-
PDF
- Split View
-
Views
-
Cite
Cite
Filippo Crea, The growing role of genetics in the understanding of cardiovascular diseases: towards personalized medicine, European Heart Journal, Volume 42, Issue 20, 21 May 2021, Pages 1929–1933, https://doi.org/10.1093/eurheartj/ehab279
Close - Share Icon Share
With thanks to Amelia Meier-Batschelet, Johanna Huggler, and Martin Meyer for help with compilation of this article.

 For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
This is a Focus Issue on genetics. Described as the ‘single largest unmet need in cardiovascular medicine’, heart failure with preserved ejection fraction (HFpEF) remains an untreatable disease currently representing 65% of new HF diagnoses. HFpEF is more frequent among women and is associated with a poor prognosis and unsustainable healthcare costs.1,2 Moreover, the variability in HFpEF phenotypes amplifies the complexity and difficulties of the approach.3–5 In this perspective, unveiling novel molecular targets is imperative. In a State of the Art Review article entitled ‘Leveraging clinical epigenetics in heart failure with preserved ejection fraction: a call for individualized therapies’, authored by Francesco Paneni from the University of Zurich in Switzerland, and colleagues,6 the authors note that epigenetic modifications—defined as changes of DNA, histones, and non-coding RNAs (ncRNAs)—represent a molecular framework through which the environment modulates gene expression.6 Epigenetic signals acquired over a lifetime lead to chromatin remodelling and affect transcriptional programmes underlying oxidative stress, inflammation, dysmetabolism, and maladaptive left ventricular (LV) remodelling, all conditions predisposing to HFpEF. The strong involvement of epigenetic signalling in this setting makes the epigenetic information relevant for diagnostic and therapeutic purposes in patients with HFpEF. The recent advances in high-throughput sequencing, computational epigenetics, and machine learning have enabled the identification of reliable epigenetic biomarkers in cardiovascular patients. In contrast to genetic tools, epigenetic biomarkers mirror the contribution of environmental cues and lifestyle changes, and their reversible nature offers a promising opportunity to monitor disease states. The growing understanding of chromatin and ncRNA biology has led to the development of several Food and Drug Administration (FDA)-approved ‘epi-drugs’ (chromatin modifiers, mimics, and anti-miRs) able to prevent transcriptional alterations underpinning LV remodelling and HFpEF. In the present review, Paneni and colleagues discuss the importance of clinical epigenetics as a new tool to be employed for a personalized management of HFpEF.
Sick sinus syndrome (SSS) is a complex cardiac arrhythmia and the leading indication for permanent pacemaker implantation worldwide. It is characterized by pathological sinus bradycardia, sinoatrial block, or alternating atrial brady- and tachyarrhythmias. Symptoms include fatigue, reduced exercise capacity, and syncope. Few studies have been conducted on the basic mechanisms of SSS, and therapeutic limitations reflect an incomplete understanding of the pathophysiology.7 In a clinical research entitled ‘Genetic insight into sick sinus syndrome’, Rosa Thorolfsdottir from deCODE genetics in Reykjavik, Iceland, and colleagues aimed to use human genetics to investigate the pathogenesis of SSS and the role of risk factors in its development.8 The authors performed a genome-wide association study (GWAS) of >6000 SSS cases and >1 000 000 controls. Variants at six loci associated with SSS. A full genotypic model best described the p.Gly62Cys association, with an odds ratio (OR) of 1.44 for heterozygotes and a disproportionally large OR of 13.99 for homozygotes. All the SSS variants increased the risk of pacemaker implantation. Their association with atrial fibrillation (AF) varied, and p.Gly62Cys was the only variant not associating with any other arrhythmia or cardiovascular disease. They also tested 17 exposure phenotypes in polygenic score (PGS) and Mendelian randomization analyses. Only two associated with risk of SSS in Mendelian randomization—AF and lower heart rate—suggesting causality. Powerful PGS analyses provided convincing evidence against causal associations for body mass index, cholesterol, triglycerides, and type 2 diabetes (P > 0.05) (Figure 1).
