-
PDF
- Split View
-
Views
-
Cite
Cite
Francesco Cosentino, Peter J Grant, Victor Aboyans, Clifford J Bailey, Antonio Ceriello, Victoria Delgado, Massimo Federici, Gerasimos Filippatos, Diederick E Grobbee, Tina Birgitte Hansen, Heikki V Huikuri, Isabelle Johansson, Peter Jüni, Maddalena Lettino, Nikolaus Marx, Linda G Mellbin, Carl J Östgren, Bianca Rocca, Marco Roffi, Naveed Sattar, Petar M Seferović, Miguel Sousa-Uva, Paul Valensi, David C Wheeler, ESC Scientific Document Group , 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD), European Heart Journal, Volume 41, Issue 2, 7 January 2020, Pages 255–323, https://doi.org/10.1093/eurheartj/ehz486
Close - Share Icon Share
 Click here to access the corresponding chapter in ESC CardioMed - Section 19 Diabetes mellitus and metabolic syndrome
Click here to access the corresponding chapter in ESC CardioMed - Section 19 Diabetes mellitus and metabolic syndrome
Table of contents
Abbreviations and acronyms 258
1 Preamble 260
2 Introduction 262
3 What is new in the 2019 Guidelines? 263
4 Diagnosis of diabetes and pre-diabetes 265
5 Cardiovascular risk assessment in patients with diabetes and pre-diabetes 266
5.1 Diabetes, pre-diabetes, and cardiovascular risk 266
5.2 Stratification of cardiovascular risk in individuals with diabetes 267
5.3 Stratification of cardiovascular risk in individuals with pre-diabetes 267
5.4 Clinical assessment of cardiovascular damage 267
5.4.1 Biomarkers 267
5.4.2 Electrocardiography 268
5.4.3 Imaging techniques 268
6 Prevention of cardiovascular disease in patients with diabetes and pre-diabetes 271
6.1 Lifestyle 271
6.1.1 Diet 271
6.1.1.1 Carbohydrate 271
6.1.1.2 Fats 271
6.1.1.3 Proteins 271
6.1.1.4 Vegetables, legumes, fruits, and wholegrain cereals 271
6.1.1.5 Alcohol consumption 271
6.1.1.6 Coffee and tea 271
6.1.1.7 Vitamins and macronutrients 271
6.1.2 Physical activity 272
6.1.3 Smoking 272
6.2 Glucose 272
6.2.1 Glycaemic targets 272
6.2.1.1 Additional glucose targets 272
6.2.2 Glucose-lowering agents 273
6.2.3 Special considerations 273
6.2.3.1 Hypoglycaemia 273
6.2.3.2 Glucose monitoring 273
6.3 Blood pressure 274
6.3.1 Treatment targets 274
6.3.2 Management of blood pressure lowering 274
6.3.2.1 Effects of lifestyle intervention and weight loss 274
6.3.2.2 Pharmacological treatments 274
6.3.2.3 Blood pressure changes with glucose-lowering treatments 274
6.4 Lipids 275
6.4.1 Lipid-lowering agents 275
6.4.1.1 Statins 275
6.4.1.2 Ezetimibe 276
6.4.1.3 Proprotein convertase subtilisin/kexin type 9 276
6.4.1.4 Fibrates 276
6.5 Platelets 277
6.5.1 Aspirin 277
6.5.1.1 Primary prevention 278
6.5.1.2 Secondary prevention 278
6.6 Multifactorial approaches 278
6.6.1 Principles of multifactorial management 278
7 Management of coronary artery disease 280
7.1 Medical treatment 280
7.1.1 Effects of intensified glucose control 280
7.1.1.1 UKPDS 280
7.1.1.2 ACCORD, ADVANCE, and VADT 280
7.1.1.3 DIGAMI 1 and 2 280
7.1.2 Glucose-lowering agents: new evidence from cardiovascular outcome trials 281
7.1.2.1 Established oral glucose-lowering drugs 281
7.1.2.2 Newer oral glucose-lowering drugs 281
7.1.2.3 Implications of recent cardiovascular outcome trials 283
7.1.3 Specific cardiovascular therapies 286
7.1.3.1 Beta-blockers 286
7.1.3.2 Blockers of the renin–angiotensin–aldosterone system 286
7.1.3.3 Lipid-lowering drugs 286
7.1.3.4 Nitrates and calcium channel blockers 286
7.1.3.5 Other anti-ischaemic drugs 286
7.1.3.6 Antiplatelet and antithrombotic drugs (see section 6.5) 287
7.2 Revascularization 288
7.2.1 Percutaneous coronary intervention vs. coronary artery bypass graft surgery 288
7.2.2 Adjunctive pharmacotherapy 289
8 Heart failure and diabetes 290
8.1 Prognostic implications of diabetes mellitus in heart failure 291
8.2 Mechanisms of left ventricular dysfunction in diabetes mellitus 291
8.3 Phenotypes of left ventricular dysfunction in diabetes mellitus 292
8.4 Treatment of heart failure in diabetes mellitus 292
8.4.1 Renin−angiotensin−aldosterone system and a neprilysin inhibitors 292
8.4.2 Beta-blockers 292
8.4.3 Ivabradine 292
8.4.4 Digoxin 292
8.4.5 Diuretics 292
8.4.6 Device therapy and surgery 292
8.5 Effect of oral glucose-lowering agents on heart failure 292
8.5.1 Metformin 292
8.5.2 Sulfonylureas 292
8.5.3 Thiazolidinediones 292
8.5.4 Dipeptidyl peptidase-4 inhibitors 292
8.5.5 Glucagon-like peptide-1 receptor agonists 292
8.5.6 Sodium-glucose co-transporter 2 inhibitors 292
9 Arrhythmias: atrial fibrillation, ventricular arrhythmias, and sudden cardiac death 294
9.1 Atrial fibrillation 294
9.1.1 Diabetes and risk of stroke in atrial fibrillation 294
9.2 Ventricular arrhythmias and sudden cardiac death 294
9.2.1 Ventricular premature beats and paroxysmal ventricular tachycardia 294
9.2.2 Sustained ventricular arrhythmias 294
9.2.3 Sudden cardiac death in diabetes 294
10 Aortic and peripheral arterial diseases 295
10.1 Aortic disease 295
10.2 Lower extremity arterial disease 295
10.2.1 Epidemiology and natural history 296
10.2.2 Screening and diagnosis 296
10.2.3 Management of lower extremity artery disease in diabetes 297
10.3 Carotid artery disease 297
11 Chronic kidney disease in diabetes 299
11.1 Management 299
11.1.1 Glycaemic control 299
11.1.2 New approaches to renoprotection 299
12 Patient-centred care 300
12.1 General aspects 300
13 ‘What to do’ and ‘what not to do’ messages from the Guidelines 302
14 Appendix 305
15 References 306
Recommendations
Recommendations for the diagnosis of disorders of glucose metabolism 266
Recommendations for the use of laboratory, electrocardiogram, and imaging testing for cardiovascular risk assessment in asymptomatic patients with diabetes 270
Recommendations for lifestyle modifications for patients with diabetes mellitus and pre-diabetes 272
Recommendations for glycaemic control in individuals with diabetes 273
Recommendations for the management of blood pressure in patients with diabetes and pre-diabetes 275
Recommendations for the management of dyslipidaemia with lipid-lowering drugs 277
Recommendations for the use of antiplatelet therapy in primary prevention in patients with diabetes 278
Recommendations for multifactorial management of patients with diabetes 280
Recommendations for glucose-lowering treatment for patients with diabetes 286
Recommendations for the management of patients with diabetes and acute or chronic coronary syndromes 287
Recommendations for coronary revascularization in patients with diabetes 289
Recommendations for the type of revascularization in patients with diabetes with stable coronary artery disease, suitable coronary anatomy for both procedures, and low predicted surgical mortality 290
Recommendations for the treatment of heart failure in patients with diabetes 293
Recommendations for the treatment of patients with type 2 diabetes to reduce heart failure risk 293
Recommendations for the management of arrhythmias in patients with diabetes 295
Recommendations for the diagnosis and management of peripheral artery disease in patients with diabetes 298
Recommendations for the prevention and management of chronic kidney disease in patients with diabetes 300
Recommendations for patient-centred care of individuals with diabetes 301
List of tables
Table 1 Classes of recommendations 261
Table 2 Levels of evidence . 261
Table 3 What is new in the 2019 Guidelines? 263
Table 4 New recommendations in the 2019 Guidelines 264
Table 5 Revised concepts in the 2019 Guidelines 265
Table 6 Diagnostic criteria for diabetes mellitus and pre-diabetes according to the 2006/2011 World Health Organization and 2019 American Diabetes Association recommendations 266
Table 7 Cardiovascular risk categories in patients with diabetes 268
Table 8 Overview of randomized controlled trials 269
Table 9 Summary of treatment targets for the management of patients with diabetes 279
Table 10 Patient characteristics of cardiovascular safety studies with glucose-lowering agents 284
Table 11 Heart failure phenotypes 291
Table 12 Assessment of the risk of amputation: the Wound, Ischaemia, and foot Infection classification 297
Table 13 Chronic kidney disease classification by estimated glomerular filtration rate and albuminuria . 299
List of figures
Figure 1 Hazard ratios for vascular outcomes in people with vs. without diabetes mellitus at baseline, based on analyses of 530 083 patients 267
Figure 2 Hazard ratios for coronary heart disease by clinically defined categories of baseline fasting blood glucose concentration 268
Figure 3 Treatment algorithm in patients with type 2 diabetes mellitus and atherosclerotic cardiovascular disease, or high/very high CV risk. 285
Figure 4 Recommendations for coronary revascularization. 291
Figure 5 Screening for lower extremity artery disease in patients with diabetes mellitus. 296
Abbreviations and acronyms
- 2hPG
2 h plasma glucose
- ABI
Ankle–brachial index
- ABPM
Ambulatory blood pressure monitoring
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- ACE
Acarbose Cardiovascular Evaluation
- ACEI
Angiotensin-converting enzyme inhibitor
- ACS
Acute coronary syndrome
- ADA
American Diabetes Association
- ADVANCE
Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation
- ADDITION
Anglo-Danish-Dutch Study of Intensive Treatment In People with Screen Detected Diabetes in Primary Care
- ADOPT
A Diabetes Outcome Progression Trial
- AF
Atrial fibrillation
- ARB
Angiotensin receptor blocker
- ART
Arterial Revascularization Trial
- ASCEND
A Study of Cardiovascular Events iN Diabetes
- ASCVD
Atherosclerotic cardiovascular disease
- ATLAS-ACS TIMI 51
Anti-Xa Therapy to Lower cardiovascular events in Addition to Standard therapy in subjects with Acute Coronary Syndromes - Thrombolysis In Myocardial Infarction 51
- BARI 2D
Bypass Angioplasty Revascularization Investigation 2 Diabetes
- BEST
Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients with Multivessel Coronary Artery Disease
- b.i.d.
Twice a day (bis in die)
- BIMA
Bilateral internal mammary artery
- BMS
Bare-metal stent
- BP
Blood pressure
- b.p.m.
Beats per minute
- CABG
Coronary artery bypass graft
- CAC
Coronary artery calcium
- CAD
Coronary artery disease
- CANVAS
Canagliflozin Cardiovascular Assessment Study
- CARDia
Coronary Artery Revascularization in Diabetes
- CARMELINA
Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus
- CAROLINA
Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Patients With Type 2 Diabetes
- CCS
Chronic coronary syndrome
- CE
Cardiac event
- CHA2DS2-VASc
Congestive heart failure, Hypertension, Age ≥75 years (Doubled), Diabetes mellitus, Stroke or transient ischaemic attack (Doubled), Vascular disease, Age 65–74 years, Sex category
- CHARISMA
Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance
- CHARM
Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity
- CHD
Coronary heart disease
- CI
Confidence interval
- CKD
Chronic kidney disease
- CLTI
Chronic limb-threatening ischaemia
- COMPASS
Cardiovascular Outcomes for People Using Anticoagulation Strategies
- CPG
Committee for Practice Guidelines
- CREDENCE
Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation
- CREST
Carotid Revascularization Endarterectomy versus Stenting Trial
- CRT
Cardiac resynchronization therapy
- CRT-D
Cardiac resynchronization therapy with an implantable defibrillator
- CT
Computed tomography
- CTCA
Computed tomography coronary angiography
- CV
Cardiovascular
- CVD
Cardiovascular disease
- CVOT
Cardiovascular outcome trial
- CVRF
Cardiovascular risk factor
- DADDY-D
Does coronary Atherosclerosis Deserve to be Diagnosed earlY in Diabetic patients?
- DAPT
Dual antiplatelet therapy
- DBP
Diastolic blood pressure
- DCCT
Diabetes Control and Complications Trial
- DECLARE- TIMI 58
Dapagliflozin Effect on Cardiovascular Events−Thrombolysis In Myocardial Infarction 58 trial
- DES
Drug-eluting stent
- DEVOTE
Trial Comparing Cardiovascular Safety of Insulin Degludec versus Insulin Glargine in Patients with Type 2 Diabetes at High Risk of cardiovascular Events
- DIAD
Detection of Ischaemia in Asymptomatic Diabetics
- DIGAMI
Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction
- DiRECT
Diabetes Remission Clinical Trial
- DM
Diabetes mellitus
- DPP4
Dipeptidyl peptidase-4
- DYNAMIT
Do You Need to Assess Myocardial Ischemia in Type 2 Diabetes
- EACTS
European Association for Cardio-Thoracic Surgery
- EAS
European Atherosclerosis Society
- EASD
European Association for the Study of Diabetes
- ECG
Electrocardiogram
- EDIC
Epidemiology of Diabetes Interventions and Complications
- EET
Exercise electrocardiogram test
- eGFR
Estimated glomerular filtration rate
- ELIXA
Evaluation of Lixisenatide in Acute Coronary Syndrome
- EMPA-REG OUTCOME
Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients–Removing Excess Glucose
- ESC
European Society of Cardiology
- EXCEL
Evaluation of XIENCE versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization trial
- EXAMINE
Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care
- EXSCEL
Exenatide Study of Cardiovascular Event Lowering
- FACTOR-64
Screening For Asymptomatic Obstructive Coronary Artery Disease Among High-Risk Diabetic Patients Using CT Angiography, Following Core 64
- FIELD
Fenofibrate Intervention and Event Lowering in Diabetes
- FOURIER
Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk
- FPG
Fasting plasma glucose
- FREEDOM
Future Revascularization Evaluation in Patients with Diabetes Mellitus
- GAMI
Glucose Abnormalities in Patients with Myocardial Infarction
- GLP1-RA
Glucagon-like peptide-1 receptor agonist
- Harmony Outcomes
Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease
- HAS-BLED
Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (>65 years), Drugs/alcohol concomitantly
- HbA1c
Haemoglobin A1c
- HEART2D
Hyperglycemia and Its Effect After Acute Myocardial Infarction on Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus
- HDL-C
High-density lipoprotein cholesterol
- HF
Heart failure
- HFmrEF
Heart failure with mid-range ejection fraction
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- HR
Hazard ratio
- hsTnT
High-sensitivity cardiac troponin T
- ICA
Invasive coronary angiography
- ICD
Implantable cardioverter defibrillator
- IFG
Impaired fasting glycaemia
- IGT
Impaired glucose tolerance
- IMPROVE-IT
Improved Reduction of Outcomes: Vytorin Efficacy International Trial
- J-DOIT3
Japan Diabetes Optimal Integrated Treatment Study for 3 Major Risk Factors of Cardiovascular Diseases
- KDIGO
Kidney Disease: Improving Global Outcomes
- LAD
Left anterior descending coronary artery
- LDL-C
Low-density lipoprotein cholesterol
- LEAD
Lower extremity artery disease
- LEADER
Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results
- Look AHEAD
Action for Health in Diabetes
- LV
Left ventricular
- LVEF
Left ventricular ejection fraction
- MACE
Major adverse cardiovascular events
- MACCE
Major adverse cardiovascular and cerebrovascular events
- MI
Myocardial infarction
- MPI
Radionuclide myocardial perfusion imaging
- MRA
Mineralcorticoid receptor antagonist
- NAVIGATOR
Nateglinide And Valsartan in Impaired Glucose Tolerance Outcomes Research
- NOAC
Non-vitamin K antagonist oral anticoagulant
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- o.d.
Once daily (omni die)
- ODYSSEY DM-INSULIN
Efficacy and Safety of Alirocumab in Insulin-treated Individuals with Type 1 or Type 2 Diabetes and High Cardiovascular Risk
- ODYSSEY OUTCOMES
Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab
- OGTT
Oral glucose tolerance test
- ORIGIN
Outcome Reduction With Initial Glargine Intervention
- PAD
Peripheral arterial disease
- PCI
Percutaneous coronary intervention
- PEGASUS- TIMI 54
Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin−Thrombolysis In Myocardial Infarction 54
- PCSK9
Proprotein convertase subtilisin/kexin type 9
- PIONEER 6
A Trial Investigating the Cardiovascular Safety of Oral Semaglutide in Subjects With Type 2 Diabetes
- PREDIMED
Prevención con Dieta Mediterránea
- PROactive
PROspective pioglitAzone Clinical Trial In macroVascular Events
- PVD
Peripheral vascular disease
- RAAS
Renin–angiotensin–aldosterone system
- RCT
Randomized controlled trial
- REDUCE-IT
Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial
- REWIND
Researching Cardiovascular Events With a Weekly Incretin in Diabetes
- SAVOR-TIMI 53
Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus−Thrombolysis In Myocardial Infarction 53
- SBP
Systolic blood pressure
- SE
Stress echocardiography
- SGLT2
Sodium-glucose co-transporter 2
- SIMA
Single internal mammary artery
- SUSTAIN-6
Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes
- SYNTAX
Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery
- T1DM
Type 1 diabetes mellitus
- T2DM
Type 2 diabetes mellitus
- TBI
Toe–brachial index
- TECOS
Trial Evaluating Cardiovascular Outcomes with Sitagliptin
- TOSCA.IT
Thiazolidinediones Or Sulfonylureas and Cardiovascular Accidents Intervention Trial
- TZD
Thiazolidinedione
- UKPDS
United Kingdom Prospective Diabetes Study
- VADT
Veterans Affairs Diabetes Trial
- VKA
Vitamin K antagonist
- VT
Ventricular tachycardia
- WHO
World Health Organization
- WIfI
Wound, Ischaemia, and foot Infection
1 Preamble
Guidelines summarize and evaluate available evidence with the aim of assisting health professionals in proposing the best management strategies for an individual patient with a given condition. Guidelines and their recommendations should facilitate decision making of health professionals in their daily practice. However, the final decisions concerning an individual patient must be made by the responsible health professional(s) in consultation with the patient and caregiver as appropriate.
A great number of guidelines have been issued in recent years by the European Society of Cardiology (ESC) and its partners such as the European Society for the Study of Diabetes (EASD), as well as by other societies and organisations. Because of their impact on clinical practice, quality criteria for the development of guidelines have been established in order to make all decisions transparent to the user. The recommendations for formulating and issuing ESC Guidelines can be found on the ESC website (http://www.escardio.org/Guidelines-&-Education/Clinical-Practice-Guidelines/Guidelines-development/Writing-ESC-Guidelines). The ESC Guidelines represent the official position of the ESC on a given topic and are regularly updated.
The ESC carries out a number of registries which are essential to assess, diagnostic/therapeutic processes, use of resources and adherence to Guidelines. These registries aim at providing a better understanding of medical practice in Europe and around the world, based on data collected during routine clinical practice.
The guidelines are developed together with derivative educational material addressing the cultural and professional needs for cardiologists and allied professionals. Collecting high-quality observational data, at appropriate time interval following the release of ESC Guidelines, will help evaluate the level of implementation of the Guidelines, checking in priority the key end points defined with the ESC Guidelines and Education Committees and Task Force members in charge.
