-
PDF
- Split View
-
Views
-
Cite
Cite
Francesco Paneni, Sarah Costantino, Nazha Hamdani, Regression of left ventricular hypertrophy with SGLT2 inhibitors, European Heart Journal, Volume 41, Issue 36, 21 September 2020, Pages 3433–3436, https://doi.org/10.1093/eurheartj/ehaa530
Close - Share Icon Share
 Listen to the podcast associated with this article, which can also befound at ESC CardioTalk https://www.escardio.org/The-ESC/Whatwe-do/news/ESC-Cardio-Talk
Listen to the podcast associated with this article, which can also befound at ESC CardioTalk https://www.escardio.org/The-ESC/Whatwe-do/news/ESC-Cardio-Talk
This editorial refers to ‘A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type 2 diabetes: the DAPA-LVH trial’†, by A.J.M. Brown et al., on page 3421.
Patients with type 2 diabetes (T2D) are at risk for both heart failure with reduced (HFrEF) and preserved ejection fraction (HFpEF), and, until recently, no breakthrough therapies were shown to be really effective in this patient population.1 , 2 The advent of sodium–glucose co-transporter-2 (SGLT2) inhibitors has profoundly changed the natural history of diabetic cardiovascular (CV) complications and, more specifically, diabetic HF.3 Four randomized clinical trials, namely the EMPA-REG OUTCOME (empagliflozin), CANVAS (canagliflozin), DECLARE‐TIMI 58, and DAPA-HF (both with dapagliflozin), have unanimously showed a striking effect of SGLT2 inhibitors in reducing HF hospitalizations in T2D patients with established atherosclerotic CV disease (CVD).4 A recent meta-analysis showed that SGLT2 inhibitors reduced the risk of CV death or HF hospitalization by 23% [hazard ratio 0.77, 95% confidence interval (CI) 0.71–0.84, P < 0.0001], with a similar benefit in patients with and without atherosclerotic CVD or HF.5 Since the publication of these trials, many experimental and pre-clinical studies appeared in rapid succession to uncover the mechanisms underpinning the beneficial effects of SGLT2 inhibitors on HF hospitalization and CV mortality. Despite recent work providing key insights in this area, SGLT2-related benefits on HF outcomes and CV mortality are still far from being understood.
Left ventricular hypertrophy (LVH) is a major feature of both HFrEF and HFpEF, although driven by different pathomechanisms.6 LVH is particularly important in HFpEF, where it promotes a concentric LV geometry, pronounced diastolic dysfunction with increased filling pressures, left atrial dilation, and impaired exercise capacity.6 In patients with HFpEF, LVH is associated with hospitalization for HF, CV death, or aborted cardiac arrest, underscoring the role of LVH as a prognostic marker in this setting.7
In the current issue of the European Heart Journal, Brown and associates present a single-centre, double-blind, placebo-controlled trial designed to investigate the effects of the SGLT2 inhibitor dapagliflozin (10 mg once daily) on LVH regression in patients with T2D (DAPA-LVH trial).8 The study included 66 T2D participants [males 57.6%, average body mass index (BMI) 32 ± 4, hypertension 77.3%, ischaemic heart disease 12.1%, stroke 10.6%] with echocardiographic evidence of LVH and no evidence of HF at baseline. The primary endpoint was defined as regression in absolute left ventricular mass (LVM) assessed by cardiac magnetic resonance imaging (cMRI). Secondary endpoints included changes in LVM indexed to body surface area (LVMi), height1 . 8, and height3 . 8. After a mean treatment period of 12 months, dapagliflozin reduced LVM as measured by cMRI in the intention-to-treat (ITT) analy sis (change in LVM: dapagliflozin group –3.95 ± 4.85 g vs. placebo group –1.13 ± 4.55 g; P =0.018), leading to an absolute mean difference of –2.82 g (95% CI –5.13 to –0.51). The reduction in LVM was even greater in the per-protocol population, with an absolute mean difference of –3.20 g (95% CI –5.62 to –0.77). Sensitivity analyses confirmed that the reduction of LVM by dapagliflozin was not affected by baseline characteristics. Moreover, dapagliflozin induced greater LVH regression in those patients with higher LVMi at baseline. The latter finding is in line with a recent subgroup analysis of the EMPA-REG OUTCOME trial, showing that the reduction of CV death, MI, and stroke was greater in patients with LVH than in those without.9 Taken together, the DAPA-LVH trial demonstrates that, as compared with placebo, the addition of dapagliflozin to standard treatment in people with T2D was associated with a significant regression of LVM as assessed by cMRI. These results provide hints to understand SGLT2 inhibitor-related benefits on structural heart disease and HF in people with T2D.
