-
PDF
- Split View
-
Views
-
Cite
Cite
Filippo Crea, The ESC Guidelines on cardiac pacing and resynchronization, and the many facets of atrial fibrillation, European Heart Journal, Volume 42, Issue 35, 14 September 2021, Pages 3411–3414, https://doi.org/10.1093/eurheartj/ehab628
Close - Share Icon Share
 For the podcast associated with this article, please visit https://academic. oup.com/eurheartj/pages/Podcasts.
For the podcast associated with this article, please visit https://academic. oup.com/eurheartj/pages/Podcasts.
This Focus Issue on arrhythmias contains the ‘2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy’.1 Pacing is an important part of electrophysiology and of cardiology in general. While some of the situations requiring pacing are clear and have not changed over the years, many others have evolved and have been the subject of extensive recent research, such as pacing after syncope, pacing following transcatheter aortic valve implantation, cardiac resynchronization therapy for heart failure and for prevention of pacing-induced cardiomyopathy, and pacing in various infiltrative and inflammatory diseases of the heart, as well as in different cardiomyopathies. Other novel topics include new diagnostic tools for decision-making on pacing, as well as a whole new area of pacing the His bundle and the left bundle branch. In addition, attention has increased on other areas, such as how to systematically minimize procedural risk and avoid complications of cardiac pacing, how to manage patients with pacemakers in special situations, such as when magnetic resonance imaging or irradiation are needed, how to follow patients with a pacemaker with emphasis on the use of remote monitoring, and how to include shared decision-making in caring for this patient population. The last pacing guidelines of the European Society of Cardiology were published in 2013; therefore, new Guidelines are timely and necessary.
In a Viewpoint article entitled ‘Does gut microbiota affect atrial rhythm? Causalities and speculations’ Dominik Linz from the University of Copenhagen in Denmark, and colleagues note that dietary intake has been shown to change the composition of the gut microbiota, and some changes in microbiota (dysbiosis) have been linked to diabetes, hypertension, and obesity, which are established risk factors for atrial fibrillation (AF).2,3 In addition, intestinal dysbiosis generates microbiota-derived bioactive metabolites that might exert proarrhythmic actions. Although emerging pre-clinical investigations and clinical observational cohort studies suggest a possible role for gut dysbiosis in AF promotion, the exact mechanisms through which dysbiosis contributes to AF remain unclear. This Viewpoint article briefly reviews evidence suggesting that abnormalities in the intestinal microbiota play an important and little-recognized role in the pathophysiology of AF and that an improved understanding of this role may open up new possibilities in the management of AF.
In another Viewpoint article entitled ‘Ageing, comorbidities, and the complex determinants of atrial fibrillation in athletes’, Eduard Guasch from the Hospital Clinic de Barcelona in Spain, and colleagues indicate that regular physical activity is one of the most powerful therapies to fight illness, including cardiovascular disease.4 However, like any other largely beneficial intervention, too much exercise can be counterproductive and the consequences of excess physical activity have often been overlooked.5,6 Emerging evidence suggests that moderate training and intense endurance exercise have different consequences for cardiovascular remodelling. Coronary calcification burden is increased in master athletes, but the causes and potential consequences are incompletely understood. In addition, the myocardium can be remodelled by intense exercise. An association between vigorous exercise training and an increased risk of AF has been recognized for >20 years. The data are compelling for highly trained endurance athletes. Lifelong strenuous endurance training is associated with an up to five- to eight-fold increased risk of AF vs. non-athletes, depending on factors such as the amount and intensity of training. Results in the general population are conflicting, however. Recent large studies and meta-analyses suggest a consistent reduction in AF with all levels of exercise in women, but a shift from protection to increased risk at high levels of exercise in men. In this Viewpoint article, the authors consider how ageing influences AF risk in trained individuals and the impact for clinical practice (Figure 1).
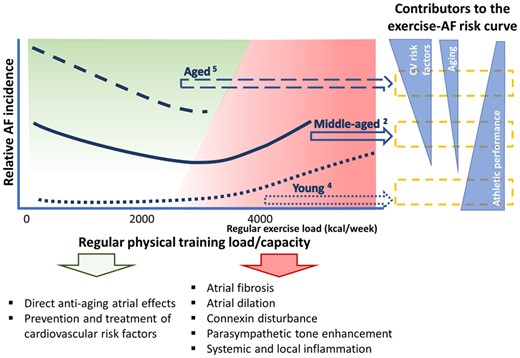
Impact of age in the relationship between exercise and atrial fibrillation (AF) risk in men. The risk of AF associated with exercise varies according to age (upper left); the effects of very high intensity exercise in aged individuals are uncertain. Atrial senescence, risk factor burden, and physical performance contribute to different impacts of exercise at different ages (right). These factors modulate the balance of potential pro- and antiarrhythmogenic mechanisms (bottom). This is a schematic with approximate regular exercise load estimations based on the literature (from Guasch E, Nattel S. Ageing, comorbidities, and the complex determinants ofatrial fibrillation in athletes. See pages 3526–3528).
