-
PDF
- Split View
-
Views
-
Cite
Cite
Filippo Crea, Mechanisms of heart failure with preserved ejection fraction, risk stratification of heart failure with reduced ejection fraction, and new light on resistance to diuretics in acute decompensated heart failure, European Heart Journal, Volume 42, Issue 43, 14 November 2021, Pages 4405–4409, https://doi.org/10.1093/eurheartj/ehab791
Close - Share Icon Share

 For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
This Focus Issue on heart failure and cardiomyopathies contains the State of the Art Review ‘Heart failure with preserved ejection fraction in humans and mice: embracing clinical complexity in mouse models’ by Coenraad Withaar from the University of Groningen in the Netherlands, and colleagues.1 The authors note that heart failure (HF) with preserved ejection fraction (HFpEF) is a multifactorial disease accounting for a large and increasing proportion of all clinical HF presentations.2–4 As a clinical syndrome, HFpEF is characterized by typical signs and symptoms of HF, a distinct cardiac phenotype, and raised natriuretic peptides. Non-cardiac comorbidities frequently co-exist and contribute to the pathophysiology of HFpEF. Recently, two clinical algorithms (HFA-PEFF and H2FPEF scores) have been developed to improve and standardize the diagnosis of HFpEF. In this review, the authors evaluate the translational utility of HFpEF mouse models in the context of these HFpEF scores. They systematically recorded evidence of symptoms and signs of HF or clinical HFpEF features and included several cardiac and extra-cardiac parameters as well as age and sex for each HFpEF mouse model. The authors found that most of the pre-clinical HFpEF models do not meet the HFpEF clinical criteria, although some multifactorial models resemble human HFpEF to a reasonable extent. Withaar et al. therefore conclude that to optimize the translational value of mouse models to human HFpEF, a novel approach for the development of pre-clinical HFpEF models is needed, taking into account the complex HFpEF pathophysiology in humans.
In a second State of the Art Review article entitled ‘Untangling the pathophysiologic link between coronary microvascular dysfunction and heart failure with preserved ejection fraction’, Aish Sinha from King’s College London in the UK, and colleagues note that coronary microvascular disease (CMD), characterized by impaired coronary flow reserve (CFR), is a common finding in patients with stable angina.5 Impaired CFR, in the absence of obstructive coronary artery disease, is also present in up to 75% of patients with HFpEF. HFpEF is a heterogeneous syndrome comprising distinct endotypes and it has been hypothesized that CMD lies at the centre of the pathogenesis of one such entity: the CMD–HFpEF endotype. This article provides a contemporary review of the pathophysiology underlying CMD, with a focus on the mechanistic link between CMD and HFpEF. The authors discuss the central role played by subendocardial ischaemia and impaired lusitropy in the development of CMD–HFpEF, as well as the clinical and research implications of the CMD–HFpEF mechanistic link. Future prospective follow-up studies detailing outcomes in patients with CMD and HFpEF are much needed to enhance our understanding of the pathological processes driving these conditions, which may lead to the development of physiology-stratified therapy to improve the quality of life and prognosis in these patients.
Regional and racial/ethnic differences in the incidence of and outcomes related to HF with reduced ejection fraction (HFrEF) have been reported in both observational studies and global clinical trials. These regional and ethnic differences may reflect differences in patient characteristics, comorbidities, medical practice, and healthcare delivery.6 In a Fast Track Clinical Research article entitled ‘Regional and ethnic influences on the response to empagliflozin in patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial’, Carolyn Lam from the National Heart Centre Singapore, and colleagues explore the influence of region and race/ethnicity on the effects of empagliflozin in the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR-Reduced) trial.7 Of 3730 patients, 1353 (36%) were enrolled in Europe, 1286 (34%) in Latin America, 425 (11%) in North America, and 493 (13%) in Asia; 2629 (70%) were White, 257 (7%) Black, and 672 (18%) Asian. Placebo event rates (per 100 patient-years) for cardiovascular death or HF hospitalization varied by region (Asia 28, North America 26, Latin America 21, and Europe 17) and race/ethnicity (Black 34, Asian 24, and White 18), driven by differences in HF hospitalization. The ratio of total HF hospitalization to cardiovascular death varied from 5.4 in Asia and 4.8 in North America to 2.1 in Europe; and from 4.8 in Black and 4.2 in Asian to 2.2 in White patients. Groups with the highest ratio had the greatest reduction in the primary outcome with empagliflozin. Inclusion of outpatient worsening HF episodes added more events in Europe vs. other regions, enhanced the placebo event rates in Europe vs. other regions, and increased the relative risk reduction with empagliflozin in Europe from 6% to 26%.
