (see Supplementary material online, Data S2 for male:female ratios in SCD causes).33–35 The larger male:female ratio in SrSCD incidence may be due to differences in sports performed and exercise intensity. Mostly, literature on SrSCD incidences for females engaging in competitive/professional and non-competitive/recreational sports or determined for specific sports is limited. Nonetheless, male:female ratios in SrSCDs occurring during, respectively, recreational and competitive sports were similar.25 During long-distance races, the SrSCD incidence was 0.01 per million female marathoners vs. 0.06 per million male marathoners.27 The SCD incidences among athletes per million female athlete-years were 20.3 in women’s volleyball, 21.2 in cross-country (vs. 23.3 in males), 17.4 in swimming (vs. 23.4 in males), and 13.0 in basketball (vs. 111.4 in males).36 Therefore, even within the same sports, a large difference persists in SCD incidence between male and female athletes. However, men do more frequently engage in moderate and vigorous exercise than women.37
Sports mainly associated with SrSCD in women in France were (in >90% of cases) jogging, cycling, and swimming. While 91% occurred during the activity, 9% occurred within the following hour.23 A retrospective study of SrSCDs in the UK, where males represented 92%, also showed a predominance of running, cycling, and swimming.38 These data suggest jogging/running, cycling, and swimming (endurance sports) are activities commonly associated with SrSCD in both sexes,6 yet popular sports in a country naturally present a higher absolute SrSCD frequency. For example, in the USA, basketball is more commonly associated with SrSCD in male and female athletes.9 , 36
While the age at SrSCD occurrence varies according to the cause of SrSCD,24 , 26 age ranges of 12–78 years in males and 15–75 years in females predominated in SrSCD cases.6 , 7 , 23 , 24 As SrSCD incidences increase with the age of sports participants,24 age categories at SrSCD should be reported separately when comparing SrSCD incidences between sexes, especially considering the apparent 10-year delayed onset of CAD and 20-year delayed onset of sudden death in women.39
SrSCDs were more common during moderate to vigorous exertion in both sexes.23 In 81% of SrSCDs in France, the woman participated in moderate to vigorous exercise, yet more SrSCDs occurred in women than men during light exertion.23 Primarily, the risk of SrSCD was in women not exercising regularly,40 consistent with the finding that, while the risk of SCD is increased during exercise,22 habitual exercise is associated with reduced mortality.24
Physiological and pathophysiological specificities in women
Specificities in female cardiac adaptation to exercise may contribute to sex differences in SrSCD incidence. Physiological and pathophysiological responses to short- and long-term exercise differ and subsequent cardiac adaptations such as degrees of hypertrophy, right ventricular remodelling, fibrosis, and atherosclerosis are sex-specific. Mechanisms for this variation include sex differences in hormones, autonomic tone, blood pressure, and presentation of acute coronary syndromes.14 Furthermore, pressures from society impact exercise habits among women and can influence their risk of atherosclerosis. Changes in cardiac morphology depend on age, sex, ethnicity, and sporting discipline.11 Nonetheless, targeted prevention of SrSCD in females demands clear differentiation between physiological and pathological cardiac changes following exercise in women.
Cardiac hypertrophy
The degree of cardiac hypertrophy caused by long-term endurance exercise partly depends on angiotensinogen gene polymorphisms in individuals, where TT homozygotes have greater myocardial mass than MM homozygotes.41 The genotype prevalences were 30% for MM, 52% for MT, and 18% for TT. There were no associations between the other ACE gene I/D or AT1 gene A1166C polymorphisms studied and left ventricular mass.41
The left ventricular mass in TT homozygotes was ∼ 20 g/m higher in males and 15 g/m higher in females vs. MM homozygotes.41 Sex plays an important role in MT heterozygotes, as male MT heterozygotes had similar mass to TT homozygotes, while female MT heterozygotes had similar mass to MM homozygotes.41 More intensive training and higher intensity during sports in males could cause the increased hypertrophy in male athletes.22 However, the findings are consistent with greater left ventricular wall thickness (LVWT) and left ventricular sizes found in males vs. females of similar age and training intensity.42
Left ventricular hypertrophy was associated with ventricular arrhythmias and found to be an independent predictor of SCD.43 While testosterone in males directly promotes hypertrophy by binding to myocyte androgen receptors44, presumably through a genomic effect involving transforming growth factor beta (TGF-) and non-genomic effect via extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin (mTOR), oestrogens inhibit angiotensin II-induced myocardial hypertrophy through estrogen receptor beta (ERβ) receptor inhibition of calcineurin.14 , 45–47 Furthermore, testosterone was a mediator in angiotensin II-induced cardiac hypertrophy in male rats.48 Thus, greater hypertrophy following exercise in males may contribute to higher risk of SrSCD and complicate distinction from hypertrophic cardiomyopathy (HCM), a common cause of SrSCD.
