-
PDF
- Split View
-
Views
-
Cite
Cite
Filippo Crea, Personalized antithrombotic treatment of cardiovascular disease: a glimpse into the future based on current knowledge, European Heart Journal, Volume 43, Issue 10, 7 March 2022, Pages 925–929, https://doi.org/10.1093/eurheartj/ehac086
Close - Share Icon Share
With thanks to Amelia Meier-Batschelet, Johanna Huggler, and Martin Meyer for help with compilation of this article.
 For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
This Focus Issue on thrombosis and antithrombotic treatment contains the State of the Art Review article ‘Towards personalized antithrombotic management with drugs and devices across the cardiovascular spectrum’ by Thomas Lüscher from the Royal Brompton & Harefield Hospitals in London, UK, and colleagues.1 The authors note that intravascular thrombus formation and embolization are among the most frequent events leading to a number of cardiovascular conditions with high morbidity and mortality.2–4 Personalized anticoagulation must consider the clinical context, the patient characteristics (including genetics where appropriate), the expected efficacy vs. risk of bleeding related to different antithrombotic medications, and the patient's preference. The fact that in many patients, several clinical conditions such as acute coronary syndrome (ACS), atrial fibrillation, valvular heart disease, and/or peripheral or carotid artery disease may co-exist complicates matters substantially. Unfortunately, many trials have excluded such multimorbid patients and hence the evidence is somewhat limited in this setting. Particularly challenging situations are patients with cerebral bleeding and ACS undergoing a percutaneous coronary intervention. Similarly, some patients may receive several devices simultaneously or in staged procedures. Undoubtedly, the risk of complications and bleeding might be considerably higher in such patients, requiring good clinical judgement and personalized decision-making within a Heart Team. Finally, patient-centred care has to consider the patient's interests and preferences, particularly as regards the risks of lifelong antithrombotic medication vs. efficacy and safety of implantable devices. A differential and personalized use of anticoagulants, platelet inhibitors, and devices is recommended and reviewed in this article.
Guidelines recommend the use of potent P2Y12 inhibitors over clopidogrel for the reduction of ischaemic events in patients with ACS.5–9 However, this comes at the expense of increased bleeding. A guided selection of P2Y12-inhibiting therapy has the potential to overcome this limitation. In a Fast Track Clinical Research article entitled ‘Comparative effects of guided vs. potent P2Y12 inhibitor therapy in acute coronary syndrome: a network meta-analysis of 61 898 patients from 15 randomized trials’, Mattia Galli from the Catholic University of Rome in Italy, and colleagues aimed to evaluate the comparative safety and efficacy of guided vs. routine selection of potent P2Y12-inhibiting therapy in patients with ACS.10 The authors performed a network meta-analysis of randomized controlled trials (RCTs) comparing different oral P2Y12 inhibitors currently recommended for the treatment of patients with ACS (clopidogrel, prasugrel, and ticagrelor). RCTs including a guided approach (i.e. platelet function or genetic testing) vs. standard selection of P2Y12 inhibitors among patients with ACS were also included. Incidence rate ratios (IRRs) and associated 95% confidence intervals (CIs) were estimated. P-scores were used to estimate hierarchies of efficacy and safety. The primary efficacy endpoint was major adverse cardiovascular events (MACE) and the primary safety endpoint was all bleeding. A total of >61 000 patients from 15 RCTs were included. Clopidogrel was used as reference treatment. A guided approach was the only strategy associated with reduced MACE (IRR 0.80, 95% CI 0.65–0.98) without any significant trade-off in all bleeding (IRR 1.22, 95% CI 0.96–1.55) (Figure 1).
