-
PDF
- Split View
-
Views
-
Cite
Cite
Ulrich Limper, Jens Tank, Tobias Ahnert, Marc Maegele, Oliver Grottke, Marc Hein, Jens Jordan, The thrombotic risk of spaceflight: has a serious problem been overlooked for more than half of a century?, European Heart Journal, Volume 42, Issue 1, 1 January 2021, Pages 97–100, https://doi.org/10.1093/eurheartj/ehaa359
Close - Share Icon Share
Abstract
The first ever venous thrombotic condition associated with spaceflight, an internal jugular vein thrombus requiring anticoagulation, has recently been reported. Systematic investigation of space travel-associated thrombotic risk has not been conducted. Cellular, animal, and human studies performed in ground-based models and in actual weightlessness revealed influences of weightlessness and gravity on the blood coagulation system. However, human study populations were small and limited to highly selected participants. Evidence in individuals with medical conditions and older persons is lacking. Evidence for thrombotic risk in spaceflight is unsatisfactory. This issue deserves further study in heterogeneous, high risk populations to find prevention strategies and to enable safe governmental and touristic human spaceflight.
Introduction
Half a century ago, the first human being set foot on the surface of the moon. Now, humankind steps up efforts to return to the moon and to go beyond to other celestial bodies. Moreover, the National Aeronautics and Space Administration (NASA) recently announced plans to open the International Space Station (ISS) for touristic space flight. Several companies in the USA are waiting in the wings to offer suborbital flights to paying customers. Previously, space was accessible to few carefully selected and highly trained individuals. In the near future, persons not meeting all these medical and psychological requirements will have the chance to travel to space. In this optimistic atmosphere, potential risks of space travel should not be neglected. Deep leg vein thrombosis following airplane travel, the so-called economy class syndrome, received much attention years ago. Now, a report on internal jugular vein thrombosis in astronauts in space,1 , 2 an unusual thrombotic site on ground,3 has startled the space medical community.
The medical problem
The complex human haemostatic system evolved under terrestrial conditions.4 The system is crucial for rapid bleeding control, immune defence, and wound healing. The human coagulation system comprises the three columns endothelial function, platelet-, leucocyte-, and microparticle-driven cellular clotting, and plasmatic coagulation. Malfunction of one or more of these columns may cause life-threatening medical conditions like spontaneous bleeding, myocardial infarction, stroke, pulmonary embolisms, or immune dysfunction.
Spaceflight causes blood stasis in the upper body
Stasis of blood flow, one of the components of the classical Virchow triad, increases thrombosis risk (Take home figure). During long-term missions to the ISS, ultrasound revealed grossly dilated internal jugular veins in resting astronauts. On average, internal jugular diameter was 9.8 mm2 while seated on Earth and 70.3 mm2 onboard ISS. The finding is unexpected given the substantial reduction of invasively measured central venous pressure in weightlessness.5 However, internal jugular vein pressure and volume increased approximately three- and four-fold in acute weightlessness, respectively.6 , 7 In addition to chronic cranial venous hypervolaemia and hypertension, strenuous physical tasks in weightlessness are likely to produce peaks in venous pressure and wall stress. Veins under pressure respond with remodelling and inflammation of their walls and with endothelial dysfunction.8 Interestingly, venous wall biomechanics at higher transmural pressures show relevant inter-individual differences.9 The venous network of the upper body was not designed to store large amount of blood over a long duration which may result in structural and functional failure6 and increased thrombogenicity. Altered ribcage shape in weightlessness, ribs are more upwards and vertical oriented, may lead to a Paget-Schroetter-like, spaceflight-associated venous obstruction syndrome.10
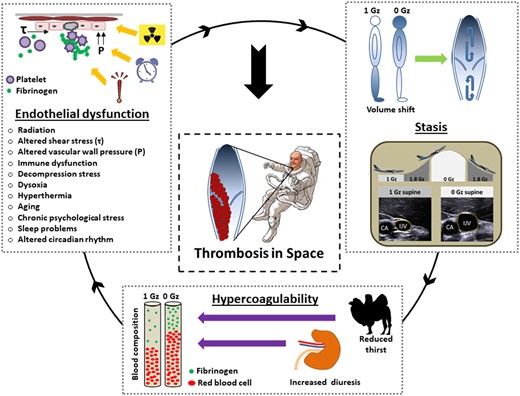
Proposed mechanisms of thrombus formation in space. The space environment is characterized by a unique combination of interacting prothrombotic factors. Several of them fall in more than one of the pathophysiological categories of the Virchow’s triad. CA, carotid artery; Gz, force of weight acting in head-to-foot direction; IJV, internal jugular vein; P, pressure; Τ, shear stress.