Physiological adaptations to exercise in females are less likely to overlap with dimensions observed in cardiomyopathies associated with SrSCD. Echocardiography showed 14–24% of male athletes exhibit a left ventricular end-diastolic diameter (LVEDD) 60 mm and 2–2.5% LVWT ≥12 mm, yet these parameters are seen in 1% of female athletes.42 , 49–51 Furthermore, males often exhibit concentric left ventricular hypertrophy, whereas females exhibit more eccentric left ventricular hypertrophy and rarely concentric hypertrophy.51 A LVEDD 60 mm, LVWT > 12 mm, or concentric hypertrophy or remodelling in females is therefore unlikely, regardless of sports discipline,52 and could represent dilated cardiomyopathy or HCM (Figure 2). However, as black female athletes show larger dimensions than white female athletes, ethnicity is important to take into consideration.11
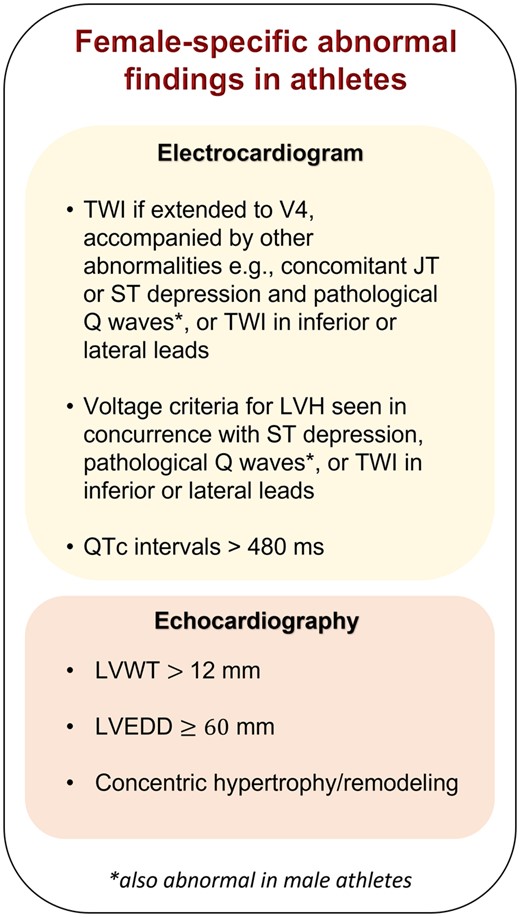
Diagram showing female-specific abnormal findings in electrocardiograms and echocardiography of athletes. LVEDD, left ventricular end-diastolic diameter; LVH, left ventricular hypertrophy; LVWT, left ventricular wall thickness; TWI, T-wave inversion.
In addition, 42% and 14% of male athletes fulfilled electrocardiographic criteria for left and right ventricular hypertrophy, respectively, vs. 14% and 0.3% of female athletes,52 , 53 reiterating that female athletes exhibit less hypertrophy than male athletes. Thus, electrocardiographic criteria for hypertrophy, particularly in concurrence with inferior or lateral T-wave inversion (TWI), ST-segment depression, or pathological Q waves in female athletes is suggestive of pathology.
Right ventricular remodelling
Intense endurance and mixed exercise are associated with more right and left ventricular remodelling than skill and power sports.52 , 54 In individuals without clinical cardiovascular disease, right ventricular ejection fraction is higher in females than males.55 Female athletes show larger right and left ventricular dimensions indexed for body surface area than male athletes in all sports,52 , 54 yet an initially higher right ventricular ejection fraction may allow females to tolerate more right ventricular dilation following exercise before right ventricular dysfunction occurs.