The Members of this Task Force were selected by the ESC and EASD, including representation from relevant ESC sub-specialty groups, in order to represent professionals involved with the medical care of patients with this pathology. Selected experts in the field from both societies undertook a comprehensive review of the published evidence for management of a given condition according to ESC Committee for Practice Guidelines (CPG) policy. A critical evaluation of diagnostic and therapeutic procedures was performed, including assessment of the risk–benefit ratio. The level of evidence and the strength of the recommendation of particular management options were weighed and graded according to predefined scales, as outlined in the tables below.
The experts of the writing and reviewing panels provided declaration of interest forms for all relationships that might be perceived as real or potential sources of conflicts of interest. These forms were compiled into one file and can be found on the ESC website (http://www.escardio.org/guidelines). Any changes in declarations of interest that arise during the writing period were notified to the ESC and EASD Chairpersons and updated. The Task Force received its entire financial support from the ESC and EASD without any involvement from the healthcare industry.
The ESC CPG supervises and coordinates the preparation of new Guidelines. The Committee is also responsible for the endorsement process of these Guidelines. The ESC Guidelines undergo extensive review by the CPG and external experts. After appropriate revisions the Guidelines are approved by all the experts involved in the Task Force. The finalized document is approved by the CPG for publication in the European Heart Journal. The Guidelines were developed after careful consideration of the scientific and medical knowledge and the evidence available at the time of their dating.
The task of developing ESC Guidelines also includes the creation of educational tools and implementation programmes for the recommendations including condensed pocket guideline versions, summary slides, booklets with essential messages, summary cards for non-specialists and an electronic version for digital applications (smartphones, etc.). These versions are abridged and thus, for more detailed information, the user should always access to the full text version of the Guidelines, which is freely available via the ESC website and hosted on the EHJ s’ website. The National Cardiac Societies of the ESC are encouraged to endorse, translate and implement all ESC Guidelines. Implementation programmes are needed because it has been shown that the outcome of disease may be favourably influenced by the thorough application of clinical recommendations.
Health professionals are encouraged to take the Guidelines fully into account when exercising their clinical judgment, as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies. However, the Guidelines do not override in any way whatsoever the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient's health condition and in consultation with that patient or the patient's caregiver where appropriate and/or necessary. It is also the health professional's responsibility to verify the rules and regulations applicable in each country to drugs and devices at the time of prescription.
2 Introduction
This is the third set of Guidelines produced by the ESC in collaboration with the EASD, designed to provide guidance on the management and prevention of cardiovascular (CV) disease (CVD) in subjects with, and at risk of developing, diabetes mellitus (DM). The last Guidelines on this subject were published in the European Heart Journal in 2013. The interval between preparing the previous Guidelines and the current document has been relatively short, but it has been a period in which we have seen an unprecedented increase in the evidence base available for practicing healthcare professionals to refer to in their daily consultations. This has been characterized by the presentation and publication of a number of CV safety trials for type 2 DM (T2DM) treatments, the results of which, to the casual observer, must seem both exciting and bewildering. Exciting, because while all the recent studies have reported CV safety, several have also reported, for the first time, clear evidence of CV benefit. Bewildering, because these trials continue to be dogged by various side effects that dull the clarity of decision-making. It is one of our aims to guide the reader through this important data set.
In other ways, and on a global scale, little has changed. The prevalence of DM worldwide continues to increase, rising to 10% of the population in countries such as China and India, which are now embracing western lifestyles. In 2017, ∼60 million adult Europeans were thought to have T2DM—half undiagnosed—and the effects of this condition on the CV health of the individual and their offspring create further public health challenges that agencies are attempting to address globally.
These massive numbers led to the prediction that >600 million individuals would develop T2DM worldwide by 2045, with around the same number developing pre-DM.1 These figures pose serious questions to developing economies, where the very individuals who support economic growth are those most likely to develop T2DM and to die of premature CVD. Awareness of specific issues associated with age at onset, sex, and race—particularly the effects of T2DM in women (including epigenetics and in utero influences on non-communicable diseases)—remains of major importance, although there is still much work to be done. Finally, the effects of advancing age and comorbidities indicate the need to manage risk in an individualized manner, empowering the patient to take a major role in the management of his or her condition.
The emphasis in these Guidelines is to provide information on the current state of the art in how to prevent and manage the effects of DM on the heart and vasculature. Our aim has been to focus mostly on the new information made available over the past 5–6 years, and to develop a shorter, concise document to this end. The need for more detailed analysis of specific issues discussed in the present Guidelines may be met by referring to the plethora of specialist Guidelines from organizations such as the ESC and the American Diabetes Association (ADA).
It has been a privilege for us to have been trusted with the opportunity to guide the development of these Guidelines and to work alongside acknowledged experts in this field. We want to extend our thanks to all members of the Task Force who gave freely of their time and expertise, to the referees who contributed a great deal to the final manuscript, and to the ESC and EASD committees that oversaw this project. Finally, we express our thanks to the Guidelines team at the European Heart House, in particular Veronica Dean, Nathalie Cameron, Catherine Despres, and Laetitia Flouret for their support in making this process run smoothly.
Francesco Cosentino and Peter J. Grant
3 What is new in the 2019 Guidelines?
 |
 |
AF = atrial fibrillation; BMS = bare-metal stent; BP = blood pressure; CABG = coronary artery bypass graft; CAD = coronary artery disease; CV = cardiovascular; CVD = cardiovascular disease; DBP = diastolic blood pressure; DES = drug-eluting stent; DM = diabetes mellitus; EACTS = European Association for Cardio-Thoracic Surgery; ESC = European Society of Cardiology; LAD = left anterior descending coronary artery; LDL-C = low-density lipoprotein cholesterol; NOAC = non-vitamin K antagonist oral anticoagulant; PCI = percutaneous coronary intervention; SBP = systolic blood pressure; SYNTAX = Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery; T2DM = type 2 diabetes mellitus; VKA = vitamin K antagonist.
 |
 |
AF = atrial fibrillation; BMS = bare-metal stent; BP = blood pressure; CABG = coronary artery bypass graft; CAD = coronary artery disease; CV = cardiovascular; CVD = cardiovascular disease; DBP = diastolic blood pressure; DES = drug-eluting stent; DM = diabetes mellitus; EACTS = European Association for Cardio-Thoracic Surgery; ESC = European Society of Cardiology; LAD = left anterior descending coronary artery; LDL-C = low-density lipoprotein cholesterol; NOAC = non-vitamin K antagonist oral anticoagulant; PCI = percutaneous coronary intervention; SBP = systolic blood pressure; SYNTAX = Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery; T2DM = type 2 diabetes mellitus; VKA = vitamin K antagonist.
 |
 |
 |
 |
ABI = ankle–brachial index; ABPM = ambulatory blood pressure monitoring; ACEI = angiotensin-converting enzyme inhibitor; b.i.d. = twice daily (bis in die); b.p.m. = beats per minute; CABG = coronary artery bypass graft; CAC = coronary artery calcium; CAD = coronary artery disease; CKD = chronic kidney disease; CRT = cardiac resynchronization therapy; CRT-D = cardiac resynchronization therapy with an implantable defibrillator; CT = computed tomography; CV = cardiovascular; CVD = cardiovascular disease; DAPT = dual antiplatelet therapy; DM = diabetes mellitus; DPP4 = dipeptidyl peptidase-4; ECG = electrocardiogram; eGFR = estimated glomerular filtration rate; GLP1-RA = glucagon-like peptide-1 receptor agonist; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter defibrillator; LEAD = lower extremity artery disease; MRA = mineralocorticoid receptor agonist; o.d. = once daily (omni die); PAD = peripheral arterial disease; PCSK9 = proprotein convertase subtilisin/kexin type 9; RAAS = renin–angiotensin–aldosterone system; SGLT2 = sodium-glucose co-transporter-2; T1DM = type 1 diabetes mellitus T2DM = type 2 diabetes mellitus.
 |
 |
 |
 |
ABI = ankle–brachial index; ABPM = ambulatory blood pressure monitoring; ACEI = angiotensin-converting enzyme inhibitor; b.i.d. = twice daily (bis in die); b.p.m. = beats per minute; CABG = coronary artery bypass graft; CAC = coronary artery calcium; CAD = coronary artery disease; CKD = chronic kidney disease; CRT = cardiac resynchronization therapy; CRT-D = cardiac resynchronization therapy with an implantable defibrillator; CT = computed tomography; CV = cardiovascular; CVD = cardiovascular disease; DAPT = dual antiplatelet therapy; DM = diabetes mellitus; DPP4 = dipeptidyl peptidase-4; ECG = electrocardiogram; eGFR = estimated glomerular filtration rate; GLP1-RA = glucagon-like peptide-1 receptor agonist; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter defibrillator; LEAD = lower extremity artery disease; MRA = mineralocorticoid receptor agonist; o.d. = once daily (omni die); PAD = peripheral arterial disease; PCSK9 = proprotein convertase subtilisin/kexin type 9; RAAS = renin–angiotensin–aldosterone system; SGLT2 = sodium-glucose co-transporter-2; T1DM = type 1 diabetes mellitus T2DM = type 2 diabetes mellitus.
| Risk assessment in DM and pre-DM |
| Classification of CV risk (moderate-to-very high risk) adapted from the 2016 ESC Guidelines on CVD prevention in clinical practice to the DM setting (see section 5.2) |
| Lifestyle |
| Moderate alcohol intake should not be promoted as a means to protect against CVD |
| BP control |
| Detailed recommendations for individualized BP targets are now provided |
| Glucose-lowering treatment (a paradigm shift after recent CVOTs) |
| For the first time, we have evidence from several CVOTs that indicate CV benefits from the use of SGLT2 inhibitors and GLP1-RAs in patients with CVD, or at very high/high CV risk |
| Revascularization |
| The recommendations have been extended following the addition of several RCTs, and the choice between CABG and PCI depends on the complexity of the CAD |
| HF |
| Treatment recommendations have been updated following positive results from CVOTs |
| PAD |
| New evidence on diagnostic methods and management |
| CKD |
| A CKD classification by eGFR and albuminuria is presented to stratify severity of disease and guide treatment |
| Risk assessment in DM and pre-DM |
| Classification of CV risk (moderate-to-very high risk) adapted from the 2016 ESC Guidelines on CVD prevention in clinical practice to the DM setting (see section 5.2) |
| Lifestyle |
| Moderate alcohol intake should not be promoted as a means to protect against CVD |
| BP control |
| Detailed recommendations for individualized BP targets are now provided |
| Glucose-lowering treatment (a paradigm shift after recent CVOTs) |
| For the first time, we have evidence from several CVOTs that indicate CV benefits from the use of SGLT2 inhibitors and GLP1-RAs in patients with CVD, or at very high/high CV risk |
| Revascularization |
| The recommendations have been extended following the addition of several RCTs, and the choice between CABG and PCI depends on the complexity of the CAD |
| HF |
| Treatment recommendations have been updated following positive results from CVOTs |
| PAD |
| New evidence on diagnostic methods and management |
| CKD |
| A CKD classification by eGFR and albuminuria is presented to stratify severity of disease and guide treatment |
BP = blood pressure; CABG = coronary artery bypass graft; CAD = coronary artery disease; CKD = chronic kidney disease; CV = cardiovascular; CVD = cardiovascular disease; CVOT = cardiovascular outcome trial; eGFR = estimated glomerular filtration rate; ESC = European Society of Cardiology; GLP1-RA = glucagon-like peptide-1 receptor agonist; HF = heart failure; PAD = peripheral arterial disease; PCI = percutaneous coronary intervention; RCT = randomized controlled trial.
| Risk assessment in DM and pre-DM |
| Classification of CV risk (moderate-to-very high risk) adapted from the 2016 ESC Guidelines on CVD prevention in clinical practice to the DM setting (see section 5.2) |
| Lifestyle |
| Moderate alcohol intake should not be promoted as a means to protect against CVD |
| BP control |
| Detailed recommendations for individualized BP targets are now provided |
| Glucose-lowering treatment (a paradigm shift after recent CVOTs) |
| For the first time, we have evidence from several CVOTs that indicate CV benefits from the use of SGLT2 inhibitors and GLP1-RAs in patients with CVD, or at very high/high CV risk |
| Revascularization |
| The recommendations have been extended following the addition of several RCTs, and the choice between CABG and PCI depends on the complexity of the CAD |
| HF |
| Treatment recommendations have been updated following positive results from CVOTs |
| PAD |
| New evidence on diagnostic methods and management |
| CKD |
| A CKD classification by eGFR and albuminuria is presented to stratify severity of disease and guide treatment |
| Risk assessment in DM and pre-DM |
| Classification of CV risk (moderate-to-very high risk) adapted from the 2016 ESC Guidelines on CVD prevention in clinical practice to the DM setting (see section 5.2) |
| Lifestyle |
| Moderate alcohol intake should not be promoted as a means to protect against CVD |
| BP control |
| Detailed recommendations for individualized BP targets are now provided |
| Glucose-lowering treatment (a paradigm shift after recent CVOTs) |
| For the first time, we have evidence from several CVOTs that indicate CV benefits from the use of SGLT2 inhibitors and GLP1-RAs in patients with CVD, or at very high/high CV risk |
| Revascularization |
| The recommendations have been extended following the addition of several RCTs, and the choice between CABG and PCI depends on the complexity of the CAD |
| HF |
| Treatment recommendations have been updated following positive results from CVOTs |
| PAD |
| New evidence on diagnostic methods and management |
| CKD |
| A CKD classification by eGFR and albuminuria is presented to stratify severity of disease and guide treatment |
BP = blood pressure; CABG = coronary artery bypass graft; CAD = coronary artery disease; CKD = chronic kidney disease; CV = cardiovascular; CVD = cardiovascular disease; CVOT = cardiovascular outcome trial; eGFR = estimated glomerular filtration rate; ESC = European Society of Cardiology; GLP1-RA = glucagon-like peptide-1 receptor agonist; HF = heart failure; PAD = peripheral arterial disease; PCI = percutaneous coronary intervention; RCT = randomized controlled trial.
4 Diagnosis of diabetes and pre-diabetes
Key messages
DM should be investigated using fasting plasma glucose (FPG) or haemoglobin A1c (HbA1c).
An oral glucose tolerance test (OGTT) is necessary to diagnose impaired glucose tolerance (IGT).
Individuals with established CVD should be screened using HbA1c and/or fasting glucose; an OGTT can be carried out if FPG and HbA1c are inconclusive.
The classification of DM and pre-DM [impaired fasting glycaemia (IFG) and IGT] is based on recommendations from the World Health Organization (WHO) and the ADA.2–5 IFG and IGT, referred to as pre-DM, reflect the natural history of progression from normoglycaemia to T2DM. It is common for such individuals to oscillate between different glycaemic states, and this needs to be considered when investigations are being carried out. Different methods may be used as a diagnostic test for DM and pre-DM (Table 6).2–5
Diagnostic criteria for diabetes mellitus and pre-diabetes according to the 2006/2011 World Health Organization and 2019 American Diabetes Association recommendations
| Diagnosis/ measurement . | WHO 20063,/20114 . | ADA 20195 . |
|---|---|---|
| DM | ||
| Can be used | Recommended | |
| HbA1c | If measured, ≥6.5% (48 mmol/mol) | ≥6.5% (48 mmol/mol) |
| Recommended | ||
| FPG | ≥7.0 mmol/L (126 mg/dL) | ≥7.0 mmol/L (126 mg/dL) |
| or | or | |
| 2hPG | ≥11.1 mmol/L (≥200 mg/dL) | ≥11.1 mmol/L (≥200 mg/dL) |
| RPG | Symptoms plus ≥11.1 mmol/L (≥200 mg/dL) | Symptoms plus ≥11.1 mmol/L (≥200 mg/dL) |
| IGT | ||
| FPG | <7.0 mmol/L (<126 mg/dL) | <7.0 mmol/L (<126 mg/dL) |
| 2hPG | ≥7.8 to <11.1 mmol/L (≥140–200 mg/dL) | ≥7.8 to <11.0 mmol/L (≥140–199 mg/dL) |
| IFG | ||
| FPG | 6.1–6.9 mmol/L (110–125 mg/dL) | 5.6–6.9 mmol/L (100–125 mg/dL) |
| 2hPG | <7.8 mmol/L (<140 mg/dL) | <7.8 mmol/L (<140 mg/dL) |
| Diagnosis/ measurement . | WHO 20063,/20114 . | ADA 20195 . |
|---|---|---|
| DM | ||
| Can be used | Recommended | |
| HbA1c | If measured, ≥6.5% (48 mmol/mol) | ≥6.5% (48 mmol/mol) |
| Recommended | ||
| FPG | ≥7.0 mmol/L (126 mg/dL) | ≥7.0 mmol/L (126 mg/dL) |
| or | or | |
| 2hPG | ≥11.1 mmol/L (≥200 mg/dL) | ≥11.1 mmol/L (≥200 mg/dL) |
| RPG | Symptoms plus ≥11.1 mmol/L (≥200 mg/dL) | Symptoms plus ≥11.1 mmol/L (≥200 mg/dL) |
| IGT | ||
| FPG | <7.0 mmol/L (<126 mg/dL) | <7.0 mmol/L (<126 mg/dL) |
| 2hPG | ≥7.8 to <11.1 mmol/L (≥140–200 mg/dL) | ≥7.8 to <11.0 mmol/L (≥140–199 mg/dL) |
| IFG | ||
| FPG | 6.1–6.9 mmol/L (110–125 mg/dL) | 5.6–6.9 mmol/L (100–125 mg/dL) |
| 2hPG | <7.8 mmol/L (<140 mg/dL) | <7.8 mmol/L (<140 mg/dL) |
2hPG = 2 h plasma glucose; ADA = American Diabetes Association; DM = diabetes mellitus; FPG = fasting plasma glucose; IFG = impaired fasting glycaemia; IGT = impaired glucose tolerance; HbA1c = haemoglobin A1c; RPG = random plasma glucose; WHO = World Health Organization.
Diagnostic criteria for diabetes mellitus and pre-diabetes according to the 2006/2011 World Health Organization and 2019 American Diabetes Association recommendations
| Diagnosis/ measurement . | WHO 20063,/20114 . | ADA 20195 . |
|---|---|---|
| DM | ||
| Can be used | Recommended | |
| HbA1c | If measured, ≥6.5% (48 mmol/mol) | ≥6.5% (48 mmol/mol) |
| Recommended | ||
| FPG | ≥7.0 mmol/L (126 mg/dL) | ≥7.0 mmol/L (126 mg/dL) |
| or | or | |
| 2hPG | ≥11.1 mmol/L (≥200 mg/dL) | ≥11.1 mmol/L (≥200 mg/dL) |
| RPG | Symptoms plus ≥11.1 mmol/L (≥200 mg/dL) | Symptoms plus ≥11.1 mmol/L (≥200 mg/dL) |
| IGT | ||
| FPG | <7.0 mmol/L (<126 mg/dL) | <7.0 mmol/L (<126 mg/dL) |
| 2hPG | ≥7.8 to <11.1 mmol/L (≥140–200 mg/dL) | ≥7.8 to <11.0 mmol/L (≥140–199 mg/dL) |
| IFG | ||
| FPG | 6.1–6.9 mmol/L (110–125 mg/dL) | 5.6–6.9 mmol/L (100–125 mg/dL) |
| 2hPG | <7.8 mmol/L (<140 mg/dL) | <7.8 mmol/L (<140 mg/dL) |
| Diagnosis/ measurement . | WHO 20063,/20114 . | ADA 20195 . |
|---|---|---|
| DM | ||
| Can be used | Recommended | |
| HbA1c | If measured, ≥6.5% (48 mmol/mol) | ≥6.5% (48 mmol/mol) |
| Recommended | ||
| FPG | ≥7.0 mmol/L (126 mg/dL) | ≥7.0 mmol/L (126 mg/dL) |
| or | or | |
| 2hPG | ≥11.1 mmol/L (≥200 mg/dL) | ≥11.1 mmol/L (≥200 mg/dL) |
| RPG | Symptoms plus ≥11.1 mmol/L (≥200 mg/dL) | Symptoms plus ≥11.1 mmol/L (≥200 mg/dL) |
| IGT | ||
| FPG | <7.0 mmol/L (<126 mg/dL) | <7.0 mmol/L (<126 mg/dL) |
| 2hPG | ≥7.8 to <11.1 mmol/L (≥140–200 mg/dL) | ≥7.8 to <11.0 mmol/L (≥140–199 mg/dL) |
| IFG | ||
| FPG | 6.1–6.9 mmol/L (110–125 mg/dL) | 5.6–6.9 mmol/L (100–125 mg/dL) |
| 2hPG | <7.8 mmol/L (<140 mg/dL) | <7.8 mmol/L (<140 mg/dL) |
2hPG = 2 h plasma glucose; ADA = American Diabetes Association; DM = diabetes mellitus; FPG = fasting plasma glucose; IFG = impaired fasting glycaemia; IGT = impaired glucose tolerance; HbA1c = haemoglobin A1c; RPG = random plasma glucose; WHO = World Health Organization.
