-
PDF
- Split View
-
Views
-
Cite
Cite
Carolyn S P Lam, João Pedro Ferreira, Egon Pfarr, David Sim, Hiroyuki Tsutsui, Stefan D Anker, Javed Butler, Gerasimos Filippatos, Stuart J Pocock, Naveed Sattar, Subodh Verma, Martina Brueckmann, Janet Schnee, Daniel Cotton, Faiez Zannad, Milton Packer, Regional and ethnic influences on the response to empagliflozin in patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial, European Heart Journal, Volume 42, Issue 43, 14 November 2021, Pages 4442–4451, https://doi.org/10.1093/eurheartj/ehab360
Close - Share Icon Share
Abstract
The aim of this article is to explore the influence of region and race/ethnicity on the effects of empagliflozin in the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR-Reduced) trial.
Of 3730 patients, 1353 (36.3%) were enrolled in Europe, 1286 (34.5%) in Latin America, 425 (11.4%) in North America, and 493 (13.2%) in Asia; 2629 (70.5%) were White, 257 (6.9%) Black, and 672 (18.0%) Asian. Placebo event rates (per 100 patient-years) for cardiovascular death or heart failure (HF) hospitalization varied by region (Asia 27.7, North America 26.4, Latin America 21.4, and Europe 17.5) and race/ethnicity (Black 34.4, Asian 24.3, and White 18.7); driven by differences in HF hospitalization. The ratio of total HF hospitalization to cardiovascular death varied from 5.4 in Asia and 4.8 in North America to 2.1 in Europe; and from 4.8 in Black and 4.2 in Asian to 2.2 in White patients. Groups with the highest ratio had the greatest reduction in the primary outcome with empagliflozin. Inclusion of outpatient worsening HF episodes added more events in Europe vs. other regions; enhanced the placebo event rates in Europe vs. other regions; and increased the relative risk reduction with empagliflozin in Europe from 6% to 26%.
There were notable differences in the placebo event rates for major HF events across diverse regions and race/ethnic groups. The benefit of empagliflozin was most pronounced in groups with the highest ratio of HF hospitalization to cardiovascular death. Regional differences were attenuated when the definition of HF events was expanded to include outpatient worsening HF events.
Effect of empagliflozin by region and race in EMPEROR-Reduced. Inclusion of outpatient events in the extended composite endpoint attenuated the between-region differences in the effect of empagliflozin seen in the primary outcome of EMPEROR-Reduced.
See page 4452 for the editorial comment for this article ‘A fourth pillar for all in the treatment of heart failure’, by E.F. Lewis, https://doi.org/10.1093/eurheartj/ehab612.
Introduction
Regional and racial/ethnic differences in the incidence of and outcomes related to heart failure (HF) have been reported in both observational studies and global clinical trials.1 These regional and ethnic differences may reflect differences in patient characteristics, comorbidities, medical practice, and healthcare delivery.2–4
In the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR-Reduced), we previously reported regional and race/ethnicity-related differences in the effect of empagliflozin on the risk of cardiovascular death and HF hospitalization in patients with chronic HF and a reduced ejection fraction, with and without diabetes.5 The treatment effect appeared to be more pronounced in North America, Latin America, and Asia (relative risk reduction of 30–50%) than in Europe (relative risk reduction of 6%). Black and Asian patients experienced a greater risk reduction with empagliflozin than White patients (∼40% vs. 12%).5 , 6 In a meta-analysis of two trials of sodium–glucose co-transporter 2 (SGLT2) inhibitors in HF and a reduced ejection fraction, the regional and race/ethnicity-related heterogeneity was still apparent.6 However, the definition of regions was based on arbitrary geographical groupings, and the contribution of differences in background medications, healthcare systems, and medical practice to this heterogeneity remained unexplored.
In the present study, we provide an in-depth study of the regional and racial/ethnic differences in the EMPEROR-Reduced trial, with a particular emphasis on the role of HF hospitalizations and outpatient HF events in influencing estimates of the magnitude of the effect of empagliflozin.
Methods
The EMPEROR-Reduced trial was a randomized, double-blind, parallel-group, placebo-controlled, and event-driven study, whose design has been described previously.5 Participants were men or women with chronic HF [New York Heart Association (NYHA) functional class II, III, or IV] with a left ventricular ejection fraction of ≤40%. Patients with an ejection fraction of ≤30% were preferentially enrolled by requiring those with a higher ejection fraction to have been hospitalized for HF within 12 months or to have markedly increased levels of N-terminal prohormone B-type natriuretic peptide (NT-proBNP), i.e. ≥1000 or ≥2500 pg/mL in those with an ejection fraction of 31–35% or 36–40%, respectively; these thresholds were doubled in patients with atrial fibrillation. The Ethics Committee of each of the 520 sites in 20 countries approved the protocol, and all patients gave written informed consent.
Randomization and endpoints
Patients were randomized double-blind (in a 1:1 ratio) to receive placebo or empagliflozin 10 mg daily, in addition to their usual therapy. The primary endpoint was the composite of adjudicated cardiovascular death and hospitalization for HF, analysed as a time-to-first event. The first secondary outcome was the occurrence of all adjudicated hospitalizations for HF (including first and recurrent events). To capture out-of-hospital worsening HF events, we analysed an extended composite outcome of the time-to-first cardiovascular death, hospitalization for HF, urgent visits for worsening of HF requiring intravenous therapy, and outpatient intensification of oral diuretic therapy.7
Definitions of region and race/ethnicity
The countries participating in the EMPEROR-Reduced trial were prospectively but arbitrarily grouped into regions: Latin America, North America, Europe, Asia, and ‘Other’ (which included India and Australia). Race/ethnicity was self-identified as White, Black, Asian, and ‘Other’ (native American, Hawaiian/Pacific Islander, and any subject who selected more than one race/ethnicity category).
Statistical analysis
Baseline characteristics were compared between regions and race/ethnicity using ANOVA for continuous variables and chi-square test for categorical variables. For time-to-first-event analyses, differences between the placebo and empagliflozin groups were assessed using a Cox proportional hazards model, with pre-specified baseline covariates of age, sex, geographic region, diabetes, left ventricular ejection fraction, and estimated glomerular filtration rate, based on all randomized patients according to the intention-to-treat principle. For the analysis of total (first and recurrent) events, between-group differences were assessed using a joint frailty model, with cardiovascular death as a competing risk. For all efficacy measures, treatment-by-subgroup (region and race/ethnicity) interaction terms were included in the models and the effect of empagliflozin was estimated for the corresponding subgroups. All analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Distribution by region and race/ethnicity
Of the 3730 patients in the EMPEROR-Reduced trial, 1353 (36.3%) were enrolled in Europe, 1286 (34.5%) in Latin America, 425 (11.4%) in North America, 493 (13.2%) in Asia, and 173 (4.6%) from India and Australia. Of these, 2629 (70.5%) were White; 257 (6.9%) were Black; 672 (18.0%) were Asian; and 114 (3.1%) were classified as ‘Other’. As expected, most Asian patients were from Asia; Black patients were primarily recruited in Latin America (n = 154) and North America (n = 100), whereas White patients were primarily enrolled in Europe (n = 1281), Latin America (n = 1026), and North America (n = 301) (Table 1).
| Race . | Overall (n = 3730) . | Region . | ||||
|---|---|---|---|---|---|---|
| North America . | Latin America . | Europe . | Asia . | Othera . | ||
| White | 2629 (70.5) | 301 (70.8) | 1026 (79.8) | 1281 (94.7) | 0 | 21 (12.1) |
| Black | 257 (6.9) | 100 (23.5) | 154 (12.0) | 3 (0.2) | 0 | 0 |
| Asian | 672 (18.0) | 17 (4.0) | 4 (0.3) | 8 (0.6) | 493 (100) | 150 (86.7) |
| Otherb | 114 (3.0) | 7 (1.6) | 102 (7.9) | 3 (0.2) | 0 | 2 (1.2) |
| Missing | 58 (1.6) | 0 | 0 | 58 (4.3) | 0 | 0 |
| Race . | Overall (n = 3730) . | Region . | ||||
|---|---|---|---|---|---|---|
| North America . | Latin America . | Europe . | Asia . | Othera . | ||
| White | 2629 (70.5) | 301 (70.8) | 1026 (79.8) | 1281 (94.7) | 0 | 21 (12.1) |
| Black | 257 (6.9) | 100 (23.5) | 154 (12.0) | 3 (0.2) | 0 | 0 |
| Asian | 672 (18.0) | 17 (4.0) | 4 (0.3) | 8 (0.6) | 493 (100) | 150 (86.7) |
| Otherb | 114 (3.0) | 7 (1.6) | 102 (7.9) | 3 (0.2) | 0 | 2 (1.2) |
| Missing | 58 (1.6) | 0 | 0 | 58 (4.3) | 0 | 0 |
Other region comprises India and Australia.
Other race comprises native American, Hawaiian/Pacific Islander, and any subject who selected more than one race category.
| Race . | Overall (n = 3730) . | Region . | ||||
|---|---|---|---|---|---|---|
| North America . | Latin America . | Europe . | Asia . | Othera . | ||
| White | 2629 (70.5) | 301 (70.8) | 1026 (79.8) | 1281 (94.7) | 0 | 21 (12.1) |
| Black | 257 (6.9) | 100 (23.5) | 154 (12.0) | 3 (0.2) | 0 | 0 |
| Asian | 672 (18.0) | 17 (4.0) | 4 (0.3) | 8 (0.6) | 493 (100) | 150 (86.7) |
| Otherb | 114 (3.0) | 7 (1.6) | 102 (7.9) | 3 (0.2) | 0 | 2 (1.2) |
| Missing | 58 (1.6) | 0 | 0 | 58 (4.3) | 0 | 0 |
| Race . | Overall (n = 3730) . | Region . | ||||
|---|---|---|---|---|---|---|
| North America . | Latin America . | Europe . | Asia . | Othera . | ||
| White | 2629 (70.5) | 301 (70.8) | 1026 (79.8) | 1281 (94.7) | 0 | 21 (12.1) |
| Black | 257 (6.9) | 100 (23.5) | 154 (12.0) | 3 (0.2) | 0 | 0 |
| Asian | 672 (18.0) | 17 (4.0) | 4 (0.3) | 8 (0.6) | 493 (100) | 150 (86.7) |
| Otherb | 114 (3.0) | 7 (1.6) | 102 (7.9) | 3 (0.2) | 0 | 2 (1.2) |
| Missing | 58 (1.6) | 0 | 0 | 58 (4.3) | 0 | 0 |
Other region comprises India and Australia.