Exercise leads to earlier arrhythmogenic right ventricular cardiomyopathy (ARVC) expression and progression in genotype-positive individuals. Males have earlier ARVC debut and more severe phenotypes, with lower right ventricular ejection fraction than females.54 Oestrogen could be protective in females, as it decreases apoptosis and lipogenesis in induced pluripotent stem cell-derived ARVC cardiomyocytes, whereas testosterone has the opposite effect.56
In addition, elevated testosterone levels are associated with higher risk of malignant ventricular arrhythmias in males with ARVC, while lower oestradiol levels are associated with higher risk of ventricular arrhythmias in females with ARVC.56 Exercise has shown to decrease free oestradiol levels in females, thus sports participation may lead to higher risk of ventricular arrhythmias in females with ARVC.57
In athletes, two-thirds of males vs. less than half of females had right ventricular dimensions meeting minor diagnostic criterion for ARVC.58 Anterior TWI is the most frequent abnormality in electrocardiograms of ARVC patients,53 yet also found in healthy athletes.11 Conversely, TWI extended to V4, in inferior or lateral leads, or accompanied by JT or ST depression or pathological Q waves, is rare in female athletes and should raise suspicion of HCM, dilated cardiomyopathy, ARVC, or myocarditis.53 , 59
Myocardial fibrosis
Prolonged high-intensity exercise in endurance athletes may cause myocardial inflammation and stimulate myocardial fibrosis,60 another potential substrate for malignant arrhythmias and SrSCD. Data on exercise-induced cardiac fatigue in females are limited, but troponin T release post-endurance exercise was higher in male than in female athletes and could signal myocardial injury.61 , 62 In accordance with this, cardiac magnetic resonance scanning showed non-ischaemic myocardial fibrosis in 17% of male athletes, yet none of the female athletes.63 Myocardial fibrosis was independently associated with higher systolic blood pressure at peak exercise, suggesting lower blood pressures in females at rest and under exercise could hinder myocardial fibrosis.63 Oestrogens may cause lower blood pressure in females by increasing nitric oxide and reducing angiotensin-converting enzyme activity, leading to peripheral vasodilation and reduced afterload.14 , 23 Conversely, testosterone increases angiotensin-converting enzyme activity. In addition, oestrogens inhibit proliferation of cardiac fibroblasts and could also explain lower myocardial fibrosis burdens in female athletes.
Female athletes with exercise-associated amenorrhoea have lower resting systolic blood pressures than eumenorrheic female athletes, assumed a result of a negative energy balance.64 Yet, post-menopausal women show similar or higher blood pressures than age-matched men, very likely to be associated with concurrent decreases in oestrogen.65–67 However, the extent of myocardial fibrosis in women with reduced oestrogen concentrations is unknown, as studies in post-menopausal and amenorrhoeic athletes are limited.
Coronary artery disease
Effects of prolonged, high-intensity endurance exercise on coronary atherosclerosis appear to differ between men and women. Coronary plaques and calcification were significantly more prominent among athletic men compared with sedentary controls.68 , 69 When the same comparison was performed in females, there was either no difference between athletic and sedentary women68 or significantly lower coronary plaque prevalence and less calcific plaque volume among female marathoners vs. sedentary controls.70 Furthermore, the aetiology behind acute coronary syndromes may differ between the sexes; while men frequently experience plaque rupture and ensuing thrombus formation, women experience plaque erosions with distal embolizations of microemboli and dysfunction of the microvascular coronary system or coronary dissections.71 , 72 In summary, these findings suggest myocardial ischaemia may present less of a threat as a SrSCD cause in women.
However, societal pressures to be thin, affecting more females than males,73 can result in insufficient caloric intake and overexertion among female athletes, leading to the female athlete triad: a syndrome of disordered eating, amenorrhoea, and osteoporosis. Amenorrhoeic athletes show unfavourable lipid profiles and less flow-mediated dilation in their arteries, which are risk factors for atherosclerosis, and presumed to be a result of decreased circulating oestrogens and reduced nitric oxide production.74 Therefore, although coronary plaques were generally less common in female than male athletes, amenorrhoeic female athletes may be a subgroup at increased risk of CAD-associated SrSCD. This is supported by preliminary data associating pre-menopausal oestrogen deficiency with increased cardiovascular events.64
Post-menopausal females or females with premature ovarian insufficiency, endometriosis, polycystic ovarian syndrome, or autoimmune disorders are at higher risk of cardiovascular events.75 Yet, there is a paucity of data on effects of autoimmune disorders on oestrogen levels. The impact of exercise on decreasing oestradiol in females may exacerbate the risk in these individuals,57 although oestradiol levels remained higher in females than males regardless of training status.57 Lack of studies in post-menopausal athletes limits knowledge on their coronary plaque prevalences.