Guidelines from the European Society of Cardiology (ESC) recommend a 3-month restriction following secondary prevention impantable cardioverter defibrillator (ICD) implantation or appropriate ICD shock, and a 1-month restriction following primary prevention ICD implantation. American Heart Association guidelines are more restrictive, recommending a 6-month driving ban following ICD implantation for secondary prevention or appropriate ICD therapy. Both groups recommend permanent restriction of professional driving and driving of vehicles heavier than 3500 kg. In a Clinical Research article entitled ‘Driving following defibrillator implantation: a nationwide register-linked survey study’, Jenny Bjerre from the Copenhagen University Hospital Herlev and Gentofte in Denmark, and colleagues sought to investigate how many patients are aware of, and adhere to, the driving restrictions, and what proportion experience an ICD shock or other cardiac symptoms while driving.7 They performed a nationwide survey of all living Danish residents 18 years or older who received a first-time ICD between 2013 and 2016 (n = 3913) and linked their responses with nationwide registers. Of 2741 respondents (47% primary prevention, 83% male, median age 67 years), 2513 (92%) held a valid driver’s licence at ICD implantation, 175 (7%) of whom had a licence for professional driving. Many drivers were unaware of driving restrictions: primary prevention 58%; secondary prevention 36%; post-appropriate shock 28%; and professional drivers 55%. Almost all (94%) resumed non-professional driving after ICD implantation, more than one-third during the restricted period; 35% resumed professional driving. During a median follow-up of 2.3 years, five (0.2%) reported receiving an ICD shock while driving, one of which resulted in a traffic accident. The estimated risk of harm was 0.0002% per person-year.
The authors conclude that in this nationwide study, many ICD patients were unaware of driving restrictions, and more than a third resumed driving during a driving restriction period. However, the rate of reported ICD shocks while driving is very low. This manuscript is accompanied by an Editorial by Harry Crijns and Kevin Vernooy from the Universiteit Maastricht Cardiovascular Research Institute Maastricht in the Netherlands.8 The authors note that changing the guidelines, including alleviation of the restrictions as well as incorporating the recommendations to assess risk mainly based on underlying cardiovascular pathology, would be most appropriate. The current ESC consensus on driving and ICD dates to 2009 and may become updated now taking new data including the present study into account. That would certainly benefit ICD patients in the near future, especially since self-driving cars are still not a daily reality.
The prevalence of chronic obstructive pulmonary disease (COPD) in AF patients is unclear, and its association with adverse outcomes is often overlooked.9,10 In a meta-analysis article entitled ‘Prevalence, management, and impact of chronic obstructive pulmonary disease in atrial fibrillation: a systematic review and meta-analysis of 4 200 000 patients’, Giulio Francesco Romiti from the University of Rome in Italy, and colleagues aimed to estimate the prevalence of COPD, its impact on clinical management, and outcomes in patients with AF, and the impact of beta-blockers on outcomes in patients with COPD.11 A systematic review and meta-analysis were conducted according to international guidelines. All studies reporting the prevalence of COPD in AF patients were included. Data on comorbidities, beta-blocker and oral anticoagulant prescription, and outcomes [all-cause death, cardiovascular (CV) death, ischaemic stroke, and major bleeding] were compared according to COPD and beta-blocker status. Among 46 studies, the pooled prevalence of COPD was 13%. COPD was associated with higher prevalence of comorbidities, higher CHA2DS2-VASc score, and significantly lower prescription of beta-blockers [odds ratio (OR) 0.77]. COPD was associated with significantly higher risk of all-cause death (OR 2.22), CV death (OR 1.84), and major bleeding (OR 1.45); no significant differences in outcomes were observed according to beta-blocker use in AF patients with COPD (Figure 2).
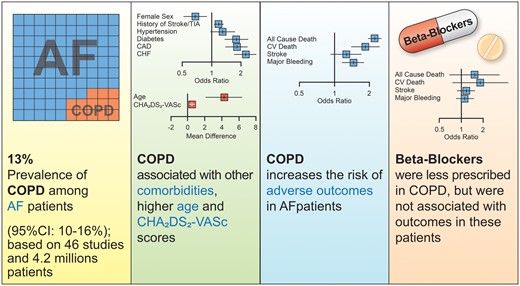
Prevalence, management, and impact of COPD in AF. AF = atrial fibrillation; CAD = coronary artery disease; CHF = chronic heart failure; CI = confidence interval; COPD = chronic obstructive pulmonary disease; TIA = transient ischaemic attack (from Romiti GF, Corica B, Pipitone E, Vitolo M, Raparelli V, Basili S, Boriani G, Harari S, Lip GYH, Proietti M. Prevalence, management, and impact of chronic obstructive pulmonary disease in atrial fibrillation: a systematic review and meta-analysis of 4,200,000 patients. See pages 3541–3554).