The authors conclude that there were notable differences in the placebo event rates for major HF events across diverse regions and race/ethnic groups. The benefit of empagliflozin was most pronounced in groups with the highest ratio of HF hospitalization to cardiovascular death. Regional differences were attenuated when the definition of HF events was expanded to include outpatient worsening HF events. The contribution is accompanied by an Editorial by Eldrin F. Lewis from the Stanford University School of Medicine in Palo Alto, CA, USA.8 Lewis concludes that as we continue to understand the variations in practice across regions of the world and investigate both racial/ethnic differences and disparities, it is imperative to not use these differences as reasons to avoid treating patients with HF optimally.
Patients with HFrEF have a variable prognosis that may depend on patients’ characteristics, healthcare and social determinants, and treatments that modify the course of the disease. Prognostic models have been developed to capture the overall risk of each individual patient.9,10 However, these prognostic models were developed before the implementation of recent therapies that impact HFrEF outcomes and they do not incorporate biomarkers with strong prognostic value, e.g. N-terminal probrain natriuretic peptide (NT-proBNP) and high-sensitivity cardiac troponin T (hs-cTnT). More recent prognostic models have included natriuretic peptides but not hs-cTnT despite the importance of both biomarkers in predicting events, and they were developed before the advent of sodium–glucose co-transporter-2 inhibitors (SGLT2is). Moreover, these models include a large number of clinical and laboratory variables as well as treatments, which necessarily limit their clinical implementation. In a Fast Track Clinical Research article entitled ‘Novel biomarker-driven prognostic models to predict morbidity and mortality in chronic heart failure: the EMPEROR-Reduced trial’, Stuart Pocock from the London School of Hygiene and Tropical Medicine in the UK, and colleagues note that the aim of their study was to generate a biomarker-driven prognostic tool for patients with chronic HFrEF.11 In EMPEROR-Reduced, 33 candidate variables were pre-selected. Multivariable Cox regression models were developed using stepwise selection for: (i) the primary composite outcome of HF hospitalization or cardiovascular death; (ii) all-cause death; and (iii) cardiovascular mortality. A total of 3730 patients were followed-up for a median of 16 months, 823 (22%) patients had a primary outcome and 515 (14%) patients died, of whom 389 (10%) died from a cardiovascular cause. NT-proBNP and hs-cTnT were the dominant predictors of the primary outcome and, in addition, a shorter time since last HF hospitalization, longer time since HF diagnosis, lower systolic blood pressure, New York Heart Association (NYHA) Class III or IV, higher heart rate, and peripheral oedema were key predictors (eight variables in total, all P < 0.001). The primary outcome risk score discriminated well (c-statistic = 0.73), with patients in the top 10 of risk having an event rate >9 times higher than those in the bottom 10. Empagliflozin benefitted patients across risk levels for the primary outcome. NT-proBNP and hs-cTnT were also the dominant predictors of all-cause and cardiovascular mortality, followed by NYHA Class III or IV and ischaemic aetiology (four variables in total, all P < 0.001). The mortality risk model presented good event discrimination for all-cause and cardiovascular mortality (c-statistic = 0.69 for both) (Figure 1). These simple models were externally validated in the BIOSTAT-CHF study, achieving similar c-statistics.
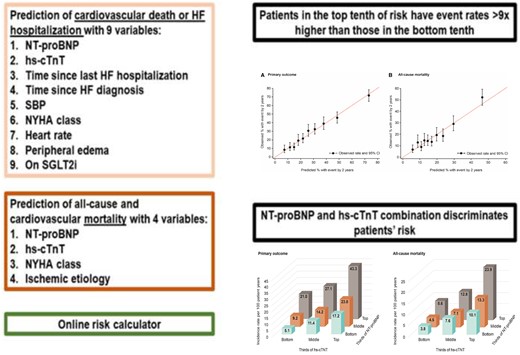
EMPEROR-Reduced risk model. CI = confidence interval; HF = heart failure; hs-cTnT = high-sensitivity cardiac troponin T; NT-proBNP = N-terminal probrain natriuretic peptide; NYHA = New York Heart Association; SBP = systolic blood pressure; SGLT2i = sodium–glucose co-transporter 2 inhibitor (from Pocock SJ, Ferreira JP, Gregson J, Anker SD, Butler J, Filippatos G, Gollop ND, Iwata T, Brueckmann M, Januzzi JL Jr., Voors AA, Zannad F, Packer M. Novel biomarker-driven prognostic models to predict morbidity and mortality in chronic heart failure: the EMPEROR-Reduced trial. See pages 4455–4464).