Other race comprises native American, Hawaiian/Pacific Islander, and any subject who selected more than one race category.
Patient characteristics by region and race/ethnicity
When compared with other regions, patients from Europe and North America were older, had worse NYHA functional class, a longer duration of HF, worse renal function and were more likely to have coronary artery disease and atrial fibrillation, but had lower NT-proBNP levels. Patients from North America were also most likely to be receiving an angiotensin receptor–neprilysin inhibitor but were least likely to be treated with a mineralocorticoid receptor antagonist (Table 2). Patients in Asia were most likely to have been hospitalized for HF within 12 months and were least likely to be treated with inhibitors of the renin–angiotensin system, beta-blockers, mineralocorticoid receptor antagonists, or cardiac devices.
EMPEROR-Reduced: baseline characteristics according to the region and race/ethnicity
| Variable . | Region . | Race/ethnicity . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Latin America (n = 1286) . | North America (n = 425) . | Europe (n = 1353) . | Asia (n = 493) . | Othera (n = 173) . | P-value . | White (n = 2629) . | Black (n = 257) . | Asian (n = 672) . | Otherb (n = 114) . | P-value . | |
| Age, years | 64.4 | 68.6 | 69.6 | 66.3 | 60.4 | <0.0001 | 68.0 | 62.1 | 64.7 | 63.1 | <0.0001 |
| Women | 398 (30.9) | 98 (23.1) | 262 (19.4) | 101 (20.5) | 34 (19.7) | <0.0001 | 609 (23.2) | 97 (37.7) | 136 (20.2) | 39 (34.2) | <0.0001 |
| Body mass index, kg/m2 | 28.1 | 29.6 | 28.9 | 24.0 | 24.6 | <0.0001 | 28.8 | 28.4 | 24.1 | 27.4 | <0.0001 |
| Heart rate, min−1 | 71.0 | 70.4 | 70.9 | 72.6 | 74.9 | <0.0001 | 70.9 | 70.8 | 73.3 | 70.9 | <0.0001 |
| Systolic blood pressure, mmHg | 121.1 | 118.8 | 124.9 | 119.3 | 121.6 | <0.0001 | 123.0 | 119.9 | 119.9 | 118.0 | <0.0001 |
| NYHA class III–IV | 299 (23.3) | 119 (28.0) | 373 (27.6) | 97 (19.7) | 42 (24.3) | 0.0025 | 677 (25.8) | 70 (27.2) | 143 (21.3) | 19 (32.8) | 0.027 |
| Duration of heart failure, years, median [IQR] | 5.7 | 7.0 | 6.8 | 5.5 | 4.0 | <0.0001 | 6.4 | 6.5 | 5.1 | 4.8 | <0.0001 |
| (1.3, 8.3) | (1.0, 10.7) | (1.8, 9.3) | (1.2, 8.0) | (0.7, 5.2) | (1.6, 9.2) | (1.5, 9.3) | (1.1, 7.3) | (1.0, 6.2) | |||
| LV ejection fraction, % | 27.5 | 26.3 | 27.5 | 28.4 | 27.2 | <0.0001 | 27.5 | 26.0 | 27.9 | 26.1 | <0.0001 |
| NT-proBNP, pg/mL, median (IQR) | 3286 | 2868 | 2820 | 3153 | 2913 | 0.0154 | 3033 | 3021 | 3122 | 3730 | 0.83 |
| (1096, 3819) | (1072, 3297) | (1147, 3212) | (1271, 3693) | (999, 3076) | (1113, 3406) | (1070, 3717) | (1163, 3637) | (1047, 3273) | |||
| HbA1c, % | 6.7 | 6.6 | 6.4 | 6.4 | 7.1 | <0.0001 | 6.5 | 6.6 | 6.6 | 7.0 | 0.0048 |
| eGFR, mL/min/1.73 m2 | 64.7 | 58.7 | 57.9 | 65.6 | 71.8 | <0.0001 | 59.8 | 70.2 | 67.2 | 65.4 | <0.0001 |
| KCCQ clinical summary score | 66.8 | 70.3 | 69.5 | 83.0 | 75.4 | <0.0001 | 68.7 | 64.6 | 80.8 | 73.0 | <0.0001 |
| Medical history | |||||||||||
| HF hospitalization within 12 months | 314 (24.4) | 134 (31.5) | 417 (30.8) | 238 (48.3) | 48 (27.7) | <0.0001 | 759 (28.9) | 59 (23.0) | 285 (42.4) | 22 (19.3) | <0.0001 |
| Coronary artery disease | 383 (29.8) | 251 (59.1) | 771 (57.0) | 230 (46.7) | 75 (43.4) | <0.0001 | 1267 (48.2) | 62 (24.1) | 314 (46.7) | 33 (28.9) | <0.0001 |
| CABG/PCI | 336 (26.1) | 212 (49.9) | 732 (54.1) | 195 (39.6) | 48 (27.7) | <0.0001 | 1161 (44.2) | 44 (17.1) | 254 (37.8) | 32 (28.1) | <0.0001 |
| Atrial fibrillation | 328 (25.5) | 187 (44.0) | 658 (48.6) | 170 (36.3) | 17 (9.8) | <0.0001 | 1059 (40.3) | 63 (24.5) | 199 (29.6) | 18 (15.8) | <0.0001 |
| Hypertension | 922 (71.7) | 345 (81.2) | 1022 (75.5) | 319 (64.7) | 90 (52.0) | <0.0001 | 1973 (75.0) | 203 (79.0) | 415 (61.8) | 81 (71.1) | <0.0001 |
| Diabetes | 625 (48.6) | 222 (52.2) | 660 (48.8) | 244 (49.5) | 105 (60.7) | 0.0323 | 1285 (48.9) | 120 (46.7) | 358 (53.3) | 68 (59.6) | 0.0316 |
| Treatments for heart failure | |||||||||||
| Loop diuretics | 1037 (80.6) | 351 (82.6) | 1195 (88.3) | 424 (86.9) | 143 (82.7) | <0.0001 | 2218 (84.4) | 217 (84.4) | 577 (85.9) | 87 (76.3) | 0.1201 |
| RASi (without neprilysin inhibitor) | 1008 (78.4) | 206 (48.5) | 971 (71.8) | 330 (66.9) | 85 (49.1) | <0.0001 | 1898 (72.2) | 176 (68.5) | 420 (62.5) | 89 (76.1) | <0.0001 |
| ACEi/ARB ≥50% target dose | 622 (48.4) | 84 (19.8) | 447 (33.0) | 34 (6.9) | 19 (11.0) | <0.0001 | 983 (37.4) | 120 (46.7) | 56 (8.3) | 40 (35.1) | <0.0001 |
| RASi (with neprilysin inhibitor) | 178 (13.8) | 161 (37.9) | 276 (20.4) | 67 (13.6) | 45 (26.0) | <0.0001 | 502 (19.1) | 60 (23.3) | 112 (16.7) | 15 (13.2) | <0.0001 |
| Beta-blocker | 1235 (96.0) | 406 (95.5) | 1302 (96.2) | 452 (91.7) | 138 (79.8) | <0.0001 | 2527 (96.1) | 249 (96.9) | 595 (88.5) | 108 (94.7) | <0.0001 |
| Beta-blocker ≥50% target dose | 780 (60.7) | 204 (48.0) | 732 (54.1) | 86 (17.4) | 23 (13.3) | <0.0001 | 1455 (55.3) | 167 (65.0) | 111 (16.5) | 57 (50.0) | <0.0001 |
| Mineralocorticoid receptor antagonist | 1032 (80.2) | 210 (49.4) | 975 (72.1) | 324 (65.7) | 120 (69.4) | <0.0001 | 1905 (72.5) | 176 (68.5) | 446 (66.4) | 90 (78.9) | 0.0057 |
| Implantable cardioverter-defibrillatorc | 94 (7.3) | 273 (64.2) | 718 (53.1) | 67 (13.6) | 18 (10.4) | <0.0001 | 953 (36.2) | 72 (28.0) | 86 (12.8) | 12 (10.5) | <0.0001 |
| Cardiac resynchronization therapyd | 39 (3.0) | 84 (19.8) | 272 (20.1) | 38 (7.7) | 5 (2.9) | <0.0001 | 354 (13.5) | 18 (7.0) | 45 (6.7) | 7 (6.1) | <0.0001 |
| Variable . | Region . | Race/ethnicity . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Latin America (n = 1286) . | North America (n = 425) . | Europe (n = 1353) . | Asia (n = 493) . | Othera (n = 173) . | P-value . | White (n = 2629) . | Black (n = 257) . | Asian (n = 672) . | Otherb (n = 114) . | P-value . | |
| Age, years | 64.4 | 68.6 | 69.6 | 66.3 | 60.4 | <0.0001 | 68.0 | 62.1 | 64.7 | 63.1 | <0.0001 |
| Women | 398 (30.9) | 98 (23.1) | 262 (19.4) | 101 (20.5) | 34 (19.7) | <0.0001 | 609 (23.2) | 97 (37.7) | 136 (20.2) | 39 (34.2) | <0.0001 |
| Body mass index, kg/m2 | 28.1 | 29.6 | 28.9 | 24.0 | 24.6 | <0.0001 | 28.8 | 28.4 | 24.1 | 27.4 | <0.0001 |
| Heart rate, min−1 | 71.0 | 70.4 | 70.9 | 72.6 | 74.9 | <0.0001 | 70.9 | 70.8 | 73.3 | 70.9 | <0.0001 |
| Systolic blood pressure, mmHg | 121.1 | 118.8 | 124.9 | 119.3 | 121.6 | <0.0001 | 123.0 | 119.9 | 119.9 | 118.0 | <0.0001 |
| NYHA class III–IV | 299 (23.3) | 119 (28.0) | 373 (27.6) | 97 (19.7) | 42 (24.3) | 0.0025 | 677 (25.8) | 70 (27.2) | 143 (21.3) | 19 (32.8) | 0.027 |
| Duration of heart failure, years, median [IQR] | 5.7 | 7.0 | 6.8 | 5.5 | 4.0 | <0.0001 | 6.4 | 6.5 | 5.1 | 4.8 | <0.0001 |