Although the WHO and ADA diagnostic criteria are clear, there are practical considerations when choosing a method to diagnose DM. In accordance with other ESC Guidelines accepting non-fasting lipids in risk scoring, most patients can have DM assessment by HbA1c at any time of day. However, there are limitations with HbA1c to be considered, such as interference as a result of haemoglobin variants, anaemia, and availability in different parts of the world.
It is recommended that diagnosis of DM is based on HbA1c or FPG, and on OGTT if still in doubt. Repeat testing is advisable to confirm the diagnosis. In patients with CVD, the methods employed for the diagnosis of DM and pre-DM are essentially the same: glucose testing with HbA1c and/or FPG first, and if inconclusive, an OGTT,6–8 which is the only means of diagnosing IGT. The high prevalence of glucose abnormalities in this setting is well established. In the Glucose Abnormalities in Patients with Myocardial Infarction (GAMI) study, OGTTs revealed that two-thirds of patients without DM had newly detected DM or pre-DM.9 The Euro Heart Survey on Diabetes and the Heart10 and EUROASPIRE IV11 demonstrated that an OGTT may diagnose a greater proportion of patients with CVD as having glucose abnormalities than FPG or HbA1c. Similar findings have been reported in patients admitted for coronary angiography.12 In acute coronary syndromes (ACS), the OGTT should not be performed earlier than 4–5 days, to minimize false-positive results.13,14
Gaps in the evidence
Measurement of glycaemia at 1 h instead of 2 h during an OGTT for the diagnosis of pre-DM and DM needs validation.
Further work needs to be carried out to establish the effects of sex, ethnicity, and age on diagnostic criteria.
Direct comparison of the predictive abilities of HbA1c- vs. OGTT-derived measures for hard outcomes in people with CVD.
Recommendations for the diagnosis of disorders of glucose metabolism
 |
 |
CVD = cardiovascular disease; DM = diabetes mellitus; FPG = fasting plasma glucose; HbA1c = haemoglobin A1c; IGT = impaired glucose tolerance; OGTT = oral glucose tolerance test; T2DM = type 2 diabetes mellitus.
Class of recommendation.
Level of evidence.
Recommendations for the diagnosis of disorders of glucose metabolism
 |
 |
CVD = cardiovascular disease; DM = diabetes mellitus; FPG = fasting plasma glucose; HbA1c = haemoglobin A1c; IGT = impaired glucose tolerance; OGTT = oral glucose tolerance test; T2DM = type 2 diabetes mellitus.
Class of recommendation.
Level of evidence.
5 Cardiovascular risk assessment in patients with diabetes and pre-diabetes
Key messages
Routine assessment of microalbuminuria should be carried out to identify patients at risk of developing renal dysfunction and/or CVD.
A resting electrocardiogram (ECG) is indicated in patients with DM and hypertension, or if CVD is suspected.
Other tests, such as transthoracic echocardiography, coronary artery calcium (CAC) score, and ankle–brachial index (ABI), may be considered to test for structural heart disease or as risk modifiers in those at moderate or high risk of CVD.
Routine assessment of novel biomarkers is not recommended for CV risk stratification.
5.1 Diabetes, pre-diabetes, and cardiovascular risk
The Emerging Risk Factor Collaboration, a meta-analysis of 102 prospective studies, showed that DM in general (data on DM type were unavailable) confers a two-fold excess risk of vascular outcomes (coronary heart disease, ischaemic stroke, and vascular deaths), independent of other risk factors (Figure 1).23 The excess relative risk of vascular events with DM was greater in women and at younger ages. Both relative and absolute risk levels will be higher in those with long-standing DM and microvascular complications, including renal disease or proteinuria. The Swedish National Diabetes Register has provided important insights into the prevalence of CVD and CV death in both type 1 DM (T1DM)24 and T2DM.25 For T1DM, 27 195 subjects were stratified by age and sex. Early onset at 1 − 10 years of age was associated with a hazard ratio (HR) of 7.38 for CV mortality, 30.95 for acute myocardial infarction (MI), and 12.9 for heart failure (HF). The corresponding figures for T1DM onset between the ages of 26 and 30 years were 3.64, 5.77, and 5.07, respectively. Development of T1DM between 1–10 years of age resulted in loss of 17.7 years of life in women and 14.2 years in men.24 For T2DM, a huge cohort of 435 369 patients was matched with controls and followed for 4.6 years. CVD mortality was 17.15/1000 patient-years for T2DM and 12.86/1000 patient-years for controls. In this cohort, age at DM diagnosis, glycaemic control, and renal complications were the major determinants of outcome.25,26 Although T2DM is far more common than T1DM, these results confirm the loss of years of life in both populations, which is particularly severe in the young in general and perhaps in young-onset female individuals with T1DM, emphasizing the need for intensive risk-factor management in these groups. In this document, we will be referring mostly to DM; this can be taken as relating to both types of DM unless otherwise specified.
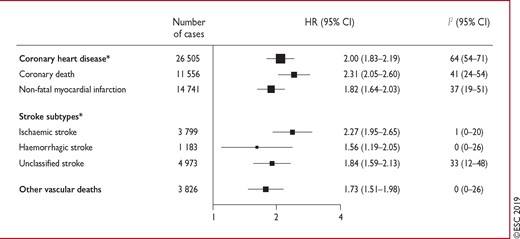
Hazard ratios for vascular outcomes in people with vs. without diabetes mellitus at baseline, based on analyses of 530 083 patients. Reproduced with permission.23 Hazard ratios were adjusted for age, smoking status, body mass index, and systolic blood pressure, and—where appropriate—stratified by sex and trial arm. The 208 coronary heart disease outcomes that contributed to the grand total could not contribute to the subtotals of coronary death or non-fatal myocardial infarction because there were <11 cases of these coronary disease subtypes in some studies. CI = confidence interval; HR = hazard ratio. aIncludes fatal and non-fatal events.
The elevated risk of coronary artery disease (CAD) starts at glucose levels below the cut-off point for DM (<7 mmol/L), and increases with increasing glucose levels (Figure 2).
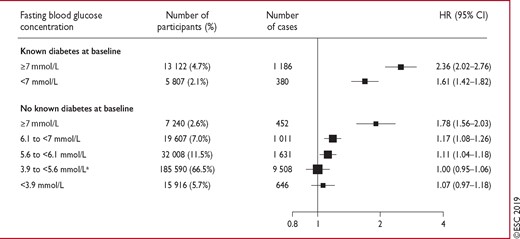
Hazard ratios for coronary heart disease by clinically defined categories of baseline fasting blood glucose concentration. Reproduced with permission.23 Analyses were based on 279 290 participants (14 814 cases). Hazard ratios were adjusted as described in Figure 1. The hazard ratio in those with fasting plasma glucose 5.60–6.99 mmol/L was 1.12 (95% confidence interval 1.06–1.18). CI = confidence interval; HR = hazard ratio. aReference group.
5.2 Stratification of cardiovascular risk in individuals with diabetes
As outlined in the 2016 European Guidelines on cardiovascular disease prevention in clinical practice,27 individuals with DM and CVD, or DM with target organ damage, such as proteinuria or kidney failure [estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2], are at very high risk (10 year risk of CVD death >10%). Patients with DM with three or more major risk factors, or with a DM duration of >20 years, are also at very high risk. Furthermore, as indicated in section 5.1, T1DM at the age of 40 years with early onset (i.e. 1 − 10 years of age) and particularly in female individuals is associated with very high CV risk.24 Most others with DM are high risk (10 year risk of CVD death 5 − 10%), with the exception of young patients (aged <35 years) with T1DM of short duration (<10 years), and patients with T2DM aged <50 years with a DM duration of <10 years and without major risk factors, who are at moderate risk. The classification of risk level applied in these Guidelines is presented in Table 7. When DM is present, female sex is not protective against premature CVD, as seen in the general population.28,29
 |
 |
CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; T1DM = type 1 diabetes mellitus; T2DM = type 2 diabetes mellitus.
Modified from the 2016 European Guidelines on cardiovascular disease prevention in clinical practice.27
Proteinuria, renal impairment defined as eGFR <30 mL/min/1.73 m2, left ventricular hypertrophy, or retinopathy.
Age, hypertension, dyslipidemia, smoking, obesity.
 |
 |
CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; T1DM = type 1 diabetes mellitus; T2DM = type 2 diabetes mellitus.
Modified from the 2016 European Guidelines on cardiovascular disease prevention in clinical practice.27
Proteinuria, renal impairment defined as eGFR <30 mL/min/1.73 m2, left ventricular hypertrophy, or retinopathy.
Age, hypertension, dyslipidemia, smoking, obesity.
5.3 Stratification of cardiovascular risk in individuals with pre-diabetes
Individuals without CVD who have pre-DM are not necessarily at elevated CV risk,23,30 but warrant risk scoring for CVD in the same way as the general population.
5.4 Clinical assessment of cardiovascular damage
5.4.1 Biomarkers
The addition of circulating biomarkers for CV risk assessment has limited clinical value.27 In patients with DM without known CVD, measurement of C-reactive protein or fibrinogen (inflammatory markers) provides minor incremental value to current risk assessment.31 High-sensitivity cardiac troponin T (hsTnT)-estimated 10 year CV mortality for individuals with undetectable (<3 ng/L), low detectable (3–14 ng/L), and increased (≥14 ng/L) levels was 4, 18, and 39%, respectively.32 However, the addition of hsTnT to conventional risk factors has not shown incremental discriminative power in this group.22 In individuals with T1DM, elevated hsTnT was an independent predictor of renal decline and CV events.33 The prognostic value of N-terminal pro-B-type natriuretic peptide (NT-proBNP) in an unselected cohort of people with DM (including known CVD) showed that patients with low levels of NT-proBNP (<125 pg/mL) have an excellent short-term prognosis.34 The value of NT-proBNP in identifying patients with DM who will benefit from intensified control of CV risk factors (CVRFs) was demonstrated in a small randomized controlled trial (RCT).21 The presence of albuminuria (30–299 mg/day) is associated with increased risk of CVD and chronic kidney disease (CKD) in T1DM and T2DM.20,35–37 Measurement of albuminuria may predict kidney dysfunction and warrant renoprotective interventions.27
5.4.2 Electrocardiography
A resting ECG may detect silent MI in 4% of individuals with DM,38 which has been associated with increased risk of CVD and all-cause mortality in men but not women.39 Additionally, prolonged corrected QT interval is associated with increased CV mortality in T1DM, whereas increasing resting heart rate is associated with risk of CVD in T1DM and T2DM.40,41 Low heart rate variability (a marker of diabetic CV autonomic neuropathy) has been associated with an increased risk of fatal and non-fatal CAD.42,43 In prospective cohorts, 20–40% of patients with DM presented silent ST-segment depression during exercise ECG.44–48 The sensitivity and specificity of exercise ECG in diagnosing significant CAD in asymptomatic DM patients were 47 and 81%, respectively.49 The combination of exercise ECG and an imaging technique provides incremental diagnostic and prognostic value patients with in DM.50–52
5.4.3 Imaging techniques
Echocardiography is the first choice to evaluate structural and functional abnormalities associated with DM. Increased left ventricular (LV) mass, diastolic dysfunction, and impaired LV deformation have been reported in asymptomatic DM and are associated with worse prognosis.53–56 A cluster analysis from two large cohorts of asymptomatic patients with DM showed that those with the lowest LV masses, smallest left atria, and lowest LV filling pressures (determined by E/e’) had fewer CV hospitalization or death events, compared with those with advanced LV systolic and diastolic dysfunctions, or greater LV masses.53,57 CV magnetic resonance and tissue characterization techniques have shown that patients with DM without CAD have diffuse myocardial fibrosis as the mechanism of LV systolic and diastolic dysfunction.55,58,59 However, the value of these advanced imaging techniques in routine practice has not yet been demonstrated.
Screening for asymptomatic CAD in patients with DM remains controversial. With computed tomography (CT), non-invasive estimation of the atherosclerotic burden (based on the CAC score) and identification of atherosclerotic plaques causing significant coronary stenosis [CT coronary angiography (CTCA)] can be performed. The presence of plaques on carotid ultrasound has been associated with increased CV events in subjects with DM.60–62 In addition, patients with DM have a higher prevalence of coronary artery calcification compared with age- and sex-matched subjects without DM.63 While a CAC score of 0 is associated with favourable prognosis in asymptomatic subjects with DM, each increment in CAC score (from 1 − 99 to 100 − 399 and ≥400) is associated with a 25 − 33% higher relative risk of mortality.63 Importantly, CAC is not always associated with ischaemia. Stress testing with myocardial perfusion imaging or stress echocardiography permits the detection of silent myocardial ischaemia. Observational studies and RCTs report the prevalence of silent myocardial ischaemia in asymptomatic DM as ∼22%.47,48,64 RCTs evaluating the impact of routine screening for CAD in asymptomatic DM and no history of CAD have shown no differences in cardiac death and unstable angina at follow-up in those who underwent stress testing, or CTCA, compared with current recommendations.47,64–68 A meta-analysis of five RCTs (Table 8) with 3299 asymptomatic subjects with DM showed that non-invasive imaging for CAD did not significantly reduce event rates of non-fatal MI (relative risk 0.65; P=0.062) and hospitalization for HF (relative risk 0.61; P=0.1).65
| Study/author | Faglia et al.69 | DIAD68 | DYNAMIT64 | FACTOR-6467 | DADDY-D70 |
| Year of publication | 2005 | 2009 | 2011 | 2014 | 2015 |
| Patients (n) | 141 (+1)a | 1123 | 615 | 899 | 520 |
| Inclusion criteria | T2DM | T2DM | T2DM | T1DM or T2DM | T2DM |
| 45–76 years | 50–75 years | 50–75 years | ♂ aged ≥50 years/♀ aged ≥55 years, DM for ≥3 years | 50–75 years | |
| ≥2 other CVRFs | ≥2 other CVRFs | ♂ aged ≥40 years/ ♀ aged ≥45 years, DM for ≥5 years | CV risk ≥10% | ||
| Sinus rhythm | |||||
| Able to do EET | |||||
| Mean age (years) | 60.1 | 60.8 | 63.9 | 61.5 | 61.9 |
| Male sex (%) | 55.6 | 53.5 | 54.5 | 52.2 | 80.0 |
| Screening test | EET and SE | MPI | EET or MPI | CTCA and CAC score | EET |
| Positive screening test (%) | 21.1 | 5.9 moderate or large defects | 21.5 positive or uncertain | 11.9 moderate; 10.7 severe | 7.6 |
| Treatment strategy | ICA and cardiac follow-up if any test was positive | At the referring physician’s discretion | According to the cardiologist’s decision | Recommendation based on stenosis severity and CAC score | ICA if EET positive |
| ICA performed after positive test (%) | 93.3 | 15.2 | 55.9 | 47.3 | 85.0 |
| Mean follow-up (years) | 4.5 | 4.8 | 3.5 | 4.0 | 3.6 |
| Annual rate of major CEs (%) | 1.9 | 0.6 | 1.0 | 0.8 | 1.4 |
| Main results of screening | Significant ↓ of major and all CEs | Non-significant ↓ of major CEs | Non-significant ↓ of MI; no effect on combined CEs | Non-significant ↓ of combined CEs | Non-significant ↓ of major CEs, but significant ↓ in those aged >60 years |
| Study/author | Faglia et al.69 | DIAD68 | DYNAMIT64 | FACTOR-6467 | DADDY-D70 |
| Year of publication | 2005 | 2009 | 2011 | 2014 | 2015 |
| Patients (n) | 141 (+1)a | 1123 | 615 | 899 | 520 |
| Inclusion criteria | T2DM | T2DM | T2DM | T1DM or T2DM | T2DM |
| 45–76 years | 50–75 years | 50–75 years | ♂ aged ≥50 years/♀ aged ≥55 years, DM for ≥3 years | 50–75 years | |
| ≥2 other CVRFs | ≥2 other CVRFs | ♂ aged ≥40 years/ ♀ aged ≥45 years, DM for ≥5 years | CV risk ≥10% | ||
| Sinus rhythm | |||||
| Able to do EET | |||||
| Mean age (years) | 60.1 | 60.8 | 63.9 | 61.5 | 61.9 |
| Male sex (%) | 55.6 | 53.5 | 54.5 | 52.2 | 80.0 |
| Screening test | EET and SE | MPI | EET or MPI | CTCA and CAC score | EET |
| Positive screening test (%) | 21.1 | 5.9 moderate or large defects | 21.5 positive or uncertain | 11.9 moderate; 10.7 severe | 7.6 |
| Treatment strategy | ICA and cardiac follow-up if any test was positive | At the referring physician’s discretion | According to the cardiologist’s decision | Recommendation based on stenosis severity and CAC score | ICA if EET positive |
| ICA performed after positive test (%) | 93.3 | 15.2 | 55.9 | 47.3 | 85.0 |
| Mean follow-up (years) | 4.5 | 4.8 | 3.5 | 4.0 | 3.6 |
| Annual rate of major CEs (%) | 1.9 | 0.6 | 1.0 | 0.8 | 1.4 |
| Main results of screening | Significant ↓ of major and all CEs | Non-significant ↓ of major CEs | Non-significant ↓ of MI; no effect on combined CEs | Non-significant ↓ of combined CEs | Non-significant ↓ of major CEs, but significant ↓ in those aged >60 years |
Reproduced/adapted with permission.
♂ = men; ♀ = women; CAC = coronary artery calcium; CE = cardiac event (major CE = cardiac death or MI); CTCA = computed tomography coronary angiography; CV = cardiovascular; CVRF = cardiovascular risk factor; DADDY-D = Does coronary Atherosclerosis Deserve to be Diagnosed earlY in Diabetic patients?; DIAD = Detection of Ischaemia in Asymptomatic Diabetics; DYNAMIT = Do You Need to Assess Myocardial Ischemia in Type 2 Diabetes; DM = diabetes mellitus; EET = exercise electrocardiogram test; FACTOR-64 = Screening For Asymptomatic Obstructive Coronary Artery Disease Among High-Risk Diabetic Patients Using CT Angiography, Following Core 64; ICA = invasive coronary angiography; MI = myocardial infarction; MPI = radionuclide myocardial perfusion imaging; RCT = randomized controlled trial; SE = stress echocardiography; T1DM = type 1 diabetes mellitus; T2DM = type 2 diabetes mellitus.