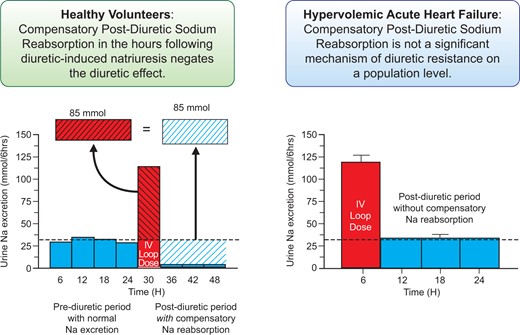
Graphical Abstract (from Cox ZL, Rao VS, Ivey-Miranda JB, Moreno-Villagomez J, Mahoney D, Ponikowski P, Biegus J, Turner JM, Maulion C, Bellumkonda L, Asher JL, Parise H, Wilson PF, Ellison DH, Wilcox CS, Testani JM. Compensatory post-diuretic renal sodium reabsorption is not a dominant mechanism of diuretic resistance in acute heart failure. See pages 4468–4477).
The authors conclude that the combination of NT-proBNP and hs-cTnT with a small number of readily available clinical variables provides prognostic assessment for patients with HFrEF. This predictive tool kit can be easily implemented for routine clinical use. This manuscript is accompanied by an Editorial by Christian Mueller and Desiree Wussler from the University Hospital Basel in Switzerland.12 The authors conclude that as with most other innovations in clinical medicine, successful implementation of the EMPEROR-Reduced score also depends on several factors beyond science. Therefore, it is highly appreciated that an Online Risk Calculator is provided in the Online Supplement that can be incorporated into the electronic medical record of HF patients.
Diuretic resistance in acute decompensated heart failure (ADHF) is common and associated with worse outcomes.13–15 Compensatory post-diuretic sodium reabsorption (CPDSR) is ubiquitously cited as a cause of diuretic resistance in ADHF. The concept of CPDSR asserts that the kidneys decrease urinary sodium excretion to very low levels over the following hours to counterbalance the diuretic-induced natriuresis. In a Clinical Research article entitled ‘Compensatory post-diuretic renal sodium reabsorption is not a dominant mechanism of diuretic resistance in acute heart failure’, Zachary Cox from the Lipscomb University College of Pharmacy in Nashville, TN, USA, and colleagues note that CPDSR is extrapolated to non-euvolaemic populations as a diuretic resistance mechanism; however, its importance in ADHF is unknown.16 Patients with ADHF receiving i.v. loop diuretics (462 administrations in 285 patients) underwent supervised urine collections entailing an immediate pre-diuretic spot urine sample, then 6-h (diuretic-induced natriuresis period) and 18-h (post-diuretic period) urine collections. The average spot urine sodium concentration immediately prior to diuretic administration was 64 ± 33 mmol/L, with only 4% of patients having low (<20 mmol/L) urine sodium consistent with CPDSR. Paradoxically, greater 6-h diuretic-induced natriuresis was associated with larger 18-h post-diuretic spontaneous natriuresis (r = 0.7, P < 0.001). A higher pre-diuretic urine sodium to creatinine ratio (r = 0.37, P < 0.001) was the strongest predictor of post-diuretic spontaneous natriuresis. In a subgroup of patients (n = 43) randomized to protocol-driven intensified diuretic therapies, the mean diuretic-induced natriuresis increased three-fold. In contrast to the substantial decrease in spontaneous natriuresis predicted by CPDSR, no change in post-diuretic spontaneous natriuresis was observed (P = 0.47). The authors conclude that on a population level, CPDSR was not an important driver of diuretic resistance in hypervolaemic ADHF. In contrast to CPDSR, a greater diuretic-induced natriuresis predicted a larger post-diuretic spontaneous natriuresis. Basal sodium avidity, rather than diuretic-induced CPDSR, appears to be the predominant determinate of both diuretic-induced and post-diuretic natriuresis in hypervolaemic ADHF. The contribution is accompanied by an Editorial by Wilfried Mullens from the Hasselt University in Diepenbeek, Belgium, and colleagues.17 The authors conclude that Cox and colleagues provide interesting data in the setting of AHF showing that CPDSR does not influence diuretic resistance. Furthermore, they provide the insight that in AHF patients with a poor diuretic response and positive sodium balance, the use of targeted diuretics can bolster the diuretic response without inflicting CPDSR. Additional studies will need to determine which diuretic strategy (an increase in loop diuretic dose, adding acetazolamide, adding an SGLT2i, adding thiazide, etc.) is most appropriate in which patients. The ongoing ADVOR (Acetazolamide in patients with Decompensated heart failure and Volume OveRload) trial, for instance, will test the effect of acetazolamide for this purpose in AHF.