| (1.3, 8.3) | (1.0, 10.7) | (1.8, 9.3) | (1.2, 8.0) | (0.7, 5.2) | (1.6, 9.2) | (1.5, 9.3) | (1.1, 7.3) | (1.0, 6.2) | |||
| LV ejection fraction, % | 27.5 | 26.3 | 27.5 | 28.4 | 27.2 | <0.0001 | 27.5 | 26.0 | 27.9 | 26.1 | <0.0001 |
| NT-proBNP, pg/mL, median (IQR) | 3286 | 2868 | 2820 | 3153 | 2913 | 0.0154 | 3033 | 3021 | 3122 | 3730 | 0.83 |
| (1096, 3819) | (1072, 3297) | (1147, 3212) | (1271, 3693) | (999, 3076) | (1113, 3406) | (1070, 3717) | (1163, 3637) | (1047, 3273) | |||
| HbA1c, % | 6.7 | 6.6 | 6.4 | 6.4 | 7.1 | <0.0001 | 6.5 | 6.6 | 6.6 | 7.0 | 0.0048 |
| eGFR, mL/min/1.73 m2 | 64.7 | 58.7 | 57.9 | 65.6 | 71.8 | <0.0001 | 59.8 | 70.2 | 67.2 | 65.4 | <0.0001 |
| KCCQ clinical summary score | 66.8 | 70.3 | 69.5 | 83.0 | 75.4 | <0.0001 | 68.7 | 64.6 | 80.8 | 73.0 | <0.0001 |
| Medical history | |||||||||||
| HF hospitalization within 12 months | 314 (24.4) | 134 (31.5) | 417 (30.8) | 238 (48.3) | 48 (27.7) | <0.0001 | 759 (28.9) | 59 (23.0) | 285 (42.4) | 22 (19.3) | <0.0001 |
| Coronary artery disease | 383 (29.8) | 251 (59.1) | 771 (57.0) | 230 (46.7) | 75 (43.4) | <0.0001 | 1267 (48.2) | 62 (24.1) | 314 (46.7) | 33 (28.9) | <0.0001 |
| CABG/PCI | 336 (26.1) | 212 (49.9) | 732 (54.1) | 195 (39.6) | 48 (27.7) | <0.0001 | 1161 (44.2) | 44 (17.1) | 254 (37.8) | 32 (28.1) | <0.0001 |
| Atrial fibrillation | 328 (25.5) | 187 (44.0) | 658 (48.6) | 170 (36.3) | 17 (9.8) | <0.0001 | 1059 (40.3) | 63 (24.5) | 199 (29.6) | 18 (15.8) | <0.0001 |
| Hypertension | 922 (71.7) | 345 (81.2) | 1022 (75.5) | 319 (64.7) | 90 (52.0) | <0.0001 | 1973 (75.0) | 203 (79.0) | 415 (61.8) | 81 (71.1) | <0.0001 |
| Diabetes | 625 (48.6) | 222 (52.2) | 660 (48.8) | 244 (49.5) | 105 (60.7) | 0.0323 | 1285 (48.9) | 120 (46.7) | 358 (53.3) | 68 (59.6) | 0.0316 |
| Treatments for heart failure | |||||||||||
| Loop diuretics | 1037 (80.6) | 351 (82.6) | 1195 (88.3) | 424 (86.9) | 143 (82.7) | <0.0001 | 2218 (84.4) | 217 (84.4) | 577 (85.9) | 87 (76.3) | 0.1201 |
| RASi (without neprilysin inhibitor) | 1008 (78.4) | 206 (48.5) | 971 (71.8) | 330 (66.9) | 85 (49.1) | <0.0001 | 1898 (72.2) | 176 (68.5) | 420 (62.5) | 89 (76.1) | <0.0001 |
| ACEi/ARB ≥50% target dose | 622 (48.4) | 84 (19.8) | 447 (33.0) | 34 (6.9) | 19 (11.0) | <0.0001 | 983 (37.4) | 120 (46.7) | 56 (8.3) | 40 (35.1) | <0.0001 |
| RASi (with neprilysin inhibitor) | 178 (13.8) | 161 (37.9) | 276 (20.4) | 67 (13.6) | 45 (26.0) | <0.0001 | 502 (19.1) | 60 (23.3) | 112 (16.7) | 15 (13.2) | <0.0001 |
| Beta-blocker | 1235 (96.0) | 406 (95.5) | 1302 (96.2) | 452 (91.7) | 138 (79.8) | <0.0001 | 2527 (96.1) | 249 (96.9) | 595 (88.5) | 108 (94.7) | <0.0001 |
| Beta-blocker ≥50% target dose | 780 (60.7) | 204 (48.0) | 732 (54.1) | 86 (17.4) | 23 (13.3) | <0.0001 | 1455 (55.3) | 167 (65.0) | 111 (16.5) | 57 (50.0) | <0.0001 |
| Mineralocorticoid receptor antagonist | 1032 (80.2) | 210 (49.4) | 975 (72.1) | 324 (65.7) | 120 (69.4) | <0.0001 | 1905 (72.5) | 176 (68.5) | 446 (66.4) | 90 (78.9) | 0.0057 |
| Implantable cardioverter-defibrillatorc | 94 (7.3) | 273 (64.2) | 718 (53.1) | 67 (13.6) | 18 (10.4) | <0.0001 | 953 (36.2) | 72 (28.0) | 86 (12.8) | 12 (10.5) | <0.0001 |
| Cardiac resynchronization therapyd | 39 (3.0) | 84 (19.8) | 272 (20.1) | 38 (7.7) | 5 (2.9) | <0.0001 | 354 (13.5) | 18 (7.0) | 45 (6.7) | 7 (6.1) | <0.0001 |
Data are given as n (%) and mean unless otherwise stated.
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HF, heart failure; IQR, interquartile range; KCCQ, Kansas City Cardiomyopathy Questionnaire; LV, left ventricular; NT-proBNP, N-terminal prohormone B-type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; RASi, renin–angiotensin system inhibitor.
Other region comprises India and Australia.
Other race comprises American Indian/Alaska native, Hawaiian/Pacific Islander, and any subject who selected more than one race category.
Includes all the patients with an implantable cardioverter-defibrillator regardless of the presence or absence of cardiac resynchronization therapy.
Includes all the patients who were receiving cardiac resynchronization therapy regardless of the presence or absence of a defibrillator.
EMPEROR-Reduced: baseline characteristics according to the region and race/ethnicity
| Variable . | Region . | Race/ethnicity . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Latin America (n = 1286) . | North America (n = 425) . | Europe (n = 1353) . | Asia (n = 493) . | Othera (n = 173) . | P-value . | White (n = 2629) . | Black (n = 257) . | Asian (n = 672) . | Otherb (n = 114) . | P-value . | |
| Age, years | 64.4 | 68.6 | 69.6 | 66.3 | 60.4 | <0.0001 | 68.0 | 62.1 | 64.7 | 63.1 | <0.0001 |
| Women | 398 (30.9) | 98 (23.1) | 262 (19.4) | 101 (20.5) | 34 (19.7) | <0.0001 | 609 (23.2) | 97 (37.7) | 136 (20.2) | 39 (34.2) | <0.0001 |
| Body mass index, kg/m2 | 28.1 | 29.6 | 28.9 | 24.0 | 24.6 | <0.0001 | 28.8 | 28.4 | 24.1 | 27.4 | <0.0001 |
| Heart rate, min−1 | 71.0 | 70.4 | 70.9 | 72.6 | 74.9 | <0.0001 | 70.9 | 70.8 | 73.3 | 70.9 | <0.0001 |
| Systolic blood pressure, mmHg | 121.1 | 118.8 | 124.9 | 119.3 | 121.6 | <0.0001 | 123.0 | 119.9 | 119.9 | 118.0 | <0.0001 |
| NYHA class III–IV | 299 (23.3) | 119 (28.0) | 373 (27.6) | 97 (19.7) | 42 (24.3) | 0.0025 | 677 (25.8) | 70 (27.2) | 143 (21.3) | 19 (32.8) | 0.027 |
| Duration of heart failure, years, median [IQR] | 5.7 | 7.0 | 6.8 | 5.5 | 4.0 | <0.0001 | 6.4 | 6.5 | 5.1 | 4.8 | <0.0001 |
| (1.3, 8.3) | (1.0, 10.7) | (1.8, 9.3) | (1.2, 8.0) | (0.7, 5.2) | (1.6, 9.2) | (1.5, 9.3) | (1.1, 7.3) | (1.0, 6.2) | |||
| LV ejection fraction, % | 27.5 | 26.3 | 27.5 | 28.4 | 27.2 | <0.0001 | 27.5 | 26.0 | 27.9 | 26.1 | <0.0001 |
| NT-proBNP, pg/mL, median (IQR) | 3286 | 2868 | 2820 | 3153 | 2913 | 0.0154 | 3033 | 3021 | 3122 | 3730 | 0.83 |
| (1096, 3819) | (1072, 3297) | (1147, 3212) | (1271, 3693) | (999, 3076) | (1113, 3406) | (1070, 3717) | (1163, 3637) | (1047, 3273) | |||
| HbA1c, % | 6.7 | 6.6 | 6.4 | 6.4 | 7.1 | <0.0001 | 6.5 | 6.6 | 6.6 | 7.0 | 0.0048 |
| eGFR, mL/min/1.73 m2 | 64.7 | 58.7 | 57.9 | 65.6 | 71.8 | <0.0001 | 59.8 | 70.2 | 67.2 | 65.4 | <0.0001 |
| KCCQ clinical summary score | 66.8 | 70.3 | 69.5 | 83.0 | 75.4 | <0.0001 | 68.7 | 64.6 | 80.8 | 73.0 | <0.0001 |
| Medical history | |||||||||||
| HF hospitalization within 12 months | 314 (24.4) | 134 (31.5) | 417 (30.8) | 238 (48.3) | 48 (27.7) | <0.0001 | 759 (28.9) | 59 (23.0) | 285 (42.4) | 22 (19.3) | <0.0001 |
| Coronary artery disease | 383 (29.8) | 251 (59.1) | 771 (57.0) | 230 (46.7) | 75 (43.4) | <0.0001 | 1267 (48.2) | 62 (24.1) | 314 (46.7) | 33 (28.9) | <0.0001 |