Spontaneous coronary artery dissection and Takotsubo syndrome
Spontaneous coronary artery dissection (SCAD) has a predilection for occurring in middle-aged, otherwise healthy women,76 yet SCAD due to intense exercise was more common in men.77 As SCAD is often misdiagnosed as acute CAD and can be missed under autopsy,78 SCAD may underlie some reported cases of CAD or blank autopsies as a SrSCD cause in men and women.
Takotsubo syndrome predominantly occurs in post-menopausal females after emotional or physical stress, presumably due to catecholamine excess, and can lead to SCD.79 There are case reports of Takotsubo cardiomyopathy in relation to exercise in females.80–82 In addition, hyponatraemia was associated with triggering Takotsubo syndrome83 and females were more likely than males to develop hyponatraemia during endurance exercise.84
Cardiomyopathies, congenital heart disease, and abnormal coronary anatomy
HCM and exercise-induced ventricular arrhythmias in HCM are more common in men than in women.85 Similarly, dilated cardiomyopathy and ARVC are more prevalent in males and more men experience malignant arrhythmias.86 , 87 Guidelines require annual risk assessments for individuals with cardiomyopathy exercising regularly. High-intensity exercise is not recommended in patients with HCM or dilated cardiomyopathy with cardiac symptoms, exercise-induced arrhythmias, prior cardiac arrest, unexplained syncope, etc.88 In individuals with an ARVC phenotype, participation in high-intensity sports is not recommended.88
SrSCD is rare in individuals with congenital heart disease,89–92 and most often due to coronary artery anomalies.3 Coronary artery anomalies were more common in females than males documented by coronary angiographies.93 Guidelines do not recommend competitive sports with moderate-to-high cardiovascular demand with an anomalous origin of a coronary artery with acutely angled take-off or anomalous course between the large vessels.88
Autonomic tone
Differences in autonomic tone between the sexes could influence their risk of SrSCD, especially in the presence of underlying cardiovascular disease. SCD can be caused by a ventricular arrhythmia, triggered by a peak in sympathetic activity, e.g. at onset or during short-term exercise. Sympathetic activation increases, as exercise intensity increases.94
Ventricular arrhythmias were less common in women with CAD and implantable cardioverter-defibrillators than in men.95 When changes in heart rate, heart rate variability, and blood pressure were analysed in patients with coronary occlusion referred for angioplasty, vagal activation was more common in females.96 Increased vagal activation may protect women against triggering fatal arrhythmias in established cardiovascular disease or channelopathies under exercise. However, women with severe menopausal symptoms show unfavourable lipid profiles and sympathetic overactivity compared with asymptomatic women, and therefore could be at higher risk of atherosclerosis and fatal arrhythmias under exercise.75
During exercise, males showed higher adrenaline concentrations than females,97 although some studies showed no difference in noradrenaline.98 , 99 Men are more competitive than women in athletics and higher pressures to win may lead to even higher levels of adrenaline in men.100
QT intervals and channelopathies
Apart from structural heart differences, men and women have inherent electrophysiological differences, which may affect their risk of SrSCD,101 as females have longer QTc intervals than males.102 , 103 Indeed, testosterone and progesterone shorten the QTc interval in humans, but oestrogen was found to prolong the QTc interval in animal studies, while human studies were inconclusive.102
While the risk of ventricular arrhythmias and fatal events in long QT syndrome (LQTS) depends on age, sex, LQTS subtype, and QTc length, there is also a female predominance in LQTS.103 Women with LQTS have longer QTc intervals than men and are generally more prone to develop polymorphic ventricular tachycardia and SCD as QTc length is a predictor of SCD regardless of LQTS subtype.103–105 Nevertheless, after adjustment for QTc duration, boys with LQTS1 have higher risk of ventricular arrhythmias and fatal events than girls during childhood, whereas females still present a greater risk in both LQTS1 and LQTS2 following puberty.106 , 107 The higher risk of SCD in females with LQTS1 is of particular importance as physical stress predominantly triggered events in this subtype, although exercise rarely also leads to SCD in LQTS2 and LQTS3.108
Further investigation is required when asymptomatic athletes present QT intervals over 470 ms in males and 480 ms in females.109 , 110 However, in a small number of athletes, physical training can induce QT prolongation mimicking congenital LQTS.111 Factors against congenital LQTS include genotype-negative and asymptomatic individuals, negative family history, and normalization following detraining.
