-
PDF
- Split View
-
Views
-
Cite
Cite
Ella Grilz, Florian Posch, Stephan Nopp, Oliver Königsbrügge, Irene M Lang, Peter Klimek, Stefan Thurner, Ingrid Pabinger, Cihan Ay, Relative risk of arterial and venous thromboembolism in persons with cancer vs. persons without cancer—a nationwide analysis, European Heart Journal, Volume 42, Issue 23, 14 June 2021, Pages 2299–2307, https://doi.org/10.1093/eurheartj/ehab171
Close - Share Icon Share
Abstract
An interrelation between cancer and thrombosis is known, but population-based studies on the risk of both arterial thromboembolism (ATE) and venous thromboembolism (VTE) have not been performed.
International Classification of Disease 10th Revision (ICD-10) diagnosis codes of all publicly insured persons in Austria (0–90 years) were extracted from the Austrian Association of Social Security Providers dataset covering the years 2006–07 (n = 8 306 244). Patients with a history of cancer or active cancer were defined as having at least one ICD-10 ‘C’ diagnosis code, and patients with ATE and/or VTE as having at least one of I21/I24 (myocardial infarction), I63/I64 (stroke), I74 (arterial embolism), and I26/I80/I82 (venous thromboembolism) diagnosis code. Among 158 675 people with cancer, 8559 (5.4%) had an ATE diagnosis code and 7244 (4.6%) a VTE diagnosis code. In contrast, among 8 147 569 people without cancer, 69 381 (0.9%) had an ATE diagnosis code and 29 307 (0.4%) a VTE diagnosis code. This corresponds to age-stratified random-effects relative risks (RR) of 6.88 [95% confidence interval (CI) 4.81–9.84] for ATE and 14.91 (95% CI 8.90–24.95) for VTE. ATE proportion was highest in patients with urinary tract malignancies (RR: 7.16 [6.74–7.61]) and lowest in patients with endocrine cancer (RR: 2.49 [2.00–3.10]). The corresponding VTE proportion was highest in cancer of the mesothelium/soft tissue (RR: 19.35 [17.44–21.47]) and lowest in oropharyngeal cancer (RR: 6.62 [5.61–7.81]).
The RR of both ATE and VTE are significantly higher in persons with cancer. Our population-level meta-data indicate a strong association between cancer, ATE and VTE, and support the concept of shared risk factors and pathobiology between these diseases.
Relative risk of ATE and VTE in persons with a cancer diagnosis code versus persons without a cancer diagnosis code.
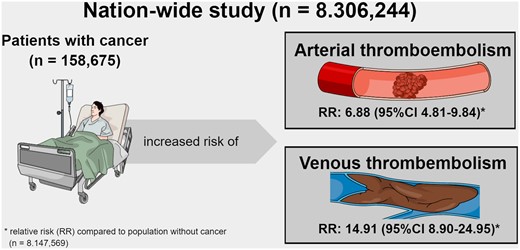
Relative risk of ATE and VTE in persons with a cancer diagnosis code versus persons without a cancer diagnosis code
See page 2308 for the editorial comment on this article (doi: 10.1093/eurheartj/ehab252)
Introduction
Thrombosis is a leading contributor to global disease burden, and one out of four deaths worldwide are caused by thrombosis.1 Prior studies suggested an interrelation between cancer and both arterial thromboembolism (ATE) and venous thromboembolism (VTE).2–7 According to recent data from the Vienna Cancer and Thrombosis Study, the 2-year cumulative incidences of ATE and VTE are 2.6% and 8.7% in patients with cancer, respectively.7 , 8 In comparison to the general population, a two-fold increased risk of ATE and a four- to seven-fold higher risk of VTE has been reported in patients with cancer in previous studies.4–6 , 9 , 10 Furthermore, prior studies have indicated that the occurrence of ATE/VTE varies by cancer type and is associated with poor prognosis.4–7 , 11 , 12 However, large-scale population-based data informing on both the risk of ATE and VTE in the overall cancer population and scrutinizing the risk according to single cancer types are currently not available.
With vascular diseases and cancer becoming more prevalent in an aging population, the proportion of cancer-associated ATE/VTE is likely to increase in the future.13–15 The emergence of novel antineoplastic therapy approaches (e.g. vascular endothelial growth factor targeted, immunotherapeutic) might further contribute to an increasing risk of cancer-associated ATE/VTE.4 , 16–19 Since data on long-term effects of cancer therapy are scarce, it is difficult to determine the exact incidence of cardiovascular complications. Furthermore, this lack of data could also result in inadequate thromboprophylaxis or unnecessary interruption of cancer therapy.20 Therefore, it is of importance to understand the associations between cancer and ATE/VTE and investigate the magnitude of these associations.