| CABG/PCI | 336 (26.1) | 212 (49.9) | 732 (54.1) | 195 (39.6) | 48 (27.7) | <0.0001 | 1161 (44.2) | 44 (17.1) | 254 (37.8) | 32 (28.1) | <0.0001 |
| Atrial fibrillation | 328 (25.5) | 187 (44.0) | 658 (48.6) | 170 (36.3) | 17 (9.8) | <0.0001 | 1059 (40.3) | 63 (24.5) | 199 (29.6) | 18 (15.8) | <0.0001 |
| Hypertension | 922 (71.7) | 345 (81.2) | 1022 (75.5) | 319 (64.7) | 90 (52.0) | <0.0001 | 1973 (75.0) | 203 (79.0) | 415 (61.8) | 81 (71.1) | <0.0001 |
| Diabetes | 625 (48.6) | 222 (52.2) | 660 (48.8) | 244 (49.5) | 105 (60.7) | 0.0323 | 1285 (48.9) | 120 (46.7) | 358 (53.3) | 68 (59.6) | 0.0316 |
| Treatments for heart failure | |||||||||||
| Loop diuretics | 1037 (80.6) | 351 (82.6) | 1195 (88.3) | 424 (86.9) | 143 (82.7) | <0.0001 | 2218 (84.4) | 217 (84.4) | 577 (85.9) | 87 (76.3) | 0.1201 |
| RASi (without neprilysin inhibitor) | 1008 (78.4) | 206 (48.5) | 971 (71.8) | 330 (66.9) | 85 (49.1) | <0.0001 | 1898 (72.2) | 176 (68.5) | 420 (62.5) | 89 (76.1) | <0.0001 |
| ACEi/ARB ≥50% target dose | 622 (48.4) | 84 (19.8) | 447 (33.0) | 34 (6.9) | 19 (11.0) | <0.0001 | 983 (37.4) | 120 (46.7) | 56 (8.3) | 40 (35.1) | <0.0001 |
| RASi (with neprilysin inhibitor) | 178 (13.8) | 161 (37.9) | 276 (20.4) | 67 (13.6) | 45 (26.0) | <0.0001 | 502 (19.1) | 60 (23.3) | 112 (16.7) | 15 (13.2) | <0.0001 |
| Beta-blocker | 1235 (96.0) | 406 (95.5) | 1302 (96.2) | 452 (91.7) | 138 (79.8) | <0.0001 | 2527 (96.1) | 249 (96.9) | 595 (88.5) | 108 (94.7) | <0.0001 |
| Beta-blocker ≥50% target dose | 780 (60.7) | 204 (48.0) | 732 (54.1) | 86 (17.4) | 23 (13.3) | <0.0001 | 1455 (55.3) | 167 (65.0) | 111 (16.5) | 57 (50.0) | <0.0001 |
| Mineralocorticoid receptor antagonist | 1032 (80.2) | 210 (49.4) | 975 (72.1) | 324 (65.7) | 120 (69.4) | <0.0001 | 1905 (72.5) | 176 (68.5) | 446 (66.4) | 90 (78.9) | 0.0057 |
| Implantable cardioverter-defibrillatorc | 94 (7.3) | 273 (64.2) | 718 (53.1) | 67 (13.6) | 18 (10.4) | <0.0001 | 953 (36.2) | 72 (28.0) | 86 (12.8) | 12 (10.5) | <0.0001 |
| Cardiac resynchronization therapyd | 39 (3.0) | 84 (19.8) | 272 (20.1) | 38 (7.7) | 5 (2.9) | <0.0001 | 354 (13.5) | 18 (7.0) | 45 (6.7) | 7 (6.1) | <0.0001 |
| Variable . | Region . | Race/ethnicity . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Latin America (n = 1286) . | North America (n = 425) . | Europe (n = 1353) . | Asia (n = 493) . | Othera (n = 173) . | P-value . | White (n = 2629) . | Black (n = 257) . | Asian (n = 672) . | Otherb (n = 114) . | P-value . | |
| Age, years | 64.4 | 68.6 | 69.6 | 66.3 | 60.4 | <0.0001 | 68.0 | 62.1 | 64.7 | 63.1 | <0.0001 |
| Women | 398 (30.9) | 98 (23.1) | 262 (19.4) | 101 (20.5) | 34 (19.7) | <0.0001 | 609 (23.2) | 97 (37.7) | 136 (20.2) | 39 (34.2) | <0.0001 |
| Body mass index, kg/m2 | 28.1 | 29.6 | 28.9 | 24.0 | 24.6 | <0.0001 | 28.8 | 28.4 | 24.1 | 27.4 | <0.0001 |
| Heart rate, min−1 | 71.0 | 70.4 | 70.9 | 72.6 | 74.9 | <0.0001 | 70.9 | 70.8 | 73.3 | 70.9 | <0.0001 |
| Systolic blood pressure, mmHg | 121.1 | 118.8 | 124.9 | 119.3 | 121.6 | <0.0001 | 123.0 | 119.9 | 119.9 | 118.0 | <0.0001 |
| NYHA class III–IV | 299 (23.3) | 119 (28.0) | 373 (27.6) | 97 (19.7) | 42 (24.3) | 0.0025 | 677 (25.8) | 70 (27.2) | 143 (21.3) | 19 (32.8) | 0.027 |
| Duration of heart failure, years, median [IQR] | 5.7 | 7.0 | 6.8 | 5.5 | 4.0 | <0.0001 | 6.4 | 6.5 | 5.1 | 4.8 | <0.0001 |
| (1.3, 8.3) | (1.0, 10.7) | (1.8, 9.3) | (1.2, 8.0) | (0.7, 5.2) | (1.6, 9.2) | (1.5, 9.3) | (1.1, 7.3) | (1.0, 6.2) | |||
| LV ejection fraction, % | 27.5 | 26.3 | 27.5 | 28.4 | 27.2 | <0.0001 | 27.5 | 26.0 | 27.9 | 26.1 | <0.0001 |
| NT-proBNP, pg/mL, median (IQR) | 3286 | 2868 | 2820 | 3153 | 2913 | 0.0154 | 3033 | 3021 | 3122 | 3730 | 0.83 |
| (1096, 3819) | (1072, 3297) | (1147, 3212) | (1271, 3693) | (999, 3076) | (1113, 3406) | (1070, 3717) | (1163, 3637) | (1047, 3273) | |||
| HbA1c, % | 6.7 | 6.6 | 6.4 | 6.4 | 7.1 | <0.0001 | 6.5 | 6.6 | 6.6 | 7.0 | 0.0048 |
| eGFR, mL/min/1.73 m2 | 64.7 | 58.7 | 57.9 | 65.6 | 71.8 | <0.0001 | 59.8 | 70.2 | 67.2 | 65.4 | <0.0001 |
| KCCQ clinical summary score | 66.8 | 70.3 | 69.5 | 83.0 | 75.4 | <0.0001 | 68.7 | 64.6 | 80.8 | 73.0 | <0.0001 |
| Medical history | |||||||||||
| HF hospitalization within 12 months | 314 (24.4) | 134 (31.5) | 417 (30.8) | 238 (48.3) | 48 (27.7) | <0.0001 | 759 (28.9) | 59 (23.0) | 285 (42.4) | 22 (19.3) | <0.0001 |
| Coronary artery disease | 383 (29.8) | 251 (59.1) | 771 (57.0) | 230 (46.7) | 75 (43.4) | <0.0001 | 1267 (48.2) | 62 (24.1) | 314 (46.7) | 33 (28.9) | <0.0001 |
| CABG/PCI | 336 (26.1) | 212 (49.9) | 732 (54.1) | 195 (39.6) | 48 (27.7) | <0.0001 | 1161 (44.2) | 44 (17.1) | 254 (37.8) | 32 (28.1) | <0.0001 |
| Atrial fibrillation | 328 (25.5) | 187 (44.0) | 658 (48.6) | 170 (36.3) | 17 (9.8) | <0.0001 | 1059 (40.3) | 63 (24.5) | 199 (29.6) | 18 (15.8) | <0.0001 |
| Hypertension | 922 (71.7) | 345 (81.2) | 1022 (75.5) | 319 (64.7) | 90 (52.0) | <0.0001 | 1973 (75.0) | 203 (79.0) | 415 (61.8) | 81 (71.1) | <0.0001 |
| Diabetes | 625 (48.6) | 222 (52.2) | 660 (48.8) | 244 (49.5) | 105 (60.7) | 0.0323 | 1285 (48.9) | 120 (46.7) | 358 (53.3) | 68 (59.6) | 0.0316 |
| Treatments for heart failure | |||||||||||
| Loop diuretics | 1037 (80.6) | 351 (82.6) | 1195 (88.3) | 424 (86.9) | 143 (82.7) | <0.0001 | 2218 (84.4) | 217 (84.4) | 577 (85.9) | 87 (76.3) | 0.1201 |
| RASi (without neprilysin inhibitor) | 1008 (78.4) | 206 (48.5) | 971 (71.8) | 330 (66.9) | 85 (49.1) | <0.0001 | 1898 (72.2) | 176 (68.5) | 420 (62.5) | 89 (76.1) | <0.0001 |
| ACEi/ARB ≥50% target dose | 622 (48.4) | 84 (19.8) | 447 (33.0) | 34 (6.9) | 19 (11.0) | <0.0001 | 983 (37.4) | 120 (46.7) | 56 (8.3) | 40 (35.1) | <0.0001 |
| RASi (with neprilysin inhibitor) | 178 (13.8) | 161 (37.9) | 276 (20.4) | 67 (13.6) | 45 (26.0) | <0.0001 | 502 (19.1) | 60 (23.3) | 112 (16.7) | 15 (13.2) | <0.0001 |
| Beta-blocker | 1235 (96.0) | 406 (95.5) | 1302 (96.2) | 452 (91.7) | 138 (79.8) | <0.0001 | 2527 (96.1) | 249 (96.9) | 595 (88.5) | 108 (94.7) | <0.0001 |
| Beta-blocker ≥50% target dose | 780 (60.7) | 204 (48.0) | 732 (54.1) | 86 (17.4) | 23 (13.3) | <0.0001 | 1455 (55.3) | 167 (65.0) | 111 (16.5) | 57 (50.0) | <0.0001 |
| Mineralocorticoid receptor antagonist | 1032 (80.2) | 210 (49.4) | 975 (72.1) | 324 (65.7) | 120 (69.4) | <0.0001 | 1905 (72.5) | 176 (68.5) | 446 (66.4) | 90 (78.9) | 0.0057 |
| Implantable cardioverter-defibrillatorc | 94 (7.3) | 273 (64.2) | 718 (53.1) | 67 (13.6) | 18 (10.4) | <0.0001 | 953 (36.2) | 72 (28.0) | 86 (12.8) | 12 (10.5) | <0.0001 |
| Cardiac resynchronization therapyd | 39 (3.0) | 84 (19.8) | 272 (20.1) | 38 (7.7) | 5 (2.9) | <0.0001 | 354 (13.5) | 18 (7.0) | 45 (6.7) | 7 (6.1) | <0.0001 |
Data are given as n (%) and mean unless otherwise stated.