One patient excluded for early non-cardiac death was reincluded.
| Study/author | Faglia et al.69 | DIAD68 | DYNAMIT64 | FACTOR-6467 | DADDY-D70 |
| Year of publication | 2005 | 2009 | 2011 | 2014 | 2015 |
| Patients (n) | 141 (+1)a | 1123 | 615 | 899 | 520 |
| Inclusion criteria | T2DM | T2DM | T2DM | T1DM or T2DM | T2DM |
| 45–76 years | 50–75 years | 50–75 years | ♂ aged ≥50 years/♀ aged ≥55 years, DM for ≥3 years | 50–75 years | |
| ≥2 other CVRFs | ≥2 other CVRFs | ♂ aged ≥40 years/ ♀ aged ≥45 years, DM for ≥5 years | CV risk ≥10% | ||
| Sinus rhythm | |||||
| Able to do EET | |||||
| Mean age (years) | 60.1 | 60.8 | 63.9 | 61.5 | 61.9 |
| Male sex (%) | 55.6 | 53.5 | 54.5 | 52.2 | 80.0 |
| Screening test | EET and SE | MPI | EET or MPI | CTCA and CAC score | EET |
| Positive screening test (%) | 21.1 | 5.9 moderate or large defects | 21.5 positive or uncertain | 11.9 moderate; 10.7 severe | 7.6 |
| Treatment strategy | ICA and cardiac follow-up if any test was positive | At the referring physician’s discretion | According to the cardiologist’s decision | Recommendation based on stenosis severity and CAC score | ICA if EET positive |
| ICA performed after positive test (%) | 93.3 | 15.2 | 55.9 | 47.3 | 85.0 |
| Mean follow-up (years) | 4.5 | 4.8 | 3.5 | 4.0 | 3.6 |
| Annual rate of major CEs (%) | 1.9 | 0.6 | 1.0 | 0.8 | 1.4 |
| Main results of screening | Significant ↓ of major and all CEs | Non-significant ↓ of major CEs | Non-significant ↓ of MI; no effect on combined CEs | Non-significant ↓ of combined CEs | Non-significant ↓ of major CEs, but significant ↓ in those aged >60 years |
| Study/author | Faglia et al.69 | DIAD68 | DYNAMIT64 | FACTOR-6467 | DADDY-D70 |
| Year of publication | 2005 | 2009 | 2011 | 2014 | 2015 |
| Patients (n) | 141 (+1)a | 1123 | 615 | 899 | 520 |
| Inclusion criteria | T2DM | T2DM | T2DM | T1DM or T2DM | T2DM |
| 45–76 years | 50–75 years | 50–75 years | ♂ aged ≥50 years/♀ aged ≥55 years, DM for ≥3 years | 50–75 years | |
| ≥2 other CVRFs | ≥2 other CVRFs | ♂ aged ≥40 years/ ♀ aged ≥45 years, DM for ≥5 years | CV risk ≥10% | ||
| Sinus rhythm | |||||
| Able to do EET | |||||
| Mean age (years) | 60.1 | 60.8 | 63.9 | 61.5 | 61.9 |
| Male sex (%) | 55.6 | 53.5 | 54.5 | 52.2 | 80.0 |
| Screening test | EET and SE | MPI | EET or MPI | CTCA and CAC score | EET |
| Positive screening test (%) | 21.1 | 5.9 moderate or large defects | 21.5 positive or uncertain | 11.9 moderate; 10.7 severe | 7.6 |
| Treatment strategy | ICA and cardiac follow-up if any test was positive | At the referring physician’s discretion | According to the cardiologist’s decision | Recommendation based on stenosis severity and CAC score | ICA if EET positive |
| ICA performed after positive test (%) | 93.3 | 15.2 | 55.9 | 47.3 | 85.0 |
| Mean follow-up (years) | 4.5 | 4.8 | 3.5 | 4.0 | 3.6 |
| Annual rate of major CEs (%) | 1.9 | 0.6 | 1.0 | 0.8 | 1.4 |
| Main results of screening | Significant ↓ of major and all CEs | Non-significant ↓ of major CEs | Non-significant ↓ of MI; no effect on combined CEs | Non-significant ↓ of combined CEs | Non-significant ↓ of major CEs, but significant ↓ in those aged >60 years |
Reproduced/adapted with permission.
♂ = men; ♀ = women; CAC = coronary artery calcium; CE = cardiac event (major CE = cardiac death or MI); CTCA = computed tomography coronary angiography; CV = cardiovascular; CVRF = cardiovascular risk factor; DADDY-D = Does coronary Atherosclerosis Deserve to be Diagnosed earlY in Diabetic patients?; DIAD = Detection of Ischaemia in Asymptomatic Diabetics; DYNAMIT = Do You Need to Assess Myocardial Ischemia in Type 2 Diabetes; DM = diabetes mellitus; EET = exercise electrocardiogram test; FACTOR-64 = Screening For Asymptomatic Obstructive Coronary Artery Disease Among High-Risk Diabetic Patients Using CT Angiography, Following Core 64; ICA = invasive coronary angiography; MI = myocardial infarction; MPI = radionuclide myocardial perfusion imaging; RCT = randomized controlled trial; SE = stress echocardiography; T1DM = type 1 diabetes mellitus; T2DM = type 2 diabetes mellitus.
One patient excluded for early non-cardiac death was reincluded.
The Detection of Ischaemia in Asymptomatic Diabetics (DIAD) study showed no difference in the prevalence of silent ischaemia between men and women (24 vs. 17%, respectively), and a significantly lower event rate for non-fatal MI and cardiac death in women compared with men (1.7 vs. 3.8%; P=0.047).71 The low event rates in RCTs and the disparities in the management of screening results (invasive coronary angiography and revascularization were not performed systematically) may explain the lack of benefit of the screening strategy. Accordingly, routine screening of CAD in asymptomatic DM is not recommended.71 However, stress testing or CTCA may be indicated in very high-risk asymptomatic individuals [with peripheral arterial disease (PAD), a high CAC score, proteinuria, or renal failure].72
Carotid intima–media thickness has been associated with CAD.73 In patients with DM, carotid intima–media thickness has not shown incremental value over the CAC score to predict CAD or CV events.73 In contrast, detection of carotid plaque has shown incremental value over carotid intima–media thickness to detect CAD in asymptomatic DM.74 Additionally, echolucent plaque and plaque thickness are independent predictors of CVD events (CAD, ischaemic stroke, and PAD).75 ABI is associated with an increased risk of all-cause and CV mortality in DM and non-DM patients76 (see further details in section 10).
Gaps in the evidence
The prognostic value of advanced imaging techniques, such as strain imaging or CV magnetic resonance with tissue characterization, needs validation in prospective cohorts.
Asymptomatic subjects with significant atherosclerosis burden (i.e. CAC score >400) may be referred for functional imaging or CTCA; however, identification of the presence of significant coronary artery stenoses has not been shown to be better than aggressive medical treatment for CVRFs.
Sex-specific differences in the diagnosis of CAD require further investigation.
The uptake of CV risk assessment in different ethnic groups requires evaluation.
Recommendations for the use of laboratory, electrocardiogram, and imaging testing for cardiovascular risk assessment in asymptomatic patients with diabetes
 |
 |
ABI = ankle–brachial index; CAC = coronary artery calcium; CAD = coronary artery disease; CT = computed tomography; CTCA = computed tomography coronary angiography; CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; ECG = electrocardiogram.
Class of recommendation.
Level of evidence.
See Table 7.
Recommendations for the use of laboratory, electrocardiogram, and imaging testing for cardiovascular risk assessment in asymptomatic patients with diabetes
 |
 |
ABI = ankle–brachial index; CAC = coronary artery calcium; CAD = coronary artery disease; CT = computed tomography; CTCA = computed tomography coronary angiography; CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; ECG = electrocardiogram.
Class of recommendation.
Level of evidence.
See Table 7.
6 Prevention of cardiovascular disease in patients with diabetes and pre-diabetes
6.1 Lifestyle
Key messages
Lifestyle changes are key to prevent DM and its CV complications.
Reduced calorie intake is recommended to lower excessive body weight in patients with DM.
A Mediterranean diet supplemented with olive oil and/or nuts reduces the incidence of major CV events.
Moderate-to-vigorous physical activity of ≥150 min/week is recommended for the prevention and control of DM.
American and European Guidelines advocate lifestyle changes as a first measure for the prevention and management of DM.27,79–81 Even modest weight loss delays progression from pre-DM to T2DM.82,83 A recent meta-analysis of 63 studies (n=17 272, mean age 49.7 years), showed that each additional kilogram loss was associated with 43% lower odds of T2DM.84 The relatively small Finnish Diabetes Prevention Study and the Da Qing Diabetes Prevention Study have both shown that lifestyle intervention in IGT significantly reduces the development of T2DM, with a reduction in vascular complications in the Chinese cohort.85,86 The 30 year results from the Da Qing study are further strengthening this conclusion.87 Results from the long-term follow-up of the Diabetes Prevention Program support the view that lifestyle intervention or metformin significantly reduce DM development over 15 years.88
In established DM, lower calorie intake causes a fall in HbA1c and improves quality of life.83 Maintenance of weight loss for 5 years is associated with sustained improvements in HbA1c and lipid levels.89 For many obese patients with DM, weight loss of >5% is needed to improve glycaemic control, lipid levels, and blood pressure (BP).90 One-year results from the Action for Health in Diabetes (Look AHEAD) trial, investigating the effects of weight loss on glycaemia and the prevention of CVD events in patients with DM, showed that an average 8.6% weight loss was associated with a significant reduction in HbA1c and CVRFs. Although these benefits were sustained for 4 years, there was no difference in CV events between groups.91 The Diabetes Remission Clinical Trial (DiRECT)—an open-label, cluster-randomized trial—assigned practices to provide either a weight-management programme (intervention) or best-practice care by guidelines (control). The results showed that at 12 months, almost one-half of the participants achieved remission to a non-diabetic state and were off glucose-lowering drugs.92 Sustained remissions at 24 months for over one-third of people with T2DM have been confirmed recently.93
Bariatric surgery causes long-term weight loss, and reduces DM and risk factor elevations, with effects that are superior to lifestyle and intensive medical management alone.94,95
6.1.1 Diet
Nutrient distribution should be based on an individualized assessment of current eating patterns, preferences, and metabolic goals.81,83 In the Prevención con Dieta Mediterránea (PREDIMED) study, among people at high CV risk (49% had DM), a Mediterranean diet supplemented with olive oil or nuts reduced the incidence of major CV events.96
6.1.1.1 Carbohydrate
The role of low-carbohydrate diets in patients DM remains unclear. A recent meta-analysis based on 10 RCTs comprising 1376 individuals has shown that the glucose-lowering effects of low- and high-carbohydrate diets are similar at 1 year or later, and have no significant effect on weight or low-density lipoprotein cholesterol (LDL-C) levels.97
6.1.1.2 Fats
The ideal amount of dietary fat for individuals with DM is controversial. Several RCTs including patients with DM have reported that a Mediterranean-style eating pattern,96,98,99 rich in polyunsaturated and monounsaturated fats, can improve both glycaemic control and blood lipids. Supplements with n-3 fatty acids have not been shown to improve glycaemic control in individuals with DM,100 and RCTs do not support recommending n-3 supplements for the primary or secondary prevention of CVD.101,102 The Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT)—using a higher dose of n3-fatty acids (4 g/day) in patients with persistent elevated triglycerides, and either established CVD or DM, and at least one other CVD risk factor—showed a significant reduction of the primary endpoint of major adverse CV events (MACE).103 Patients with DM should follow guidelines for the general population for the recommended intakes of saturated fat, dietary cholesterol, and trans fat. In general, trans fats should be avoided.
6.1.1.3 Proteins
Adjusting daily protein intake is not indicated in patients with DM unless kidney disease is present, at which point less protein is recommended.
6.1.1.4 Vegetables, legumes, fruits, and wholegrain cereals
Vegetables, legumes, fruits, and wholegrain cereals should be part of a healthy diet.104
6.1.1.5 Alcohol consumption
A recent meta-analysis indicated that whilst low levels of alcohol (≤100 g/week) were associated with a lower risk of MI, there were no clear thresholds below which lower alcohol consumption stopped being associated with a lower disease risk for other CV outcomes such as hypertension, stroke, and HF. Moderate alcohol intake should not be promoted as a means to protect against CVD.27,105
6.1.1.6 Coffee and tea
Consumption of more than four cups of coffee per day was associated with a lower risk of CVD in Finnish patients with DM.106 An exception should be made for coffee brewed by boiling ground coffee, which increases cholesterol levels.107 In a meta-analysis of 18 observational studies, increasing coffee or tea consumption appeared to reduce the risk of DM.108
6.1.1.7 Vitamins and macronutrients
Vitamin or micronutrient supplementation to reduce the risk of DM or CVD in patients with DM is not recommended.96,97
6.1.2 Physical activity
Physical activity delays conversion of IGT to T2DM, and improves glycaemic control and CVD complications.109 Aerobic and resistance training improve insulin action, glycaemic control, lipid levels, and BP.110 RCTs support the need for exercise reinforcement by healthcare workers,111 and structured aerobic exercise or resistance exercise has been shown to reduce HbA1c by ∼0.6% in patients with DM.111 Clinical trials in adults with DM have provided evidence of the HbA1c-lowering value of resistance training, and of an additive benefit of combined aerobic and resistance exercise.112 Patients with pre-DM and DM should do two sessions per week of resistance exercise; pregnant women with DM should engage in regular moderate physical activity.113 Encouragement to increase activity by any level yields benefits; even an extra 1000 steps of walking per day would be advantageous and may be a good starting point for many patients.
6.1.3 Smoking
Smoking increases the risk of DM,114 CVD, and premature death115 and should be avoided, including passive smoking.116 If advice, encouragement, and motivation are insufficient, then drug therapies should be considered early, including nicotine replacement therapy followed by bupropion or varenicline.117 Electronic cigarettes (e-cigarettes) are an emerging smoking cessation aid worldwide; however, consensus regarding their efficacy and safety has yet to be reached. Smoking cessation programmes have low efficacy at 12 months.118
Recommendations for lifestyle modifications in patients with diabetes and pre-diabetes
 |
 |
CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; IGT = impaired glucose tolerance; T2DM = type 2 diabetes mellitus.
Class of recommendation.
Level of evidence.
A commonly stated goal for obese patients with DM is to lose ∼5% of baseline weight.
It is recommended that all individuals reduce the amount of sedentary time by breaking up periods of sedentary activity with moderate-to-vigorous physical activity in bouts of ≥10 min (broadly equivalent to 1000 steps).
Recommendations for lifestyle modifications in patients with diabetes and pre-diabetes
 |
 |
CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; IGT = impaired glucose tolerance; T2DM = type 2 diabetes mellitus.
Class of recommendation.
Level of evidence.
A commonly stated goal for obese patients with DM is to lose ∼5% of baseline weight.
It is recommended that all individuals reduce the amount of sedentary time by breaking up periods of sedentary activity with moderate-to-vigorous physical activity in bouts of ≥10 min (broadly equivalent to 1000 steps).
Gaps in the evidence
Adherence to lifestyle changes.
Ethnicity and diet.
Effects of lifestyle measures on clinical outcomes.
Lifestyle advice in different stages of life, e.g. in frail and elderly patients.
Tailored exercise interventions in different ethnic groups and patient categories.
6.2 Glucose
Key messages
Glucose control to target a near-normal HbA1c (<7.0% or <53 mmol/mol) will decrease microvascular complications in patients with DM.
Tighter glucose control initiated early in the course of DM in younger individuals leads to a reduction in CV outcomes over a 20 year timescale.
Less-rigorous targets should be considered in elderly patients on a personalized basis and in those with severe comorbidities or advanced CVD.
6.2.1 Glycaemic targets
A meta-analysis of three major studies—Action to Control Cardiovascular Risk in Diabetes (ACCORD), Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE), and the Veterans Affairs Diabetes Trial (VADT)—suggested that in T2DM, an HbA1c reduction of ∼1% is associated with a 15% relative risk reduction in non-fatal MI, without beneficial effects on stroke, CV, or all-cause mortality121 or hospitalization for HF.122 Intensive glucose control was beneficial for CV events in patients with a short duration of DM, lower HbA1c at baseline, and no CVD.122 In addition, the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC) (T1DM), the UK Prospective Diabetes Study (UKPDS), and VADT (T2DM) showed that long follow-up (≤20 years) is necessary to demonstrate a beneficial effect on macrovascular complications, and that early glucose control is associated with long-term CV benefits (legacy effect).123 An HbA1c target of <7% (<53 mmol/mol) reduces microvascular complications, while evidence for an HbA1c target to reduce macrovascular risk is less compelling. However, HbA1c targets should be individualized, with more-stringent goals [6.0–6.5% (42–48 mmol/mol)] in younger patients with a short duration of DM and no evidence of CVD, if achieved without significant hypoglycaemia. Less-stringent HbA1c goals [e.g. <8% (64 mmol/mol) or ≤9% (75 mmol/mol)] may be adequate for elderly patients with long-standing DM and limited life expectancy, and frailty with multiple comorbidities, including hypoglycaemic episodes.
6.2.1.1 Additional glucose targets
Post-prandial glucose testing should be recommended for patients who have pre-meal glucose values at target but HbA1c above target. Several epidemiological studies have shown that high post-challenge (2 h OGTT) or post-prandial glucose values are associated with greater CV risk, independent of FPG.124–126 Intervention trials have failed to support the role of post-prandial glucose as a CVRF independent of HbA1c. The Hyperglycemia and Its Effect After Acute Myocardial Infarction on Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus (HEART2D) trial, an RCT that assigned patients with DM within 21 days after an acute MI to insulin regimens targeting either post-prandial or pre-prandial glucose, reported differences in FPG, less-than-expected differences in post-prandial PG, similar levels of HbA1c, and no difference in risk of future CV events.127 However, in a post hoc analysis, this risk was significantly lower in older patients treated with an insulin regimen targeting post-prandial glycaemia.128 The ACE (Acarbose Cardiovascular Evaluation) trial, in Chinese patients with CAD and IGT, showed that acarbose did not reduce the risk of MACE, but did reduce the incidence of DM by 18%.129
FPG variability has been reported to be a strong predictor of all-cause and CVD-related mortality in patients with DM, suggesting that management of glucose variability may become an additional goal.130 In the intensive arm of the ADVANCE study, an increase in HbA1c and fasting glucose variability was associated with the risk of macrovascular events.131 In insulin-treated DM, an association between fasting glucose variability and total mortality was also reported in the pooled population of the Trial Comparing Cardiovascular Safety of Insulin Degludec versus Insulin Glargine in Patients with Type 2 Diabetes at High Risk of cardiovascular Events (DEVOTE).132 Glucose variability increases in the presence of pre-DM.133 However, the role of glucose variability in CVD is difficult to dissect. In patients with DM, mean blood glucose and HbA1c are more strongly associated with CVD risk factors than FPG, post-prandial glucose levels, or measures of glucose variability using continuous glucose monitoring.134 Drugs that reduce post-prandial glucose excursions, including glucagon-like peptide-1 receptor agonists (GLP1-RAs), dipeptidyl peptidase-4 (DPP4) inhibitors, and sodium-glucose co-transporter 2 (SGLT2) inhibitors, seem an attractive way to reduce glucose variability.135
6.2.2 Glucose-lowering agents
Therapeutic agents that manage hyperglycaemia can be broadly characterized as belonging to one of five groups: (i) insulin sensitizers (metformin and pioglitazone); (ii) insulin providers (insulin, sulfonylureas, and meglitinides); (iii) incretin-based therapies (GLP1-RAs and DPP4 inhibitors); (iv) gastrointestinal glucose absorption inhibitor (acarbose); and (v) renal glucose reuptake inhibitors (SGLT2 inhibitors). For further details see sections 7.1.1 and 7.1.2.
