-
PDF
- Split View
-
Views
-
Cite
Cite
Filippo Crea, The growing population of patients with adult congenital heart disease: novel insight into treatment, participation in competitive sport, and care planning, European Heart Journal, Volume 41, Issue 43, 14 November 2020, Pages 4149–4152, https://doi.org/10.1093/eurheartj/ehaa916
Close - Share Icon Share
 For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
This is a Focus Issue on congenital heart disease (CHD). The population of adults with CHD has risen dramatically over the last 60 years, in large part due to the success of cardiac surgery and paediatric cardiac care. In most western civilizations, >85% of babies born with CHD can now be expected to survive to adulthood. Almost 1 in 100 babies are born with CHD, and the adult population of patients in Europe is estimated at 2.3 million and in the USA at >1 million, both outnumbering the paediatric CHD population.1,2 This leads to unique challenges that the surgical and medical community, together with the patients themselves, face.3,4 Some have largely been overcome, while others remain to be solved. In addition, there are unexpected new challenges which have emerged. This issue addresses some of these challenges regarding treatment, participation in competitive sports, and advance care planning in adults with congenital heart disease (ACHD).
The first contribution is a clinical research article entitled ‘Current use and safety of novel oral anticoagulants in adults with congenital heart disease: results of a nationwide analysis including more than 44 000 patients’ by Gerhard-Paul Diller from the University Hopital Münster in Germany and colleagues.5 Although the use of novel oral anticoagulants (NOACs) is well established in patients with atrial fibrillation and pulmonary thrombo-embolism,6–8 their value in patients with ACHD is still largely unexplored. The authors evaluated the use of NOACs compared with vitamin K antagonists (VKAs) in ACHD patients and assessed the outcome in a nationwide analysis. Using data from one of Germany’s largest health insurers, all ACHD patients treated with VKAs or NOACs were identified and changes in prescription patterns assessed. Furthermore, the association between anticoagulation regimen and complications including mortality was studied. About 44 000 ACHD patients were included. Between 2005 and 2018, the use of oral anticoagulants in those with ACHD increased from 6.3% to 12.4%. Since NOACs became available their utilization has increased continually, accounting for 45% of prescribed anticoagulants in ACHD patients in 2018. ACHD patients on NOACs had higher thrombo-embolic events (3.8% vs. 2.8%), major cardiovascular events (7.8% vs. 6.0%), bleeding rates (11.7% vs. 9.0%), and all-cause mortality (4.0% vs. 2.8%; all P < 0.05) after 1 year of therapy compared with VKAs. After comprehensive adjustment for patient characteristics, NOACs were still associated with increased risk of major cardiovascular events [hazard ratio (HR) 1.22] and increased all-cause mortality (HR 1.43) during long-term follow-up (Figure 1).
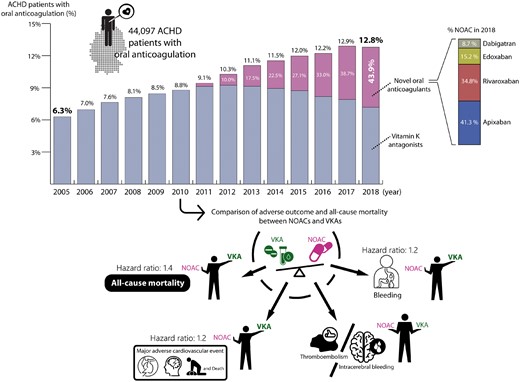
Upper panel: Increased use of (novel) oral anticoagulants in adults with congenital heart disease over time. The figure displays the annual prescription of vitamin K antagonists (VKAs) and novel oral anticoagulants (NOACs) in adults with congenital heart disease (ACHD) patients between 2005 and 2018 covering 521 493 patient-years in a total cohort size of n = 44 097 ACHD patients. The proportion of ACHD patients on oral anticoagulation increased from 6.3% in 2005 to 12.8% in 2018. Vitamin K antagonists were supplemented but also increasingly replaced by novel oral anticoagulants, with the latter accounting for 45% of all oral anticoagulants prescribed in 2018. The numbers over the bars represent the proportion of ACHD patients on oral anticoagulation during the respective year, while the white numbers represent the percentage of anticoagulated patients receiving novel oral anticoagulants. Lower panel: Results of the adjusted multivariable time-dependent Cox regression analysis. The figure illustrates vitamin K antagonists were superior to novel oral anticoagulants regarding all-cause mortality, major adverse cardiovascular events and bleeding, whereas no statistical difference could be established for thromboembolic events (from Freisinger E, Gerβ J, Makowski L, Marschall U, Reinecke H, Baumgartner H, Koeppe J, Diller G-P. Current use and safety of novel oral anticoagulants in adults with congenital heart disease: results of a nationwide analysis including more than 44 000 patients. See pages 4168–4177).
The authors conclude that despite the lack of prospective studies in ACHD patients, NOACs are increasingly replacing VKAs and now account for almost half of all oral anticoagulant prescriptions. In particularly, NOACs were associated with excess long-term risk of major cardiovascular events and mortality in this nationwide analysis, emphasizing the need for prospective studies before solid recommendations for their use in ACHD patients can be provided. The manuscript is accompanied by an Editorial by Frans Van de Werf from KU Leuven in Belgium and colleagues.9 They note that while awaiting the results of controlled studies, it is wise to use VKAs as the standard anticoagulant therapy in ACHD patients and consider NOACs for selected cases after consultation with a multidisciplinary team.