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HF, heart failure; IQR, interquartile range; KCCQ, Kansas City Cardiomyopathy Questionnaire; LV, left ventricular; NT-proBNP, N-terminal prohormone B-type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; RASi, renin–angiotensin system inhibitor.
Other region comprises India and Australia.
Other race comprises American Indian/Alaska native, Hawaiian/Pacific Islander, and any subject who selected more than one race category.
Includes all the patients with an implantable cardioverter-defibrillator regardless of the presence or absence of cardiac resynchronization therapy.
Includes all the patients who were receiving cardiac resynchronization therapy regardless of the presence or absence of a defibrillator.
Compared with other race/ethnic groups, Black patients were the youngest and had the highest prevalence of hypertension, but the most preserved renal function. Asian and other race/ethnic group patients had the lowest body mass index but the highest prevalence of diabetes and the highest values for the Kansas City Cardiomyopathy Questionnaire (Table 2).
Outcomes in the placebo group by region and race/ethnicity
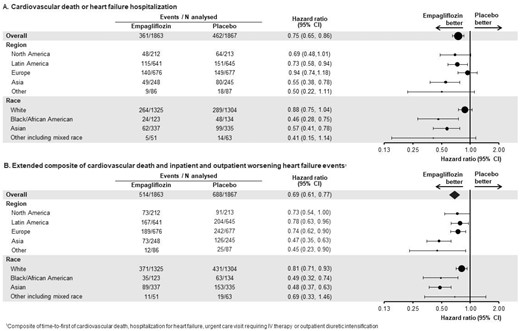
The placebo arm event rate (per 100 person-years) for the primary outcome of cardiovascular death or HF hospitalization was highest in Asia (27.7) and North America (26.4) and lowest in Europe (17.5) (Table 3 and Graphical abstract). These regional differences were driven by differences in the incidence of HF hospitalization, since there were no meaningful differences among regions in the incidence of cardiovascular death (Asia 7.7, North America 6.7, Latin America 9.1, and Europe 7.7 per 100 patient-years). Total HF hospitalizations (per 100 patient-years) in the placebo arm were highest in Asia (41.4) and North America (31.9), followed by Latin America (18.9), and lowest in Europe (16.3). Thus, the rate ratio of total HF hospitalizations to cardiovascular death was highest in Asia and North America (5.4 and 4.8, respectively) and lowest in Europe (2.1).
| . | Latin America . | North America . | Europe . | Asia . | Other . | Interaction . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 1286) . | (n = 425) . | (n = 1353) . | (n = 493) . | (n = 173) . | P-value . | |||||||||||
| . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | |
| Cardiovascular death or heart failure hospitalization | ||||||||||||||||
| Placebo | 151 | 21.4 | 0.73 (0.58, 0.94) | 64 | 26.4 | 0.69 (0.48, 1.01) | 149 | 17.5 | 0.94 (0.74, 1.18) | 80 | 27.7 | 0.55 (0.38, 0.78) | 18 | 16.1 | 0.50 (0.22, 1.11) | 0.10 |
| Empagliflozin | 115 | 15.7 | 48 | 17.4 | 140 | 16.5 | 49 | 15.1 | 9 | 8.2 | ||||||
| Total (first and recurrent) hospitalizations for heart failurea | ||||||||||||||||
| Placebo | 147 | 18.9 | 0.65 (0.46, 0.93) | 92 | 31.9 | 0.71 (0.43, 1.18) | 152 | 16.3 | 0.96 (0.70, 1.33) | 145 | 41.4 | 0.41 (0.26, 0.66) | 17 | 14.6 | 0.48 (0.16, 1.40) | 0.055 |
| Empagliflozin | 91 | 11.9 | 84 | 27.4 | 144 | 15.5 | 61 | 17.5 | 8 | 7.1 | ||||||
| Extended composite of cardiovascular death and inpatient and outpatient worsening heart failure eventsb | ||||||||||||||||
| Placebo | 204 | 31.2 | 0.78 (0.63, 0.96) | 91 | 41.9 | 0.73 (0.54, 1.00) | 242 | 31.6 | 0.74 (0.62, 0.90) | 126 | 52.5 | 0.47 (0.35, 0.63) | 25 | 23.8 | 0.45 (0.23, 0.90) | 0.037 |
| Empagliflozin | 167 | 24.3 | 73 | 29.5 | 189 | 23.5 | 73 | 24.6 | 12 | 11.0 | ||||||
| Cardiovascular death | ||||||||||||||||
| Placebo | 71 | 9.1 | 1.01 (0.73, 1.40) | 20 | 6.7 | 0.81 (0.42, 1.54) | 72 | 7.7 | 0.98 (0.71, 1.36) | 27 | 7.7 | 0.87 (0.50, 1.52) | 12 | 10.1 | 0.33 (0.11, 1.02) | 0.43 |
| Empagliflozin | 72 | 9.4 | 17 | 5.5 | 71 | 7.6 | 23 | 6.6 | 4 | 3.4 | ||||||
| . | Latin America . | North America . | Europe . | Asia . | Other . | Interaction . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 1286) . | (n = 425) . | (n = 1353) . | (n = 493) . | (n = 173) . | P-value . | |||||||||||
| . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | |
| Cardiovascular death or heart failure hospitalization | ||||||||||||||||
| Placebo | 151 | 21.4 | 0.73 (0.58, 0.94) | 64 | 26.4 | 0.69 (0.48, 1.01) | 149 | 17.5 | 0.94 (0.74, 1.18) | 80 | 27.7 | 0.55 (0.38, 0.78) | 18 | 16.1 | 0.50 (0.22, 1.11) | 0.10 |
| Empagliflozin | 115 | 15.7 | 48 | 17.4 | 140 | 16.5 | 49 | 15.1 | 9 | 8.2 | ||||||
| Total (first and recurrent) hospitalizations for heart failurea | ||||||||||||||||
| Placebo | 147 | 18.9 | 0.65 (0.46, 0.93) | 92 | 31.9 | 0.71 (0.43, 1.18) | 152 | 16.3 | 0.96 (0.70, 1.33) | 145 | 41.4 | 0.41 (0.26, 0.66) | 17 | 14.6 | 0.48 (0.16, 1.40) | 0.055 |
| Empagliflozin | 91 | 11.9 | 84 | 27.4 | 144 | 15.5 | 61 | 17.5 | 8 | 7.1 | ||||||
| Extended composite of cardiovascular death and inpatient and outpatient worsening heart failure eventsb | ||||||||||||||||
| Placebo | 204 | 31.2 | 0.78 (0.63, 0.96) | 91 | 41.9 | 0.73 (0.54, 1.00) | 242 | 31.6 | 0.74 (0.62, 0.90) | 126 | 52.5 | 0.47 (0.35, 0.63) | 25 | 23.8 | 0.45 (0.23, 0.90) | 0.037 |
| Empagliflozin | 167 | 24.3 | 73 | 29.5 | 189 | 23.5 | 73 | 24.6 | 12 | 11.0 | ||||||
| Cardiovascular death | ||||||||||||||||
| Placebo | 71 | 9.1 | 1.01 (0.73, 1.40) | 20 | 6.7 | 0.81 (0.42, 1.54) | 72 | 7.7 | 0.98 (0.71, 1.36) | 27 | 7.7 | 0.87 (0.50, 1.52) | 12 | 10.1 | 0.33 (0.11, 1.02) | 0.43 |
| Empagliflozin | 72 | 9.4 | 17 | 5.5 | 71 | 7.6 | 23 | 6.6 | 4 | 3.4 | ||||||
Rate, incidence rate per 100 person-years.
Total heart failure hospitalization event rates were derived from an unadjusted negative binomial model.