We have compared the relative risk (RR) of ATE and VTE (i.e. history or concurrent ATE or VTE diagnosis), respectively, in persons with cancer vs. subjects without cancer, and analysed the association of ATE and VTE with cancer in a nationwide cross-sectional study. Our aim was to estimate the magnitude of the relationship between cancer and ATE/VTE and provide epidemiological background for further investigations to gain insights into the interrelation of cancer and thrombosis.
Methods
Patient data
Data were extracted from the Austrian Association of Social Security Providers (AASSP) dataset. The AASSP dataset includes anonymized medical claims data for all publically insured persons aged 0–90 years in Austria in the calendar years 2006–07, comprising a total population of 8 306 244 people. This cohort represents 99.998% of the total population of the country, as permanent residents in Austria have public insurance per law.21
For this analysis, we have extracted International Classification of Disease 10th Revision (ICD-10) diagnosis codes of all persons included in the AASSP. Both main and side diagnosis codes were extracted (i.e. if a patient had been hospitalized for cancer and additionally developed VTE, cancer is the main diagnosis and VTE the side diagnosis). Patients with a history of cancer or active cancer were defined as having at least one ICD-10 diagnosis code starting with the letter ‘C’. Cancer types were classified according to the ICD-10 classification system: ‘C00–C14’—malignant neoplasms of lip, oral cavity and pharynx; ‘C15–C26’—malignant neoplasms of digestive organs; ‘C30–C39’—malignant neoplasms of respiratory and intrathoracic organs; ‘C40–C41’—malignant neoplasms of bone and articular cartilage; ‘C43–C44’—melanoma and other malignant neoplasms of skin; ‘C45–C49’—malignant neoplasms of mesothelial and soft tissue; ‘C50’—malignant neoplasm of breast; ‘C51–C58’—malignant neoplasms of female genital organs; ‘C60–C63’—malignant neoplasms of male genital organs; ‘C64–C68’—malignant neoplasms of urinary tract; ‘C69–C72’—malignant neoplasms of eye, brain and other parts of central nervous system; ‘C73–C75’—malignant neoplasms of thyroid and other endocrine glands; ‘C76–C80’—malignant neoplasms of ill-defined, secondary and unspecified sites; and ‘C81–C96’—malignant neoplasms, stated or presumed to be primary, of lymphoid, haematopoietic, and related tissue.
Patients with ATE were defined as having at least one of the following ICD-10 diagnosis codes: (1) ‘I21’—acute myocardial infarction, (2) ‘I24’—other acute ischaemic heart diseases, (3) ‘I63’—cerebral infarction, (4) ‘I64’—stroke, not specified as haemorrhage or infarction, and (5) ‘I74’—arterial embolism and thrombosis, respectively. Patients with VTE were defined as having at least one of the following ICD-10 diagnosis code: (1) ‘I26’—pulmonary embolism, (2) ‘I80’—thrombosis, phlebitis, and thrombophlebitis, and (3) ‘I82’—other venous thromboembolism, and thrombosis.
Specific approval from the local ethics committee was not required for conducting the analysis.
Statistical analysis
Continuous variables were summarized as median [25th–75th percentile], and count data as absolute frequency (%). The proportion of subjects with cancer, ATE and VTE diagnosis code was summarized using a random-effects (RE) model for proportions with exact 95% confidence intervals (CI) (Stata routine metaprop). To stabilize the variance of the proportions in these analyses, a Freeman–Tukey double arcsine transformation was performed.22 The RR of an ATE or VTE diagnosis code between patients with and without cancer was estimated with an RE model for RR (Stata routine metan). Throughout, proportions and RR were estimated/stratified by age groups. In all analyses, the I 2-statistic was used to quantify heterogeneity, with I 2 values >0.30 considered indicative of heterogeneity. Stata 15.0 (Stata Corp., Houston, TX, USA) and SPSS 25 (SPSS Inc, Chicago IL, USA) were used to perform all statistical analyses.
Results
Description of the study cohort
The study cohort consists of 4 255 119 (51.23%) female, and 4 051 125 (48.77%) male persons. The median age of the Austria population was 41 (25th–75th percentile [interquartile range (IQR)]: 23–58) years. Male persons were younger than female persons (median age [IQR]: 40 [22–55] vs. 42 [24–60] years).
Overall, 158 675 (77 853 [49.06%] women, 80 822 [50.94%] men) persons, who were included in this study, had a cancer diagnosis code, and 42 484 (26.77%) of them had at least two different ‘C’ diagnosis codes. A detailed description of the study cohort is presented in Table 1.
