-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas F Lüscher, Typical and atypical acute coronary syndromes: inflammation, vasoconstriction, and dissection as major mechanisms, European Heart Journal, Volume 41, Issue 23, 14 June 2020, Pages 2135–2139, https://doi.org/10.1093/eurheartj/ehaa533
Close - Share Icon Share
 For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
The mechanisms and triggers of acute coronary syndromes (ACS) are increasingly understood. While initially it was considered mainly a thrombotic event, we now know that plaque rupture1 and endothelial erosion2 are the substrate for initiating intravascular clot formation.3 More recent research has shown that inflammatory mechanisms lead to plaque rupture and endothelial erosion, and eventually clinical events. This experimental concept was confirmed clinically in the Canakinumab Anti-inflammatory Thrombosis Outcomes (CANTOS) Study establishing the role of interleukin-1β (IL-1β).4,5 In their Fast Track ‘Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1β inhibition with canakinumab: further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis’6 Paul Ridker from the Brigham and Women’s Hospital in Boston, USA and colleagues remind us that both interleukin-18 (IL-18), which like IL-1β requires the NLRP3 inflammasome7 for activation, and interleukin-6 (IL-6), a proinflammatory cytokine downstream of IL-1β, may contribute to recurrent events that occur even on canakinumab therapy, and thus represent novel targets for treating atherothrombosis.
The NLRP3 inflammasome, a multicomponent assembly of proteins in the cell, senses a variety of danger signals that yield activation of its constituent enzyme caspase-1. This proteinase cleaves both pro-IL-18 and pro-IL-1β to their active forms. IL-18 or IL-1β can induce the production of copious amounts of IL-6 from a variety of cell types, serving as an amplifier of proinflammatory signalling. IL-6 in turn triggers hepatocytes to alter their programme of protein synthesis to augment production of the acute phase reactants including C-reactive protein. IL-1 can induce its own gene expression, providing another amplification loop. Existing therapeutic agents (in italics) can inhibit many components of this pathway.
In 4848 post-ACS patients of CANTOS, IL-18 and IL-6 were measured. Compared with placebo, canakinumab significantly reduced IL-6 levels in a dose-dependent manner, yielding placebo-subtracted median percentage reductions in IL-6 at 3 months of 25–43% with 50, 150, and 300 mg of canakinumab. In contrast, canakinumab did not change IL-18 levels. Yet, despite this, baseline and on-treatment levels of IL-18 or IL-6 were associated with major adverse cardiac events (MACE). Thus, there remains substantial residual inflammatory risk related to both IL-18 and IL-6 after interleukin-1β inhibition, supporting the development of anti-cytokine therapies for atherothrombosis that simultaneously inhibit IL-1β and IL-18, such as NLRP3 inhibitors, as well as agents that directly target IL-6. These stimulating findings are further discussed in an insightful Editorial by Luigi M. Biasucci from the Policlinico A. Gemelli of the Università Cattolica del S. Cuore in Rome, Italy.8
Besides inflammatory pathways, coronary vasomotion may also contribute to coronary vascular occlusion.9 Indeed, ST-elevation myocardial infarction (STEMI) is associated with high levels of cardiac sympathetic drive and release of the co-transmitter neuropeptide Y.10 In their article entitled ‘The cardiac sympathetic co-transmitter neuropeptide Y is pro-arrhythmic following ST-elevation myocardial infarction despite beta-blockade’, Neil Herring and colleagues from the University of Oxford in the UK hypothesized that despite beta-blockade, neuropeptide Y promotes arrhythmogenesis via ventricular myocyte receptors.11 In 78 patients treated with primary percutaneous coronary intervention (PCI), sustained ventricular tachycardia or fibrillation occurred in 7.7%. These patients showed higher neuropeptide Y levels despite absence of cardiovascular risk factors including late presentation, larger infarct size and beta-blocker usage. RT-qPCR demonstrated neuropeptide