-
PDF
- Split View
-
Views
-
Cite
Cite
Ricardo Sanz-Ruiz, Francisco Fernández-Avilés, Cardiovascular regenerative and reparative medicine: is myocardial infarction the model?, European Heart Journal, Volume 41, Issue 36, 21 September 2020, Pages 3459–3461, https://doi.org/10.1093/eurheartj/ehaa557
Close - Share Icon Share
This editorial refers to ‘Intracoronary ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): randomized, placebo-controlled, double-blind trial’†, by R.R. Makkar et al., on page 3451.
Very few cardiovascular diseases have experienced more advances that ST-segment elevation myocardial infarction (STEMI). Since the times of thrombolysis and later on primary percutaneous coronary interventions, important breakthroughs have decreased the in-hospital mortality rates of such a devastating condition. However, up to 25% of survivors from STEMI may develop chronic heart failure (CHF), the leading cause of disability and death in developed countries.1 Among others, reparative therapies with stem cell-derived products emerged 20 years ago in an attempt to improve the cardiovascular prognosis of millions of patients worldwide.2
In this issue of the European Heart Journal, Makkar and colleagues report the results of ALLSTAR, a multicentre, randomized, double-blind, placebo-controlled clinical trial to assess the safety and efficacy of intracoronary delivery of cardiosphere-derived cells (CDCs) in patients with STEMI and at high risk of developing CHF.3 A total of 134 patients with reperfused STEMI and large left ventricular (LV) scars as assessed by magnetic resonance imaging (MRI) were randomized 2:1 to receive intracoronary injections of 25 × 109 allogeneic CDCs from six different master cell banks or placebo. The time for infusion ranged from 1 to 12 months (mean 4.6 ± 3.1 months) after the index STEMI. Of note, a small proportion of patients with non-STEMI (NSTEMI) were included, but those patients had to have the same LV ischaemic consequences (scar size ≥15% of total LV mass). Two primary endpoints were defined, a comprehensive one for safety and an MRI-based one for efficacy. Whereas the former was met, with no events at the time of CDC infusion or during the first month, including immunological surveillance, the latter was not. CDCs did not reduce scar size when compared with placebo at 6-month follow-up, and thus the trial was stopped due to the high chance of futility, despite the fact that the primary efficacy endpoint was to be assessed at 12-month follow-up. However, some benefits in terms of LV remodelling and laboratory parameters were observed.
Two years ago, CAREMI was the first randomized clinical trial that demonstrated the safety of allogeneic cardiac stem cells (CSCs) in the acute phase of STEMI, the paradigm of myocardial tissue destruction because of ischaemia.4 ALLSTAR further investigates the role of this type of cells in more advanced phases of the ischaemic insult, once cellular and tissue adaptative mechanisms become established and subsequently definitive (Figure 1). It constitutes an example of a well-designed clinical trial, with rigorous and meaningful selection criteria, a promising stem cell-based product with high-quality manufacturing standards, and appropriate readouts. Indeed, several important conclusions may be drawn from ALLSTAR. First of all, and in accordance with those previous experiences with allogeneic CSCs in STEMI patients,4 the safety of non-autologous donor-derived cellular products can hardly be denied, especially when immunological profiles have been so meticulously monitored. Of note, the very rare cases of allosensitization did not show any immunological adverse event. This is of relevance for the design of future clinical trials in more chronic conditions in which reparative therapies have shown better outcomes (i.e. CHF and refractory angina),5 , 6 given the unquestionable advantages of allogeneic sources.
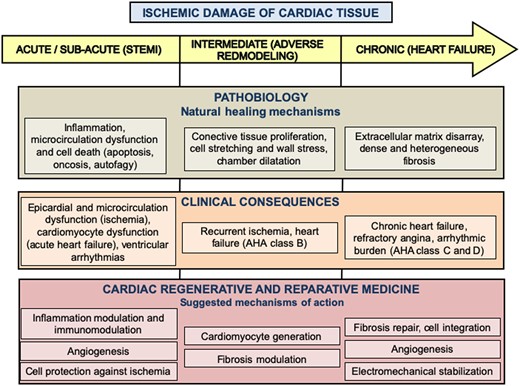
Evolution of myocardial tissue destruction after ischaemic damage as a way to understand left ventricular dysfunction, from the pathobiological and the clinical points of view. The main objectives of regenerative and reparative products according to suggested mechanisms of action are also depicted for each phase. AHA, American Heart Association; STEMI, ST-segment elevation myocardial infarction.