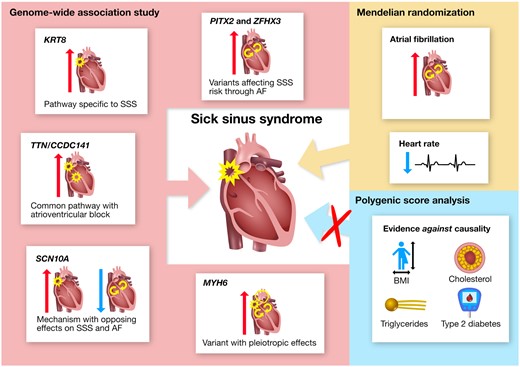
Summary of genetic insight into the pathogenesis of sick sinus syndrome (SSS) and the role of risk factors in its development. Variants at six loci (named by corresponding gene names) were identified through genome-wide association study (GWAS), and their unique phenotypic associations provide insight into distinct pathways underlying SSS. Investigation of the role of risk factors in SSS development supported a causal role for atrial fibrillation (AF) and heart rate, and provided convincing evidence against causality for body mass index (BMI), cholesterol (HDL and non-HDL), triglycerides, and type 2 diabetes (T2D). Mendelian randomization did not support causality for coronary artery disease, ischaemic stroke, heart failure, PR interval, or QRS duration (not shown in the figure). Red and blue arrows represent positive and negative associations, respectively (from Thorolfsdottir RB, Sveinbjornsson G, Aegisdottir HM, Benonisdottir S, Stefansdottir L, Ivarsdottir EV, Halldorsson GH, Sigurdsson JK, Torp-Pedersen C, Weeke PE, Brunak S, Westergaard D, Pedersen OB, Sorensen E, Nielsen KR, Burgdorf KS, Banasik K, Brumpton B, Zhou W, Oddsson A, Tragante V, Hjorleifsson KE, Davidsson OB, Rajamani S, Jonsson S, Torfason B, Valgardsson AS, Thorgeirsson G, Frigge ML, Thorleifsson G, Norddahl GL, Helgadottir A, Gretarsdottir S, Sulem P, Jonsdottir I, Willer CJ, Hveem K, Bundgaard H, Ullum H, Arnar DO, Thorsteinsdottir U, Gudbjartsson DF, Holm H, Stefansson K. Genetic insight into sick sinus syndrome. See pages 1959–1971.).
Thorolfsdottir et al. conclude that they report the associations of variants at six loci with SSS, including a missense variant in KRT8 that confers high risk in homozygotes and points to a mechanism specific to SSS development. Mendelian randomization supports a causal role for AF in the development of SSS. The article is accompanied by an Editorial by Stefan Kääb from LMU Klinikum in Munich, Germany, and colleagues.9 The authors conclude that the limitations of the work challenge clinical translation, but do not diminish the multiple interesting findings of Thorolfsdottir et al., bringing us closer to the finishing line of unlocking SSS genetics to develop new therapeutic strategies. They also highlight that this study represents a considerable accomplishment for the field, but also clearly highlights upcoming challenges and indicates areas where further research is warranted on our way on the translational road to personalized medicine.
Duchenne muscular dystrophy (DMD) is an X-linked genetic disorder that affects ∼1 in every 3500 live-born male infants, making it the most common neuromuscular disease of childhood. The disease is caused by mutations in the dystrophin gene, which lead to dystrophin deficiency in muscle cells, resulting in decreased fibre stability and continued degeneration. The patients present with progressive muscle wasting and loss of muscle function, develop restrictive respiratory failure and dilated cardiomyopathy, and usually die in their late teens or twenties from cardiac or respiratory failure.10 In a clinical research article ‘Association between prophylactic angiotensin-converting enzyme inhibitors and overall survival in Duchenne muscular dystrophy: analysis of registry data’ Raphaël Porcher from the Université de Paris in France, and colleagues estimate the effect of prophylactic angiotensin-converting enzyme (ACE) inhibitors on survival in DMD.11 The authors analysed the data from the French multicentre DMD-Heart-Registry. They estimated the association between the prophylactic prescription of ACE inhibitors and event-free survival in 668 patients between the ages of 8 and 13 years, with normal left ventricular function, using (i) a Cox model with intervention as a time-dependent covariate; (ii) a propensity-based analysis comparing ACE inhibitor treatment vs. no treatment; and (iii) a set of sensitivity analyses. The study outcomes were (i) overall survival and (ii) hospitalizations for HF or acute respiratory failure. Among the patients included in the DMD-Heart-Registry, 576 were eligible for this study, of whom 390 were treated with an ACE inhibitor prophylactically. Death occurred in 53 patients (13.5%) who were and 60 patients (32.3%) who were not treated prophylactically with an ACE inhibitor. In a Cox model, with intervention as a time-dependent variable, the hazard ratio (HR) associated with ACE inhibitor treatment was 0.49 for overall mortality after adjustment for baseline variables. In the propensity-based analysis, with 278 patients included in the treatment group and 302 in the control group, ACE inhibitors were associated with a lower risk of death (HR 0.32) and hospitalization for HF (HR 0.16) (Figure 2). All sensitivity analyses yielded similar results.

