-
PDF
- Split View
-
Views
-
Cite
Cite
Mrinal Kanti Das, A Novel Concept of the Sigma (∑) value for BP Surge Differentials: A discussion of the total burden of BP surge differentials presents a new concept for the understanding of hypertension and end organ damage, European Heart Journal, Volume 41, Issue 48, 21 December 2020, Pages 4541–4542, https://doi.org/10.1093/eurheartj/ehaa725
Close - Share Icon Share
Introduction
The chronobiology or 24-h load of blood pressure (BP) in an individual with BP variability including circadian variations is going to cause a paradigm shift in BP management. An array of events revolves around the surge in BP throughout days, months, and years. They significantly influence the mechano-kinetics and dynamics of the heart and also the prognosis for cardiovascular illness. The correct concept and appropriate diagnosis of the variability are essential to guide specific treatment of hypertension (HTN). The scenario is changing fast with different guidelines which are recommending a shift in treatment of HTN from single-dose multiple drug therapy to single-dose multi-drug combination therapy.
Sigma (∑) of the hypertensive episodes and consequent haemodynamic changes
The standard concept is that office BP is checked thrice at a particular time and the average value is the guide for treating or not treating a person for at least 4–6 weeks. Neither the patient nor the physician knows the BP status during that period, until the BP level at the next office visit.1 The 24-h ambulatory BP monitoring (ABPM) has taught us some lessons. The BP plot is never linear throughout 24 h, there is BP variability and in some persons there can be surges of high BP many times a day. In some persons, there may be non-dipping of BP at night, a normal physiological phenomenon, and in extremes, there may be reverse-dipping and a morning surge of BP. Sustained raised BP can be recognized by 24-h ABPM2–4 a tool not only for research but also an accepted technique for multiple BP measurements.
The average BP level by ABPM better predicts major cardiovascular events compared to single office BP checks.5 However, the method is associated with nocturnal discomfort from sleep disturbance and unwanted skin reaction. To overcome these, home BP monitoring (HBPM) was developed and had similar predicatabilty.6
Missing in HBPM is the total burden of different surge values [total burden of BP surge differential (TBBPSD) values] throughout 24 h or an even longer period. The average or mean value focuses on a single value with a wide range of deviation.
What is BP surge differential?
It is the difference (Z) between the index BP recorded with surge (X) and the normal value of BP (Y) or {Index BP (X) − normal BP (Y) = Z}.
Ideally, the S or ∑ value should be zero. However, in real life due to various extraneous stimuli, it becomes more than zero. ‘S’ is an index number of HTN exerting a burden on the heart over a period of specified time. It can be 24 h if monitoring is over 24 h, or it can be in years as per the ability of the recording. The huge number derived is sustained by the individual’s heart if not intervened at the right time and with appropriate drugs, alone or in combination. Initially, there will be a physiological response to the surge in BP, but only to a certain limit. When the stimuli are continuous, the responses result in pathological sequalae.
Why is this Sigma value or S important?
The heart is a unique organ where the ventricular myocardium contracts in unison. It has in addition to the longitudinal and circumferential fibres, a third distribution of fibres arranged obliquely, intermingled with the other fibres. All these fibres acting in different directions contribute to a churning and twisting effect which ensures complete blood ejection from the ventricles. This is important because when various factors come into play acting on the muscle, all fibres take part in the process. Therefore, the in vitro testing of muscle fibre kinetics may not replicate the unified myocardium organ.