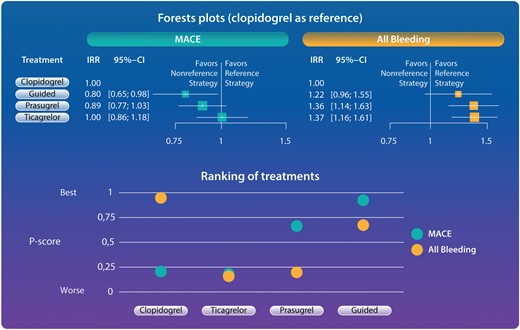
Forest plots and ranking of treatments for primary outcomes. Guided selection of P2Y12 inhibiting was the only strategy associated with reduced major adverse cardiovascular events without any increase of bleeding (forest plots). Ranking of treatments according to P-scores showed guided therapy to be the strategy with the best balance between safety and efficacy. P-scores range between 0 and 1: the higher the P-score value, the higher the likelihood that a therapy is more effective or safe. A guided approach showed the best P-score for MACE (0.931) and the second best P-score for all bleeding (0.569). Clopidogrel showed the best P-score for all bleeding (0.983) but the poorest P-score for MACE (0.199). Prasugrel showed the second best P-score for MACE (0.673) but a poor P-score for all bleeding (0.233). Ticagrelor showed a poor P-score for both MACE and all bleeding (0.198 and 0.214, respectively). CI, confidence interval; IRR, incidence rate ratio; MACE, major adverse cardiovascular events.10
The authors conclude that in patients with ACS, compared with routine selection of potent P2Y12-inhibiting therapy (prasugrel or ticagrelor), a guided selection of P2Y12-inhibiting therapy is associated with the most favourable balance between safety and efficacy. These findings support a broader adoption of a guided approach for the selection of P2Y12-inhibiting therapy in patients with ACS. The contribution is accompanied by an Editorial by Michelle O’Donoghue and Nicholas Marston from the Brigham and Women's Hospital, Boston, MA, USA.11 The authors highlight that physicians are encouraged to tailor the duration of double antiplatelet therapy based on individual patient characteristics. By extension of logic and based on the clinical trial evidence, it would be appropriate for the medical community to also shift toward a more guided selection of antiplatelet therapy based on patients’ perceived bleeding and ischaemic risk, as currently supported by some updated guideline documents. Ultimately, an era of more personalized medicine is helping clinical practice to evolve beyond the ‘one size fits all’ approach that has failed many in the past.
Traditional atherosclerotic cardiovascular disease (ASCVD) risk factors fail to address the full spectrum of the complex interplay of atherosclerotic and atherothrombotic factors integral to ASCVD events.12–15 In a Clinical Research article entitled ‘Atherothrombotic factors and atherosclerotic cardiovascular events: the multi-ethnic study of atherosclerosis’, Andrew DeFilippis from the Vanderbilt University Medical Center in Nashville, TN, USA, and colleagues sought to examine the association between atherothrombotic biomarkers and ASCVD events.16 The association between atherothrombotic biomarkers and 877 ASCVD events with and without adjustment for traditional risk factors was evaluated via Cox proportional hazards models and factor analysis in 5789 participants in the Multi-Ethnic Study of Atherosclerosis over a median follow-up of 14.7 years. Factor analysis accounted for multi-dimensional relationships and shared variance among study biomarkers, which identified two new variables: a thrombotic factor (Factor 1), principally defined by shared variance in fibrinogen, plasmin–antiplasmin complex, factor VIII, D-dimer, and lipoprotein(a), and a fibrinolytic factor (Factor 2), principally defined by shared variance of plasminogen and oxidized phospholipids on plasminogen. In a model including both factors, the thrombotic factor was associated with the higher risk of ASCVD events [hazard ratio (HR) 1.57], while the fibrinolytic factor was associated with the lower risk of ASCVD events (HR 0.76), with estimated ASCVD-free survival highest for low atherothrombotic Factor 1 and high atherothrombotic Factor 2.
