-
PDF
- Split View
-
Views
-
Cite
Cite
Andrew H Baker, Mauro Giacca, Antagonism of miRNA in heart failure: first evidence in human, European Heart Journal, Volume 42, Issue 2, 7 January 2021, Pages 189–191, https://doi.org/10.1093/eurheartj/ehaa967
Close - Share Icon Share
This editorial refers to ‘Novel antisense therapy targeting microRNA-132 in patients with heart failure: results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study’†, by T. Thum et al., on page 178.
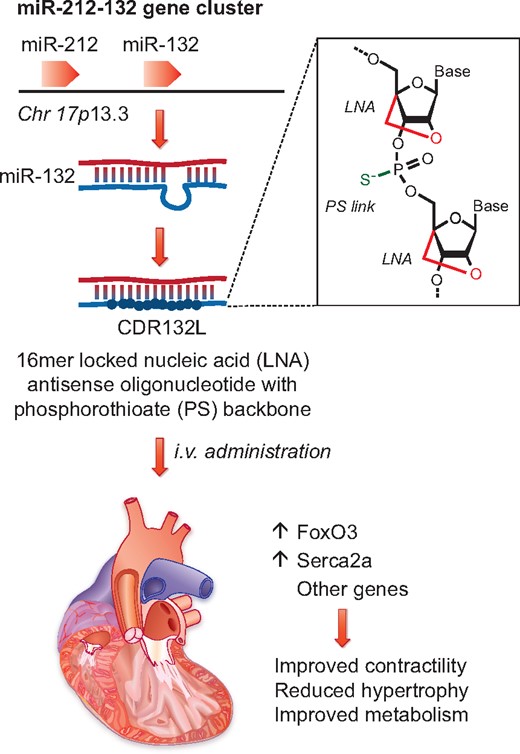
Thum and co-workers6 show that inhibition of miR-132, which is expressed from the miR-212-132 locus on human chromosome 17, results in increased levels of FoxO3 and Serca2a and improved cardiac function. Inhibition is achieved by administering a chemically modified oligonucleotide (CDR132L), which contains locked nucleic acid (LNA) nucleotides and phosphonothioate (PS) linkages to increase in vivo stability.
Heart failure (HF) is frequent, lethal, and expensive.1 In spite of remarkable progress in clinical management of patients and use of devices, the prognosis of this condition remains poor, with mortality rates estimated at 6–7% at 1 year in patients with chronic HF and at ≥25% in those hospitalized with acute HF.2 HF is also tremendously expensive, representing 2–3% of national health expenditures in high-income countries3 and a projection to more than double in the next 20 years as a result of the population ageing.4 Current drug treatment for HF has not evolved significantly recently. Standard of care includes drugs [angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), beta-blockers, mineralocorticoid receptor antagonists, ivabradin, and, more recently, combined ARB–neprilysin inhibitors5] that have been developed based on patho-physiological discoveries dated decades ago. Most notably, all the available drugs are small molecules. At this present time, not a single biological drug (recombinant protein, peptide, nucleic acid, or monoclonal antibody) is available for a disease as prevalent as HF.
It is in this setting that the recent development of novel nucleic acid therapeutics is most welcome news. In this issue of the European Heart Journal, Thum and collaborators report the first-in-human data for an antisense locked nucleic acid (LNA) oligonucleotide (CDR132L) targeting microRNA (miR)-132-3p for HF patients.6 That non-coding nucleic acids, and miRNAs in particular, play a fundamental role in the pathophysiology of HF is a notion that has gained progressive ground over the last decade. Several miRNAs are known to be expressed in a dysregulated manner during disease progression, hence the therapeutic opportunity of manipulating their levels through the design of antisense molecules. An attractive mechanistic consideration for miRNA targeting is the ability of a single intervention targeting one miRNA to influence the expression of multiple genes both within and between important pathways relevant to the disease. This is mediated through the well-known mechanism of action of miRNAs, by which an individual molecule can target multiple mRNAs, inducing their degradation or blocking their translation.