Graphical Abstract (from Porcher R, Desguerre I, Amthor H, Chabrol B, Audic F, Rivier F, Isapof A, Tiffreau V, Campana-Salort E, Leturcq F, Tuffery-Giraud S, Ben Yaou R, Annane D, Amédro P, Barnerias C, Bécane HM, Béhin A, Bonnet D, Bassez G, Cossée M, de La Villéon G, Delcourte C, Fayssoil A, Fontaine B, Godart F, Guillaumont S, Jaillette E, Laforêt P, Leonard-Louis S, Lofaso F, Mayer M, Morales RJ, Meune C, Orlikowski D, Ovaert C, Prigent H, Saadi M, Sochala M, Tard C, Vaksmann G, Walther-Louvier U, Eymard B, Stojkovic T, Ravaud P, Duboc D, Wahbi K. Association between prophylactic angiotensin-converting enzyme inhibitors and overall survival in Duchenne muscular dystrophy: analysis of registry data. See pages 1976–1984.).
Porcher et al. conclude that prophylactic treatment with ACE inhibitors in DMD is associated with a significantly higher overall survival and lower rate of hospitalization for management of HF. The manuscript is accompanied by an Editorial by Mariell Jessup and colleagues from the American Heart Association in Dallas, Texas, USA.12 The authors describe how cardioprotective strategies have been investigated in a number of cardiovascular disorders and successfully incorporated into treatment regimens for selected patients, including ACE inhibitors in patients with and without diabetes and coronary artery disease, angiotensin receptor blockers and beta-blockers in Marfan syndrome, and ACE inhibitors and beta-blockers in patients at risk for chemotherapy-related toxicity. They conclude that Porcher et al. have now convincingly demonstrated that even very young patients with DMD can benefit from the life-saving intervention of ACE inhibition.
Hypertrophic cardiomyopathy (HCM) is characterized by unexplained LV hypertrophy and often caused by pathogenic variants in genes that encode the sarcomere apparatus. Patients with HCM may experience atrial and ventricular arrhythmias and HF; however, disease expression and severity are highly variable. Furthermore, there is marked diversity in the age of diagnosis. Although childhood-onset disease is well documented, it is far less common. Owing to its rarity, the natural history of childhood-onset HCM is not well characterized.12–14 In a clinical research article entitled ‘Clinical characteristics and outcomes in childhood-onset hypertrophic cardiomyopathy’, Nicholas Marston from the Harvard Medical School in Boston, MA, USA, and colleagues aimed to describe the characteristics and outcomes of childhood-onset HCM.15 They performed an observational cohort study of >7500 HCM patients. HCM patients were stratified by age at diagnosis [<1 year (infancy), 1–18 years (childhood), >18 years (adulthood)] and assessed for composite endpoints including HF, life-threatening ventricular arrhythmias, AF, and an overall composite that also included stroke and death. Stratifying by age of diagnosis, 2.4% of patients were diagnosed in infancy, 14.7% in childhood, and 2.9% in adulthood. Childhood-onset HCM patients had an ∼2%/year event rate for the overall composite endpoint, with ventricular arrhythmias representing the most common event in the first decade following the baseline visit, and HF and AF more common by the end of the second decade. Sarcomeric HCM was more common in childhood-onset HCM (63%) and carried a worse prognosis than non-sarcomeric disease, including a >2-fold increased risk of HF and 67% increased risk of the overall composite outcome. When compared with adult-onset HCM, those with childhood-onset disease were 36% more likely to develop life-threatening ventricular arrhythmias and twice as likely to require transplant or a ventricular assist device.
The authors conclude that patients with childhood-onset HCM are more likely to have sarcomeric disease, carry a higher risk of life-threatening ventricular arrythmias, and have greater need for advanced HF therapies. The manuscript is accompanied by an Editorial by Juan Pablo Kaski from the University College London (UCL) Institute of Cardiovascular Science in London, UK.16 Kaski concludes that the field of HCM is now entering the era of personalized medicine, with the advent of gene therapy programmes and a focus on treatments targeting the underlying pathophysiology. Pre-clinical data suggesting that small molecule myosin inhibitors may attenuate or even prevent disease expression provide cause for optimism, and nowhere more so than for childhood-onset HCM. An international collaborative approach involving basic, translational, and clinical science is now needed to characterize disease expression and progression and develop novel therapies for childhood HCM.