The authors conclude that two atherothrombotic factors, one representative of thrombotic propensity and the other representative of fibrinolytic propensity, are significantly and complementarily associated with incident ASCVD events, remained significantly associated with incident ASCVD after controlling for traditional risk factors, and have promise for identifying patients at high ASCVD event risk specifically due to their atherothrombotic profile. The contribution is accompanied by an Editorial by Artur Fedorowski from the Karolinska University Hospital in Stockholm, Sweden, and colleagues.17 The authors note that taken together, these observations support the idea that further improvement of CV risk prevention may be reached by implementation of multi-biomarker tools. The pathogenesis of atherothrombosis is multi-factorial, and different mechanisms may concur (e.g. thrombosis, inflammation, and lipid plaque formation), with varying roles of each of these mechanisms in different individuals. Thus, the optimal prediction model would integrate information from the coagulation system, lipid metabolism, and inflammation. Consequently, a two-step approach might be valuable: first, individual stratification of clinical risks to select subjects with moderate to high risk of CV events, followed by multi-biomarker evaluation indicating which pathogenetic mechanism (i.e. thrombosis vs. inflammation vs. lipid disorders) is predominant. The latter may inform the clinician of which individually tailored preventive strategies should be applied.
Direct oral anticoagulants (DOACs) are now recognized as the first-line treatments for stroke prevention in atrial fibrillation and for the acute treatment and long-term prevention of recurrent venous thrombo-embolism.18,19 Ciraparantag is a reversal agent for anticoagulants including DOACs. In a Clinical Research article entitled ‘Ciraparantag reverses the anticoagulant activity of apixaban and rivaroxaban in healthy elderly subjects’, Jack Ansell from the Hofstra Northwell School of Medicine in New York, NY, USA, and colleagues evaluate the efficacy and safety of ciraparantag to reverse anticoagulation induced by apixaban or rivaroxaban in healthy elderly adults.20 The authors report the results of two randomized, placebo-controlled, dose-ranging trials conducted in healthy subjects aged 50–75 years. Subjects received apixaban (Study 1) 10 mg orally twice daily for 3.5 days or rivaroxaban (Study 2) 20 mg orally once daily for 3 days. At steady state, anticoagulation subjects were randomized 3:1 to a single intravenous dose of ciraparantag (Study 1: 30, 60, or 120 mg; Study 2: 30, 60, 120, or 180 mg) or placebo. Efficacy was based on correction of the whole blood clotting time (WBCT) at multiple time points over 24 h. Complete reversal of WBCT within 1 h post-dose and sustained through 5 h (apixaban) or 6 h (rivaroxaban) was dose related and observed with apixaban in 67, 100, 100, and 17% of subjects receiving ciraparantag 30, 60, or 120 mg, or placebo, respectively; and with rivaroxaban in 58, 75, 67, 100, and 13% of subjects receiving ciraparantag 30, 60, 120, or 180 mg, or placebo, respectively. Adverse events related to ciraparantag were mild, transient hot flashes or flushing.
Ansell et al. conclude that ciraparantag provides a dose-related reversal of anticoagulation induced by steady-state dosing of apixaban or rivaroxaban. Sustained reversal was achieved with 60 mg ciraparantag for apixaban and 180 mg ciraparantag for rivaroxaban. All doses of ciraparantag were well tolerated. The manuscript is accompanied by an Editorial by Noel Chan and Jeffrey Weitz from McMaster University in Hamilton, Ontario, Canada.21 Chan and Weitz conclude that although the road forward is challenging and much work remains to be done, the study by Ansell and colleagues provides the impetus to study ciraparantag reversal in patients taking apixaban or rivaroxaban.
According to the 2019 European Society of Cardiology (ESC) guidelines on chronic coronary syndromes (CCS), adding a P2Y12 inhibitor or rivaroxaban to aspirin should be considered in high-risk patients.22 In a Clinical Research article entitled ‘Dual antithrombotic treatment in chronic coronary syndrome: European Society of Cardiology criteria vs. CHADS-P2A2RC score’, Morten Würtz from the Aarhus University Hospital in Denmark, and colleagues estimated the proportion of patients eligible for treatment with the ESC criteria and examined if a recently validated risk score (CHADS-P2A2RC) could improve risk prediction.23 The authors included >61 000 CCS patients undergoing first-time coronary angiography in Western Denmark (2003–2016) and classified them according to the ESC criteria and the CHADS-P2A2RC score. The ESC criteria identified 34% as high risk, 53% as moderate risk, and 13% as low risk. The CHADS-P2A2RC score identified 25% as high risk, 48% as moderate risk, and 27% as low risk. MACE per 100 person-years were 4.8 in patients considered high risk with both schemes, 2.1 in patients considered high risk with the ESC but low to moderate risk with the CHADS-P2A2RC criteria, 3.8 in patients considered low to moderate risk with the ESC but high risk with the CHADS-P2A2RC criteria, and 1.5 in patients considered low to moderate risk with both schemes. The CHADS-P2A2RC score enabled correct downward risk reclassification of 5161 patients (8%) without events (Figure 2).