While the original proposal of creating an ‘antisense’ oligonucleotide-based drug to inhibit RNA dates back three decades,7 the field of RNA-targeted therapeutics has now matured significantly, with several RNA-targeting drugs already approved for commercial use and several others in the final phases of the clinical trial pipeline.8,9 This progress was rendered possible by significant improvements in the chemical modification of antisense nucleic acids to increase their pharmacological properties while maintaining Watson–Crick base pairing. In particular, the inclusion of LNA nucleotides in the antisense oligonucleotide increases both stability and thermodynamic strength of duplex formation with complementary target RNAs.10 The efficacy of this approach in the cardiovascular field is also highlighted by recent evidence in humans injected with an LNA antagonist to miR-92a-3p, an agent that is being developed for intervention in cardiovascular disease and wound healing. At doses up to 1.5 mg/kg, this LNA molecule showed dose-dependent decreases in circulating miR-92a levels and target derepression in the peripheral blood compartment. This encouraging study has provided early evidence of target-specific activity following injection of miRNA antagonists into humans.11
The work by Thum and collaborators on miR-132-3p inhibition in HF has progressively built momentum from pre-clinical studies to the first-in-human trial reported in this issue.6 First, the miR-132 stem–loop is part of a cluster (the miR-212/132 family). This stem–loop cluster is responsive to HF cues and its expression is activated in cells and experimental models mimicking HF conditions. Second, two miRNAs from this cluster (i.e. miR-212 and miR-132) both independently regulate similar target genes in the heart, in particular a member of the FoxO gene family (both miRNAs directly target FoxO312), which contributes antihypertrophic signals by regulating calcineurin signalling in cardiomyocytes as well as autophagy.12–14 Third, and the most important finding from a translational context, inhibition of miR-132-3p alone (i.e independent of miR-212) via an antisense oligonucleotide approach is sufficient to block the development of cardiac hypertrophy and transition to heart failure in multiple mouse models.12 With this is at hand, a subsequent expansive large animal study has provided definitive pre-clinical evidence of dosing regimens, safety, and efficacy.12 In this randomized study, which assessed both intravenous and intracoronary delivery, medium and high doses of the miR-132-3p LNA inhibitor (5 mg/kg and 10 mg/kg, respectively) were effective at improving cardiac function and reducing brain natriuretic peptide (BNP) levels, while not affecting evolution of the fibrotic scar or cardiac revascularization. It was previously shown that deletion of the same axis in mice positively affects angiogenesis in vivo,15 thus it is important to note that this mechanism of action is not obviously relevant in the context of revascularization in the heart post-injury.
Now, the same team of investigators report the first-in-human data from this antisense oligonucleotide strategy for HF patients with New York Heart Association (NYHA) class I–III and left ventricular ejection fraction (LVEF) >30% (Take home figure).6 The study design was a prospective, randomized, and placebo-controlled phase 1b dose-escalation study. The dosing regimen involving 28 patients at four dose cohorts and placebo (5:2 design) up to a maximum of 10 mg/kg intravenously 4 weeks apart. The dosing regimen, in general, appeared to be safe and well tolerated. The study drug was readily detected in the plasma of all dosed patients in the absence of drug accumulation. Critically, plasma levels of miR-132-3p were dose-dependently reduced in patients receiving the drug. Based on predictive pharmacokinetic modelling, the team pooled the placebo and low dose groups and three higher dose groups into two pools [non-pharmacodynamic (PD)-active and PD-active, respectively; 27 patients in total]. Using this strategy, a number of efficacy measures were assessed, including NT-proBNP, LVEF and NT-proBNP combined, biomarkers of HF, and cardiac fibrosis, as well as QRS complex narrowing. Although some trends and significance were noted, the study design at this phase 1b stage was clearly underpowered for efficacy measures. What is striking, however, is the encouraging safety, tolerability, and dose-dependent target reduction in the plasma observed, supported by valuable prediction modelling of dosing from porcine efficacy and safety studies. There is indeed great encouragement embedded within this important dataset.
More broadly, this study is also important to the field to guide additional investigations targeting miRNAs and other RNA species using oligonucleotide approaches in cardiovascular patients. The 4-weekly dosing regimen, the chemistry, and the pharmacokinetics all show encouraging signs for use in humans. In summary, this first-in-human study targeting miR-132-3p described here represents a considerable advance in the field of miRNA therapeutics in cardiovascular disease. While it is too early to indicate whether the strategy will be efficacious in human, the safety and feasibility herein described, when combined with the detailed evidencing of efficacy in small and large animal models, provides tremendous encouragement for progression of further studies in patients with HF. The results of the next phase in this exciting journey will be eagerly awaited by the field.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
Footnotes
† doi:10.1093/eurheartj/ehaa898.
Acknowledgements
A.H.B. is supported by the British Heart Foundation programme grant RG/14/3/30706 and Chair of Translational Cardiovascular Sciences CH/11/2/28733, European Research Council Advanced Grant 338991 VASCMIR, and from the European Union’s Horizon 2020 Programme for Research and Innovation (Cardioregenix: 825670). M.G. is supported by the European Research Council (ERC) Advanced Grant 787971 ‘CuRE’, by the British Heart Foundation (BHF) Programme Grant RG/19/11/34633, and the King’s College London BHF Centre of Research Excellence grant RE/18/2/34213; and by grants 825670 ‘CardioReGenix’ and 874764 ‘REANIMA’ from the European Commission Horizon 2020 programme.
Conflicts of interest: none declared.