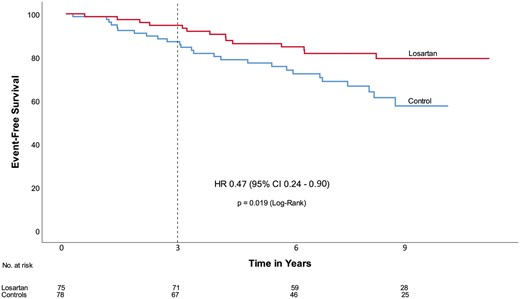
Event free survival. Time = 0 refers to the date of randomization. The dotted line indicates the end of the initial COMPARE trial period. CI, confidence interval; HR, hazard ratio (from van Andel MM, Indrakusuma R, Jalalzadeh H, Balm R, Timmermans J, Scholte AJ, van den Berg MP, Zwinderman AH, Mulder BJM, de Waard V, Groenink M. Long-term clinical outcomes of losartan in patients with Marfan syndrome: follow-up of the multicentre randomized controlled COMPARE trial. See pages 4181–4187).
The COMPARE trial showed a small but significant beneficial effect of 3-year losartan treatment on aortic root dilatation rate in adults with Marfan syndrome (MFS).10 However, no significant effect was found on clinical endpoints, possibly due to a short follow-up period. In a clinical research manuscript entitled ‘Long-term clinical outcomes of losartan in patients with Marfan syndrome: follow-up of the multicentre randomized controlled COMPARE trial’, Mitzi van Andel from the University of Amsterdam in the Netherlands and colleagues investigate the long-term clinical outcomes after losartan treatment.11 In the original COMPARE study (inclusion 2008–2009), 233 adult patients with MFS were randomly allocated to either the angiotensin II receptor blocker losartan on top of regular treatment (beta-blockers in 71% of the patients) or no additional medication. After the COMPARE trial period of 3 years, study subjects chose to continue their losartan medication or not. In a median follow-up period of 8 years, 75 patients continued losartan medication, whereas 78 patients, originally allocated to the control group, never used losartan after inclusion. No differences existed between baseline characteristics of the two groups except for age at inclusion and beta-blocker use (losartan 81%, control 64%). Clinical endpoints, defined as all-cause mortality, aortic dissection/rupture, elective aortic root replacement, reoperation, and vascular graft implantation beyond the aortic root, were compared between the two groups. A per patient composite endpoint was also analysed. Patients who used losartan during the entire follow-up period showed a reduced number of events compared with the control group and exhibited a significantly lower number of deaths (0 vs. 5) and aortic dissections (3 vs. 11). They also experienced a non-significant lower number of elective aortic root replacement (10 vs. 13), reoperation (1 vs. 2), and vascular graft implantation beyond the aortic root (0 vs. 3) (Figure 2). These results remained similar when corrected for age and beta-blocker use in a multivariate analysis.
Van Andel et al. conclude that these results suggest a clinical benefit of combined losartan and beta-blocker treatment in patients with MFS. The manuscript is accompanied by an Editorial by Guillaume Jondeau from the Hôpital Bichat in Paris, France.12 Jondeau and colleagues hope that a forthcoming meta-analysis combining all of the randomized studies already published or unpublished will confirm the early results of this study.
The issue continues with the Special Article ‘Recommendations for participation in competitive sport in adolescent and adult athletes with Congenital Heart Disease (CHD): position statement of the Sport Cardiology & Exercise Section of the European Association of Preventive Cardiology (EAPC), The European Society of Cardiology (ESC) Working Group on Adult Congenital Heart Disease, and the Sports Cardiology, Physical Activity and Prevention Working Group of The Association for European Paediatric and Congenital Cardiology (AEPC)’ by Werner Budts from the Catholic University Leuven in Belgium and colleagues.13 The authors note that improved clinical care has led to an increase in the number of ACHD patients engaging in leisure time and competitive sports activities. Although the benefits of exercise in patients with ACHD are well established, there is a low but appreciable risk of exercise-related complications. Published exercise recommendations for individuals with ACHD are predominantly centred on anatomic lesions, hampering an individualized approach to exercise advice in this heterogeneous population. This document presents an update of the recommendations for competitive sports participation in athletes with cardiovascular disease. It introduces an approach which is based on assessment of haemodynamic, electrophysiological, and functional parameters, rather than anatomical lesions. The recommendations provide a comprehensive assessment algorithm which allows for patient-specific assessment and risk stratification of athletes with ACHD who wish to participate in competitive sports.
Finally, this issue also contains the Special Article ‘Recommendations for advance care planning in adults with congenital heart disease: a position paper from the ESC Working Group of Adult Congenital Heart Disease, the Association of Cardiovascular Nursing and Allied Professions (ACNAP), the European Association for Palliative Care (EAPC), and the International Society for Adult Congenital Heart Disease (ISACHD)’ by Markus Schwerzmann from the University of Bern in Switzerland and colleagues.14 The authors remind us that survival prospects in ACHD, although improved in recent decades, still remain below expectations for the general population. Patients and their loved ones benefit from preparation for both unexpected and predictable deaths, sometimes preceded by a prolonged period of declining health. Hence, advance care planning (ACP) is an integral part of comprehensive care in those with ACHD. This position paper summarizes evidence regarding benefits of and patients’ preferences for ACP and provides practical advice regarding the implementation of ACP processes within clinical ACHD practice. They suggest that ACP be delivered as a structured process across different stages, with content dependent upon the anticipated disease progression. They also acknowledge potential barriers to initiate ACP discussions and emphasize the importance of a sensitive and situation-specific communication style. Conclusions presented in this paper reflect agreed expert opinions, and include both patient and provider perspectives.
The editors hope that this issue of the European Heart Journal will be of interest to its readers.
With thanks to Amelia Meier-Batschelet, Johanna Huggler, and Martin Meyer for help with compilation of this article.