The authors conclude that COPD is common in AF, being found in 13% of patients, and is associated with an increased burden of comorbidities, differential management, and worse outcomes, with a more than two-fold higher risk of all-cause death and increased risk of CV death and major bleeding. Therapy with beta-blockers does not increase the risk of adverse outcomes in patients with AF and COPD. The contribution is accompanied by an Editorial by James Reiffel from Columbia University in New York City, USA.12 The author concludes that these are important observations that have major implications for clinical practice.
Arrhythmogenic cardiomyopathy (ACM) is an inherited heart disease affecting 1 in ∼2500 individuals. Defects in genes encoding desmosome proteins or their binding partners lead to malfunction of intercellular junctions in cardiac muscle, which normally absorb mechanical stress and favour membrane stability during cardiac contraction. Desmosome dysfunction in cardiomyocytes produces membrane frailty, mitochondrial damage, disrupted cell signalling, and myocardial injury, which triggers progressive myocyte death, inflammatory activation, and ultimately fibrosis. The fibrosis in turn leads to conduction slowing and re-entry circuits, favouring ventricular arrhythmias (VAs). This complex pathophysiology results in heterogeneous clinical manifestations ranging from mild symptoms such as palpitations, to heart failure, and premature death.13,14 Small extracellular vesicles (EVs), secreted by cardiosphere-derived cells (CDCs), are stromal/progenitor cells with anti-inflammatory, immunomodulatory, antifibrotic, and cardiomyogenic properties. In a Translational Research article entitled ‘Extracellular vesicles from immortalized cardiosphere-derived cells attenuate arrhythmogenic cardiomyopathy in desmoglein-2 mutant mice’, Yen-Nien Lin from the China Medical University and Hospital in Taiwan, and colleagues sought to assess the efficacy of EVs in an ACM murine model.15 Four-week-old homozygous knock-in mutant desmoglein-2 (Dsg2mt/mt) mice were randomized to receive weekly EVs or vehicle for 4 weeks. After 4 weeks, Dsg2mt/mt mice receiving EVs showed improved biventricular function (left, P < 0.0001; right, P = 0.0037) and less left ventricular dilation (P < 0.0179). Electrocardiography revealed abbreviated QRS duration (P = 0.0003) and QTc interval (P = 0.0006) in EV-treated Dsg2mt/mt mice. Further electrophysiology testing in the EV group showed decreased burden (P = 0.0042) and inducibility of ventricular arrhythmias (P = 0.0037). Optical mapping demonstrated accelerated repolarization (P = 0.0290) and faster conduction (P = 0.0274) in Dsg2mt/mt mice receiving EVs. Dsg2mt/mt hearts exhibited reduced fibrosis, less cell death, and preserved connexin 43 expression after EV treatment. Hearts of Dsg2mt/mt mice expressed markedly increased levels of inflammatory cytokines that were, in part, attenuated by EV therapy. The pan-inflammatory transcription factor nuclear factor-κB (NF-κB), the inflammasome sensor NLRP3, and the macrophage marker CD68 were all reduced in EV-treated animals. Blocking EV hsa-miR-4488 in vitro and in vivo reactivates NF-κB and blunts the beneficial effects of EVs.
The authors conclude that EV treatment improves cardiac function, reduces cardiac inflammation, and suppresses arrhythmogenesis in ACM. The manuscript is accompanied by an Editorial by Cristina Basso from the University of Padua in Italy, and colleagues.16 The authors note that putting together the results of Lin and colleagues with our novel concept of ACM as a multicellular and multiorgan disorder, it would be interesting to evaluate whether the EVs target other cardiac and extracardiac cell types, which may be involved in the disease. Thus, many questions need to be addressed before moving EV therapy from bench to bedside.
The issue is also complemented by two Discussion Forum contributions. In a commentary entitled ‘Methodological considerations when discussing the fracture risk of atrial fibrillation patients taking different drug’, Ping-Hao Chiang from the Chung Shan Medical University in Taiwan and colleagues comment on the recent publication ‘Fracture risks among patients with atrial fibrillation receiving different oral anticoagulants: a real-world nationwide cohort study’ by Huei-Kai Huang from the Tzu Chi University in Taiwan.17,18 Huang et al. respond in a separate comment.19
The editors hope that this issue of the European Heart Journal will be of interest to its readers.
With thanks to Amelia Meier-Batschelet, Johanna Huggler, and Martin Meyer for help with compilation of this article.