6.2.3 Special considerations
6.2.3.1 Hypoglycaemia
Although studies suggest an association between hypoglycaemia and CV events, there is no clear evidence for causality. Prevention of hypoglycaemia remains critical, particularly with advanced disease or CVD (including HF), to reduce the risk of arrhythmias and myocardial ischaemia.136 Several studies, including Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction 2 (DIGAMI 2),137 ADVANCE,138 and Outcome Reduction With Initial Glargine Intervention (ORIGIN), have indicated that severe hypoglycaemia is associated with increased risk of death and an impaired CV prognosis,139 whilst DEVOTE reported decreased hypoglycaemia but failed to show a difference in MACE.140
6.2.3.2 Glucose monitoring
Structured self-monitoring of blood glucose and continuous glucose monitoring are valuable tools to improve glycaemic control.141 Electronic ambulatory glucose142 has been shown to reduce the time spent in hypoglycaemia and to increase the time when glucose is within the recommended range.142–144
Recommendations for glycaemic control in patients with diabetes
 |
 |
DM = diabetes mellitus; HbA1c = haemoglobin A1c.
Class of recommendation.
Level of evidence.
Recommendations for glycaemic control in patients with diabetes
 |
 |
DM = diabetes mellitus; HbA1c = haemoglobin A1c.
Class of recommendation.
Level of evidence.
Gaps in the evidence
More research is needed to define a ‘personalized’ target for patients with DM.
The role of new glucose-monitoring technologies (continuous glucose monitoring and electronic ambulatory glucose) in the control of post-prandial glycaemia and glucose variability needs to be defined.
The roles of these new technologies in the prevention of DM complications needs to be tested.
6.3 Blood pressure
Key messages
The BP goal is to target systolic BP (SBP) to 130 mmHg in patients with DM and <130 mmHg if tolerated, but not <120 mmHg. In older people (aged >65 years), the SBP goal is to a range of 130 − 139 mmHg.
The diastolic BP (DBP) target is <80 mmHg, but not <70 mmHg.
Optimal BP control reduces the risk of micro- and macrovascular complications.
Guidance on lifestyle changes must be provided for patients with DM and hypertension.
Evidence strongly supports the inclusion of an angiotensin-converting enzyme inhibitor (ACEI), or an angiotensin receptor blocker (ARB) in patients who are intolerant to ACEI.
BP control often requires multiple drug therapy with a renin–angiotensin–aldosterone system (RAAS) blocker, and a calcium channel blocker or diuretic. Dual therapy is recommended as first-line treatment.
The combination of an ACEI and an ARB is not recommended.
In pre-DM, the risk of new-onset DM is lower with RAAS blockers than with beta-blockers or diuretics.
Patients with DM on combined antihypertensive treatments should be encouraged to self-monitor BP.
The prevalence of hypertension is high in patients with DM, reaching ≤67% after 30 years of T1DM152 and >60% in T2DM. Mediators of increased BP in patients with DM involve factors linked to obesity, including hyperinsulinaemia.153
6.3.1 Treatment targets
RCTs have shown the benefit (reduction of stroke, coronary events, and kidney disease) of lowering SBP to <140 mmHg and DBP to <90 mmHg in DM patients. In a meta-analysis of 13 RCTs involving patients with DM or pre-DM, a SBP reduction to 131–135 mmHg reduced the risk of all-cause mortality by 13%, whereas more-intensive BP control (≤130 mmHg) was associated with a greater reduction in stroke but did not reduce other events.154 In a meta-analysis, antihypertensive treatment significantly reduced mortality, CAD, HF, and stroke, with an achieved mean SBP of 138 mmHg, whereas only stroke was reduced significantly, with a mean SBP of 122 mmHg.155 Reducing SBP to <130 mmHg may benefit patients with a particularly high risk of a cerebrovascular event, such as those with a history of stroke.154–157 The UKPDS post-trial 10 year follow-up study reported no persistence of the benefits of the earlier period of tight BP control with respect to macrovascular events, death, and microvascular complications, while initial between-group BP differences were no longer maintained.149 In the ADVANCE trial, the combination of perindopril and indapamide reduced mortality, and the benefit was still present, but attenuated, at the end of the 6 year post-trial follow-up, without evidence of a sex difference.159 Thus, optimal BP control is important in reducing the risk of micro- and macrovascular complications, and must be maintained if these benefits are to be sustained.
In patients with DM receiving BP-lowering drugs, it is recommended that office BP should be targeted to an SBP of 130 mmHg, and lower if tolerated. In older patients (aged ≥65 years) the SBP target range should be 130–140 mmHg if tolerated. In all patients with DM, SBP should not be lowered to <120 mmHg and DBP should be lowered to <80 mmHg.160
6.3.2 Management of blood pressure lowering
6.3.2.1 Effects of lifestyle intervention and weight loss
Reduction of sodium intake (to <100 mmol/day); diets rich in vegetables, fruits, and low-fat dairy products; and Mediterranean diets have all been demonstrated to improve BP control.161–163 As a result of long-term exercise training intervention, modest but significant reductions in systolic (by −7 mmHg) and diastolic (by −5 mmHg) BP are observed. Ideally, an exercise prescription aimed at lowering BP in individuals with normal BP or hypertension would include a mix of predominantly aerobic exercise training supplemented with dynamic resistance exercise training.164
A marked improvement in CVRFs (hypertension, dyslipidaemia, inflammation, and DM), associated with marked weight loss, was observed after bariatric surgery.165 In the Look AHEAD trial, those who lost 5 to <10% of body weight had increased odds of achieving a 5 mmHg decrease in SBP and DBP.166
6.3.2.2 Pharmacological treatments
If office SBP is ≥140 mmHg and/or DBP is ≥90 mmHg, drug therapy is necessary in combination with non-pharmacological therapy. All available BP-lowering drugs (except beta-blockers) can be used, but evidence strongly supports the use of a RAAS blocker, particularly in patients with evidence of end-organ damage (albuminuria and LV hypertrophy).167–170 BP control often requires multiple drug therapy with a RAAS blocker, and a calcium channel blocker or a diuretic, while the combination of an ACEI with an ARB is not recommended.171 A combination of two or more drugs at fixed doses in a single pill should be considered, to improve adherence. The beta-blocker/diuretic combination favours the development of DM, and should be avoided in pre-DM, unless required for other reasons. Among beta-blockers, nebivolol has been shown not to affect insulin sensitivity in patients with metabolic syndrome.172
A meta-analysis in which ACEIs or ARBs were compared with placebo reported a reduced incidence of new-onset DM [odds ratio 0.8, 95% confidence interval (CI) 0.8–0.9; P < 0.01] and CV mortality (odds ratio 0.9, 95% CI 0.8–1.0; P < 0.01) on active therapy.173 In patients with pre-DM, ramipril did not significantly reduce the incidence of DM, but significantly increased regression to normoglycaemia.174 In patients with IGT, valsartan significantly reduced the incidence of new-onset DM.175
6.3.2.3 Blood pressure changes with glucose-lowering treatments
Trials testing GLP1-RAs have shown evidence of a slight, but significant, BP decrease, partly due to weight loss. In the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial, a sustained decrease was observed (SBP/DBP –1.2/–0.6 mmHg) with a slight increase in heart rate (3 b.p.m.).176 SGLT2 inhibitors induced a larger BP decrease (SBP/DBP –2.46/–1.46 mmHg) without heart rate changes.177 The BP-lowering effects of these drugs have to be taken into consideration when managing BP.
Recommendations for the management of blood pressure in patients with diabetes and pre-diabetes
 |
 |
ABPM = ambulatory blood pressure monitoring; ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BP = blood pressure; DBP = diastolic blood pressure; DM = diabetes mellitus; GLP1-RA = glucagon-like peptide-1 receptor agonist; IFG = impaired fasting glycaemia; IGT = impaired glucose tolerance; LV = left ventricular; RAAS = renin–angiotensin–aldosterone system; SBP = systolic blood pressure; SGLT2 = sodium-glucose co-transporter 2.
Class of recommendation.
Level of evidence.
Recommendations for the management of blood pressure in patients with diabetes and pre-diabetes
 |
 |
ABPM = ambulatory blood pressure monitoring; ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BP = blood pressure; DBP = diastolic blood pressure; DM = diabetes mellitus; GLP1-RA = glucagon-like peptide-1 receptor agonist; IFG = impaired fasting glycaemia; IGT = impaired glucose tolerance; LV = left ventricular; RAAS = renin–angiotensin–aldosterone system; SBP = systolic blood pressure; SGLT2 = sodium-glucose co-transporter 2.
Class of recommendation.
Level of evidence.
Gaps in the evidence
Optimal BP targets are unknown, particularly in young patients with T1DM, recent-onset T2DM, and DM with CAD.
The role of stabilization or reversal of end-organ damage (including albuminuria, LV hypertrophy, and arterial stiffness), beyond BP control, is poorly known.
Is treatment with GLP-RAs and SGLT2 inhibitors affecting the current treatment algorithms for BP lowering?
The interaction of GLP1-RAs and SGLT2 inhibitors with BP-lowering treatments, in terms of CV prognosis, is unknown.
6.4 Lipids
Key messages
Statins effectively prevent CV events and reduce CV mortality, and their use is associated with a limited number of adverse events. Because of the high-risk profile of patients with DM, intensive statin treatment should be used on an individualized basis.
Currently, statins remain state-of-the-art therapy in lipid-lowering treatment in patients with DM.
Ezetimibe or a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor on top of a statin—or alone, in case of documented intolerance to statins—further contribute to LDL-C reduction in patients with DM, thus improving CV outcomes and reducing CV mortality.
A cluster of lipid and apolipoprotein abnormalities accompanies DM. The two core components are moderate elevation of fasting and non-fasting triglycerides, and low high-density lipoprotein cholesterol (HDL-C). Other features comprise elevation of triglyceride-rich lipoproteins, including chylomicron and very low-density lipoprotein remnants, and normal-to-mildly elevated levels of LDL-C, with small dense low-density lipoprotein particles. In well-controlled T1DM, HDL-C levels tend to be normal (or even slightly elevated), as do serum triglyceride levels.186
6.4.1 Lipid-lowering agents
6.4.1.1 Statins
Consistent data have demonstrated the efficacy of statins in preventing CV events and reducing CV mortality in patients with DM, with no evidence for sex differences. A meta-analysis including 18 686 patients with DM demonstrated that a statin-induced reduction of LDL-C by 1.0 mmol/L (40 mg/dL) was associated with a 9% reduction in all-cause mortality and a 21% reduction in the incidence of major CV events.187 Similar benefits were seen in both T1DM and T2DM. In patients with an ACS, intensive statin treatment led to a reduction in all-cause and CV death, and contributed to a reduction in atheroma progression.188 In both T1DM and young-onset T2DM, there is a paucity of evidence to indicate the age at which statin therapy should be initiated. To guide an approach, statins are not indicated in pregnancy,189,190 and should be avoided in women with T1DM or T2DM who are planning pregnancy. In the absence of vascular damage, and in particular microalbuminuria, it seems reasonable to delay statin therapy in asymptomatic patients with DM until the age of 30 years. Below this age, statin therapy should be managed on a case-by-case basis taking into account the presence of microalbuminuria, end-organ damage, and ambient LDL-C levels.
Statins are safe and generally well tolerated. Adverse events, except for muscle symptoms, are rare. In the majority of cases of myopathy or rhabdomyolysis, there are drug interactions with a higher-than-standard dose of statin or combination with gemfibrozil.191,192 Evidence indicates that most patients (70–90%) who report statin intolerance are able to take a statin when rechallenged.193–196 Patients may be rechallenged with the same statin unless they have creatine kinase elevation. Evidence supports a lower rate of side effects with low-dose rosuvastatin or pravastatin.193–196
Statin therapy has been associated with new-onset DM: for every 40 mmol/L (mg/dL) reduction of LDL-C by statins, conversion to DM is increased by 10%.197,198 The risk of new-onset DM increases with age and is confined to those already at risk of developing DM.199 Nevertheless, the benefits in terms of CV event reduction greatly exceed the risks of statin therapy, and this has been confirmed in patients at low CV risk.187
6.4.1.2 Ezetimibe
Further intensification of LDL-C lowering occurs by adding ezetimibe to a statin. In the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT), a significant reduction of the primary endpoint event rate (HR 0.85, 95% CI 0.78–0.94) for post-ACS patients with DM receiving simvastatin plus ezetimibe was reported, with a stronger beneficial effect on outcome than in non-DM. The results in this subgroup were mainly driven by a lower incidence of MI and ischaemic stroke.200,201 The combination of ezetimibe with a statin should be recommended to patients with DM with a recent ACS, particularly when the statin alone is not sufficient to reduce LDL-C levels to <1.4 mmol/L (55 mg/dL).
6.4.1.3 Proprotein convertase subtilisin/kexin type 9
The new entry among lipid-lowering therapies is the PCSK9 inhibitors, which reduce LDL-C to an unprecedented extent. In the Efficacy and Safety of Alirocumab in Insulin-treated Individuals with Type 1 or Type 2 Diabetes and High Cardiovascular Risk (ODYSSEY DM-INSULIN) trial, alirocumab, compared with placebo, reduced LDL-C by 50% in patients with DM after 24 weeks of treatment.202 In the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial, patients with atherosclerotic CVD on statin therapy were randomly assigned to a fixed dose of evolocumab or placebo. The results demonstrated that the primary composite endpoint (CV death, MI, stroke, hospital admission for unstable angina, or coronary revascularization) was significantly reduced.203,204 Similar results were obtained from the ODYSSEY OUTCOMES (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) trial, which randomly assigned patients with CVD and LDL-C >1.8 mmol/L (70 mg/dL), despite high-intensity statins, to alirocumab or placebo, with dose titration of the active drug targeting an LDL-C level of 0.6 − 1.3 mmol/L (25 − 50 mg/dL). Alirocumab significantly reduced the risk of the primary composite endpoint (CV death, MI, stroke, or hospital admission for unstable angina) compared with placebo, with the greatest absolute benefit of alirocumab seen in patients with baseline LDL-C levels >2.6 mmol/L (100 mg/dL).205 In a subgroup analysis of the ODYSSEY OUTCOMES trial, patients with DM (n=5444) had double the absolute risk reduction compared with pre-DM (n=8246) and non-DM (n=5234) subjects (2.3 vs. 1.2%, respectively).206 At present, these results should be regarded as exploratory.
6.4.1.4 Fibrates
In patients with high triglyceride levels [≥2.3 mmol/L (200 mg/dL)], lifestyle advice (with a focus on weight reduction and alcohol abuse, if relevant) and improved glucose control are the main targets. Both the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) and ACCORD studies demonstrated that administration of fenofibrate on top of statins significantly reduced CV events, but only in patients who had both elevated triglyceride and reduced HDL-C levels.191,207 Gemfibrozil should be avoided because of the risk of myopathy. A meta-analysis of fibrate trials reported a significant reduction in non-fatal MI, with no effect on mortality.208 Fibrates may be administered in patients with DM who are statin intolerant and have high triglyceride levels. If triglycerides are not controlled by statins or fibrates, high-dose omega-3 fatty acids (4 g/day) of icosapent ethyl may be used.209,103
Recommendations for the management of dyslipidaemia with lipid-lowering drugs
 |
 |
CV = cardiovascular; DM = diabetes mellitus; EAS = European Atherosclerosis Society; ESC = European Society of Cardiology; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; PCSK9 = proprotein convertase subtilisin/kexin type 9; T1DM = type 1 diabetes mellitus; T2DM = type 2 diabetes mellitus.
Class of recommendation.
Level of evidence.
See Table 7.
See the 2019 ESC/EAS Guidelines for the management of dyslipidaemias for non-HDL-C and apolipoprotein B targets.
Recommendations for the management of dyslipidaemia with lipid-lowering drugs
 |
 |
CV = cardiovascular; DM = diabetes mellitus; EAS = European Atherosclerosis Society; ESC = European Society of Cardiology; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; PCSK9 = proprotein convertase subtilisin/kexin type 9; T1DM = type 1 diabetes mellitus; T2DM = type 2 diabetes mellitus.
Class of recommendation.
Level of evidence.
See Table 7.
See the 2019 ESC/EAS Guidelines for the management of dyslipidaemias for non-HDL-C and apolipoprotein B targets.
Gaps in the evidence
The optimal LDL-C level needs to be established.
The effects of fibrates on CV outcomes in patients with triglycerides >2.3 mmol/L are unclear.
The role of PCSK9 inhibitors in patients with DM remains to be further elucidated.
6.5 Platelets
Key messages
Patients with DM and symptomatic CVD should be treated no differently to patients without DM.
In patients with DM at moderate CV risk, aspirin for primary prevention is not recommended.
In patients with DM at high/very high risk, aspirin may be considered in primary prevention.
Several abnormalities have been described concerning in vivo and/or ex vivo platelet function, and increased platelet activation in patients with DM. Hyperglycaemia,216 low-degree inflammation,217 and increased oxidation may contribute to in vivo platelet activation and altered responsiveness to antithrombotic drugs in patients with DM. However, platelet abnormalities and poor antiplatelet drug responsiveness have also been described in patients with DM with good metabolic control.218,–220 A dysmegakaryopoiesis may characterize DM, resulting in increased platelet mass,221 an altered ratio between platelet count and volume,221,222 megakaryocyte aneuploidy,223 and increased reticulated platelets in the peripheral blood.219 In addition, platelet thrombin generation appears enhanced, clot type appears to be altered, and fibrinolysis reduced in patients with DM.224
6.5.1 Aspirin
Aspirin permanently inhibits cyclooxygenase 1 activity and thromboxane A2-dependent platelet aggregation.225 Small, proof-of-concept, pharmacodynamic, randomized studies have consistently shown that once-daily low-dose aspirin may be insufficient to fully inhibit platelet cyclooxygenase 1 activity in patients with DM218–220,226 and increased platelet turnover.219 This would support testing different regimens [e.g. b.i.d. (twice daily)] of low-dose aspirin in patients with DM in RCTs.
6.5.1.1 Primary prevention
Although aspirin has unquestionable benefits in the secondary prevention of CVD (see section 6.5.1.2), the situation is less clear in primary prevention. In 2009, the Antithrombotic Trialists’ Collaboration published a meta-analysis of primary prevention trials including 95 000 individuals at low risk.227 They reported a 12% reduction in CVD outcomes with aspirin, but a significant increase in major bleeds, which cast doubt on the value of aspirin under these circumstances. Since then, further trials have reported similar or no reduction in CV outcomes, but the risk of major bleeds is consistent across studies.228,229 Gender studies of aspirin use have revealed similar bleeding risks in men and women, and similar 12% reductions in CV events in both sexes, driven by a decrease in ischaemic stroke in women and of MI in men.229 Recent large trials in patients at moderate risk, which (i) excluded DM230 and (ii) specifically recruited patients with DM,231 were unable to progress the argument that aspirin should be used in primary prevention. The A Study of Cardiovascular Events iN Diabetes (ASCEND) trial randomized 15 480 patients with DM with no evident CVD to aspirin 100 mg once daily [o.d. (onmi die)] or placebo.231 The primary efficacy outcome (MI, stroke, transient ischaemic attack, or death from any cause) occurred in 658 patients (8.5%) on aspirin vs. 743 (9.6%) on placebo (rate ratio 0.88, 95% CI 0.79–0.97; P=0.01). Major bleeding occurred in 314 (4.1%) patients on aspirin vs. 245 (3.2%) on placebo (rate ratio 1.29, 95% CI 1.09–1.52; P=0.003). There were no differences in fatal or intracranial bleeding, and a substantial proportion (≈25%) of the major bleeds defined according to ASCEND were in the upper gastrointestinal tract. The number needed to treat/number needed to harm ratio was 0.8. A recent meta-analysis demonstrated that the proton pump inhibitors provide substantial protection from upper gastrointestinal bleeding with an odds ratio of ∼0.20.232 It should be emphasized that only one in four patients in the ASCEND trial were being treated with a proton pump inhibitor at the end of the study, and wider use in trials could potentially amplify the benefit of aspirin in primary prevention.
It has been recently suggested that body weight233 or size can lower responsiveness to aspirin, as well as to clopidogrel, requiring higher daily doses.234 Pharmacokinetic data suggest a lower degree of platelet inhibition, especially in moderate-to-severely obese patients.234 However, the benefit of intensified antiplatelet regimens in obese DM patients remains to be established.