Composite of time-to-first of cardiovascular death, heart failure hospitalization, urgent visit for worsening heart failure requiring intravenous therapy, or outpatient diuretic intensification.
| . | Latin America . | North America . | Europe . | Asia . | Other . | Interaction . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 1286) . | (n = 425) . | (n = 1353) . | (n = 493) . | (n = 173) . | P-value . | |||||||||||
| . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | |
| Cardiovascular death or heart failure hospitalization | ||||||||||||||||
| Placebo | 151 | 21.4 | 0.73 (0.58, 0.94) | 64 | 26.4 | 0.69 (0.48, 1.01) | 149 | 17.5 | 0.94 (0.74, 1.18) | 80 | 27.7 | 0.55 (0.38, 0.78) | 18 | 16.1 | 0.50 (0.22, 1.11) | 0.10 |
| Empagliflozin | 115 | 15.7 | 48 | 17.4 | 140 | 16.5 | 49 | 15.1 | 9 | 8.2 | ||||||
| Total (first and recurrent) hospitalizations for heart failurea | ||||||||||||||||
| Placebo | 147 | 18.9 | 0.65 (0.46, 0.93) | 92 | 31.9 | 0.71 (0.43, 1.18) | 152 | 16.3 | 0.96 (0.70, 1.33) | 145 | 41.4 | 0.41 (0.26, 0.66) | 17 | 14.6 | 0.48 (0.16, 1.40) | 0.055 |
| Empagliflozin | 91 | 11.9 | 84 | 27.4 | 144 | 15.5 | 61 | 17.5 | 8 | 7.1 | ||||||
| Extended composite of cardiovascular death and inpatient and outpatient worsening heart failure eventsb | ||||||||||||||||
| Placebo | 204 | 31.2 | 0.78 (0.63, 0.96) | 91 | 41.9 | 0.73 (0.54, 1.00) | 242 | 31.6 | 0.74 (0.62, 0.90) | 126 | 52.5 | 0.47 (0.35, 0.63) | 25 | 23.8 | 0.45 (0.23, 0.90) | 0.037 |
| Empagliflozin | 167 | 24.3 | 73 | 29.5 | 189 | 23.5 | 73 | 24.6 | 12 | 11.0 | ||||||
| Cardiovascular death | ||||||||||||||||
| Placebo | 71 | 9.1 | 1.01 (0.73, 1.40) | 20 | 6.7 | 0.81 (0.42, 1.54) | 72 | 7.7 | 0.98 (0.71, 1.36) | 27 | 7.7 | 0.87 (0.50, 1.52) | 12 | 10.1 | 0.33 (0.11, 1.02) | 0.43 |
| Empagliflozin | 72 | 9.4 | 17 | 5.5 | 71 | 7.6 | 23 | 6.6 | 4 | 3.4 | ||||||
| . | Latin America . | North America . | Europe . | Asia . | Other . | Interaction . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 1286) . | (n = 425) . | (n = 1353) . | (n = 493) . | (n = 173) . | P-value . | |||||||||||
| . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | |
| Cardiovascular death or heart failure hospitalization | ||||||||||||||||
| Placebo | 151 | 21.4 | 0.73 (0.58, 0.94) | 64 | 26.4 | 0.69 (0.48, 1.01) | 149 | 17.5 | 0.94 (0.74, 1.18) | 80 | 27.7 | 0.55 (0.38, 0.78) | 18 | 16.1 | 0.50 (0.22, 1.11) | 0.10 |
| Empagliflozin | 115 | 15.7 | 48 | 17.4 | 140 | 16.5 | 49 | 15.1 | 9 | 8.2 | ||||||
| Total (first and recurrent) hospitalizations for heart failurea | ||||||||||||||||
| Placebo | 147 | 18.9 | 0.65 (0.46, 0.93) | 92 | 31.9 | 0.71 (0.43, 1.18) | 152 | 16.3 | 0.96 (0.70, 1.33) | 145 | 41.4 | 0.41 (0.26, 0.66) | 17 | 14.6 | 0.48 (0.16, 1.40) | 0.055 |
| Empagliflozin | 91 | 11.9 | 84 | 27.4 | 144 | 15.5 | 61 | 17.5 | 8 | 7.1 | ||||||
| Extended composite of cardiovascular death and inpatient and outpatient worsening heart failure eventsb | ||||||||||||||||
| Placebo | 204 | 31.2 | 0.78 (0.63, 0.96) | 91 | 41.9 | 0.73 (0.54, 1.00) | 242 | 31.6 | 0.74 (0.62, 0.90) | 126 | 52.5 | 0.47 (0.35, 0.63) | 25 | 23.8 | 0.45 (0.23, 0.90) | 0.037 |
| Empagliflozin | 167 | 24.3 | 73 | 29.5 | 189 | 23.5 | 73 | 24.6 | 12 | 11.0 | ||||||
| Cardiovascular death | ||||||||||||||||
| Placebo | 71 | 9.1 | 1.01 (0.73, 1.40) | 20 | 6.7 | 0.81 (0.42, 1.54) | 72 | 7.7 | 0.98 (0.71, 1.36) | 27 | 7.7 | 0.87 (0.50, 1.52) | 12 | 10.1 | 0.33 (0.11, 1.02) | 0.43 |
| Empagliflozin | 72 | 9.4 | 17 | 5.5 | 71 | 7.6 | 23 | 6.6 | 4 | 3.4 | ||||||
Rate, incidence rate per 100 person-years.
Total heart failure hospitalization event rates were derived from an unadjusted negative binomial model.
Composite of time-to-first of cardiovascular death, heart failure hospitalization, urgent visit for worsening heart failure requiring intravenous therapy, or outpatient diuretic intensification.
The inclusion of outpatient worsening HF events added 93 additional placebo events in Europe, 53 in Latin America, 46 in Asia, and 27 in North America to the time-to-first-event analysis. As a result, the incidence of the extended composite outcome in the placebo group in Europe became similar to Latin America (31.6 and 31.2 per 100 patient-years, respectively), but remained lower than in North America and Asia (with 41.9 and 52.5 events per 100 patient-years, respectively) (Table 3 and Graphical abstract).
The event rate (per 100 person-years) for the primary composite outcome of cardiovascular death or HF hospitalization in the placebo group was highest among Black patients (34.4) and lowest in White patients (18.7) (Table 4 and Graphical abstract). These racial/ethnic differences were driven by differences in total hospitalizations for HF, with no meaningful differences among races/ethnicities in the incidence of cardiovascular death (Black 7.6, Asian 8.0, and White 8.1). The incidence of total HF hospitalizations (per 100 patient-years) in the placebo arm was highest in Black and lowest in White patients (36.3 and 17.6 per 100 patient-years, respectively). Accordingly, the ratio of total HF hospitalizations to cardiovascular death was highest in Black patients and lowest in White patients (4.8 and 2.2, respectively). The extended composite outcome (which included both inpatient and outpatient worsening HF events) remained highest among Black patients and lowest in White patients (51.5 and 30.4 per 100 patient-years, respectively) (Table 4 and Graphical abstract).
| . | White . | Black . | Asian . | Other . | Interaction . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 2629) . | (n = 257) . | (n = 672) . | (n = 114) . | P-value . | |||||||||
| . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | . |
| Cardiovascular death or heart failure hospitalization | |||||||||||||
| Placebo | 289 | 18.7 | 0.88 (0.75, 1.04) | 48 | 34.4 | 0.46 (0.28, 0.75) | 99 | 24.3 | 0.57 (0.41, 0.78) | 14 | 20.2 | 0.41 (0.15, 1.14) | 0.008 |
| Empagliflozin | 264 | 16.6 | 24 | 15.3 | 62 | 14.0 | 5 | 8.0 | |||||
| Total (first and recurrent) hospitalizations for heart failurea | |||||||||||||
| Placebo | 297 | 17.6 | 0.90 (0.71, 1.13 | 65 | 36.3 | 0.39 (0.19, 0.80) | 160 | 33.6 | 0.45 (0.29, 0.70 | 15 | 20.1 | 0.08 (0.01, 0.70) | 0.002 |
| Empagliflozin | 287 | 16.7 | 23 | 13.8 | 70 | 14.9 | 1 | 1.6 | |||||
| Extended composite of cardiovascular death and inpatient and outpatient worsening heart failure eventsb | |||||||||||||
| Placebo | 431 | 30.4 | 0.81 (0.71, 0.93) | 63 | 51.5 | 0.49 (0.32, 0.74) | 153 | 43.9 | 0.48 (0.37, 0.63) | 19 | 28.4 | 0.69 (0.33, 1.46) | 0.002 |
| Empagliflozin | 371 | 24.8 | 35 | 23.9 | 89 | 21.6 | 11 | 19.1 | |||||
| Cardiovascular death | |||||||||||||
| Placebo | 137 | 8.1 | 1.00 (0.79, 1.27) | 14 | 7.6 | 0.68 (0.30, 1.58) | 39 | 8.0 | 0.82 (0.51, 1.31) | 9 | 11.9 | 0.57 (0.17, 1.84) | 0.60 |
| Empagliflozin | 142 | 8.2 | 9 | 5.3 | 31 | 6.5 | 4 | 6.2 | |||||
| . | White . | Black . | Asian . | Other . | Interaction . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 2629) . | (n = 257) . | (n = 672) . | (n = 114) . | P-value . | |||||||||
| . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | . |
| Cardiovascular death or heart failure hospitalization | |||||||||||||
| Placebo | 289 | 18.7 | 0.88 (0.75, 1.04) | 48 | 34.4 | 0.46 (0.28, 0.75) | 99 | 24.3 | 0.57 (0.41, 0.78) | 14 | 20.2 | 0.41 (0.15, 1.14) | 0.008 |
| Empagliflozin | 264 | 16.6 | 24 | 15.3 | 62 | 14.0 | 5 | 8.0 | |||||
| Total (first and recurrent) hospitalizations for heart failurea | |||||||||||||
| Placebo | 297 | 17.6 | 0.90 (0.71, 1.13 | 65 | 36.3 | 0.39 (0.19, 0.80) | 160 | 33.6 | 0.45 (0.29, 0.70 | 15 | 20.1 | 0.08 (0.01, 0.70) | 0.002 |
| Empagliflozin | 287 | 16.7 | 23 | 13.8 | 70 | 14.9 | 1 | 1.6 | |||||
| Extended composite of cardiovascular death and inpatient and outpatient worsening heart failure eventsb | |||||||||||||
| Placebo | 431 | 30.4 | 0.81 (0.71, 0.93) | 63 | 51.5 | 0.49 (0.32, 0.74) | 153 | 43.9 | 0.48 (0.37, 0.63) | 19 | 28.4 | 0.69 (0.33, 1.46) | 0.002 |
| Empagliflozin | 371 | 24.8 | 35 | 23.9 | 89 | 21.6 | 11 | 19.1 | |||||
| Cardiovascular death | |||||||||||||
| Placebo | 137 | 8.1 | 1.00 (0.79, 1.27) | 14 | 7.6 | 0.68 (0.30, 1.58) | 39 | 8.0 | 0.82 (0.51, 1.31) | 9 | 11.9 | 0.57 (0.17, 1.84) | 0.60 |
| Empagliflozin | 142 | 8.2 | 9 | 5.3 | 31 | 6.5 | 4 | 6.2 | |||||
Rate, incidence rate per 100 person-years.