Female sex is an independent risk factor for drug-induced LQTS102 , 112 and females show higher risk of drug-induced LQTS during the first half of the menstrual cycle.113 Therefore, usage of QT-prolonging medications, such as sotalol, amiodarone, macrolides, and fluconazole, during the vulnerable menses and ovulation phases of the menstrual cycle and sports participation may prove a risky combination in relation to SrSCD in women.
Literature is limited on sex differences in catecholaminergic polymorphic ventricular tachycardia; however, increased vagal tone may be protecting women from fatal arrhythmias associated with this condition. Brugada syndrome has male predominance and the risk of SCD is higher in post-pubertal men with Brugada syndrome than in women.114–116 To date, no evidence shows sport increases the risk of SCD in Brugada syndrome, despite reports of exercise-induced syncope and ventricular arrhythmias in Brugada patients.116
Causes of sports-related sudden cardiac death in women
Causes of SrSCD vary in different countries according to the prevalence of underlying cardiac diseases, e.g. HCM, in different ethnicities and types of pre-participation screening implemented.22 Combined with low incidence of SrSCD in females, this limits the identification of recurring causes of SrSCD in women; however, the following studies demonstrate overall patterns in SrSCD causes.
SrSCD in women is less likely to be seen in concurrence with structural heart disease compared with men.7 , 23 , 26 , 38 For instance, the prospective study of SCDs (including resuscitated cases) from France established that 42% of SrSCDs in women were non-structural, vs. 4% in men.23 Causes of death in these female SrSCD cases without structural heart disease included LQTS, early repolarization syndrome, and a malignant accessory pathway. Similarly, in a retrospective study from the UK, post-mortem evaluation revealed a normal heart in 55% of women after SrSCD vs. 42% in the whole cohort consisting of 92% men.38 Accumulating data from various studies showed that, overall, 32% of female SrSCDs (at ages 15–75) were unexplained and 7% were labelled as due to sudden arrhythmic death syndrome (SADS)/channelopathies (Figure 3).7–9 , 23 , 24 , 26 , 27 , 29 , 38
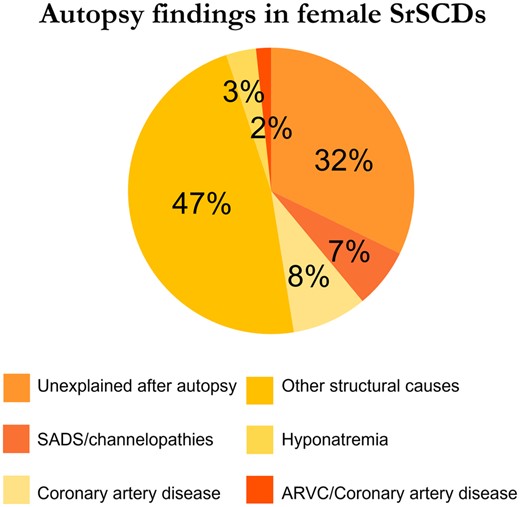
Although CAD is the culprit in most SrSCDs in athletes over 35 years, it is less common in females.117 While 13–78% of male SrSCDs presented with CAD (including some 35 years), 0–33% of the female cases did.23 , 25 , 26 Thus, CAD is much less common in female than male SrSCDs—a finding consistent with coronary plaques being less common in athletic women than athletic men.68 While exercise is recommended in individuals with CAD to limit mortality and re-hospitalizations, competitive sports are not recommended in individuals at high risk of exercise-induced adverse events or with residual ischaemia.88
Other structural conditions often associated with SrSCD in women were HCM, coronary artery anomalies, ARVC, idiopathic fibrosis, and myocarditis.7 , 9 , 23 , 25 , 26 , 38
HCM and coronary artery anomalies were the most frequent causes of SrSCD in high school and college athletes in the USA.9 HCM was seen in 14% of female SrSCDs vs. 51% of male SrSCDs. However, 29% of female SrSCDs were due to coronary artery anomalies vs. 14% of male SrSCDs.