6.5.1.2 Secondary prevention
The best available evidence for aspirin in secondary prevention remains that discussed in the 2013 ESC Guidelines on DM, pre-diabetes, and CVDs, developed in collaboration with the EASD72 (see section 7.1).
Recommendations for the use of antiplatelet therapy in primary prevention in patients with diabetes
 |
 |
CV = cardiovascular; DM = diabetes mellitus.
Class of recommendation.
Level of evidence.
See Table 7.
Gastrointestinal bleeding, peptic ulceration within the previous 6 months, active hepatic disease, or history of aspirin allergy.
Recommendations for the use of antiplatelet therapy in primary prevention in patients with diabetes
 |
 |
CV = cardiovascular; DM = diabetes mellitus.
Class of recommendation.
Level of evidence.
See Table 7.
Gastrointestinal bleeding, peptic ulceration within the previous 6 months, active hepatic disease, or history of aspirin allergy.
Gaps in the evidence
More data on CV prevention are needed for T1DM where in vivo platelet activation has been reported.236
There is a need to assess the effect of body mass, especially of moderate-to-severe obesity on antiplatelet drug responsiveness and effectiveness in patients with DM, and to investigate higher dose strategies.
Whether antithrombotic preventive strategy effects in pre-DM and DM are similar should be explored.
6.6 Multifactorial approaches
Key messages
Combined reduction in HbA1c, SBP, and lipids decreases CV events by 75%.
Multifactorial treatment is still underused.
6.6.1 Principles of multifactorial management
Patients with glucose perturbations may benefit from the early identification and treatment of comorbidities and factors that increase CV risk.237 However, many patients are not achieving risk factor goals for CVD prevention (Table 9). In EUROASPIRE IV, a BP target <140/90 mmHg was achieved in 68% of patients with CAD without DM, in 61% of patients with newly detected DM, and in 54% of patients with previously known DM. An LDL-C target <1.8 mmol/L was achieved in 16, 18, and 28% of these groups, respectively. Furthermore, the combined use of four cardioprotective drugs (antiplatelets, beta-blockers, RAAS blockers, and statins) in these groups was only 53, 55, and 60%, respectively.238
| Risk factor | Target |
| BP |
|
| Glycaemic control: HbA1c |
|
| Lipid profile: LDL-C |
|
| Platelet inhibition | In DM patients at high/very high CV risk |
| Smoking | Cessation obligatory |
| Physical activity | Moderate-to-vigorous, ≥150 min/week, combined aerobic and resistance training |
| Weight | Aim for weight stabilization in overweight or obese patients with DM, based on calorie balance, and weight reduction in subjects with IGT, to prevent the development of DM. |
| Dietary habits | Reduction of caloric intake is recommended in obese patients with T2DM to lower body weight; there is no ideal percentage of calories from carbohydrate, protein, and fat for all people with DM. |
| Risk factor | Target |
| BP |
|
| Glycaemic control: HbA1c |
|
| Lipid profile: LDL-C |
|
| Platelet inhibition | In DM patients at high/very high CV risk |
| Smoking | Cessation obligatory |
| Physical activity | Moderate-to-vigorous, ≥150 min/week, combined aerobic and resistance training |
| Weight | Aim for weight stabilization in overweight or obese patients with DM, based on calorie balance, and weight reduction in subjects with IGT, to prevent the development of DM. |
| Dietary habits | Reduction of caloric intake is recommended in obese patients with T2DM to lower body weight; there is no ideal percentage of calories from carbohydrate, protein, and fat for all people with DM. |
BP = blood pressure; CV = cardiovascular; DM = diabetes mellitus; HbA1c = haemoglobin A1c; IGT = impaired glucose tolerance; LDL-C = low-density lipoprotein cholesterol; SBP = systolic blood pressure; T2DM = type 2 diabetes mellitus.
See Table 7.
| Risk factor | Target |
| BP |
|
| Glycaemic control: HbA1c |
|
| Lipid profile: LDL-C |
|
| Platelet inhibition | In DM patients at high/very high CV risk |
| Smoking | Cessation obligatory |
| Physical activity | Moderate-to-vigorous, ≥150 min/week, combined aerobic and resistance training |
| Weight | Aim for weight stabilization in overweight or obese patients with DM, based on calorie balance, and weight reduction in subjects with IGT, to prevent the development of DM. |
| Dietary habits | Reduction of caloric intake is recommended in obese patients with T2DM to lower body weight; there is no ideal percentage of calories from carbohydrate, protein, and fat for all people with DM. |
| Risk factor | Target |
| BP |
|
| Glycaemic control: HbA1c |
|
| Lipid profile: LDL-C |
|
| Platelet inhibition | In DM patients at high/very high CV risk |
| Smoking | Cessation obligatory |
| Physical activity | Moderate-to-vigorous, ≥150 min/week, combined aerobic and resistance training |
| Weight | Aim for weight stabilization in overweight or obese patients with DM, based on calorie balance, and weight reduction in subjects with IGT, to prevent the development of DM. |
| Dietary habits | Reduction of caloric intake is recommended in obese patients with T2DM to lower body weight; there is no ideal percentage of calories from carbohydrate, protein, and fat for all people with DM. |
BP = blood pressure; CV = cardiovascular; DM = diabetes mellitus; HbA1c = haemoglobin A1c; IGT = impaired glucose tolerance; LDL-C = low-density lipoprotein cholesterol; SBP = systolic blood pressure; T2DM = type 2 diabetes mellitus.
See Table 7.
In the Swedish national DM registry, the excess risk of outcomes decreased by each risk factor within the target range (HbA1c, LDL-C, albuminuria, smoking, and SBP). In T2DM with variables at target, the HR for all-cause death was 1.06 (95% CI 1.00–1.12), 0.84 (95% CI 0.75–0.93) for acute MI, and 0.95 (95% CI 0.84–1.07) for stroke. The risk of hospitalization for HF was consistently higher among patients with DM than controls (HR 1.45, 95% CI 1.34–1.57).239
Intensified, multifactorial treatment for DM in primary care and early in the disease trajectory was evaluated in the Anglo-Danish-Dutch Study of Intensive Treatment In People with Screen Detected Diabetes in Primary Care (ADDITION).240 Follow-up (1 and 5 year) did not show significant reductions in the frequencies of microvascular241 or macrovascular events.242 Interestingly, modelled 10 year CVD risk calculated with the UKPDS risk engine was lower in the intensive-treatment group after adjustment for baseline CV risk (–2.0, 95% CI –3.1 to 0.9).243
A beneficial effect of a multifactorial intervention in patients with DM and established microalbuminuria was demonstrated by the Steno-2 study, in which 160 very high-risk patients with DM were randomized to intensive, target-driven, multifactorial therapy or conventional management. The targets in the intensively treated group were HbA1c <6.5% (48 mmol/mol), total cholesterol <4.5 mmol/L (175 mg/dL), and BP <130/80 mmHg. All patients in this group received RAAS blockers and low-dose aspirin. This approach resulted in a reduction in microvascular and macrovascular events of ∼50% after 7.8 years of follow-up. Long-term follow-up (21 years from baseline) showed that intensive treatment significantly reduced end-stage renal disease combined with death to HR 0.53 (95% CI 0.35–0.8), and induced a 7.9-year gain of life matched by time free from incident CVD.37,244 This study also showed that the risk of hospitalization for HF reduced by 70%.245
The Japan Diabetes Optimal Integrated Treatment Study for 3 Major Risk Factors of Cardiovascular Diseases (J-DOIT3) studied the effect of an intensive multifactorial intervention with stringent goals in Japanese patients with DM aged 45–69 years with risk factors. Results showed significantly improved HbA1c, SBP, DBP, and LDL-C compared with conventional therapy. There was a non-significant trend towards reduction of the primary composite outcome, comprising non-fatal MI, stroke, revascularization, or all-cause death (HR 0.81, 95% CI 0.63–1.04; P=0.094). Post hoc analysis showed that cerebrovascular events were reduced in the intensive-therapy group (HR 0.42, 95% CI 0.24–0.74; P=0.002), while no differences were seen for all-cause death and coronary events.246
Among 1425 patients with known DM and CAD participating in the Euro Heart Survey, 44% received a combination of aspirin, a beta-blocker, a RAAS blocker, and a statin. Patients on this combination had significantly lower all-cause death (3.5 vs. 7.7%; P=0.001) and fewer combined CV events (11.6 vs. 14.7%; P=0.05) after 1 year of follow-up.247
Recommendations for multifactorial management of patients with diabetes
 |
 |
CVD = cardiovascular disease; DM = diabetes mellitus.
Class of recommendation.
Level of evidence.
Recommendations for multifactorial management of patients with diabetes
 |
 |
CVD = cardiovascular disease; DM = diabetes mellitus.
Class of recommendation.
Level of evidence.
Gaps in the evidence
The optimal strategy for multifactorial treatment in primary and secondary intervention has not been established.
Sex differences have not been evaluated in the setting of multifactorial intervention.
7 Management of coronary artery disease
Key messages
T2DM and pre-DM are common in individuals with ACS and chronic coronary syndromes (CCS), and are associated with an impaired prognosis.
Glycaemic status should be systematically evaluated in all patients with CAD.
Intensive glycaemic control may have more favourable CV effects when initiated early in the course of DM.
Empagliflozin, canagliflozin, and dapagliflozin reduce CV events in patients with DM and CVD, or in those who are at very high/high CV risk.
Liraglutide, semglutide and dulaglutide reduce CV events in patients with DM and CVD, or who are at very high/high CV risk.
Intensive secondary prevention is indicated in patients with DM and CAD.
Antiplatelet drugs are the cornerstone of secondary CV prevention.
In high-risk patients, the combination of low-dose rivaroxaban and aspirin may be beneficial for CAD.
Aspirin plus reduced-dose ticagrelor may be considered for ≤3 years post-MI.
Antithrombotic treatment for revascularization does not differ according to DM status.
In patients with DM and multivessel CAD, suitable coronary anatomy for revascularization, and low predicted surgical mortality, coronary artery bypass graft (CABG) is superior to percutaneous coronary intervention (PCI).
7.1 Medical treatment
Glucose abnormalities are common in patients with acute and stable CAD, and are associated with a poor prognosis.16,18,249 Approximately 20–30% of patients with CAD have known DM, and of the remainder, up to 70% have newly detected DM or IGT when investigated with an OGTT.9,250,251 Patients with CAD, without known glucose abnormalities, should have their glycaemic state evaluated as outlined in sections 4 and 5.
It is important to acknowledge that recommendations for the secondary prevention of CAD in patients with DM are mostly based on evidence from subgroup analyses of trials that enrolled patients with and without DM.72 Because of the higher CV event rates consistently observed in patients with DM, the absolute benefit often appears amplified while the relative benefit remains similar.238,247 General recommendations for patients with CCS and ACS are outlined in other ESC Guidelines.252–255
There is evidence that improved glycaemic control defers the onset, reduces the progression, and (in some circumstances) may partially reverse markers of microvascular complications in patients with DM. Accordingly, early, effective, and sustained glycaemic control is advocated in all DM guidelines to mitigate the risks of hyperglycaemia. Achieving this without detriment and with benefit to the CV system is an important challenge, particularly when selecting glucose-lowering therapies to suit the individual. Key clinical trials that delineate the effects of glucose-lowering therapies on CV outcomes are considered below.
7.1.1 Effects of intensified glucose control
7.1.1.1 UKPDS
In UKPDS, 5102 patients with newly diagnosed drug-naïve DM were randomly assigned to intensive glucose control with a sulfonylurea or insulin, or to management with diet alone, for a median 10.7 years. Although a clear reduction in microvascular complications was evident, the reduction in MI was marginal at 16% (P=0.052).145 In the study extension phase, the risk reduction in MI remained at 15%, which became significant as the number of cases increased.149 Furthermore, the beneficial effects persisted for any DM-related endpoint, including death from any cause, which was reduced by 13%. Of note, this study was performed when modern aspects of multifactorial management (lipid lowering and BP) were unavailable.
7.1.1.2 ACCORD, ADVANCE, and VADT
Three trials reported the CV effects of more-intensive vs. standard glucose control in patients with DM at high CV risk.138,256–258 They included >23 000 patients treated for 3–5 years and showed no CVD benefit from intensified glucose control. ACCORD was terminated after a mean follow-up of 3.5 years because of higher mortality in the intensive arm (14/1000 vs. 11/1000 patient deaths/year), which was pronounced in those with multiple CVRFs and driven mainly by CV mortality. A further analysis found that individuals with poor glycaemic control within the intensive arm accounted for the excess CV mortality.259
7.1.1.3 DIGAMI 1 and 2
DIGAMI 1260 reported that insulin-based intensified glycaemic control reduced mortality in patients with DM and acute MI (mortality after 3.4 years was 33% in the insulin group vs. 44% in the control group; P=0.011).261 The effect of intensified glycaemic control remained 8 years after randomization, increasing survival by 2.3 years.262 These results were not reproduced in DIGAMI 2, which was stopped prematurely due to slow recruitment of patients.263 In pooled data, an insulin–glucose infusion did not reduce mortality in acute MI and DM.264 If it is felt necessary to improve glycaemic control in patients with ACS, this should be carried out cognisant of the risk of hypoglycaemia, which is associated with poor outcomes in patients with CAD.265,266 The strategy of metabolic modulation by glucose−insulin−potassium, to stabilize the cardiomyocyte and improve energy production, regardless of the presence of DM, has been tested in several RCTs without a consistent effect on morbidity or mortality.267,268
In patients undergoing cardiac surgery, glucose control should be considered.269 Observational data in patients undergoing CABG suggest that the use of continuous insulin infusion achieving moderately tight glycaemic control is associated with lower mortality, and fewer major complications, than tighter or more lenient glycaemic control.270 In the CABG stratum in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial, long-term insulin-providing treatment was associated with more CV events than insulin-sensitization medications.271
The glycaemic targets for people with CAD, and the preferred classes of drugs for DM, are outlined in section 6.2 and below.
7.1.2 Glucose-lowering agents: new evidence from cardiovascular outcome trials
7.1.2.1 Established oral glucose-lowering drugs
The CV effects of long-established oral glucose-lowering drugs have not been evaluated in large RCTs, as with more recent drugs.
7.1.2.1.1 Metformin
In a nested study of 753 patients in UKPDS comparing conventional therapy with metformin, metformin reduced MI by 39%, coronary death by 50%, and stroke by 41% over a median period of 10.7 years in newly diagnosed overweight patients with T2DM without previous CVD.146 Metformin also reduced MI and increased survival when the study was extended for a further 8–10 years of intensified therapy, including the use of other drugs.149 Observational and database studies provide supporting evidence that long-term use of metformin improves CV prognosis.272,273 Still, there have been no large-scale randomized CV outcome trials (CVOTs) designed to assess the effect of metformin on CV events.
7.1.2.1.2 Sulfonylureas and meglinides
CV risk reduction with a sulfonylurea is more effective than modest lifestyle interventions alone, but is less effective than metformin.145,146,274–276 Sulfonylureas carry the risk of hypoglycaemia and, since the 1960s, there has been an ongoing debate on the CV safety of sulfonylureas. However, the CAROLINA (CARdiovascular Outcome Study of LINAgliptin Versus Glimepiride in Type 2 Diabetes) study, comparing the DPP4 inhibitor linagliptin vs. the sulfonylurea glimiperide, showed comparable CV safety of both drugs in patients with T2DM over 6.2 years.277 Nateglinide did not reduce major CV events in the Nateglinide And Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR) trial, a 5 year prospective study of IGT and CVD, or high CV risk.278
7.1.2.1.3 Alpha-glucosidase inhibitor
Acarbose did not alter MACE in patients with IGT and CVD during the large, 5 year, prospective ACE trial.129
7.1.2.1.4 Thiazolidinediones
The PROspective pioglitAzone Clinical Trial In macroVascular Events (PROactive) of pioglitazone was a neutral trial for its composite primary outcome (HR 0.90, 95% CI 0.80–1.02; P=0.095).279 Because of this, reported secondary outcomes should be viewed as hypothesis generating only. These included a nominally significant reduction of the secondary composite endpoint by 16% (HR 0.84, 95% CI 0.72–0.98; P=0.027),279 and the risk of subsequent MI and recurrent stroke by 16 and 47%, respectively,280,281 with a reduction in the risk of recurrent stroke in non-DM.282 The occurrence of HF was significantly higher with pioglitazone than with placebo in the PROactive trial, but without increased mortality.283 The Thiazolidinediones Or Sulfonylureas and Cardiovascular Accidents Intervention Trial (TOSCA.IT)—a large, randomized, but unblinded comparison of pioglitazone vs. sulfonylurea as add-on to metformin—was stopped prematurely because of futility. The composite endpoint and the individual components of the composite endpoint were similar in the two groups.284 In the IRIS trial of insulin-resistant subjects without DM, pioglitazone reduced the combined endpoint of recurrent stroke and MI by 24% vs. placebo over a median follow-up of 4.8 years.282 Following a meta-analysis of CV events with the thiazolidinedione rosiglitazone,285 the regulatory landscape for DM drugs underwent a major change in 2008,286 after which all future DM drugs were required to demonstrate designated margins of CV safety to achieve or maintain regulatory approval. This led to an increase in trials to assess CV outcomes with these therapies,287,288 most of which were designed to confirm non-inferiority of the experimental therapy vs. placebo added to background antihyperglycaemic treatment.