In line with DAPA-LVH, the recent EMPA-HEART Cardiolink-6 trial showed that 6-month treatment with the SGLT2 inhibitor empagliflozin reduced cMRI-detected LVMi in T2D patients with CVD [adjusted difference −3.35 g/m2, 95% CI −5.9 to −0.81 g/m2, P = 0.01].10 Notably, the EMPA-HEART Cardiolink-6 trial recruited patients without LVH at baseline, thus indicating that empagliflozin induces LVM regression even in the absence of hypertrophic remodelling. These early effects of both dapagliflozin and empagliflozin on LV remodelling fit well with the early separation of the Kaplan–Meier curves for CV death and HF hospitalization noted in the respective clinical trials. In contrast to the EMPA-HEART, in the DAPA-LVH trial the effects of dapagliflozin were observed only on LVM indexed to height, height1 . 8, and height3 . 8, but not on LVM indexed to body surface area. This is probably because patients recruited in the DAPA-LVH trial were mainly obese as compared with the EMPA-HEART study (average BMI 32.4 vs. 26.6, respectively). Indeed, LVH defined by LVM/height1 . 8 is more sensitive than LVMi for the quantification of hypertrophy in obesity, and more accurate in predicting CV events in these patients.11
Albeit DAPA-LVH and EMPA-HEART trials have demonstrated potentially important effects of SGLT2 inhibitors on LVH regression, the exact mechanisms underlying the reduction in wall thickness remain to be elucidated. Several factors deserve to be considered in the antihypertrophic role of SGLT2 inhibitors (Figure 1). First, SGLT2 inhibitors significantly reduce blood pressure, and this may contribute to LVH regression by decreasing afterload. In DAPA-LVH, dapagliflozin determined a significant reduction of 24-h ambulatory systolic BP (adjusted difference –3.63 mmHg, P = 0.012) and, most notably, nocturnal systolic BP (adjusted difference –4.38 mmHg, P = 0.017), the latter being a potent predictor of target organ damage.12 The observed changes in ambulatory and nocturnal SBP showed modest correlations with LVM change (r = 0.415, P = 0.001 and r = 0.321, P = 0.012, respectively), thus suggesting that the BP drop might participate in LVM reduction. Similarly, in the EMPA-HEART trial, empagliflozin significantly reduced overall ambulatory systolic BP (adjusted difference −6.8 mmHg, P = 0.003) and diastolic BP (adjusted difference −3.2 mmHg, P = 0.02). However, in the latter study, the change in 24-h ambulatory BP did not correlate with the change in LVM, implying that mechanisms other than BP reduction might be involved. This hypothesis is also supported by the notion that in patients with cardiometabolic alterations, LVM often exceeds the amount needed to compensate haemodynamic load.13
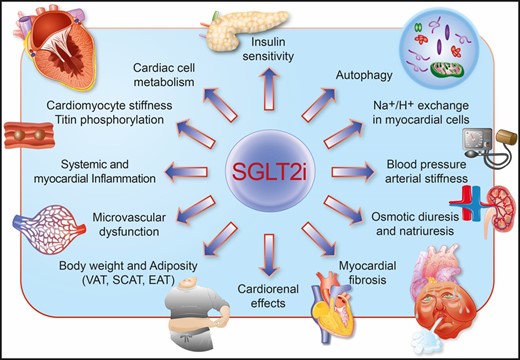
Schematic illustrating the principal mechanisms underlying LVH regression with SGLT2 inhibitors.