Few ARVC caused SCD in female athletes, yet were the most frequent cause of SCD in male athletes in Italy.22 As cardiac penetrance for ARVC is higher in men and males with ARVC have higher likelihood of SCD, ARVC may pose a greater threat in male athletes.118 , 119
To summarize, these findings suggest a large share of SrSCDs in women are non-structural or unexplained after autopsy and could be attributed to SADS, e.g. LQTS, Brugada syndrome, or catecholaminergic polymorphic ventricular tachycardia. Female predominance in LQTS and greater susceptibility to SCD in LQTS1 and LQTS2 following puberty104 compared with men support that the high proportion of non-structural SrSCDs in women could partially be explained by LQTS.31
Accordingly, this suggests greater attention should be paid to long QT in female athletes, especially those with known LQTS (particularly LQTS1), albeit athletes tend to present longer QT intervals.109 , 111
Sports-related sudden cardiac death: a multiple hit model?
Differences in exercise-related cardiac adaptations in men and women due to the genetic polymorphisms mentioned and hormones could partially explain the difference in SrSCD incidence; however, unexplored genetic polymorphisms may also play a role. SrSCD may indeed represent a multiple hit model, where biological, lifestyle-related, and psychological factors accumulate and culminate in SrSCD (Figure 4).
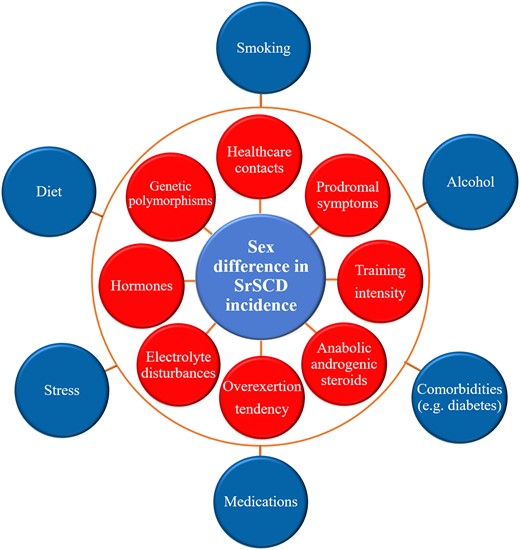
A multiple hit model for sports-related sudden cardiac death in women. SCD, sudden cardiac death; SrSCD, sports-related sudden cardiac death. The outermost blue factors are relevant for the sex difference in SCD incidence in the general population as well as SrSCD, whereas the red factors may specifically impact the sex difference in SrSCD.
Some factors affect the sex difference in SCD in the general population as well as SrSCD, i.e. diet, smoking, alcohol consumption, stress, medication usage, and comorbidities like diabetes10 and psychiatric disease.120 Other factors specifically impact SrSCD and could account for the amplified sex difference in SrSCD incidence, i.e. attention to prodromal symptoms under exercise and frequency of healthcare contacts,121 training intensity, tendency to overexert during exercise,23 electrolyte disturbances, and anabolic steroid usage.122
While men consume more fat, red meat, and alcohol, women consume more fruits, vegetables, and whole grains.123 Yet, even with similar dietary intake of fat and cholesterol, men primarily transported excess cholesterol as low-density lipoprotein, while women responded with a greater rise in high-density lipoprotein2.124 Men have higher low-density lipoprotein levels, yet lower high-density lipoprotein levels than women until the age of 50, where women experience an increase in low-density lipoprotein.72 Additionally, the global prevalence of men who smoke is substantially higher than in women.125 These factors could elevate the risk of atherosclerosis, CAD, and related SCD in men. Moreover, a disproportion between the sexes in the prevalence of comorbidities with cardiac involvement or sequelae should be considered. Diabetes is more prevalent in men,10 , 126 whereas stress, anxiety, and depression are more prevalent in women.120 , 127 , 128 Stress is associated with Takotsubo cardiomyopathy and anxiety and depression with CAD.129
Higher risk of SrSCD in men may result from men being more physically active,130 engaging in more intensive training and higher intensity levels during sports.22 SrSCD in males occurring towards the end of races could imply a tendency for men to overexert themselves, disregarding prodromal symptoms and pushing beyond usual limits.32 Women reportedly utilize healthcare services more frequently than men,131 which could enable earlier detection of exertion-related symptoms due to underlying cardiovascular disease and implementation of preventive measures to avoid SrSCD. Men, however, show a tendency to delay help-seeking.132
Anabolic androgenic steroid abuse is associated with cardiac hypertrophy, arrhythmias, and SCD.133 Multiple studies showed women were less likely than men to use anabolic steroids,134 placing women at lower risk of anabolic steroid-associated SrSCD.