Age and sex distribution of the study cohort overall and by arterial thromboembolism, venous thromboembolism, and cancer diagnosis code
| . | Absolute number . | Median age (95% CI) . | % female . |
|---|---|---|---|
| Total study cohort | 8 306 244 | 41 (23–58) | 51.23 |
| Persons with ATE | 77 940 | 76 (65–83) | 47.25 |
| Persons with VTE | 36 551 | 71 (57–81) | 58.17 |
| Persons with cancera | 158 675 | 69 (59–79) | 49.06 |
| Oropharyngeal (C00–14) | 4720 | 63 (55–71) | 28.69 |
| Gastrointestinal (C15–29) | 35 490 | 72 (63–80) | 43.78 |
| Respiratory (C30–39) | 16 827 | 68 (60–76) | 34.17 |
| Bone/cartilage (C40–41) | 1177 | 61 (44–72) | 46.13 |
| Skin (C43–44) | 19 368 | 74 (63–81) | 50.34 |
| Mesothelium/soft tissue (C45–49) | 3897 | 68 (57–78) | 57.22 |
| Breast (C50) | 23 164 | 66 (54–76) | 98.69 |
| Gynecological (C51–58) | 9416 | 66 (56–77) | 99.77 |
| Male genital (C60–63) | 21 232 | 70 (64–79) | 2.06 |
| Urinary tract (C64–68) | 14 927 | 73 (64–80) | 31.61 |
| Central nervous system (C69–72) | 3639 | 60 (44–71) | 48.28 |
| Endocrine (C73–75) | 3219 | 58 (45–69) | 66.95 |
| Unspecified (C76–80) | 36 890 | 69 (60–78) | 50.53 |
| Haematological (C81–96) | 16 372 | 69 (58–79) | 48.12 |
| . | Absolute number . | Median age (95% CI) . | % female . |
|---|---|---|---|
| Total study cohort | 8 306 244 | 41 (23–58) | 51.23 |
| Persons with ATE | 77 940 | 76 (65–83) | 47.25 |
| Persons with VTE | 36 551 | 71 (57–81) | 58.17 |
| Persons with cancera | 158 675 | 69 (59–79) | 49.06 |
| Oropharyngeal (C00–14) | 4720 | 63 (55–71) | 28.69 |
| Gastrointestinal (C15–29) | 35 490 | 72 (63–80) | 43.78 |
| Respiratory (C30–39) | 16 827 | 68 (60–76) | 34.17 |
| Bone/cartilage (C40–41) | 1177 | 61 (44–72) | 46.13 |
| Skin (C43–44) | 19 368 | 74 (63–81) | 50.34 |
| Mesothelium/soft tissue (C45–49) | 3897 | 68 (57–78) | 57.22 |
| Breast (C50) | 23 164 | 66 (54–76) | 98.69 |
| Gynecological (C51–58) | 9416 | 66 (56–77) | 99.77 |
| Male genital (C60–63) | 21 232 | 70 (64–79) | 2.06 |
| Urinary tract (C64–68) | 14 927 | 73 (64–80) | 31.61 |
| Central nervous system (C69–72) | 3639 | 60 (44–71) | 48.28 |
| Endocrine (C73–75) | 3219 | 58 (45–69) | 66.95 |
| Unspecified (C76–80) | 36 890 | 69 (60–78) | 50.53 |
| Haematological (C81–96) | 16 372 | 69 (58–79) | 48.12 |
ATE, arterial thromboembolism; CI, confidence interval; VTE, venous thromboembolism.
Multiple cancer diagnoses were possible when data were broken down into separate cancer types.
