-
PDF
- Split View
-
Views
-
Cite
Cite
Filippo Crea, Dyslipidaemias in stroke, chronic kidney disease, and aortic stenosis: the new frontiers for cholesterol lowering, European Heart Journal, Volume 42, Issue 22, 7 June 2021, Pages 2137–2140, https://doi.org/10.1093/eurheartj/ehab295
Close - Share Icon Share
 For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
This Focus Issue on dyslipidaemias contains a State of the Art Review article entitled ‘How low is safe? The frontier of very low (<30 mg/dL) LDL cholesterol’ by Angelos Karagiannis from Emory University School of Medicine in Atlanta, GA, USA, and colleagues.1 The authors note that LDL cholesterol (LDL-C) is a proven causative factor for developing atherosclerotic cardiovascular disease (CVD), and its reduction substantially reduces cardiovascular risk.2–4 Individuals with genetic conditions associated with lifelong very low LDL-C levels can be healthy. We now possess the pharmacological armamentarium [statins, ezetimibe, and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors] to reduce LDL-C to an unprecedented extent. Increasing numbers of patients are expected to achieve very low (<30 mg/dL) LDL-C. Cardiovascular event reduction increases log linearly in association with lowering LDL-C, without reaching any clear plateau even when very low LDL-C levels are achieved. It is still controversial whether lower LDL-C levels are associated with significant clinical adverse effects (e.g. new-onset diabetes mellitus and possibly haemorrhagic stroke), and long-term data are needed to address safety concerns. This review presents the familial conditions characterized by very low LDL-C, analyses trials with lipid-lowering agents where patients attained very low LDL-C, and summarizes the benefits and potential adverse effects associated with achieving very low LDL-C. Given the potential for cardiovascular benefit and the short-term safety profile of very low LDL-C, it may be advantageous to attain such low levels in specific high-risk populations. Further studies are needed to compare the net clinical benefit of non-LDL-C-lowering interventions with very low LDL-C approaches, in addition to comparing the efficacy and safety of very low LDL-C levels vs. current recommended targets (Figure 1).
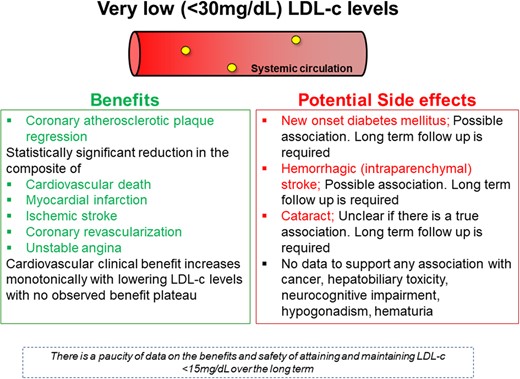
Summary of all the potential benefits and side effects associated with very low LDL-C. (from Karagiannis AD, Mehta A, Dhindsa DS, Virani SS, Orringer CE, Blumenthal RS, Stone NJ, Sperling LS. How low is safe? The frontier of very low (<30 mg/dL) LDL cholesterol. See pages 2154–2169)
Chronic kidney disease (CKD) is associated with high cardiovascular risk.5,6 CKD patients exhibit a specific lipoprotein pattern termed ‘uraemic dyslipidaemia’, which is characterized by rather normal/low LDL-C, low HDL cholesterol (HDL-C), and high triglyceride plasma levels. In a second State of the Art Review article entitled ‘Lipoproteins in chronic kidney disease: from bench to bedside’, Thimoteus Speer from the Saarland University Hospital in Germany, and colleagues provide an update on uraemic dyslipidaemia.7 The authors indicate that several lipoprotein classes are involved in the pathogenesis of CKD-associated CVDs. Uraemia leads to several modifications of the structure of lipoproteins such as changes of the proteome and the lipidome, post-translational protein modifications (e.g. carbamylation), and accumulation of small molecular substances within the lipoprotein moieties, which affect their functionality. Lipoproteins from CKD patients interfere with lipid transport and promote inflammation, oxidative stress, endothelial dysfunction, as well as other features of atherogenesis, thus contributing to the development of CKD-associated CVD (Figure 2). While lipid-modifying therapies play an important role in the management of CKD patients, their efficacy is modulated by kidney function. Novel therapeutic agents to prevent the adverse remodelling of lipoproteins in CKD and to improve their functional properties are highly desirable and partially under development.
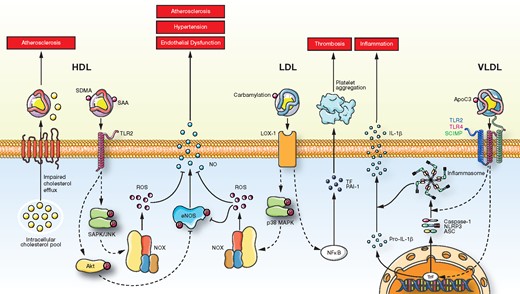
Cellular effects of HDL, LDL, and VLDL from patients with chronic kidney disease. SDMA, symmetric dimethylarginine; SAA, serum amyloid A; SAPK/JNK, protein kinase/Janus kinase; SCIMP, SLP adaptor and CSK interacting membrane protein; SDMA, symmetric dimethylarginine; TF, tissue factor; TLR2, toll-like receptor-2; TLR4, toll-like receptor-4; TrF, transcription factor (from Speer T, Ridker PM, von Eckardstein A, Schunk SJ, Fliser D. Lipoproteins in chronic kidney disease: from bench to bedside. See pages 2170–2185).