Classically, HTN causes increased afterload. To overcome this physiologically, the heart re-adjust itself to a new milieu through the lengthening of muscle fibres. This generates a greater force inside the chamber to eject blood, based on Laplace’s Law and the Frank–Starling mechanism of working with an elastic container filled with viscous fluid.7,8 There are many other factors influencing this process such as the resistance of the peripheral vasculature, the pulmonary vasculature, the skeletal muscles, pericardium, heart rate and rhythm, integrity of different parts of the heart, interplay of the various receptors including baroreceptors, and lastly, the sustainability of BP during the 24 h. Hypertrophy is the mechanism by which the heart increases in size and mass. Growth, pregnancy, and exercise result in a physiological hypertrophy and cause no lasting pathology. Various extraneous stimuli, drugs, or volume changes, trigger beta-adrenergic receptors which in turn aid in phosphorylation or dephosphorylation of myofilament proteins, influencing the excitation–contraction coupling. If the rise in BP is sustained, the signal transduction network is adapted and reprogrammes the gene expression. The various microRNAs, long non-coding RNAs, or circular RNAs actively participate in the process of transcriptional regulation.
Initially, the activated perpetuating pathways act as an adaptive mechanism but later is converted to mal-adaptive mechanism. This may be in the form of cellular hypertrophy either concentric (by increased number of sarcomere in parallel) or eccentric (by increase in sarcomeres in series) initiated by a continuous state of lengthening, cell loss or apoptosis, increased interstitial tissue genesis, causing a stiff heart and high end-diastolic pressure, elastic and contractile failure, leading to remodelling of the heart and vasculature. Similarly, the renin–angiotensin–aldosterone–muscarine pathways acting through huge number of receptors also take part in the adaptive or mal-adaptive process.
Sustained HTN is the sole mechanism for translation to ventricular or atrial hypertrophy, resulting in dilatation and ultimately to heart failure and also sudden death.9
These responses are intricately mediated by external stimuli causing cell-membrane stretching which in turn leads to gene transcription and protein synthesis. In order to reprogramme gene expression, the multitude of signals must reach the intracellular nucleus, a process called transduction. This receptor-mediated signal transduction mostly by protein kinases, protein phosphatase calcineurin also interact amongst themselves, and then post-transduction modulation, protein degradation and generation of transcription factors work almost in a cascade, as a symphony. Via several genes these transcription factors promote gene transcription. Thus, several genes ultimately promote and perpetuate the hypertrophy. The whole process is comparable to reverting back to foetal cellular activity.
The most cited pathway for regulation of hypertrophy is the calcineurin pathway. It is a calcium-calmodulin-activated protein phosphatase 2B(PP2B), which is activated by sustained elevation of intracellular calcium.10
These pathways may therefore be the target for a therapeutic approach especially by inhibiting the various genomes and their pathways (Figure 1).
Therapeutic implication of the ∑ value or S
The ∑ being a huge value of mmHg and many times more than a single value of BP may be responsible for not only cardiac hypertrophy but also vascular remodelling by hypertrophy of the arterial media followed by atherosclerotic changes in the vascular tree such as intima proliferation, macrophage stimulation, plaque formation, calcification, etc. These vascular phenomena may be localized to a region or organ in a pan vascular fashion. The affected organs are together called HTN-mediated target organ damage.11
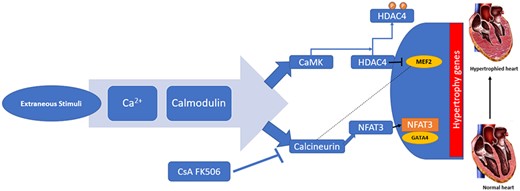
Calcium pathway in hypertrophy. Ca2+, calcium; Calcineurin, also called protein phosphatase 2B (PP2B); CaM, calcium-calmodulin complex; CaMK, CaM-dependent kinases; CsA and FK596, cytotoxic drugs inhibits calcineurin; CsA, cyclosporin A; HDAC, histone deacetylase; MEF, myocyte enhancer factor; NFAT, nuclear factor of activated T-cells. Modified from Passier R, Zeng H, et al. CaM kinase signalling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. J Clin Invest 2000;105(10):1395–1406. doi:10.1172/JCI8551. PMD:10811847, PMCID: PMC315462.
The Sigma (∑) value of BP surge differential values or, as the TBBPSD is based on currently available information. Robust clinical studies in the future can provide more evidence for possible inclusion in international guidelines.
References
References are available as supplementary material at European Heart Journal online.
Conflict of interest: none declared. 