Age and sex distribution of the study cohort overall and by arterial thromboembolism, venous thromboembolism, and cancer diagnosis code
| . | Absolute number . | Median age (95% CI) . | % female . |
|---|---|---|---|
| Total study cohort | 8 306 244 | 41 (23–58) | 51.23 |
| Persons with ATE | 77 940 | 76 (65–83) | 47.25 |
| Persons with VTE | 36 551 | 71 (57–81) | 58.17 |
| Persons with cancera | 158 675 | 69 (59–79) | 49.06 |
| Oropharyngeal (C00–14) | 4720 | 63 (55–71) | 28.69 |
| Gastrointestinal (C15–29) | 35 490 | 72 (63–80) | 43.78 |
| Respiratory (C30–39) | 16 827 | 68 (60–76) | 34.17 |
| Bone/cartilage (C40–41) | 1177 | 61 (44–72) | 46.13 |
| Skin (C43–44) | 19 368 | 74 (63–81) | 50.34 |
| Mesothelium/soft tissue (C45–49) | 3897 | 68 (57–78) | 57.22 |
| Breast (C50) | 23 164 | 66 (54–76) | 98.69 |
| Gynecological (C51–58) | 9416 | 66 (56–77) | 99.77 |
| Male genital (C60–63) | 21 232 | 70 (64–79) | 2.06 |
| Urinary tract (C64–68) | 14 927 | 73 (64–80) | 31.61 |
| Central nervous system (C69–72) | 3639 | 60 (44–71) | 48.28 |
| Endocrine (C73–75) | 3219 | 58 (45–69) | 66.95 |
| Unspecified (C76–80) | 36 890 | 69 (60–78) | 50.53 |
| Haematological (C81–96) | 16 372 | 69 (58–79) | 48.12 |
| . | Absolute number . | Median age (95% CI) . | % female . |
|---|---|---|---|
| Total study cohort | 8 306 244 | 41 (23–58) | 51.23 |
| Persons with ATE | 77 940 | 76 (65–83) | 47.25 |
| Persons with VTE | 36 551 | 71 (57–81) | 58.17 |
| Persons with cancera | 158 675 | 69 (59–79) | 49.06 |
| Oropharyngeal (C00–14) | 4720 | 63 (55–71) | 28.69 |
| Gastrointestinal (C15–29) | 35 490 | 72 (63–80) | 43.78 |
| Respiratory (C30–39) | 16 827 | 68 (60–76) | 34.17 |
| Bone/cartilage (C40–41) | 1177 | 61 (44–72) | 46.13 |
| Skin (C43–44) | 19 368 | 74 (63–81) | 50.34 |
| Mesothelium/soft tissue (C45–49) | 3897 | 68 (57–78) | 57.22 |
| Breast (C50) | 23 164 | 66 (54–76) | 98.69 |
| Gynecological (C51–58) | 9416 | 66 (56–77) | 99.77 |
| Male genital (C60–63) | 21 232 | 70 (64–79) | 2.06 |
| Urinary tract (C64–68) | 14 927 | 73 (64–80) | 31.61 |
| Central nervous system (C69–72) | 3639 | 60 (44–71) | 48.28 |
| Endocrine (C73–75) | 3219 | 58 (45–69) | 66.95 |
| Unspecified (C76–80) | 36 890 | 69 (60–78) | 50.53 |
| Haematological (C81–96) | 16 372 | 69 (58–79) | 48.12 |
ATE, arterial thromboembolism; CI, confidence interval; VTE, venous thromboembolism.
Multiple cancer diagnoses were possible when data were broken down into separate cancer types.
The proportion of cancer in the total study population, in women, and men was 1.91% (95% CI 1.90–1.92), 1.83% (1.82–1.84), and 2.00% (1.98–2.01), respectively. The cancer proportion increased with age (Supplementary material online, Figure S1). Details of proportions of cancer types are given in Supplementary material online, Table S1.
The proportions of ATE in the total study population, in women, and men were 0.94% (95% CI 0.93–0.94), 0.87% (0.86–0.87), and 1.01% (1.01–1.02), respectively, and increased with age (Supplementary material online, Figure S2 and Supplementary material online, Table S2).
The proportion of VTE in the total study population, in women, and men was 0.44% (95% CI 0.44–0.44), 0.50% (0.49–0.51), and 0.38% (0.37–0.38), respectively. As expected, the proportion of VTE increased with higher age, ranging from 0.01% (0.01–0.01) in subjects ≤12 years to 2.78% (2.73–2.83) in subjects aged 80–90 (Supplementary material online, Figure S3 and Supplementary material online, Table S2).
Relative risk of arterial thromboembolism in persons with vs. without a cancer diagnosis code
Among 158 675 patients with a cancer diagnosis code, 8559 also had a documented ATE diagnosis (RE–RR: 0.05 [95% CI 0.05–0.06]). The corresponding RR for females and males were 0.04 (0.04–0.05) and 0.06 (0.06–0.06), respectively. In comparison, among 8.147 569 persons without a documented cancer diagnosis, 69 381 also had a documented ATE diagnosis (RE proportion: 0.01 [95% CI 0.01–0.01]). The RE proportion for females and males was 0.01 (0.01–0.01) each. Detailed information on the RR of ATE in subjects with and without cancer stratified by age groups is summarized in Supplementary material online, Table S2.
Overall, the RE–RR ratio of ATE in persons with a cancer diagnosis code was 6.88 (95% CI 4.81–9.84, P < 0.001; Figure 1) compared to persons without a cancer diagnosis code, and in the age-stratified analysis the RE–RR increased with younger age. The corresponding RE–RR for women and men were 6.43 (4.24–9.76; Supplementary material online, Figure S4) and 5.81 (4.05–8.35; Supplementary material online, Figure S5), respectively. Compared to patients without cancer or other cancers, ATE proportion was highest in patients with urinary tract malignancies (RE–RR: 7.16 [6.74–7.61]) followed by patients with cancer of the respiratory system (RE–RR: 7.12 [6.73–7.54]) and cancer of the male genitals (RE–RR: 7.03 [6.68–7.41]). Patients with endocrine cancer had the lowest ATE proportion (RE–RR: 2.49 [2.00–3.10]). RR for all cancer types are shown in Figure 2.