Lipoprotein(a) [Lp(a)] is a recognized causal risk factor for atherosclerotic cardiovascular disease.8–10 Its role in acute ischaemic stroke (AIS), however, remains controversial. In a clinical research article entitled ‘Lipoprotein(a) is associated with large artery atherosclerosis stroke aetiology and stroke recurrence among patients below the age of 60 years: results from the BIOSIGNAL study’, Markus Arnold from the University Hospital Zurich in Switzerland, and colleagues evaluated the association of Lp(a) with large artery atherosclerotic (LAA) stroke and risk of recurrent cerebrovascular events in AIS patients.11 For this analysis of the prospective, observational, multicentre BIOSIGNAL cohort study, the authors measured Lp(a) levels in plasma samples of >1700 primarily Caucasian AIS patients, collected within 24 h after symptom onset. Primary outcomes were LAA stroke aetiology and recurrent cerebrovascular events (ischaemic stroke or transient ischaemic attack) within 1 year. Arnold et al. showed that Lp(a) levels were independently associated with LAA stroke aetiology [adjusted odds ratio (adj OR) 1.48, per unit log 10 Lp(a) increase] and identified age as a potent effect modifier of this association (P for interaction = 0.031). The adj OR for LAA stroke in patients aged <60 years was 3.64. The authors did not find a significant association in the whole cohort for 152 recurrent cerebrovascular events. However, Lp(a) levels ≥100 nmol/L were associated with an increased risk for recurrent events among patients who were either <60 years (adj HR 2.40), had evident LAA stroke aetiology (adj HR 2.18), or had no known atrial fibrillation (adj HR 1.60).
The authors conclude that elevated Lp(a) is independently associated with LAA stroke aetiology and risk of recurrent cerebrovascular events among primarily Caucasian individuals aged <60 years. The manuscript is accompanied by an Editorial by Sotirios Tsimikas from the University of California-San Diego in La Jolla, CA, USA.12 The author concludes that this study may provide useful data on whether the frequency of first or recurrent stroke events can be reduced by lowering plasma Lp(a).
Familial hypercholesterolaemia (FH) and elevated Lp(a) are inherited disorders associated with premature atherosclerotic cardiovascular disease (ASCVD).13,14 Aortic valve stenosis (AVS) is the most prevalent valvular heart disease, and LDL-C and Lp(a) may be involved in its pathobiology. In a clinical research article ‘Lipoprotein(a), LDL-cholesterol, and hypertension: predictors of the need for aortic valve replacement in familial hypercholesterolaemia’ Leopoldo Pérez de Isla from the Universidad Complutense in Madrid, Spain, and colleagues investigated the frequency and predictors of severe AVS requiring aortic valve replacement (AVR) in molecularly defined patients with FH.15 The authors analysed the frequency and predictors of the need for AVR due to AVS in this cohort. More than 5000 were enrolled [3712 with FH; 1310 non-affected relatives (NARs)]. Fifty patients with FH (1.48%) and three NARs (0.27%) required AVR (OR 5.71) after a mean follow-up of 7.48 years. Cox regression analysis demonstrated an association between FH and AVR (HR 3.89), with older age, previous ASCVD, hypertension, and elevated Lp(a) being independently predictive of an event.
Pérez de Isla and colleagues conclude that the need for AVR due to AVS is significantly increased in FH patients, particularly in those who are older and have previous ASCVD, hypertension, and elevated Lp(a). Reduction in LDL-C and Lp(a) together with control of hypertension could retard the progression of AVS in FH, but this needs testing in clinical trials. The article is accompanied by an Editorial by Florian Kronenberg from the Medical University of Innsbruck in Austria.16 The author concludes that we will need additional pieces of evidence to come to a definite conclusion considering that the incidence of aortic valve stenosis will markedly increase over the next decades due to increasing life expectancy.
The issue is complemented by two Discussion Forum articles. In a contribution entitled ‘Neutrophil–lymphocyte ratio in the immune checkpoint inhibitors-related atherosclerosis’, Nan Zhang from the Second Hospital of Tianjin Medical University in Tianjin, China, and colleagues comment on the recent contribution entitled ‘The neutrophil–lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials’ by Nicholas H. Adamstein from the Brigham and Women’s Hospital in Boston, MA, USA, and colleagues.17,18 Adamstein et al. respond to this message in a separate contribution.19
The editors hope that readers of this issue of the European Heart Journal will find it of interest.
With thanks to Amelia Meier-Batschelet, Johanna Huggler, and Martin Meyer for help with compilation of this article.