Y mRNA in human and rat stellate ganglia. In Langendorff perfused rat hearts, stimulation of stellate ganglia released neuropeptide Y. Despite beta-blockade with metoprolol, optical mapping of ventricular voltage and calcium demonstrated an increase in magnitude and shortening in duration of the calcium transient and a significant lowering of the ventricular fibrillation threshold. This was prevented by the Y1 receptor antagonist BIBO3304. Neuropeptide Y increased ventricular tachycardia and fibrillation by 60% and 10%, respectively during experimental ischaemia and reperfusion, again prevented by BIBO3304. Thus, neuropeptide Y is released during sympathetic stimulation and acts as a novel arrhythmic trigger. Y1 receptor antagonists may represent novel anti-arrhythmics, a conclusion that is put into context in an Editorial by Peter Schwartz from the Istituto Auxologico Italiano Istituto di Ricovero e Cura a Carattere Scientifico in Milan, Italy.12
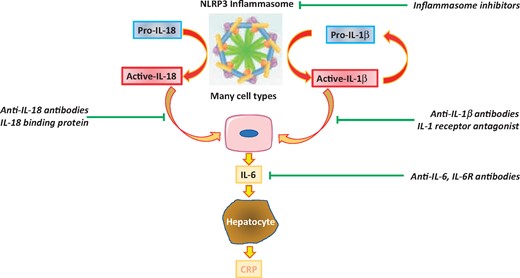
The inflammasome interleukin-18, interleukin-1β, interleukin-6 pathway. The NLRP3 inflammasome, a multicomponent assembly of proteins in the cell, senses a variety of danger signals that yield activation of its constituent enzyme caspase-1. This proteinase cleaves both pro-interleukin-18 and pro-interleukin-1β to their active forms. Interleukin-18 or interleukin-1β can induce the production of copious interleukin-6 from a variety of cell types, serving as an amplifier of pro-inflammatory signalling. Interleukin-6 in turn triggers hepatocytes to alter their programme of protein synthesis to augment production of the acute phase reactants including C-reactive protein. Interleukin-1 can induce its own gene expression providing another amplification loop. Existing therapeutic agents (in italics) can inhibit many components of this pathway and include direct NLRP3 inhibitors as well as antibodies targeting interleukin-1, interleukin-6, and interleukin-18 (from Ridker PM, MacFadyen JG, Thuren T, Libby P, on behalf of the CANTOS Trial Group. Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1β inhibition with canakinumab: further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. See pages 2153–2163).
In both ACS and cancer, inflammatory pathways are involved, as documented by experimental and clinical studies13 and the CANTOS trial.14 Cardiovascular risk is increased in cancer patients.15,16 The interaction between the two are investigated in the article ‘Acute myocardial infarction treatments and outcomes in 6.5 million patients with a current or historical diagnosis of cancer in the USA’17 by Mohamed Mohamed and colleagues from Keele University in Newcastle Under Lyme, UK. They evaluated temporal trends, treatment, and clinical outcomes of patients with ACS and a current or historical diagnosis of cancer. Out of 6 563 255 patients, 186 604 had current cancer and 409 697 a history of cancer, mainly prostate, breast, colon, and lung cancer. Patients with cancer were older with more comorbidities. Of patients without cancer, 43.9% underwent PCI, whilst only 21.0% of those with lung cancer did. Lung cancer was associated with the highest in-hospital mortality, with an odds ratio of 2.71, MACE, cerebrovascular complications, and stroke, while colon cancer had the highest risk of bleeding, with an odds ratio of 2.82. Irrespective of the type of cancer, metastasis was associated with worse in-hospital outcomes, while historical cancer did not affect survival. Thus, concomitant cancer is associated with a conservative medical management strategy for ACS and worse outcomes with survival, and MACE vary with the type of cancer and metastasis status. These findings are further evaluated in an Editorial by Marc Bonaca from the University of Colorado School of Medicine in Aurora, Colorado, USA.18
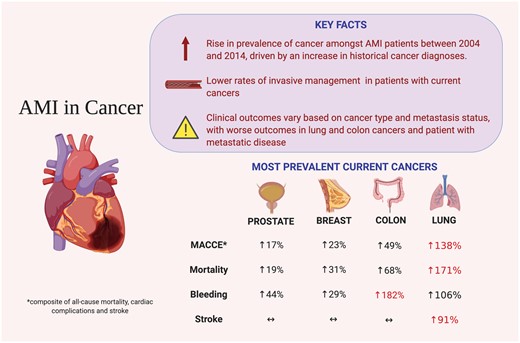
Summary of study findings (from Bharadwaj A, Potts J, Mohamed MO, Parwani P, Swamy P, Lopez-Mattei JC, Rashid M, Kwok CS, Fischman DL, Vassiliou VS, Freeman P, Michos ED, Mamas MA. Acute myocardial infarction treatments and outcomes in 6.5 million patients with a current or historical diagnosis of cancer in the USA. See pages 2183–2193).