On the other hand, females are at greater risk of torsade de pointes with usage of QT-prolonging medications and more vulnerable to ventricular arrhythmias during specific menstrual phases, presumably due to hormones. These pro-arrhythmic medications include sotalol, amiodarone, macrolides, and fluconazole.135 Elderly females are at higher risk of hypokalaemia,136 which can be caused by intense endurance exercise due to sweat loss. Conversely, high-intensity training can cause hyperkalaemia due to rhabdomyolysis.137 , 138 Female athletes were also more likely than male athletes to develop hyponatraemia during endurance exercise.84 Therefore, sports participation during the first half of the menstrual cycle139 combined with QT-prolonging drugs and eventual electrolyte derangements could provide multiple hits, collectively increasing the risk of SrSCD in women.
Future directions
The lower incidence of SrSCD in females could be partially due to distinct cardiac adaptations following exercise in males and females. Future studies should investigate the prevalence of various cardiac adaptations by examining echocardiographic parameters and electrocardiograms in female athletes and comparing these with dimensions and findings in autopsied SrSCDs in females. Hence, it may be possible to differentiate between pathological changes associated with SrSCD and physiological changes following exercise in women.
Performing sports pre-participation evaluations for all athletes to prevent SrSCD remains a controversial topic. A prospective register over cardiac events during long-distance races including more than 1 000 000 participants over 10 years recorded only one life-threatening cardiac event in a female, despite females representing 22% of runners.140 Consequently, the low incidence of SrSCD in women challenges the cost-effectiveness of screening in female athletes.141 Rather, the high proportion of non-structural SrSCD causes in female athletes appeals to drawing attention to prodromal symptoms upon exertion, allowing for the implementation of near-term prevention,142 and family history to uncover potential channelopathies. However, if screening programmes were to be implemented for all athletes, this review demonstrates the importance of these being sex-specific to account for differences in the most common male and female SrSCD causes.
Conclusion
Despite increasing female participation in sports, this review reinforces that SrSCD seems to be less of a threat in females than males, emphasizing the importance of reporting sex-specific SrSCD incidences. Sex-specific cardiac adaptations to exercise may explain the difference in SrSCD incidence, where female athletes exhibit less hypertrophy, dilation, right ventricular remodelling, coronary atherosclerosis, myocardial fibrosis, and sympathetic tone than males, reducing sources for fatal arrhythmias. Social or behavioural differences between the genders, i.e. a proclivity for men to use performance-enhancing drugs or overexert, could also contribute to the dissimilarity in SrSCD incidence. Nonetheless, in female athletes, a LVWT > 12 mm, LVEDD ≥ 60 mm, concentric hypertrophy/remodelling, inferior or lateral TWI may represent cardiac pathology, warranting investigation for structural abnormalities. However, as a high proportion of female SrSCDs are non-structural, preventive measures in women should lay emphasis on exertion-related symptoms and family history to uncover potential channelopathies. Future studies of molecular autopsies among autopsy-negative SrSCD cases in women are required to determine the significance of cardiac channelopathies.
Limitations
Differences in outcomes following sudden cardiac arrest (chance of resuscitation, survival, and primary rhythms) in male and female athletes were not investigated in this review.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The authors report no specific funding related to this article.
Conflict of interest: none declared.
Data availability
No new data were generated or analysed in support of this research.
Permissions information
The authors do hereby declare that all illustrations and figures in the manuscript are entirely original and do not require reprint permission.
References
Author notes
Jesper Svane, Jacob Tfelt-Hansen contributed equally to this work