7.1.2.1.5 Insulin
In the ORIGIN trial, 12 537 people (mean age 63.5 years) at high CVD risk—with IFG, IGT, or DM—were randomized to long-acting insulin glargine [targeting an FPG level of 5.3 mmol/L (≤95 mg/dL)] or standard care. After a median follow-up of 6.2 years, the rates of CV outcomes were similar in the two groups.289 In DEVOTE, a double-blind comparison of ultra-long-acting degludec o.d. (n=3818) with insulin glargine U100 (n=3819) for 1.8 years in patients with DM at high CV risk found no significant differences in MACE (composite of CV death, non-fatal MI, or non-fatal stroke).290 A significant reduction in the frequency of hypoglycaemia was observed in the degludec arm. 290
7.1.2.2 Newer oral glucose-lowering drugs
7.1.2.2.1 Dipeptidyl peptidase-4 inhibitors
Five large prospective trials in T2DM populations with different CV risk (Table 10) that assessed the CV effects of DPP4 inhibitors have reported to date: saxagliptin [Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–thrombolysis in myocardial infarction 53 (SAVOR-TIMI 53)]291 alogliptin [Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE)],292 sitagliptin [Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS)],293 and linagliptin [Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus [CARMELINA]294 and CAROLINA277). Four of these trials confirmed statistical non-inferiority vs. placebo (which included alternative glucose-lowering medication to achieve glycaemic equipoise) for the primary composite CV outcome examined. However, none of the DPP4 inhibitors were associated with significant CV benefits in their trial populations, which comprised patients with a long history of DM and CVD, or clustered CVD risk factors. In the SAVOR-TIMI 53 trial, saxagliptin was associated with an increase in risk of hospitalization for HF,291 compared with a numerical, non-significant increase with alogliptin in EXAMINE,292 and no HF signal with sitagliptin in TECOS293 and with linagliptin in CARMELINA.294,295 Subgroup analyses of SAVOR-TIMI 53 suggested that high baseline NT-proBNP, pre-existing HF, or CKD conferred a greater risk of hospitalization for HF in saxagliptin-treated subjects.296 Only the CAROLINA study compared linagliptin vs. glimiperide as an active comparator and showed comparable CV safety of both drugs.277
Patient characteristics of cardiovascular safety studies with glucose-lowering agentsa
| SGLT2 inhibitors | GLP1-RAs | DPP4 inhibitors | ||||||||||||||
| Trial | EMPA-REG OUTCOME306 | CANVAS309 | DECLARE– TIMI 58311 | CREDENCE313 | ELIXA297 | LEADER176 | SUSTAIN-6299 | EXSCEL158 | Harmony Outcomes301 | REWIND303 | PIONEER 6300 | SAVOR− TIMI 53291 | EXAMINE292 | TECOS293 | CARMELINA294 | CAROLINA277 |
| Baseline | Empagliflozin vs. placebo | Canagliflozin vs. placebo | Dapagliflozin vs. placebo | Canaglifozin vs. placebo | Lixisenatide vs. placebo | Liraglutide vs. placebo | Semaglutide vs. placebo | Exenatide vs. placebo | Albiglutide vs. placebo | Dulaglutide vs. placebo | Oral Semaglutide vs. placebo | Saxagliptin vs. placebo | Alogliptin vs. placebo | Sitagliptin vs. placebo | Linagliptin vs. placebo | Linagliptin vs. glimiperide |
| n | 7020 | 10 142 | 17160 | 4401 | 6068 | 9340 | 3297 | 14 752 | 9463 | 9901 | 3182 | 16 492 | 5400 | 14 671 | 6979 | 6033 |
| Age (years) | 63 | 63 | 63 | 63 | 60 | 64 | 64 | 62 | 64 | 66 | 66 | 65 | 61 | 66 | 65 | 64 |
| DM (years) | 57% >10 | 13.5 | 11.8 | 15.8 | 9.3 | 12.8 | 13.9 | 12.0 | 14.1 | 10.5 | 14.9 | 10 | 7.2 | 9.4 | 14.7 | 6.2 |
| Body mass index (kg/m2) | 30.6 | 32.0 | 32.1 | 31.3 | 30.1 | 32.5 | 32.8 | 31.8 | 32 | 32.3 | 32.3 | 31 | 29 | 30 | 31.3 | 30.1 |
| Insulin (%) | 48 | 50 | ∼40 | 65 | 39 | 44 | 58 | 46 | 60 | 24 | 61 | 41 | 30 | 23 | 58 | 0 |
| HbA1c (%) | 8.1 | 8.2 | 8.3 | 8.3 | 7.7 | 8.7 | 8.7 | 8.0 | 8.7 | 7.2 | 8.2 | 8.0 | 8.0 | 7.3 | 7.9 | 7.2 |
| Previous CVD (%) | 99 | 65 | 40 | 50.4 | 100 | ∼81 | ∼83 | 73 | 100 | 31 | 35 | 78 | 100 | 100 | 57 | 42 |
| CV risk inclusion criteria | MI, CHD, CVD, or PVD | MI, CHD, CVD, or PVD | CVD or at least one CVRF | CKD | ACS <180 days | Age ≥50 years and CVD,bor CKD, or age ≥60 years and at least one CVRF | CHD, CVD, or PVD27% no previous CV event | MI, CHD, CVD, or PVD | Age ≥50 years and CVD or CVRFs | Age ≥50 years and CVD, or CKD, or age ≥60 years and CVRFs | Age ≥40 years and CVD (CHD, CVD, or PVD), or age ≥55 years and at least one CVRF | ACS <90 days | CHD, CVD, or PVD | CVD and/or CKD | CVD or evidence of vascular- related end-organ damage, or age ≥70 years, or at least two CVRFs | |
| Hypertension (%) | 94 | 89 | 89 | 96.8 | 76 | 92 | 92 | 90 | 86 | 93 | 94 | 81 | 83 | 86 | 95 | 90 |
| Follow-up (years) | 3.1 | 2.4 | 4.5 | 2.6 | 2.1 | 3.8 | 2.1 | 3.2 | 1.6 | 5.4 | 1.3 | 2.1 | 1.5 | 2.8 | 2.2 | 6.3 |
| SGLT2 inhibitors | GLP1-RAs | DPP4 inhibitors | ||||||||||||||
| Trial | EMPA-REG OUTCOME306 | CANVAS309 | DECLARE– TIMI 58311 | CREDENCE313 | ELIXA297 | LEADER176 | SUSTAIN-6299 | EXSCEL158 | Harmony Outcomes301 | REWIND303 | PIONEER 6300 | SAVOR− TIMI 53291 | EXAMINE292 | TECOS293 | CARMELINA294 | CAROLINA277 |
| Baseline | Empagliflozin vs. placebo | Canagliflozin vs. placebo | Dapagliflozin vs. placebo | Canaglifozin vs. placebo | Lixisenatide vs. placebo | Liraglutide vs. placebo | Semaglutide vs. placebo | Exenatide vs. placebo | Albiglutide vs. placebo | Dulaglutide vs. placebo | Oral Semaglutide vs. placebo | Saxagliptin vs. placebo | Alogliptin vs. placebo | Sitagliptin vs. placebo | Linagliptin vs. placebo | Linagliptin vs. glimiperide |
| n | 7020 | 10 142 | 17160 | 4401 | 6068 | 9340 | 3297 | 14 752 | 9463 | 9901 | 3182 | 16 492 | 5400 | 14 671 | 6979 | 6033 |
| Age (years) | 63 | 63 | 63 | 63 | 60 | 64 | 64 | 62 | 64 | 66 | 66 | 65 | 61 | 66 | 65 | 64 |
| DM (years) | 57% >10 | 13.5 | 11.8 | 15.8 | 9.3 | 12.8 | 13.9 | 12.0 | 14.1 | 10.5 | 14.9 | 10 | 7.2 | 9.4 | 14.7 | 6.2 |
| Body mass index (kg/m2) | 30.6 | 32.0 | 32.1 | 31.3 | 30.1 | 32.5 | 32.8 | 31.8 | 32 | 32.3 | 32.3 | 31 | 29 | 30 | 31.3 | 30.1 |
| Insulin (%) | 48 | 50 | ∼40 | 65 | 39 | 44 | 58 | 46 | 60 | 24 | 61 | 41 | 30 | 23 | 58 | 0 |
| HbA1c (%) | 8.1 | 8.2 | 8.3 | 8.3 | 7.7 | 8.7 | 8.7 | 8.0 | 8.7 | 7.2 | 8.2 | 8.0 | 8.0 | 7.3 | 7.9 | 7.2 |
| Previous CVD (%) | 99 | 65 | 40 | 50.4 | 100 | ∼81 | ∼83 | 73 | 100 | 31 | 35 | 78 | 100 | 100 | 57 | 42 |
| CV risk inclusion criteria | MI, CHD, CVD, or PVD | MI, CHD, CVD, or PVD | CVD or at least one CVRF | CKD | ACS <180 days | Age ≥50 years and CVD,bor CKD, or age ≥60 years and at least one CVRF | CHD, CVD, or PVD27% no previous CV event | MI, CHD, CVD, or PVD | Age ≥50 years and CVD or CVRFs | Age ≥50 years and CVD, or CKD, or age ≥60 years and CVRFs | Age ≥40 years and CVD (CHD, CVD, or PVD), or age ≥55 years and at least one CVRF | ACS <90 days | CHD, CVD, or PVD | CVD and/or CKD | CVD or evidence of vascular- related end-organ damage, or age ≥70 years, or at least two CVRFs | |
| Hypertension (%) | 94 | 89 | 89 | 96.8 | 76 | 92 | 92 | 90 | 86 | 93 | 94 | 81 | 83 | 86 | 95 | 90 |
| Follow-up (years) | 3.1 | 2.4 | 4.5 | 2.6 | 2.1 | 3.8 | 2.1 | 3.2 | 1.6 | 5.4 | 1.3 | 2.1 | 1.5 | 2.8 | 2.2 | 6.3 |
ACS = acute coronary syndromes; CANVAS = Canagliflozin Cardiovascular Assessment Study; CARMELINA = Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus; CAROLINA = Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Patients With Type 2 Diabetes; CHD = coronary heart disease; CKD = chronic kidney disease > stage 3; CREDENCE = Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation trial; CV = cardiovascular; CVD = cardiovascular disease; CVRF = cardiovascular risk factor; DECLARE–TIMI 58 = Dapagliflozin Effect on Cardiovascular Events−Thrombolysis In Myocardial Infarction 58 trial; DM = diabetes mellitus; DPP4 = dipeptidyl peptidase-4; ELIXA = Evaluation of Lixisenatide in Acute Coronary Syndrome; EMPA-REG OUTCOME = Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients–Removing Excess Glucose; EXAMINE = Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care; EXSCEL = Exenatide Study of Cardiovascular Event Lowering; GLP1-RA = glucagon-like peptide-1 receptor agonist; Harmony Outcomes = Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease; HbA1c = haemoglobin A1c; HF = heart failure (New York Heart Association class II or III); LEADER = Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results; MI = myocardial infarction; PIONEER 6 = A Trial Investigating the Cardiovascular Safety of Oral Semaglutide in Subjects With Type 2 Diabetes; PVD = peripheral vascular disease; REWIND = Researching Cardiovascular Events With a Weekly Incretin in Diabetes; SAVOR−TIMI 53 = Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus−Thrombolysis In Myocardial Infarction 53; SGLT2 = sodium-glucose co-transporter 2; SUSTAIN-6 = Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes; TECOS = Trial Evaluating Cardiovascular Outcomes with Sitagliptin.
Follow-up is median years.
Modified after.318
CVD in LEADER and SUSTAIN-6 included CHD, CVD, PVD and HF.
Patient characteristics of cardiovascular safety studies with glucose-lowering agentsa
| SGLT2 inhibitors | GLP1-RAs | DPP4 inhibitors | ||||||||||||||
| Trial | EMPA-REG OUTCOME306 | CANVAS309 | DECLARE– TIMI 58311 | CREDENCE313 | ELIXA297 | LEADER176 | SUSTAIN-6299 | EXSCEL158 | Harmony Outcomes301 | REWIND303 | PIONEER 6300 | SAVOR− TIMI 53291 | EXAMINE292 | TECOS293 | CARMELINA294 | CAROLINA277 |
| Baseline | Empagliflozin vs. placebo | Canagliflozin vs. placebo | Dapagliflozin vs. placebo | Canaglifozin vs. placebo | Lixisenatide vs. placebo | Liraglutide vs. placebo | Semaglutide vs. placebo | Exenatide vs. placebo | Albiglutide vs. placebo | Dulaglutide vs. placebo | Oral Semaglutide vs. placebo | Saxagliptin vs. placebo | Alogliptin vs. placebo | Sitagliptin vs. placebo | Linagliptin vs. placebo | Linagliptin vs. glimiperide |
| n | 7020 | 10 142 | 17160 | 4401 | 6068 | 9340 | 3297 | 14 752 | 9463 | 9901 | 3182 | 16 492 | 5400 | 14 671 | 6979 | 6033 |
| Age (years) | 63 | 63 | 63 | 63 | 60 | 64 | 64 | 62 | 64 | 66 | 66 | 65 | 61 | 66 | 65 | 64 |
| DM (years) | 57% >10 | 13.5 | 11.8 | 15.8 | 9.3 | 12.8 | 13.9 | 12.0 | 14.1 | 10.5 | 14.9 | 10 | 7.2 | 9.4 | 14.7 | 6.2 |
| Body mass index (kg/m2) | 30.6 | 32.0 | 32.1 | 31.3 | 30.1 | 32.5 | 32.8 | 31.8 | 32 | 32.3 | 32.3 | 31 | 29 | 30 | 31.3 | 30.1 |
| Insulin (%) | 48 | 50 | ∼40 | 65 | 39 | 44 | 58 | 46 | 60 | 24 | 61 | 41 | 30 | 23 | 58 | 0 |
| HbA1c (%) | 8.1 | 8.2 | 8.3 | 8.3 | 7.7 | 8.7 | 8.7 | 8.0 | 8.7 | 7.2 | 8.2 | 8.0 | 8.0 | 7.3 | 7.9 | 7.2 |
| Previous CVD (%) | 99 | 65 | 40 | 50.4 | 100 | ∼81 | ∼83 | 73 | 100 | 31 | 35 | 78 | 100 | 100 | 57 | 42 |
| CV risk inclusion criteria | MI, CHD, CVD, or PVD | MI, CHD, CVD, or PVD | CVD or at least one CVRF | CKD | ACS <180 days | Age ≥50 years and CVD,bor CKD, or age ≥60 years and at least one CVRF | CHD, CVD, or PVD27% no previous CV event | MI, CHD, CVD, or PVD | Age ≥50 years and CVD or CVRFs | Age ≥50 years and CVD, or CKD, or age ≥60 years and CVRFs | Age ≥40 years and CVD (CHD, CVD, or PVD), or age ≥55 years and at least one CVRF | ACS <90 days | CHD, CVD, or PVD | CVD and/or CKD | CVD or evidence of vascular- related end-organ damage, or age ≥70 years, or at least two CVRFs | |
| Hypertension (%) | 94 | 89 | 89 | 96.8 | 76 | 92 | 92 | 90 | 86 | 93 | 94 | 81 | 83 | 86 | 95 | 90 |
| Follow-up (years) | 3.1 | 2.4 | 4.5 | 2.6 | 2.1 | 3.8 | 2.1 | 3.2 | 1.6 | 5.4 | 1.3 | 2.1 | 1.5 | 2.8 | 2.2 | 6.3 |
| SGLT2 inhibitors | GLP1-RAs | DPP4 inhibitors | ||||||||||||||
| Trial | EMPA-REG OUTCOME306 | CANVAS309 | DECLARE– TIMI 58311 | CREDENCE313 | ELIXA297 | LEADER176 | SUSTAIN-6299 | EXSCEL158 | Harmony Outcomes301 | REWIND303 | PIONEER 6300 | SAVOR− TIMI 53291 | EXAMINE292 | TECOS293 | CARMELINA294 | CAROLINA277 |
| Baseline | Empagliflozin vs. placebo | Canagliflozin vs. placebo | Dapagliflozin vs. placebo | Canaglifozin vs. placebo | Lixisenatide vs. placebo | Liraglutide vs. placebo | Semaglutide vs. placebo | Exenatide vs. placebo | Albiglutide vs. placebo | Dulaglutide vs. placebo | Oral Semaglutide vs. placebo | Saxagliptin vs. placebo | Alogliptin vs. placebo | Sitagliptin vs. placebo | Linagliptin vs. placebo | Linagliptin vs. glimiperide |
| n | 7020 | 10 142 | 17160 | 4401 | 6068 | 9340 | 3297 | 14 752 | 9463 | 9901 | 3182 | 16 492 | 5400 | 14 671 | 6979 | 6033 |
| Age (years) | 63 | 63 | 63 | 63 | 60 | 64 | 64 | 62 | 64 | 66 | 66 | 65 | 61 | 66 | 65 | 64 |
| DM (years) | 57% >10 | 13.5 | 11.8 | 15.8 | 9.3 | 12.8 | 13.9 | 12.0 | 14.1 | 10.5 | 14.9 | 10 | 7.2 | 9.4 | 14.7 | 6.2 |
| Body mass index (kg/m2) | 30.6 | 32.0 | 32.1 | 31.3 | 30.1 | 32.5 | 32.8 | 31.8 | 32 | 32.3 | 32.3 | 31 | 29 | 30 | 31.3 | 30.1 |
| Insulin (%) | 48 | 50 | ∼40 | 65 | 39 | 44 | 58 | 46 | 60 | 24 | 61 | 41 | 30 | 23 | 58 | 0 |
| HbA1c (%) | 8.1 | 8.2 | 8.3 | 8.3 | 7.7 | 8.7 | 8.7 | 8.0 | 8.7 | 7.2 | 8.2 | 8.0 | 8.0 | 7.3 | 7.9 | 7.2 |
| Previous CVD (%) | 99 | 65 | 40 | 50.4 | 100 | ∼81 | ∼83 | 73 | 100 | 31 | 35 | 78 | 100 | 100 | 57 | 42 |
| CV risk inclusion criteria | MI, CHD, CVD, or PVD | MI, CHD, CVD, or PVD | CVD or at least one CVRF | CKD | ACS <180 days | Age ≥50 years and CVD,bor CKD, or age ≥60 years and at least one CVRF | CHD, CVD, or PVD27% no previous CV event | MI, CHD, CVD, or PVD | Age ≥50 years and CVD or CVRFs | Age ≥50 years and CVD, or CKD, or age ≥60 years and CVRFs | Age ≥40 years and CVD (CHD, CVD, or PVD), or age ≥55 years and at least one CVRF | ACS <90 days | CHD, CVD, or PVD | CVD and/or CKD | CVD or evidence of vascular- related end-organ damage, or age ≥70 years, or at least two CVRFs | |
| Hypertension (%) | 94 | 89 | 89 | 96.8 | 76 | 92 | 92 | 90 | 86 | 93 | 94 | 81 | 83 | 86 | 95 | 90 |
| Follow-up (years) | 3.1 | 2.4 | 4.5 | 2.6 | 2.1 | 3.8 | 2.1 | 3.2 | 1.6 | 5.4 | 1.3 | 2.1 | 1.5 | 2.8 | 2.2 | 6.3 |
ACS = acute coronary syndromes; CANVAS = Canagliflozin Cardiovascular Assessment Study; CARMELINA = Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus; CAROLINA = Cardiovascular Outcome Study of Linagliptin Versus Glimepiride in Patients With Type 2 Diabetes; CHD = coronary heart disease; CKD = chronic kidney disease > stage 3; CREDENCE = Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation trial; CV = cardiovascular; CVD = cardiovascular disease; CVRF = cardiovascular risk factor; DECLARE–TIMI 58 = Dapagliflozin Effect on Cardiovascular Events−Thrombolysis In Myocardial Infarction 58 trial; DM = diabetes mellitus; DPP4 = dipeptidyl peptidase-4; ELIXA = Evaluation of Lixisenatide in Acute Coronary Syndrome; EMPA-REG OUTCOME = Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients–Removing Excess Glucose; EXAMINE = Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care; EXSCEL = Exenatide Study of Cardiovascular Event Lowering; GLP1-RA = glucagon-like peptide-1 receptor agonist; Harmony Outcomes = Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease; HbA1c = haemoglobin A1c; HF = heart failure (New York Heart Association class II or III); LEADER = Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results; MI = myocardial infarction; PIONEER 6 = A Trial Investigating the Cardiovascular Safety of Oral Semaglutide in Subjects With Type 2 Diabetes; PVD = peripheral vascular disease; REWIND = Researching Cardiovascular Events With a Weekly Incretin in Diabetes; SAVOR−TIMI 53 = Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus−Thrombolysis In Myocardial Infarction 53; SGLT2 = sodium-glucose co-transporter 2; SUSTAIN-6 = Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes; TECOS = Trial Evaluating Cardiovascular Outcomes with Sitagliptin.
Follow-up is median years.
Modified after.318
CVD in LEADER and SUSTAIN-6 included CHD, CVD, PVD and HF.
7.1.2.2.2 Glucagon-like peptide-1 receptor agonists
Seven CVOTs have examined the effects of GLP1-RAs on CV events in patients with DM and high CV risk. In the Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial, lixisenatide 10 or 20 μg o.d. was non-inferior to placebo, but did not significantly affect a four-point MACE (three-point MACE plus hospitalization for unstable angina) in patients with DM post-ACS.297 In the Exenatide Study of Cardiovascular Event Lowering (EXSCEL) study of a DM population in whom 73% had experienced a previous CV event, exenatide 2 mg once weekly showed non-inferiority vs. placebo and a numerical, but non-significant, 14% reduction of the primary three-point MACE.158 The intention-to-treat analysis revealed a significant reduction in all-cause death by exenatide of 14% (P=0.016), but this result has to be considered exploratory given the hierarchical statistical testing. However, in the subgroup with known CVD, those treated with exenatide demonstrated a 10% relative risk reduction for MACE (HR 0.90, 95% CI, 0.816–0.999; nominal P=0.047).