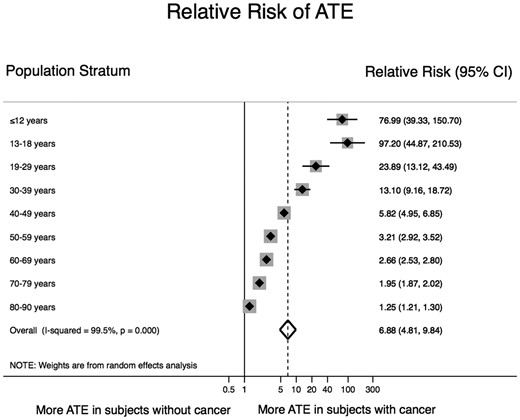
Relative risk of an arterial thromboembolism diagnosis in subjects with and without a cancer diagnosis code. The risk of having an arterial thromboembolism diagnosis code was 7 times higher in patients with a concurrent cancer diagnosis code than in subjects without a cancer diagnosis code. The relative risk of arterial thromboembolism declined with increasing age, due to the increase in arterial thromboembolism proportion with age in subjects without a cancer diagnosis code. The overall pooled risk ratio was estimated with a random-effects model. Individual estimates per age category are depicted as diamonds with 95% confidence intervals as bars. Grey boxes surrounding the diamonds are proportional to the weight of the individual age strata within the overall pooled risk ratio.
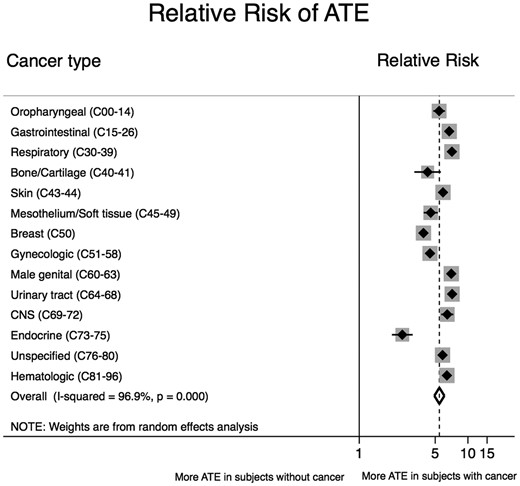
Relative risk of an arterial thromboembolism diagnosis code divided by cancer type. The overall pooled risk ratio was estimated with a random-effects model. Individual estimates per cancer type are depicted as diamonds with 95% confidence intervals as bars. Grey boxes surrounding the diamonds are proportional to the weight of the individual strata within the overall pooled risk ratio.
Relative risk of venous thromboembolism in persons with vs. without a cancer diagnosis code
Overall, 7244 of the 158 675 people with cancer (RE proportion: 0.05 [95% CI 0.04–0.05]) and 29 307 of the 8 147 569 people without cancer (RE proportion: <0.01 [95% CI 0.00–0.00]) had a VTE diagnosis. The RE proportion for female and male subjects with a cancer diagnosis code was 0.05 (0.05–0.05) and 0.04 (0.04–0.04), respectively. The RE proportion for females and males without cancer was <0.01 (0.00–0.00) each. Detailed information regarding the RR of VTE in subjects with and without cancer is given in Supplementary material online, Table S2.
Overall, the RE–RR of VTE in persons with a cancer diagnosis code was 14.91 (95% CI 8.90–24.95, P < 0.001; Figure 3) compared to persons without a cancer diagnosis code, and in the age-stratified analysis the RE–RR increased with younger age. The corresponding RE–RR for women and men was 13.97 (8.28–23.55, Supplementary material online, Figure S6) and 14.60 (8.64–24.68, Supplementary material online, Figure S7), respectively. Compared to patients without cancer or other cancers, the RR of VTE was highest in patients with cancer of the mesothelium/soft tissue (RR: 19.32 [17.41–21.43]) and lowest in patients with oropharyngeal cancer (RR: 6.62 [5.61–7.81]) (Figure 4).