Secondly, natriuresis and osmotic diuresis induced by SGLT2 inhibitors reduce plasma volume, as outlined by the increase in haematocrit consistently observed across different studies.4 However, end-diastolic volume was not affected in both DAPA-LVH and EMPA-HEART trials. The same applied to left atrial area, which remained unchanged after a 1 year treatment with dapagliflozin.8 The lack of differences in cardiac volumes cannot even be attributed to insensitive measurements, as cMRI can detect small variations (up to 5 mL) of LV systolic and diastolic volumes. Moreover, the lack of correlation between haematocrit and LVM regression as well as the lack of changes in N-terminal pro brain natriuretic peptide (NT-proBNP) may further suggest a pre-load-independent reduction of LVH by SGLT2 inhibitors.
Thirdly, emerging evidence suggests that a cross-talk between adipose tissue and the heart is a major culprit fostering cardiac structural changes and HF. Visceral (VAT), subcutaneous (SCAT), and epicardial adipose tissue may all contribute to secrete proinflammatory, pro-oxidant factors, as well as gaseous messengers which may exert significant effects on the myocardium, both in a paracrine and an endocrine fashion.14 Adipocyte-derived production of tumour necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) has been shown to promote transcriptional programmes inducing LVH.14 Interestingly, in the DAPA-LVH trial, both VAT and SCAT were clearly reduced after treatment with dapagliflozin, with a strong correlation between VAT reduction and LVM regression. These findings were also associated with improved insulin sensitivity (HOMA-IR) and reduced levels of systemic inflammation, as assessed by high sensitivity C-reactive protein (hsCRP). The amelioration of systemic insulin response might also contribute to the LVH regression observed with dapagliflozin. Further studies investigating how SGLT2 inhibitors impact adipose tissue phenotype (browning) and functionality (secretome) as well as cardiac insulin signalling are greatly anticipated.
Fourthly, SGLT2 inhibitors have also shown direct effects at the level of the myocardium, both on cardiomyocytes and on the microvasculature. For example, empagliflozin has been shown to prevent cardiac microvascular endothelial cell dysfunction by preserving nitric oxide signalling and cardiomyocyte functionality in experimental HFpEF.15 , 16 Indeed, microvascular endothelial dysfunction contributes to elevated passive tension of cardiomyocytes, diastolic dysfunction, and LVH, eventually leading to HFpEF. Of interest, empagliflozin was also found to cause direct pleiotropic effects on the myocardium by improving diastolic stiffness. In myocardial fibres from patients and rats with HFpEF, empagliflozin reduced cardiomyocyte passive stiffness by enhancing phosphorylation levels of myofilament regulatory proteins, namely titin.16 Given the emerging role of titin in the regulation of hypertrophic signalling,17 one may speculate that the observed effects of SGLT2 inhibitors on LVH regression can be partially attributed to this phenomenon. Most importantly, the effects of empagliflozin on diastolic dysfunction are independent of diabetes.16
The above-mentioned effects of SGLT2 inhibitors set the ground for a possible beneficial effect of these drugs in patients with HFpEF, where microvascular dysfunction, cardiomyocyte inflammation, and cardiometabolic alterations take centre stage. In EMPA-HEART and DAPA-LVH trials, SGLT2 inhibitors exerted their beneficial effects in patients with T2D, obesity, hypertension, oxidative stress, and systemic inflammation, all conditions that, when in a cluster, amplify the risk of HFpEF.18 Whether SGLL2 inhibitors exert similar effects in patients without diabetes remains elusive. Ongoing studies will answer this relevant question.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
Footnotes
† doi:10.1093/eurheartj/ehaa419.
Acknowledgements
F.P. is the recipient of a H.H. Sheikh Khalifa bin Hamad Al Thani Foundation Assistant Professorship at the Faculty of Medicine, University of Zurich.
Conflict of interest: none declared.
References