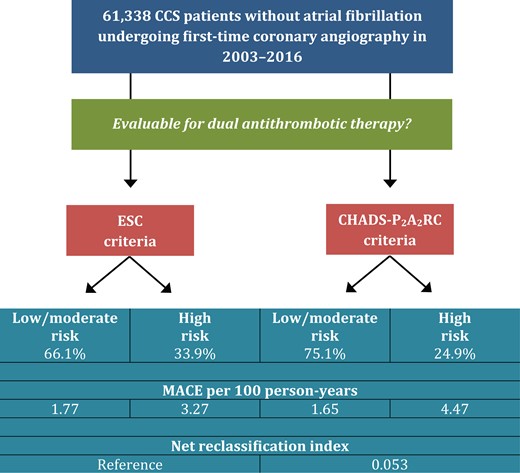
Risk stratification strategies to guide extended dual antithrombotic therapy. Use of the CHADS-P2A2RC score instead of the European Society of Cardiology (ESC) criteria improved overall risk classification. The CHADS-P2A2RC score enabled downward risk reclassification of 8% of the total population without major adverse cardiovascular events (MACE), yielding an overall net reclassification index of 0.053 (P < 0.0001).23
Würtz et al. conclude that based on the 2019 ESC guidelines, dual antithrombotic treatment should be considered in one-third of CCS patients. The CHADS-P2A2RC score improves risk classification and may particularly identify low-risk patients with limited benefit from treatment. This manuscript is accompanied by an Editorial by Julinda Mehilli and Maximilian Winhard from the Landshut-Achdorf Hospital in Germany.24 The authors highlight that cardiac risk scores are considered by physicians as valuable tools to improve uniformity in care delivery, educating interns, conducting research, and quantifying physicians’ own risk assessment, while their perception regarding the influence of cardiac risk scores on treatment decision pathways remains contradictory. Yet, cardiac risk scores have an important potential to enhance the quality of care of delivery. Providing randomized evidence of risk score use to guide care delivery in addition to guideline-directed treatment strategies is the most important step to increase their adoption in clinical routine.
In a Viewpoint article entitled ‘Heparin use in acute coronary syndromes and cardiovascular interventions: habit or evidence based?’, Sean Tan, Harvey White, and Jamie Layland from the Victorian Heart Institute in Melbourne, Australia, and colleagues note that unfractionated heparin (UFH) remains the only parenteral anticoagulant with a short half-life, safety in kidney disease, and full reversibility, and hence continues to be commonly used in contemporary cardiovascular procedures and in critically ill patients.25 However, certain applications of UFH remain guided by historical experience rather than robust clinical trials. There remains scope for further investigative studies to evaluate the indication, optimal dosing, and monitoring required for UFH use, as well as other alternative agents to UFH, in routine cardiovascular procedures. This viewpoint article aims to highlight evidence-based practice and gaps in evidence in UFH use for the treatment of coronary artery disease and support of cardiovascular procedures.
The issue is also complemented by two Discussion Forum contributions. In a commentary entitled ‘Myocardial infarction after elective percutaneous coronary intervention—which cardiac troponin cut-off to use?’ Kai Eggers from the Uppsala University in Sweden and colleagues comment on the recent publication ‘Procedural myocardial injury, infarction and mortality in patients undergoing elective PCI: a pooled analysis of patient-level data’ by Johanne Silvain from the Hôpital Pitié-Salpêtrière (AP-HP) in Paris, France.26,27 Silvain et al. respond in a separate comment.28
The editors hope that readers of this issue of the European Heart Journal will find it of interest.