Secondly, STEMI does not seem to be the most favourable pathobiological scenario for reparative therapies. Even with a sound rationale from preclinical and clinical phase I trials,7 a 15% relative reduction in scar size seems to be far away from the eventual effective impact of any currently available biological therapy. The degree of myocardial damage (the loss of billions of cells and extracellular matrix disarray), the adverse inflammatory milieu during the first weeks, and massive fibrotic tissue replacement thereafter make the scar a pretty difficult substrate to modify. We should bear in mind that there are already available mechanical reperfusion and pharmacological therapies which have dramatically improved LV post-infarction performance after decades of research. Interestingly, positive results in terms of LV remodelling and CHF markers (brain natriuretic peptide) were observed, but the real clinical benefit of those cannot be assured due to the small sample size, a major limitation of every phase I and II clinical trial. This evidence on the biological activity of CDCs must be interpreted as exploratory and hypothesis-generating.
Other remarkable insights that can be extracted from ALLSTAR include the pre-defined analysis of two important stratified subgroups. The first one, based on the age of the myocardial infarction (the authors’ Supplementary material), demonstrated no changes in scar size between patients with ‘recent’ (<3 months) compared with ‘chronic’ (>3 months) infarctions. This is relevant because one may think that no matter when the moment of CDC infusion is, the effect on myocardial scarring is equally neutral, and these findings complement those of the previous study with a more acute administration of CSC (5–7 days after the index STEMI),4 which was also negative in terms of infarct size. The second insight refers to the presence or absence of pre-existing donor-specific antibodies in all cell batches in included patients. As it is important that the results of CDCs were the same in both cases, it is outstanding and reassuring that the cells elicited just a mild humoral response with no cellular rejection and with no anaphylactic clinical consequences, something which is also in agreement with previous experiences in humans.4
A critical issue that Makkar and colleagues mention is the surprising discordance between the results of their phase I pivotal trial7 and ALLSTAR. Although the CDC dose and delivery method were the same, the reasons that the authors hypothesize are convincing. Indeed, the source of the cells was different and it is now well known that the performance of autologous cells importantly depends on the health status of the donor and recipient. Also, the timing for administration was much broader in ALLSTAR, and the pathobiological milieu may have been very different. In additions, other confounding factors such as differences in both study populations (CADUCEUS started enrolment in 2009), medications, and treatment strategies, or in the CDC manufacturing and supply protocols may have played a role.
After 20 years of research, we can conclude that there are already stem cell-based products with clinical evidence of benefits, as is the case of CD34+ bone marrow-derived stem cells for patients with refractory angina. Although phase I, II, and III clinical trials8–10 and a meta-analysis have been published6 with demonstrated important symptomatic and mortality rate improvements, these products are surprisingly not being used in clinical practice. In the case of CHF, the results of reparative therapies are promising but less definitive.5 Thus, their incorporation into the standard of care cannot be claimed yet. Finally, in the case of STEMI, and despite the fact that we are still waiting for the final results of BAMI (ClinicalTrials.gov Identifier: NCT01569178),11 it seems clear that no further research is warranted in this particular scenario.
In our opinion, all efforts of the scientific community devoted to biological reparative therapies must be strongly focused nowadays on other aspects of research and not on clinical trials, above all in STEMI patients. Keeping in mind the results of meaningful and well-conducted trials such as ALLSTAR and others,4 maybe it is the time for a thorough reflection and a more cautious approach to future clinical investigations. Although we do not think it is a compulsory requisite, a more complete understanding of the mechanisms of action of reparative products is advisable, potentiating mechanistic studies and eventually improving their results when applied to biological living systems (Figure 1). Basic bench research should also put all effort into the design and manufacturing of more potent reparative effectors (i.e. induced pluripotent stem cells and derived lineages, and tissue engineering solutions). In the field of preclinical research, key points include the application of the same rigorous methodology as clinical trials (i.e. pre-registration and strict regulatory requirements), the development of better large-animal models, and a peer-reviewed publishing policy to increase honesty and scientific quality for the whole field. Once all these objectives are met, more basic and animal studies should be undertaken to assess optimal dosages and timings, the effect of repetitive administrations, and ideally comparative studies with different cell or cell-free approaches. We really think that all these points need to be better addressed before proceeding again to clinical trials, and it is our responsibility to accomplish them if we are ever to definitely move the field of cardiovascular regenerative and reparative medicine forward, for the sake of the cardiovascular health of millions of patients.
Conflict of interest: none declared.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
Footnotes
† doi:10.1093/eurheartj/ehaa541.
References