The spectrum of ACS has expanded considerably, and now not only involves STEMI and NSTEMI, but also Takotsubo syndrome,19,20 myocarditis, and spontaneous coronary artery dissection or SCAD.21 While outcomes have been characterized recently,22 infarct size has not been determined properly, as outlined in the article ‘Chronic infarct size after spontaneous coronary artery dissection: implications for pathophysiology and clinical management’23 by David Adlam et al. from the University of Leicester in the UK. They assessed myocardial injury and left ventricular (LV) systolic function with cardiac magnetic resonance (CMR) imaging following SCAD and predictors of myocardial injury. Of 158 SCAD survivors, 98% were female and they were compared with 59 mainly female healthy controls. SCAD presented with non-STEMI in 60%, as STEMI in 33%, and as cardiac arrest in 7%. LV function was well preserved, with small reductions in LV ejection fraction of 57% in SCAD and 60% in controls, and small increases in LV end-systolic and diastolic volumes. Infarcts were generally small, averaging 4% of LV mass and 39% having no detectable late gadolinium enhancement. Multivariate modelling showed STEMI at presentation, initial TIMI 0/1 flow, multivessel SCAD, and a Beighton score >4 to be associated with larger infarcts of >10% LV mass. Thus, the majority of SCAD patients have no or small infarctions and preserved LV ejection fraction, which has important implications for their management, as outlined further in an Editorial by Jacqueline Saw from the University of British Columbia in Vancouver, Canada.24
Besides classical type 1 infarction, myocardial injury and infarction may occur in different contexts, as defined in the 4th Universal Definition of Myocardial Infarction.25 However, the clinical application of these definitions has not been evaluated yet. This is addressed in the article ‘Clinical application of the 4th Universal Definition of Myocardial Infarction’ by Johannes Neumann et al. from the University Heart Center Hamburg in Germany in 2302 patients presenting to the emergency department with symptoms suggestive of ACS.26 After re-adjudication, around one-third had to be reclassified. Most of them had acute or chronic myocardial injury. One-fifth were diagnosed with myocardial infarction (MI), compared with 22% when adjudication was based on the 3rd definition.27 In the non-MI population, patients with myocardial injury were older, more often female, and had worse renal function compared with those without myocardial injury. Application of diagnostic algorithms for patients with suspected MI revealed a high accuracy after re-adjudication. Reclassified patients had a substantially higher rate of MACE compared with those not reclassified, particularly those reclassified to the category of myocardial injury. Thus, the categories of acute and chronic myocardial injury in the 4th Universal Definition succeed in identifying patients with higher risk of MACE and poorer outcome, and thus the new definition seems to improve risk assessment.
The issue is complemented by Discussion Forum articles on the subject. In a contribution entitled ‘Is Escherichia coli involved in the myocardial infarction?’, Angelo Zullo and colleagues from the Poliambulatorio Nuovo Regina Margherita in Rome, Italy comment on the recent publication entitled ‘Low-grade endotoxaemia enhances artery thrombus growth via Toll-like receptor 4: implication for myocardial infarction’ by Francesco Violi of Sapienza University in Rome, Italy.28,29 Violi et al. respond in a separate comment.30
In a contribution entitled ‘Conclusions of complete revascularization meta-analysis are challenged by state-of-the-art methods’ Alexander Jobs and colleagues from the Herzzentrum Leipzig Universitatsklinik Klinik für Kardiologie in Germany comment on the recent contribution ‘Complete revascularization reduces cardiovascular death in patients with ST-segment elevation myocardial infarction and multivessel disease: systematic review and meta-analysis of randomized clinical trials’ by Gianluca Campo and colleagues from the Cardiology Unit in Cona, Ferrara, Italy.31,32 Campo et al. respond in a separate comment.33
The editors hope that readers of this issue of the European Heart Journal will find it of interest.
With thanks to Amelia Meier-Batschelet for help with compilation of this article.
References