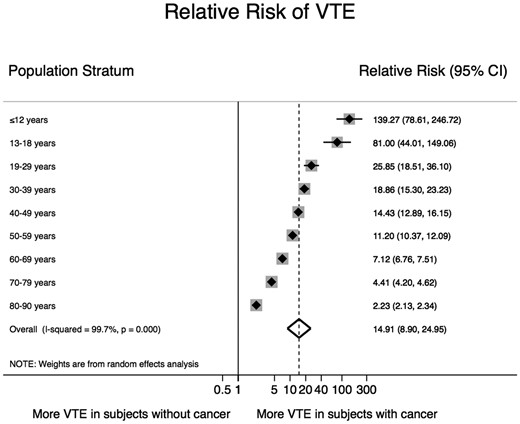
Relative risk of a venous thromboembolism diagnosis code in subjects with and without a cancer diagnosis code. The risk of having a venous thromboembolism diagnosis code was 15 times higher in patients with a concurrent cancer diagnosis code than in subjects without a cancer diagnosis code. The relative risk of venous thromboembolism declined with increasing age, due to the increase in venous thromboembolism proportion with age in subjects without a cancer diagnosis code. The overall pooled risk ratio was estimated with a random-effects model. Individual estimates per age category are depicted as diamonds with 95% confidence intervals as bars. Grey boxes surrounding the diamonds are proportional to the weight of the individual age strata within the overall pooled risk ratio.
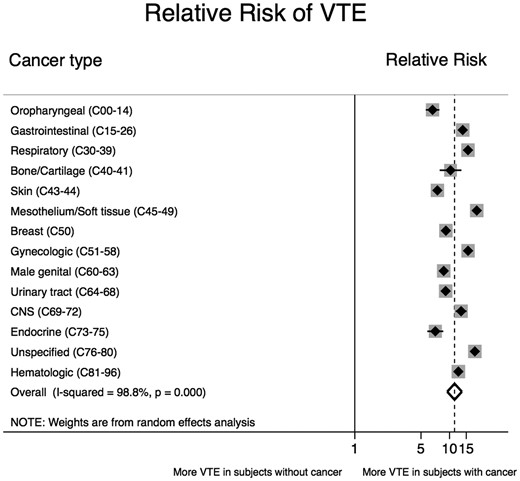
Relative risk of a venous thromboembolism diagnosis code separated by cancer type. The overall pooled risk ratio was estimated with a random-effects model. Individual estimates per cancer type are depicted as diamonds with 95% confidence intervals as bars. Grey boxes surrounding the diamonds are proportional to the weight of the individual strata within the overall pooled risk ratio.
Proportions of patients with cancer of all patients with an arterial thromboembolism or venous thromboembolism diagnosis code
Overall, 8559 of the 77 940 (10.98%; 95% CI 10.76–11.20) patients with an ATE diagnosis code also had a documented cancer diagnosis (Figure 5 and Supplementary material online, Table S3). The RR of cancer in women and men with an ATE diagnosis was 9.42% (9.13–9.73) and 12.38% (12.06–12.70), respectively (Supplementary material online, Figure S8 and Supplementary material online, Table S3). Within the group of patients with ATE, gastrointestinal cancer was most prevalent (2.81%; 2.69–2.93) and bone/cartilage cancer was the least prevalent cancer type (0.06%; 0.04–0.08) (Figure 6 and Supplementary material online, Table S4).
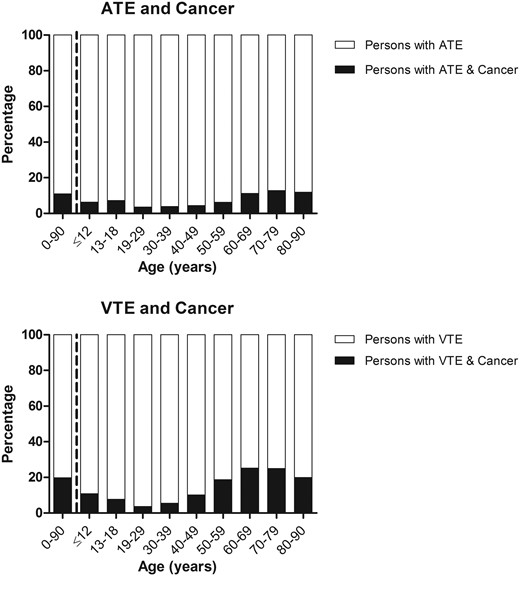
Proportion of cancer diagnosis codes in persons with a diagnosis code for arterial thromboembolism and venous thromboembolism separated by age groups.