Total heart failure hospitalization event rates were derived from an unadjusted negative binomial model.
Composite of time-to-first of cardiovascular death, heart failure hospitalization, urgent visit for worsening heart failure requiring intravenous therapy, or outpatient diuretic intensification.
| . | White . | Black . | Asian . | Other . | Interaction . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 2629) . | (n = 257) . | (n = 672) . | (n = 114) . | P-value . | |||||||||
| . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | . |
| Cardiovascular death or heart failure hospitalization | |||||||||||||
| Placebo | 289 | 18.7 | 0.88 (0.75, 1.04) | 48 | 34.4 | 0.46 (0.28, 0.75) | 99 | 24.3 | 0.57 (0.41, 0.78) | 14 | 20.2 | 0.41 (0.15, 1.14) | 0.008 |
| Empagliflozin | 264 | 16.6 | 24 | 15.3 | 62 | 14.0 | 5 | 8.0 | |||||
| Total (first and recurrent) hospitalizations for heart failurea | |||||||||||||
| Placebo | 297 | 17.6 | 0.90 (0.71, 1.13 | 65 | 36.3 | 0.39 (0.19, 0.80) | 160 | 33.6 | 0.45 (0.29, 0.70 | 15 | 20.1 | 0.08 (0.01, 0.70) | 0.002 |
| Empagliflozin | 287 | 16.7 | 23 | 13.8 | 70 | 14.9 | 1 | 1.6 | |||||
| Extended composite of cardiovascular death and inpatient and outpatient worsening heart failure eventsb | |||||||||||||
| Placebo | 431 | 30.4 | 0.81 (0.71, 0.93) | 63 | 51.5 | 0.49 (0.32, 0.74) | 153 | 43.9 | 0.48 (0.37, 0.63) | 19 | 28.4 | 0.69 (0.33, 1.46) | 0.002 |
| Empagliflozin | 371 | 24.8 | 35 | 23.9 | 89 | 21.6 | 11 | 19.1 | |||||
| Cardiovascular death | |||||||||||||
| Placebo | 137 | 8.1 | 1.00 (0.79, 1.27) | 14 | 7.6 | 0.68 (0.30, 1.58) | 39 | 8.0 | 0.82 (0.51, 1.31) | 9 | 11.9 | 0.57 (0.17, 1.84) | 0.60 |
| Empagliflozin | 142 | 8.2 | 9 | 5.3 | 31 | 6.5 | 4 | 6.2 | |||||
| . | White . | Black . | Asian . | Other . | Interaction . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 2629) . | (n = 257) . | (n = 672) . | (n = 114) . | P-value . | |||||||||
| . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | N . | Rate . | Hazard ratio . | . |
| Cardiovascular death or heart failure hospitalization | |||||||||||||
| Placebo | 289 | 18.7 | 0.88 (0.75, 1.04) | 48 | 34.4 | 0.46 (0.28, 0.75) | 99 | 24.3 | 0.57 (0.41, 0.78) | 14 | 20.2 | 0.41 (0.15, 1.14) | 0.008 |
| Empagliflozin | 264 | 16.6 | 24 | 15.3 | 62 | 14.0 | 5 | 8.0 | |||||
| Total (first and recurrent) hospitalizations for heart failurea | |||||||||||||
| Placebo | 297 | 17.6 | 0.90 (0.71, 1.13 | 65 | 36.3 | 0.39 (0.19, 0.80) | 160 | 33.6 | 0.45 (0.29, 0.70 | 15 | 20.1 | 0.08 (0.01, 0.70) | 0.002 |
| Empagliflozin | 287 | 16.7 | 23 | 13.8 | 70 | 14.9 | 1 | 1.6 | |||||
| Extended composite of cardiovascular death and inpatient and outpatient worsening heart failure eventsb | |||||||||||||
| Placebo | 431 | 30.4 | 0.81 (0.71, 0.93) | 63 | 51.5 | 0.49 (0.32, 0.74) | 153 | 43.9 | 0.48 (0.37, 0.63) | 19 | 28.4 | 0.69 (0.33, 1.46) | 0.002 |
| Empagliflozin | 371 | 24.8 | 35 | 23.9 | 89 | 21.6 | 11 | 19.1 | |||||
| Cardiovascular death | |||||||||||||
| Placebo | 137 | 8.1 | 1.00 (0.79, 1.27) | 14 | 7.6 | 0.68 (0.30, 1.58) | 39 | 8.0 | 0.82 (0.51, 1.31) | 9 | 11.9 | 0.57 (0.17, 1.84) | 0.60 |
| Empagliflozin | 142 | 8.2 | 9 | 5.3 | 31 | 6.5 | 4 | 6.2 | |||||
Rate, incidence rate per 100 person-years.
Total heart failure hospitalization event rates were derived from an unadjusted negative binomial model.
Composite of time-to-first of cardiovascular death, heart failure hospitalization, urgent visit for worsening heart failure requiring intravenous therapy, or outpatient diuretic intensification.
Influence of region and race/ethnicity on effect of empagliflozin
The magnitude of the effect of empagliflozin on the primary composite outcome and total hospitalizations for HF was most pronounced in Asia (hazard ratios of 0.55 and 0.41, respectively); intermediate in North America (hazard ratios of 0.69 and 0.71, respectively) and Latin America (hazard ratios of 0.73 and 0.65, respectively); and least pronounced in Europe (hazard ratios of 0.94 and 0.96, respectively). The treatment-by-region interaction was P = 0.10 for the primary outcome and was P = 0.055 for total hospitalizations for HF. In general, the higher the ratio of HF hospitalization to cardiovascular death, the greater the magnitude of the treatment effect of empagliflozin on the primary and first secondary outcome measures (Table 3).
When outpatient HF events were included in the extended composite endpoint, the magnitude of the effect of empagliflozin was amplified in Europe where outpatient events were most numerous (the hazard ratio declined from 0.94 to 0.74), but the inclusion of outpatient events did not influence the effect size in Latin America or other regions, where outpatient events were less frequent (Table 3). A region-by-treatment interaction was apparent, even when adjusting for baseline covariates reflecting background therapy with drugs and devices for HF or a recent history of an HF hospitalization (interaction P = 0.031).
The magnitude of the effect of empagliflozin on the primary composite outcome and on total hospitalizations for HF was most pronounced in Black patients (hazard ratios of 0.46 and 0.39, respectively) and Asian patients (hazard ratios of 0.57 and 0.45, respectively) and least pronounced in White patients (hazard ratios of 0.88 and 0.90, respectively). The treatment-by-race interaction was P = 0.008 for the primary outcome, P = 0.002 for total hospitalizations for HF and 0.002 for the extended composite outcome (Table 4). Adjusting for baseline covariates reflecting background therapy with drugs and devices for HF or a recent history of an HF hospitalization did not alter these interactions. Interestingly, the effect of empagliflozin in Black patients was more pronounced than in White patients in the two regions where meaningful numbers of both Black and White patients were recruited (North America and Latin America) (Supplementary material online, Figure S1). When outpatient worsening HF events were included in the analysis, the effect size for empagliflozin in White patients increased from 12% to 19% relative risk reduction.
Discussion
Even though the inclusion and exclusion criteria in the EMPEROR-Reduced trial were standardized, there were notable differences in the clinical characteristics in patients who were recruited in different regions and among patients from different racial and ethnic backgrounds. These differences may be related to contrasts in medical practice and healthcare access that may occur across different regions (or even within the same city or country). Many of the patterns that we observed in the EMPEROR-Reduced trial are very similar to patterns that have been noted in previous trials and registries. We were not surprised to find that coronary artery disease (and coronary artery interventions) was particularly common in patients recruited in North America and Europe. Similarly, it is well-known that hypertension is particularly prevalent in Black patients and that atrial fibrillation is more common in White patients. Asian patients are especially prone to diabetes and to coronary artery disease (the prevalence of which was nearly as high as in Whites), despite a lower body mass index. Neprilysin inhibitors are most frequently prescribed in North America (and less frequently prescribed in Latin America and Asia), whereas mineralocorticoid receptor antagonists are popular in Latin America. Heart failure device therapy was more commonly utilized in North America and Europe than in Latin America or Asia. Many of these differences reflect known associations of cardiovascular risk factors with specific racial groups. Other patterns represent differences in the availability of healthcare resources, which encourage the utilization of expensive interventions in regions that have substantial financial resources to devote to healthcare.8–14
Did these differences in clinical characteristics, medical and device therapy yield differences in patient outcomes? The rate of total HF hospitalizations was strikingly different across regions, being nearly three-fold higher in Asia than in Europe. Specifically, when treated with placebo, the rate of total HF hospitalization was 41.4 in Asia, but only 16.3 in Europe, with intermediate rates in the other regions. The difference in HF hospitalization rates in Asia and Europe was not related to the severity of the underlying HF, since the values for ejection fraction and natriuretic peptides were similar in the two regions, and patients recruited in Europe were more likely to have had class III/IV HF at the start of the trial. Furthermore, the risk of cardiovascular death was identical in Europe and Asia, suggesting a similar severity of the underlying disease at baseline. However, in a time-to-first-event analysis, it is noteworthy that the inclusion of outpatient events provided 62% additional worsening HF events to the primary outcome in the placebo group in Europe as compared with only 35% additional events in Latin America, 42% in North America, and 58% in Asia. Thus, worsening HF was more likely to be treated as an outpatient in Europe than in other regions.15 , 16 These findings suggest that European physicians prefer to treat worsening HF as an outpatient rather than in a hospitalized setting.7 , 12 , 17 , 18
Differences in the way that worsening HF events are managed in different regions may influence estimates of the effect of a drug that reduces the risk of worsening HF events. This is especially true if the treatment effect is described using time-to-first-event methods that are applied to composite endpoints; in such analyses, non-fatal events take precedence over deaths. Empagliflozin reduces the risk of the composite of hospitalization for HF or cardiovascular death, a benefit that is determined primarily by its ability to reduce hospitalizations. Therefore, in a time-to-first-event analysis, the effect of empagliflozin would be expected to be greater in regions where the ratio of HF hospitalizations to cardiovascular mortality is higher. The ratio of total HF hospitalizations to cardiovascular death in the placebo group was >4 in Asia and North America, and in these regions, empagliflozin reduces the risk of the primary endpoint by 30–45%; in contrast, the ratio of total HF hospitalizations to cardiovascular death was only 2 in Europe, where the risk reduction produced by empagliflozin was ≈5%. However, when outpatient events were included in the analysis, the ratio of worsening HF events to cardiovascular death increased dramatically in Europe, and at the same time, the risk reduction by empagliflozin increased from ≈5% to 26%. In contrast, an increase in the estimate of the treatment effect was not seen in regions where outpatient treatment of worsening HF events was uncommon. Therefore, the modest response to the effect of SGLT2 inhibition on cardiovascular death and HF hospitalization in Europe appeared to be related to the exclusion of outpatient worsening HF events from the analysis of the primary endpoint. Many of the outpatient events in Europe were likely treated in a hospital setting in other geographical regions.