Six out of 11 astronauts showed static or reversed blood flow in the right internal jugular vein. In terrestrial medicine, the phenomenon is seen in patients with severe tricuspid valve insufficiency. One astronaut developed clinically silent thrombosis of the internal jugular vein discovered on flight day 50. Following this incidental finding an internal jugular vein mass was retrospectively discovered on the ultrasound images of a second astronaut.2 The first astronaut was anticoagulated according to current ESC guidelines for the management of deep vein thrombosis11 adapted to the unique spaceflight context.1 Possibly, venous congestion of the head and lung embolism, driven by a headward shift of 2000 mL blood and extracellular volume in weightlessness,12 could pose a serious health risk in professional and recreational astronauts. The high impact forces imposed on the returning capsule at ground contact could conceivably dislodge preformed venous thrombi, thus, provoking embolic events.
Blood coagulability and rheology is altered in space
Blood hypercoagulability in space is mainly driven by haemoconcentration (Take home figure). After an initial increase in central blood volume when entering weightlessness, reduced thirst and increased urine output13 lead to intravascular volume contraction. The mechanisms contributes to relative increases in fibrinogen levels and elevated red blood cell and platelet counts.14 While the distribution of red blood cell morphologies in peripheral blood was changed in astronauts on the Skylab space station, in vitro red cell aggregation of blood samples from healthy donors and from patients with cardiovascular disease was lower on the space shuttle compared to ground.15
Spaceflight is associated with triggers of vascular endothelial dysfunction
During spaceflight, astronauts may experience vascular endothelial dysfunction for different reasons (Take home figure). Mild homocysteinaemia, with its potentially toxic effects on the blood clotting cascade and on vascular endothelium, increases thrombo-embolic risk.16 Due to genetic predisposition, some astronauts show altered 1-carbon-metabolism resulting in mild homocysteinaemia in space.17 Radiation induced vascular damage through DNA double strand breaks, oxidative stress, and inflammation18 could become particularly relevant during exploration missions to the moon and Mars.19 Chronic stasis and dilation of the upper body venous network may lead to shedding of the intravascular microlayer, which restrains coagulation at vascular interface.20 Aging individuals are increasingly assigned to commercial and professional spaceflight missions. Aging is associated with alterations of vascular endothelial function21 and an increased thrombotic risk.22 A low-grade chronic inflammatory state due to muscle tissue wasting23 and altered immune function in space,24 accompanied by chronically increased body temperature,25 could also affect the vascular endothelium. Spaceflight specific atmospheric threats, like hypobaric decompression stress during extravehicular activity,26 hyperoxia or hypoxia of future lunar and Martian habitats,27 may lead to endothelial dysfunction. Finally, high levels of psychosocial stress related to confinement,28 lack of natural light,29 chronic time shifting,30 and disturbed sleep31 may perturb the vascular endothelium and jeopardize the vulnerable haemostasic balance.
Given the importance of the haemostatic system for human survival, it is surprising that weightlessness has been overlooked as thrombotic risk factor for over half of a century. Many physiological circuits including cardiovascular, pulmonary, and musculoskeletal systems have been intensively investigated in space and in terrestrial space analogues. However, human blood coagulation and rheology have been somewhat neglected. Nevertheless, the idea that spaceflight could impact human coagulation has long been postulated.
Inflight evidence for thrombotic risk
As far as we know, clinically apparent thromboembolic events have not been described in astronauts. A cranial magnetic resonance imaging (MRI) study in 27 astronauts, designed to detect thrombotic masses of the cerebral and ocular veins did not depict any thrombotic condition after landing.32 Moreover, decades of vascular ultrasound experiments aboard the Russian space station MIR and ISS did not reveal thrombotic structures.6 However, ultrasound is highly operator dependent and particularly challenging under space conditions. Moreover, techniques that are applied to detect thrombi during ultrasound scans, particularly venous compression, have not been routinely applied. Finally, the ultrasound scans on ISS only covered selected venous segments for physiological analysis rather than clinical thrombus detection.