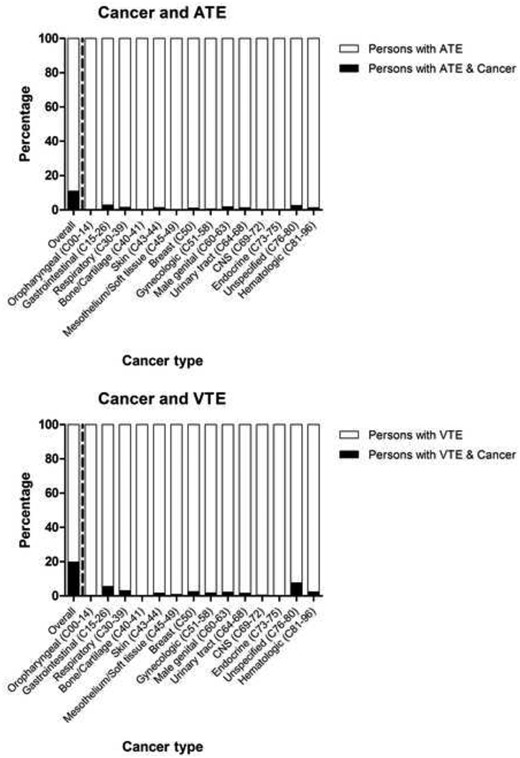
Proportion of cancer diagnosis codes in persons with a diagnosis code for arterial thromboembolism and venous thromboembolism separated by cancer type.
A cancer diagnosis code was present in 7244 of the 36 551 patients with a VTE diagnosis code. Therefore, the proportion of patients with cancer in the group of all patients with VTE was 19.82% (19.41–20.23) (Figure 5 and Supplementary material online, Table S3). The corresponding proportions for women and men were 18.10% (17.58–18.62) and 22.22% (21.56–22.88) (Supplementary material online, Figure S8 and Supplementary material online, Table S3). Within the group of patients with VTE, unspecified cancer was the most common cancer type (7.62%; 7.35–7.90) and bone/cartilage cancer was the least common cancer type (0.15%; 0.11–0.19) (Figure 6 and Supplementary material online, Table S4).
Supplementary material online, Figure S9 and Supplementary material online, Table S4 provide gender-segregated data regarding the proportion of patients with certain cancer types and an ATE or VTE diagnosis code.
Discussion
In this nationwide analysis, we utilized medical claims data to quantify the association between cancer and a history of concurrent ATE or VTE diagnosis. Persons with a cancer diagnosis code were seven times more likely to have an ATE diagnosis code and 15 times more likely to have a VTE diagnosis code compared to persons without a diagnosis of cancer (Graphical Abstract). The RR of both ATE and VTE varied by cancer type. ATE proportion was highest in patients with cancer of the urinary tract, while VTE proportion was highest in patients with cancer of the mesothelium/soft tissue.
We followed two different approaches to clarify the link between cancer and ATE or VTE, respectively. First, we analysed the RR of both ATE and VTE in subjects with a diagnosis code for cancer and found a higher proportion compared to people who did not have a cancer diagnosis. Second, we investigated the proportion of subjects with a cancer code in all people with a diagnosis of ATE or VTE. Of note, 11% with an ATE and 20% with a VTE also had a cancer diagnosis code. Although similar numbers have been repeatedly reported in previous publications and reviews, the evidence from large population-based studies was scarce.6 , 7 , 23 , 24 Here, we provide epidemiological data, which specifically support the assumption that one in five VTE cases are related to cancer.
These population-level meta-data support the concept of a close interrelation between cancer and both ATE and VTE. While the increased risk of VTE in patients with cancer is well documented, data on the risk of ATE are scarce. Recently, Navi et al.25 showed that patients with cancer have a six times higher risk of ATE in the first 30 days after a cancer diagnosis than age, sex, race, and education matched controls. The risk of VTE in patients with cancer has been reported to be 4 to 7 times higher than in persons without cancer, based on older studies.26 , 27 Interestingly, in our study cohort, the RR of VTE was 15 times higher in patients with cancer compared to subjects without cancer. The higher RR could be explained by the fact that our analysis is based on proportion data and not incidence data and it is very likely that some patients developed VTE before the diagnosis of cancer.28
Despite the relatively high prevalence of cancer and thrombosis in the general population, the magnitude of their association has not been reflected in previous studies. Moreover, the majority of recent interventional studies investigating antithrombotic therapy for ATE or VTE have excluded patients with active cancer.29–31 For instance, in phase III trials comparing direct oral anticoagulants with vitamin K antagonists for treatment of acute VTE, the proportion of patients with cancer varies between 2% and 9%, which is much lower than the proportion of patients with VTE and cancer in our present study.32–35 Interestingly, the RIETE registry, where patients with acute VTE are enrolled and prospectively followed, supports our result that 20% of all venous thromboembolic events are related to cancer.36 Active cancer is generally an exclusion criterion for an interventional trial investigating treatments for ATE; therefore, no controlled data exist on the treatment of cancer patients with ATE. Recent cohort studies have demonstrated that the risk of a cancer diagnosis is increased after an arterial event.37 , 38
Some limitations due to the cross-sectional design of the study have to be acknowledged. In contrast to previous studies, which reported incidence, we were only able to estimate RR, which limits direct comparisons.2–7 Due to the nature of our medical claims database, which did not provide the exact dates of diagnosis, we could not identify the temporal connection between the occurrence of cancer and ATE or VTE. This directly extends to the next major limitation, namely our inability to meaningfully include anticoagulant, antithrombotic, and antineoplastic therapy prescriptions into our analysis. A careful analysis of these treatments may have allowed for a more refined estimation of the association between malignancy and thrombosis.