The inclusion of outpatient worsening HF events narrowed, but did not eliminate, the between-region differences in the effect of empagliflozin on the primary endpoint of the EMPEROR-Reduced trial. A region-by-treatment interaction persisted because Black and Asian patients had a more pronounced response to empagliflozin (when compared with White patients), regardless of the region in which they were recruited. Intriguingly, as in the case of region, the magnitude of the benefit of empagliflozin paralleled the frequency of hospitalizations for HF. The placebo arm ratio of total HF hospitalizations to cardiovascular death was 4.8 and 4.2 in Black and Asian patients, respectively, and in these racial groups, empagliflozin reduced the risk of the primary endpoint by 40–55%; in contrast, the ratio of total HF hospitalizations to cardiovascular death was only 2.2 in White patients, and in this cohort, the risk reduction produced by empagliflozin was 12%. Importantly, the inclusion of outpatient worsening HF events increased the magnitude of the treatment effect in White patients to a 19% risk reduction (hazard ratio 0.81; 95% confidence interval 0.71, 0.93), without a meaningful change in the risk reduction in Black and Asian patients.
Our finding of a race-by-treatment interaction is intriguing, but it is not clear that it represents a replicable finding. Racial groups are not homogeneous and varied meaningfully with respect to baseline characteristics, and it is likely that unmeasured confounders may have contributed to the differences that we observed. A recently published meta-analysis that focused on cardiovascular death (and not HF hospitalizations) reported a greater effect size in trials with a higher recruitment of patients from Asia, but this conclusion was based on a small effect that was discerned by the application of meta-regression methods to trials that often had zero events.19 Another recent meta-analysis reported a greater effect of SGLT2 inhibitors in Asian patients, but this study did not analyse HF hospitalizations in the large-scale cardiovascular outcomes trials in type 2 diabetes.20 A registry report has suggested a smaller treatment effect of SGLT2 inhibitors on HF hospitalizations in Asia than in North America, but this study used observational methods without the protection of randomization.21 Most importantly, other large-scale randomized trials in patients with HF and patients with type 2 diabetes did not report an attenuated effect of SGLT2 inhibitors in general or of empagliflozin specifically in White patients.22–24 In the EMPA-REG OUTCOME trial, the hazard ratios and 95% confidence intervals for the effect of empagliflozin to reduce the risk of a first HF hospitalization were 0.67 (0.49, 0.92), 0.49 (0.18, 1.31), and 0.70 (0.37, 1.33) in White, Black, and Asian patients, respectively (interaction P = 0.82). This lack of external validation in trials that are comparable in size and design to the current study suggests that the race-by-treatment interaction found in EMPEROR-Reduced may not represent a replicable finding, particularly given the multiplicity of comparisons performed in the current trial.
These findings should be considered in the context of the primary analyses of EMPEROR-Reduced showing robust benefits of empagliflozin on the primary endpoint in the entire study population. Specific acknowledged limitations include our definition of regions based on arbitrary geographical groupings and our analysis of race/ethnicity based on the self-report of participants. Therefore, our results may not be replicable if different criteria were used to define these patient subgroups. Certain subgroups (particularly those in categories labelled as ‘other’) were small and exceptionally heterogeneous, and thus, the estimates in these groups were necessarily imprecise. While outpatient treatment intensification events were not independently adjudicated, ascertainment of these events was prespecified and prospectively collected in the case report form. In addition to the adjudication and characterization of hospitalizations for HF, at each scheduled study visit, patients were prospectively asked about interval events and about changes in the use of diuretics that reflected the occurrence of worsening HF since the last visit. We performed multiple statistical tests, which may have increased the possibility of chance findings. Finally, our analyses were not pre-planned, and thus, we did not assess socioeconomic status, medical practice, or access to healthcare, which represent potentially important but unmeasured confounding variables.
Conclusions
We observed notable differences in the baseline characteristics, the placebo event rates for major HF events and the treatment effect estimates across the diverse regions and racial/ethnic groups that participated in the EMPEROR-Reduced trial. The benefits of empagliflozin were most pronounced in regions and racial/ethnic groups with the highest ratios of HF hospitalization to cardiovascular death. Differences among regions and among racial/ethnic groups in the effect size estimates were attenuated when the analyses captured both outpatient and inpatient worsening HF events.
Supplementary material
Supplementary material is available at European Heart Journal online.
Acknowledgements
Graphical assistance was provided by Matthew Smith of Elevate Scientific Solution and supported financially by Boehringer Ingelheim.
Funding
The EMPEROR-Reduced trial was funded by Boehringer Ingelheim and Eli Lilly (EMPEROR-Reduced ClinicalTrials.gov number, NCT03057977).
Conflict of interest: C.S.P.L. reports research support from AstraZeneca, Bayer, Boston Scientific, and Roche Diagnostics; fees as consultant or on the Advisory Board/Steering Committee/Executive Committee for Actelion, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Darma Inc., Us2.ai, Janssen Research & Development LLC, Medscape, Merck, Novartis, Novo Nordisk, Radcliffe Group Ltd, Roche Diagnostics, Sanofi, and WebMD Global LLC; and position as co-founder and non-executive director of Us2.ai. J.P.F. reports consulting fees from Boehringer Ingelheim, during the conduct of the study. D.S. reports personal fees from Boehringer Ingelheim, during the conduct of the study. H.T. reports personal fees from Boehringer Ingelheim, during the conduct of the study; grants from Actelion Pharmaceuticals Japan Ltd, Japan Tobacco Inc, Daiichi Sankyo Co., IQVIA Services Japan, Omron Healthcare, Astellas Pharma Inc, and Teijin Pharma Ltd; grants and personal fees from Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Company Limited, and MSD K K; grants, personal fees, and other from Nippon Boehringer Ingelheim Co., Ltd and Novartis Pharma K.K; personal fees from Astellas Pharma Inc, Pfizer Japan Inc, Bristol Myers Squibb Company, Otsuka Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd, Kowa Pharmaceutical Co. Ltd, and Teijin Pharma Ltd; personal fees and other from Bayer Yakuhin, Ltd, outside the submitted work. S.D.A. reports grants from Vifor; personal fees from Vifor, Bayer, Boehringer Ingelheim, Novartis, Servier, Impulse Dynamics, Cardiac Dimensions, and Thermo Fisher Scientific; and grants and personal fees from Abbott Vascular, outside the submitted work. F.Z. reports personal fees from Boehringer Ingelheim, during the conduct of the study; personal fees from Janssen, Novartis, Boston Scientific, Amgen, CVRx, AstraZeneca, Vifor Fresenius, Cardior, Cereno pharmacuetical, Applied Therapeutics, Merck, Bayer, and Cellprothera, outside the submitted work; and other support from CVCT and Cardiorenal, outside the submitted work. J.B. reports consultancy fees from Boehringer Ingelheim, during the conduct of the study; and consultancy fees from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, BerlinCures, Boehringer Ingelheim, Cardior, CVRx, Foundry, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, Sequana Medical, V-Wave, and Vifor, outside the submitted work. G.F. reports receiving payment from Boehringer Ingelheim for being a trial committee member during the conduct of the study and from Medtronic, Vifor, Servier, and Novartis for being a trial committee member, outside the submitted work. S.J.P. reports personal fees from Boehringer Ingelheim, during the conduct of the study. N.S. reports personal fees and non-financial support from Boehringer Ingelheim, during the conduct of the study; personal fees from Amgen, Astra Zeneca, Eli Lilly, Novo Nordisk, Novartis, and Sanofi; grants and personal fees from Boehringer Ingelheim, outside the submitted work. S.V. reports personal fees from Boehringer Ingelheim, during the conduct of the study; grants from Tier 1 Canada Research Chair in Cardiovascular Surgery and Amarin; grants and personal fees from Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, EOCI Pharmacomm Ltd, HTLS Therapeutics, Janssen, Merck, Novartis, Novo Nordisk, Sanofi, and Sun; other from Canadian Medical and Surgical Knowledge Translation Research Group, outside the submitted work. M.P. reports personal fees from Boehringer Ingelheim, during the conduct of the study; personal fees from AbbVie, Akcea, Amarin, AstraZeneca, Amgen, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Johnson & Johnson, Lilly, Novartis, Pfizer, Relypsa, Sanofi, Synthetic Biologics, Theravance, and NovoNordisk, outside the submitted work. E.P., J.S., D.C., and M.B. are employees of Boehringer Ingelheim.
Data availability
Data will be made available upon request in adherence to transparency conventions in medical research and through requests to the corresponding author. The executive committee of EMPEROR has developed a comprehensive analysis plan and numerous pre-specified analyses, which will be presented in future scientific meetings and publications. At a later time-point, the full database will be made available in adherence with the transparency policy of the sponsor (available at https://trials.boehringer-ingelheim.com/transparency_policy.html).
References
Author notes
Carolyn S.P. Lam and João Pedro Ferreira are co-first authors.