Cardiac injury and remodelling are associated with the rearrangement of cardiac lipids. Glycosphingolipids are membrane lipids that are important for cellular structure and function, and cardiac dysfunction is a characteristic of rare monogenic diseases with defects in glycosphingolipid synthesis and turnover. However, it is not known how cardiac glycosphingolipids regulate cellular processes in the heart. In a Translational Research article entitled, ‘Glucosylceramide synthase deficiency in the heart compromises β1-adrenergic receptor trafficking’, Linda Andersson from the Sahlgrenska Academy at University of Gothenburg and Sahlgrenska University Hospital in Sweden, and colleagues note that the aim of their study is to determine the role of cardiac glycosphingolipids in heart function.18 Using human myocardial biopsies, they showed that the glycosphingolipids glucosylceramide and lactosylceramide are present at very low levels in the non-ischaemic human heart with normal function and are elevated during remodelling. Similar results were observed in mouse models of cardiac remodelling. They also generated mice with cardiomyocyte-specific deficiency in Ugcg, the gene encoding glucosylceramide synthase (hUgcg–/– mice). In 9- to 10-week-old hUgcg–/– mice, contractile capacity in response to dobutamine stress was reduced. Older hUgcg–/– mice developed severe HF and left ventricular dilatation even under baseline conditions, and died prematurely. Using RNA-seq and cell culture models, they showed defective endolysosomal retrograde trafficking and autophagy in Ugcg-deficient cardiomyocytes. They also showed that responsiveness to β-adrenergic stimulation was reduced in cardiomyocytes from hUgcg–/– mice and that Ugcg knockdown suppressed the internalization and trafficking of β1-adrenergic receptors.
The authors conclude that their findings suggest that cardiac glycosphingolipids are required to maintain β-adrenergic signalling and contractile capacity in cardiomyocytes and to preserve normal heart function. The manuscript is accompanied by an Editorial by Jean-Luc Balligand and Lauriane Michel from the University of Louvain Medical School in Brussels, Belgium.19 The authors note that many studies have highlighted the protective role of several forms of autophagy in the face of cardiac stress, and ask several questions. Would the levels of glycosphingolipids observed in cardiac muscle from ischaemic patients drive autophagy at levels that protect the heart? Also, could any pharmacological intervention on the Ugcg pathway be harnessed in a sufficiently precise manner to avoid deleterious effects of exaggerated autophagy? They highlight that these questions will need to be answered before the intriguing observations in the study of Anderson et al. can be translated therapeutically.
The issue is also complemented by two Discussion Forum contributions. In a commentary entitled ‘Efficacy of the Biosync trial, or when facts prompt a reconsideration of theories’, J. Gert van Dijk from the University of Amsterdam in the Netherlands, and colleagues comment on the recent publications ‘Cardiac pacing in severe recurrent reflex syncope and tilt-induced asystole’ by Michele Brignole et al. and ‘Effectiveness of closed loop stimulation pacing in patients with cardio-inhibitory vasovagal reflex syncope is questionable’ by Wouter Wieling et al.20–22 Two Discussion Forum responses have been supplied: one each by Wieling and by Brignole.23,24
The editors hope that this issue of the European Heart Journal will be of interest to its readers.
With thanks to Amelia Meier-Batschelet, Johanna Huggler, and Martin Meyer for help with compilation of this article.