Dilated cardiomyopathy (DCM) is a heart muscle disease characterized by LV dilatation and systolic dysfunction in the absence of abnormal loading conditions or coronary artery disease. It is a major cause of systolic HF, the leading indication for heart transplantation, and therefore a major public health problem due to the important cardiovascular morbidity and mortality.17,18 Understanding of the genetic basis of DCM has improved in recent years, with a role for both rare and common variants resulting in a complex genetic architecture of the disease. In a translational research article entitled ‘Genome-wide association analysis in dilated cardiomyopathy reveals two new players in systolic heart failure on chromosomes 3p25.1 and 22q11.23’, Sophie Garnier from the Sorbonne Université in Paris, France, and colleagues conducted the largest genome-wide association study performed so far in DCM, with >2500 cases and >4000 controls in the discovery population.19 They identified and replicated two new DCM-associated loci, on chromosome 3p25.1 and chromosome 22q11.23, while confirming two previously identified DCM loci on chromosomes 10 and 1, BAG3 and HSPB7. A PGS constructed from the number of risk alleles at these four DCM loci revealed a 27% increased risk of DCM for individuals with eight risk alleles compared with individuals with five risk alleles (median of the referral population). In silico annotation and functional 4C-sequencing analysis on induced pluripotent stem cell (iPSC)-derived cardiomyocytes identified SLC6A6 as the most likely DCM gene at the 3p25.1 locus. This gene encodes a taurine transporter whose involvement in myocardial dysfunction and DCM is supported by numerous observations in humans and animals. At the 22q11.23 locus, in silico and data mining annotations, and to a lesser extent functional analysis, strongly suggested SMARCB1 as the candidate culprit gene.
Garnier et al. conclude that their study provides a better understanding of the genetic architecture of DCM and sheds light on novel biological pathways underlying HF. The manuscript is accompanied by an Editorial by Elizabeth McNally from the Northwestern University Feinberg School of Medicine in Chicago, USA, and colleagues.20 The authors conclude that methods to integrate common and rare genetic information will continue to evolve and provide insight on disease progression, potentially providing biomarkers and clues for useful therapeutic pathways to guide drug development. At present, rare cardiomyopathy variants have clinical utility in predicting risk, especially arrhythmic risk. PGS analyses for HF or DCM progression are expected to come to clinical use, especially with the addition of broader GWAS-derived data. Combining genetic risk data with clinical and social determinants should help identify those at greatest risk, offering the opportunity for risk reduction.
In a Special Article entitled ‘Influenza vaccination: a ‘shot’ at INVESTing in cardiovascular health’, Scott Solomon from the Brigham and Women’s Hospital, Harvard Medical School in Boston, MA, USA, and colleagues note that the link between viral respiratory infection and non-pulmonary organ-specific injury has become increasingly appreciated during the current coronavirus disease 2019 (COVID-19) pandemic.21 Even prior to the pandemic, however, the association between acute infection with influenza and elevated cardiovascular risk was evident. The recently published results of the NHLBI-funded INVESTED trial, a 5200-patient comparative effectiveness study of high-dose vs. standard-dose influenza vaccine to reduce cardiopulmonary events and mortality in a high-risk cardiovascular population, found no difference between strategies. However, the broader implications of influenza vaccine as a strategy to reduce morbidity in high-risk patients remains extremely important, with randomized control trial and observational data supporting vaccination in high-risk patients with cardiovascular disease. Given a favourable risk–benefit profile and widespread availability at generally low cost, the authors contend that influenza vaccination should remain a centrepiece of cardiovascular risk mitigation and describe the broader context of underutilization of this strategy. Few therapeutics in medicine offer seasonal efficacy from a single administration with generally mild, transient side effects and exceedingly low rates of serious adverse effects. Infection control measures such as physical distancing, hand washing, and the use of masks during the COVID-19 pandemic have already been associated with substantially curtailed incidence of influenza outbreaks across the globe. Appending annual influenza vaccination to these measures represents an important public health and moral imperative.
The issue is complemented by two Discussion Forum articles. In a contribution entitled ‘Management of acute coronary syndromes in patients presenting without persistent ST-segment elevation and coexistent atrial fibrillation’, Paolo Verdecchia from the Hospital S. Maria della Misericordia in Perugia, Italy, and colleagues comment on the recently published contribution ‘2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC)’.22,23 A response to Verdecchia’s comment has been supplied by Collet et al.24
The editors hope that readers of this issue of the European Heart Journal will find it of interest.
References