Another important limitation due to the study design is the impossibility to assess the temporal relationship between cancer and thrombosis or to identify the underlying cause of thromboembolism. Furthermore, by using medical claims data, our study has the possibility of incorrect or missed diagnoses.
Nevertheless, our study provides a cross-sectional analysis of the total population and the results are supported by previous studies.6 , 7
Whether interrelation between cancer and ATE/VTE is mainly causally related or confounded by common risk factors is still not entirely understood. Smoking, for example a strong risk factor for both lies on the causal pathway for ATE and most cancer types and might potentially confound the association between cancer and ATE.39 However, the RR of ATE and VTE was highest in younger patients with a cancer diagnosis. This might suggest a direct effect of cancer and/or its treatments (e.g. chemotherapy and radiation) on risk of ATE and VTE. Because the proportion of thrombosis is very low in the general population at a younger age, a higher RR is observed in patients with cancer at a younger age, and the RR decreases with increasing age.40 Furthermore, these patients might be survivors of childhood cancer and we know from prior studies that childhood cancer survivors are at an increased risk of cardiovascular disease compared with age-matched peers, due to long-term effects of cancer therapy.41 Since improved anti-cancer treatments prolong survival of patients with cancer, long-term effects of cancer therapies are becoming more relevant for adults as well. As the number of patients with a history of cancer is expected to further increase, these patients need special follow-up, which has led to the establishment of cardio-oncology clinics. This medical sub-specialty is dedicated to clinical care and research in patients with cancer and cardiovascular diseases.42 The 2016 position paper by Zamorano et al.20 state that the implementation of cardio-oncology teams and regular screening for cardiovascular disease in cancer survivors are useful measures to improve the cardiovascular outcome of cancer patients. In addition to higher morbidity, previous studies have shown that the occurrence of ATE/VTE in patients with cancer increases mortality.6 , 7 , 43 Therefore, it is necessary to further investigate the coincidence of cancer and thrombosis to improve the clinical management of patients with cancer and ATE/VTE. This applies in particular when ATE occurs in patients with cancer, for which there is less awareness than cancer-associated VTE.
In conclusion, the RR of ATE and VTE was substantially higher in all age groups in persons with cancer, whereby the concept of shared risk factors and pathobiology between these diseases is supported. Our population-level meta-data not only describe the burden of VTE in patients with cancer but also support the concept that a diagnosis of cancer is associated with ATE. Further research is needed to better understand the epidemiology of cancer-associated thrombosis and to gain more knowledge on long-term complications of anti-cancer therapies. Finally, it is necessary to increase the awareness of medical professionals about cancer-associated ATE and VTE, as it is becoming more prevalent in an aging population.
Supplementary material
Supplementary material is available at European Heart Journal online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Acknowledgments
We thank the Austrian Science Fund [FWF, Special Research Program (SFB) 5405-B21] for supporting this project.
Authorship contributions
Ella Grilz acquired, analysed, and interpreted data, performed statistical analyses, contributed to the study design, and drafted the manuscript; Florian Posch performed statistical analyses, contributed to the study design and critically revised the manuscript; Stephan Nopp critically revised the manuscript for important intellectual content, and contributed to the study design; Oliver Königsbrügge critically revised the manuscript for important intellectual content, and contributed to the study design; Irene M. Lang critically revised the manuscript for important intellectual content, and contributed to the study design; Peter Klimek acquired data, and critically revised the manuscript for important intellectual content; Stefan Thurner acquired data, and critically revised the manuscript for important intellectual content; Ingrid Pabinger designed and conceived the study, obtained funding, critically revised the manuscript for important intellectual content, and provided administrative support; Cihan Ay designed and supervised the study, obtained funding, critically revised the manuscript for important intellectual content, and provided administrative support; and all authors reviewed and edited the manuscript and finally approved the article.
Data access and responsibility
Ella Grilz and Florian Posch had full access to all the data in the study and take the responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest: E.G., F.P., S.N., O.K., I.M.L., P.K., S.T., and I.P. have no conflict of interest to declare. C.A. received a grant from the Austrian science fund [FWF, Special Research Program (SFB) 5405-B21].
References
Day ISCfWT.
Statistics Austria. Bevölkerung zu Jahres-und Quartalsanfang;