Thus far, only 565 highly selected individuals travelled to space spending overall 150 man-years under spaceflight conditions. This relatively small group may not be sufficient to gauge the incidence of thrombo-embolic events in space. Thus, reliable estimates of spaceflight-associated thrombotic risk cannot be ascertained. For comparison, the risk for thrombotic events on Earth is age-related.22 , 33 In individuals 25–30 years old—the average age of the US astronauts is in their forties—the risk for venous thrombosis is approximately 1:10 000/year.33 In comparison, passengers of long distance airline flights develop symptomatic, or silent venous thrombosis and pulmonary embolism, respectively, on 1 per 4600 flights.34
Strenuous space flight missions have previously been associated with serious cardiovascular events in astronauts pointing towards enhanced thrombus formation and inflammation.35 The first day in space and the return day, which are particularly challenging, are associated with signs of an acute phase stress response, which is known to promote blood coagulation.36–38 These mission phases are, however, dominated by the multiple stresses imposed by launch and landing. Accordingly, space shuttle astronauts showed transient increases in fibrinogen synthesis rate on landing day39 and on the first day in space likely caused by a metabolic stress response.40 Whether this increase represents only a short lasting elevation of fibrinogen concentration or an actual coagulation activation with clinical implications, is unknown. Increased orthostatic stress following return to Earth may have contributed to the response.41 Further spaceflight-associated conditions, like sustained immobilization in the narrow Russian Cheget cosmonaut chair during taxi flights to the ISS could conceivably increase the risk for haemostatic imbalance. Haemoconcentration accompanied by an increased platelet count, which could promote thrombosis, has been documented for long-duration missions aboard ISS.14 Coagulation protein expression, in particular the fibrinogen beta chain, was changed in cosmonauts after long-term spaceflight but recovered within 1 week after return to Earth.42 The majority of US female astronauts take oral contraceptives for cycle suppression during their space mission, which would double their risk of sustaining deep venous thrombosis on Earth. However, this risk calculation may not be simply transferable to their stay in space.43
Evidence from human ground-based models
Bedrest studies in the −6° head-down position are commonly applied as ground-based analogues for weightlessness. The approach models musculoskeletal and cardiovascular decondition, and volume shifts observed in space. Reduced red blood cell deformability and increased aggregation have been reported in such studies.44 Yet, platelet activation was reduced.45 6° head-down bedrest led to an increase in fibrinogen concentration without clinically relevant changes in blood coagulation capacity.46 Thrombelastographic clotting times point rather towards an extenuated coagulation during chronic bedrest47 which is in line with the fact that singular stasis has been questioned as general thrombotic risk factor.48 Though, orthostatic stress during reambulation was associated with a significant shift towards hypercoagulopathy pointing towards an increased risk for thrombotic events after the end of bed rest.49 Increased hydrostatic pressure and shear stress in the maladapted vessels50 of the lower body likely induce endothelial cell-based activation of coagulation.4 In addition to intact haematological cells, gravity-driven effects on cell fragments, so-called microparticles, could affect haemostasis. Circulating endothelial microparticles, a sign of endothelial dysfunction, were increased during dry immersion, which is another ground-based analogue for weightlessness.51
Evidence from animal and cell models
Data from animal and cell models on influences of real or simulated spaceflight related conditions on haemostasis is scarce. The models show a relevant influence of gravity on cellular haemostasis in the sense that hypergravity augments and reduced gravity attenuates blood coagulation.52–55
Conclusions and clinical outlook
Internal jugular vein thrombosis in 2 out of 11 astronauts is unexpected.2 Yet, whether and to what degree spaceflight puts astronauts at an increased thrombotic risk cannot be ascertained. Data from ground-based studies are not conclusive. Given the potential for life-threatening complications and limited access to medical care in space, thrombotic risks associated with spaceflight should be assessed soon to protect the health of professional and recreational astronauts.
As far as we know, systematic testing for hereditary or acquired thrombophilia risk factors is currently neither part of the European astronaut selection processes nor is it yet recommended for commercial space flight. Thus, individuals with risk factors for thrombophilia may fly into space. The validity of thrombophilia screening as part of the commercial astronaut selection should be re-evaluated in the light of the recent thrombotic reports from space. Thrombosis of the jugular internal vein, as recently reported in two astronauts on the ISS, is uncommon on ground but has been reported as the primary representation of thrombophilia. Moreover, strenuous exercise with the upper extremities, unpreventable during life in weightlessness, is a known risk factor for thrombotic events of the upper body so-called effort thrombosis.56 In general, thrombotic events of the upper parts of the body may develop more often in weightlessness compared to ground. In any case, systematic, robust data on global effects of space flight on human haemostasis are still lacking but are now urgently needed. Technical limitations hamper the success of this kind of study. For example, point of care testing techniques like viscoelastic methods (e.g. thrombelastometry, thrombelastography) and evaluation of platelet function (e.g. aggregometry) are technically not feasible in weightlessness. Furthermore coagulation testing is extremely susceptible for error and, both, adequate sample storage in space and safe sample return to Earth are substantial challenges.
Preventive and therapeutic approaches for spaceflight-associated thrombosis have not yet been tested. Mechanistic studies are required to guide such interventions. Lower body negative pressure could serve as countermeasure for venous stasis thereby lowering thrombotic risk in weightlessness. However, severe orthostatic challenges could promote thrombotic risk.4 Whether and how spaceflight-associated thrombosis requires treatment such as therapeutic anticoagulation is unknown. Potential benefits on thrombus formation have to be weighed against increased bleeding risk which is difficult to manage onboard a spacecraft. Manned space exploration and commercial space flight require comprehensive understanding of human haemostasis in space to provide safer surgical procedures, adequate wound healing, and thrombo-embolic prevention, especially in older astronauts and individuals with relevant pre-existing conditions.
Funding
This research received funding from the internal grant program (project IFF 2020-26) of the Faculty of Health at Witten/Herdecke University, Germany.
Conflict of interest: none declared.
References