In the LEADER trial, 9340 patients with DM at high CV risk (81% with previous CVD) were randomized to liraglutide 0.6 − 1.8 mg o.d. vs. placebo as add-on to other glucose-lowering drugs. All patients had a long history of DM and CVRFs that were well controlled. After a follow-up of 3.1 years, liraglutide significantly reduced the composite three-point primary endpoint (CV death, non-fatal MI, or non-fatal stroke) by 13%. In addition, liraglutide significantly reduced CV death and total death by 22 and 15%, respectively, and produced a non-significant numerical reduction in non-fatal MI and non-fatal stroke.176 Pre-specified secondary analyses showed lower rates of development and progression of CKD with liraglutide compared with placebo.298 The Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) was a phase III pre-approval study in which a smaller population of 3297 patients with DM and high CV risk (73% with CVD) were randomized to semaglutide 0.5–1.0 mg once weekly vs. placebo. After 2.1 years, semaglutide significantly reduced the three-point MACE by 26%, an effect driven mainly by a 39% significant reduction of non-fatal stroke. Moreover, semaglutide led to a non-significant numerical reduction of non-fatal MI. Semaglutide also reduced the secondary endpoint of new or worsening nephropathy.299 The Peptide Innovation for Early Diabetes Treatment (PIONEER)-6 trial, also a phase III pre-approval CVOT, examined the effect of oral semaglutide o.d. (target dose 14 mg) vs. placebo on CV outcomes in patients with T2DM and high CV risk. Non-inferiority for CV safety of oral semaglutide was confirmed with an HR of 0.79 (P < 0.001) in favour of oral semaglutide compared with placebo over a median follow-up of 16 months. Moreover, semaglutide significantly reduced the risk for CV death [15 (0.9%) events with oral semaglutide vs. 30 (1.9%) events with placebo, HR 0.49, P=0.03] and all-cause death [23 (1.4%) events in the semaglutide vs. 45 (2.8%) events in the placebo group, HR 0.51, P=0.008].300 However, albeit low in absolute numbers, there was a significant increase in retinopathy complications, including vitreous haemorrhage, blindness, or requirement for intravitreal agent or photocoagulation, the implications of which require further study. In the Albiglutide and CV outcomes in patients with type 2 DM and CVD (Harmony Outcomes) trial, once weekly albiglutide, a no-longer marketed GLP1-RA, led to a significant 22% reduction of three-point MACE compared with placebo in patients with DM and manifest CVD. In addition, albiglutide significantly reduced MI by 25%.301 A recent meta-analysis of five of these trials suggests that GLP-RAs reduce three-point MACE by 12% (HR 0.88, 95% CI 0.84–0.94; P < 0.001).302 The Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND) trial assessed the effect of once weekly subcutaneous dulaglutide (1.5 mg) vs. placebo on three-point MACE in 9901 subjects with T2DM, who had either a previous CV event or CVRFs. During a median follow-up of 5.4 years, the primary composite outcome occurred in 594 (12.0%) participants in the dulaglutide group and in 663 (13.4%) participants in the placebo group (HR 0.88, 95% CI 0.79–0·99; P=0.026).303
Although the mechanisms through which some of these GLP-RAs reduced CV outcomes have not been established, their long half-lives may be contributing to their CV benefits. In addition, GLP1-RAs improve several CV parameters, including a small reduction in SBP and weight loss, and have direct vascular and cardiac effects that may contribute to the results.304 The gradual divergence of the event curves in the trials suggests that the CV benefit is mediated by a reduction in atherosclerosis-related events.
7.1.2.2.3 Sodium-glucose co-transporter 2 inhibitors
Four CVOTs with SGLT2 inhibitors [Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients–Removing Excess Glucose (EMPA-REG OUTCOME), the Canagliflozin Cardiovascular Assessment Study (CANVAS) Program, Dapagliflozin Effect on Cardiovascular Events−Thrombolysis In Myocardial Infarction (DECLARE−TIMI 58), and the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial] have been published. In EMPA-REG OUTCOME, 7020 patients with DM of long duration (57% >10 years) and CVD were randomized to empagliflozin 10 or 25 mg o.d., or placebo; patients were followed for a mean of 3.1 years.305 The patient population was well treated with good management of risk factors (mean BP 135/77 mmHg and mean LDL-C 2.2 mmol/L). Empagliflozin significantly reduced the risk of the three-point composite primary outcome (CV death, non-fatal MI, or non-fatal stroke) by 14% compared with placebo. This reduction was driven mainly by a highly significant 38% reduction in CV death (P < 0.0001), with separation of the empagliflozin and placebo arms evident as early as 2 months into the trial. There was a non-significant 13% reduction of non-fatal MI (P=0.30) and a non-significant 24% increased risk of non-fatal stroke.306 In a secondary analysis, empagliflozin was associated with a 35% reduction in hospitalization for HF (P < 0.002), with separation of the empagliflozin and placebo groups evident almost immediately after treatment initiation, suggesting a very early effect on HF risk. Empagliflozin also reduced overall mortality by 32% (P < 0.0001), a highly significant effect, translating into a number needed to treat of 39 over 3 years to prevent one death. These findings were consistent in all subgroups. Additional analyses from EMPA-REG OUTCOME revealed that the CV benefit was gained by those with and without HF at baseline, the latter comprising ∼10% of the study cohort.307
The CANVAS Program integrated data from two RCTs (CANVAS and CANVAS-R), in which 10 142 patients with DM at high CV risk were randomized to canagliflozin 100–300 mg o.d. vs. placebo.308 After 3.1 years, canagliflozin significantly reduced a composite three-point MACE by 14% (P=0.02), but did not significantly alter CV death or overall death.309 Similar to the findings in EMPA-REG OUTCOME, canaglifozin significantly reduced HF hospitalization. However, canagliflozin led to an unexplained increased incidence in lower limb fractures and amputations (albeit low numbers), a finding that was not replicated in a recent large cohort study.310
DECLARE–TIMI 58 examined the effect of 10 mg dapagliflozin o.d. vs. placebo in 17 160 patients with DM and CVD, or multiple CVRFs, among them 10 186 without atherosclerotic CVD.311 After a median follow-up of 4.2 years, dapagliflozin met the pre-specified criterion for non-inferiority for the composite three-point MACE compared with placebo. In the two primary efficacy analyses, dapaglifozin did not significantly reduce MACE, but resulted in a lower rate of the combined endpoint of CV death or HF hospitalization (4.9 vs. 5.8%; HR 0.83, 95% CI 0.73 − 0.95; P=0.005). This was driven by a lower rate of HF hospitalizations (HR 0.73, 95% CI 0.61 − 0.88), but no between-group difference in CV death (HR 0.98, 95% CI 0.82 − 1.17). The benefit of dapagliflozin with respect to CV death or HF hospitalization was similar in the subgroup with CVD, as well as those with multiple risk factors only. A meta-analysis of the three trials suggested consistent benefits on reducing the composite of HF hospitalization or CV death, as well as on the progression of kidney disease, regardless of existing atherosclerotic CVD or a history of HF, while the reduction in MACE was only apparent in patients with established CVD.312 The CREDENCE trial313 randomized 4401 patients with T2DM and albuminuric CKD (eGFR 30 to <90 mL/min/1.73 m2) to canagliflozin or placebo, and showed a relative reduction of the primary renal outcome of 30% by canagliflozin after a median follow-up of 2.6 years. In addition, canagliflozin significantly reduced the pre-specified secondary CV outcomes of three-point MACE (HR 0.80, 95% CI 0.67 − 0.95; P=0.01) and hospitalization for HF (HR 0.61, 95% CI 0.47–0.80; P < 0.001) compared with placebo in this very high-CV risk group of patients (see section 11).313
The CV benefits of SGLT2 inhibitors are mostly unrelated to the extent of glucose lowering and occur too early to be the result of weight reduction. The rapid separation of placebo and active arms in the four studies in terms of reduction in HF hospitalizations indicates that the beneficial effects achieved in these trials are more likely the result of a reduction in HF-associated events. They could involve effects on haemodynamic parameters, such as reduced plasma volume, direct effects on cardiac metabolism and function, or other CV effects.314–317
7.1.2.3 Implications of recent cardiovascular outcome trials
For the first time in the history of DM, we have data from several CVOTs that indicate CV benefits from the use of glucose-lowering drugs in patients with CVD or at very high/high CV risk. The results obtained from these trials, using both GLP1-RAs (LEADER, SUSTAIN-6, Harmony Outcomes, REWIND, and PIONEER 6) and SGLT2 inhibitors (EMPA-REG OUTCOME, CANVAS, DECLARE-TIMI 58, and CREDENCE), strongly suggest that these drugs should be recommended in patients with T2DM with prevalent CVD or very high/high CV risk, such as those with target-organ damage or several CVRFs (see Table 7), whether they are treatment naïve or already on metformin. In addition, based on the mortality benefits seen in LEADER and EMPA-REG OUTCOME, liraglutide is recommended in patients with prevalent CVD or very high/high CV risk, and empagliflozin is recommended in patients with prevalent CVD, to reduce the risk of death. The recommendation for empagliflozin is supported by a recent meta-analysis which found high heterogeneity between CVOTs in mortality reduction.312 The benefits seen with GLP1-RAs are most likely derived through the reduction of arteriosclerosis-related events, whereas SGLT2 inhibitors seem to reduce HF-related endpoints. Thus, SGLT2 inhibitors are potentially of particular benefit in patients who exhibit a high risk for HF. In subjects with newly diagnosed T2DM without CVD and at moderate risk, the results of UKPDS suggest a beneficial effect of metformin in primary prevention. Although the trial-based evidence for metformin monotherapy from UKPDS is not as strong as with the novel drugs tested in recent CVOTs, it is supported by extensive observations from everyday clinical practice. In the recent CVOTs, a majority of patients received metformin before and concurrently with the newer drug under test. However, because metformin was similarly present in the active and placebo groups, it is unlikely to explain the beneficial effects of the newer drugs under test. Thus, the choice of drug to reduce CV events in patients with T2DM should be prioritized based on the presence of CVD and CV risk (Figure 3).
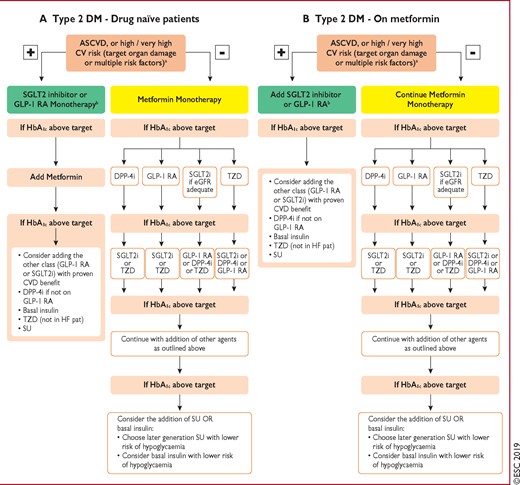
Treatment algorithm in patients with type 2 diabetes mellitus and atherosclerotic cardiovascular disease, or high/very high CV risk Treatment algorithms for (A) drug-naïve and (B) metformin-treated patients with diabetes mellitus. ASCVD = atherosclerotic cardiovascular disease: CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; DPP4i = dipeptidyl peptidase-4 inhibitor; eGFR = estimated glomerular filtration rate; GLP1-RA = glucagon-like peptide-1 receptor agonist; HbA1c = haemoglobin A1c; HF = heart failure; SGLT2i = sodium-glucose co-transporter 2 inhibitor; SU = sulphonylureas; T2DM = type 2 diabetes mellitus; TZD = thiazolidinedione. aSee Table 7. bUse drugs with proven CVD benefit.
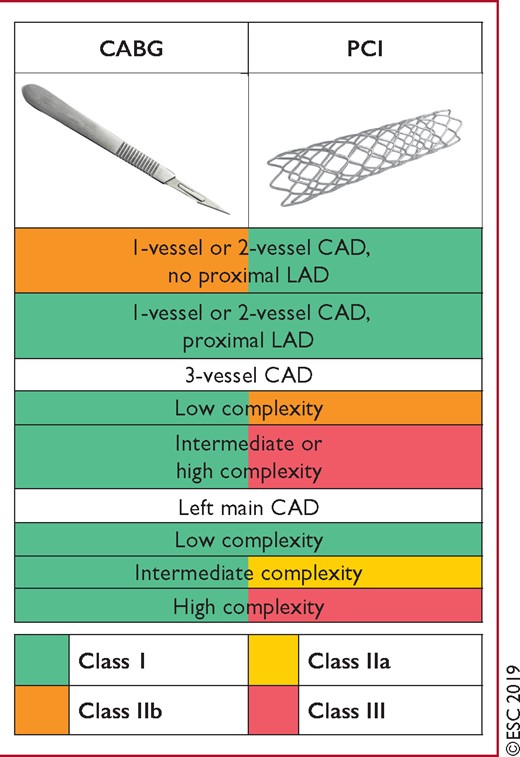
Recommendations for coronary revascularization. SYNTAX score calculation: http://www.syntaxscore.com. CABG = coronary artery bypass grafting; CAD = coronary artery disease; High complexity = SYNTAX score ≥33; Intermediate complexity = SYNTAX score 23–32; LAD = left anterior descending coronary artery; Low complexity = SYNTAX score 0–22; PCI = percutaneous coronary intervention; SYNTAX = Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery.
Recommendations for glucose-lowering treatment for patients with diabetes
 |
 |
ACS = acute coronary syndromes; CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; DPP4 = dipeptidyl peptidase-4; GLP1-RA = glucagon-like peptide-1 receptor agonist; HF = heart failure; SGLT2 = sodium-glucose co-transporter 2; T2DM = type 2 diabetes mellitus.
Class of recommendation.
Level of evidence.
See Table 7.
Recommendations for glucose-lowering treatment for patients with diabetes
 |
 |
ACS = acute coronary syndromes; CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; DPP4 = dipeptidyl peptidase-4; GLP1-RA = glucagon-like peptide-1 receptor agonist; HF = heart failure; SGLT2 = sodium-glucose co-transporter 2; T2DM = type 2 diabetes mellitus.
Class of recommendation.
Level of evidence.
See Table 7.
7.1.3 Specific cardiovascular therapies
7.1.3.1 Beta-blockers
In CCS, beta-blockers are effective at reducing both exercise-induced angina and asymptomatic ischaemic episodes, while improving exercise capacity.254 Their favourable impact on prognosis is questionable, and was not confirmed by a propensity score-matched analysis of patients included in a large observational study.320 Long-term beta-blocker administration in patients with DM has recently been questioned by a prospective observational study, as well as a post hoc analysis from the ACCORD study, suggesting increased all-cause death in DM patients treated with beta-blockers.321,322 Further assessment is needed in the future.
In contrast, the benefit of long-term administration of oral beta-blockers in the post-MI phase is established in patients with HF and LV ejection fraction (LVEF) <40%, as outlined in section 8.4.2.252,323 Carvedilol and nebivolol may be preferred because of their ability to improve insulin sensitivity, with no negative effects on glycaemic control.324,325
7.1.3.2 Blockers of the renin–angiotensin–aldosterone system
Treatment with ACEIs is recommended to prevent major CV events, and HF, in all patients with CCS or ACS and systolic LV dysfunction, based on a systematic review of RCTs.326 An ARB should be administered in patients intolerant of ACEIs. Finally, mineralocorticoid receptor antagonists (MRA) are recommended in patients with LV systolic dysfunction or HF after MI.252,327
7.1.3.3 Lipid-lowering drugs
Details of lipid-lowering drugs are outlined in section 6.4.1.
7.1.3.4 Nitrates and calcium channel blockers
Nitrates (preferably short-acting) and calcium channel blockers are indicated for relief of angina symptoms,255 and are frequently used when beta-blockers are contraindicated or not tolerated, or in addition to beta-blockers if patients remain symptomatic, but offer no prognostic benefit.255
7.1.3.5 Other anti-ischaemic drugs
Ranolazine is a selective inhibitor of the late sodium current, effective in the treatment of chronic angina.255 When added to one or more antianginal drugs in patients with DM, ranolazine further reduced the number of ischaemic episodes and the use of nitrates compared with placebo.328 Ranolazine also has metabolic effects and may lower HbA1c levels in patients with DM.329 Trimetazidine is an anti-ischaemic metabolic modulator that improves glucose control and cardiac function in patients with DM,330,331 as well as effort-induced myocardial ischaemia in patients with CCS.332,333 The drug was reviewed by the European Medicines Agency in 2012, and is contraindicated in Parkinson’s disease and motion disorders.334 Ivabradine inhibits the If current—the primary modulator of spontaneous diastolic depolarization in the sinus node—resulting in heart rate lowering and antianginal effects. These drugs should be considered as second line treatment.255,335
7.1.3.6 Antiplatelet and antithrombotic drugs
There is no evidence at the moment to support different antiplatelet strategies in patients with ACS or CCS with vs. without DM (see also section 6.5).72,252,253,336
7.1.3.6.1 Aspirin
In secondary prevention, low-dose (75–160 mg) aspirin, alone or in combination (see section 7.1.3.6.2 below), remains the recommended drug in patients with DM.72
7.1.3.6.2 P2Y12 receptor blockers
Clopidogrel provides an alternative for aspirin-intolerant patients, and is combined with low-dose aspirin as dual antiplatelet therapy (DAPT) (clopidogrel 75 mg o.d. and aspirin 75–160 mg o.d.) in patients with ACS and those undergoing PCI, with unchanged evidence since the 2013 Guidelines.72 A post hoc analysis of the CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance) trial suggested that clopidogrel, added to background aspirin, may increase overall and CV death in DM patients with microalbuminuria (≥30 μg/mL).337 In patients with ACS, DAPT with prasugrel338 or ticagrelor339 on a background of low-dose aspirin was superior to DAPT with clopidogrel in the DM subgroup, with a benefit similar to that in the population without DM. Patients with DM tended to have a greater reduction in ischaemic events with prasugrel than clopidogrel,338 without an increase in major bleeding. The Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin−Thrombolysis In Myocardial Infarction 54 (PEGASUS−TIMI 54) trial compared adding ticagrelor 60 or 90 mg b.i.d. vs. placebo to a background of low-dose aspirin in patients who experienced an MI 1 − 3 years before recruitment into the study.340 The relative risk reduction of MACE with ticagrelor was similar in the DM and non-DM cohorts (HR 0.84, 95% CI 0.72–0.99 and HR 0.84, 95% CI 0.74–0.96, respectively). Ticagrelor was associated with an increase in major bleeding, which was similar in the two groups (HR 2.56, 95% CI 1.52–4.33 and HR 2.47, 95% CI 1.73–3.53 in DM vs. non-DM, respectively).340
7.1.3.6.3 Novel oral anticoagulant drugs
In the Anti-Xa Therapy to Lower cardiovascular events in Addition to Standard therapy in subjects with Acute Coronary Syndrome-TIMI 51 (ATLAS-ACS_TIMI 51) trial in patients with a recent ACS (32% DM), a low dose of the activated factor Xa blocker rivaroxaban (2.5 mg b.i.d.) added to DAPT significantly reduced CV death, MI, or stroke compared with placebo (9.1 vs. 10.7%; HR 0.84, 95% CI 0.72–0.97; P=0.02).341 This benefit was associated with a significant increase in major, non-CABG-related bleeding (1.8 vs. 0.6%) and intracranial haemorrhage (0.4 vs. 0.2%) in the rivaroxaban arm, with no difference in fatal bleeding.341 The Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial recruited 27 395 patients with stable atherosclerotic disease and showed that low-dose aspirin (100 mg o.d.) combined with a low dose of rivaroxaban (2.5 mg b.i.d.) was superior to aspirin alone in preventing MI, stroke, or CV death (4.1 vs. 5.4%, respectively; HR 0.76, 95% CI 0.66–0.86; P < 0.001).342 Major bleeding, but not fatal or intracranial bleeding, was increased (HR 1.7, 95% CI 1.7–2.05; P<0.001). The net clinical benefit favoured the combination (HR 0.80, 95% CI 0.70–0.91; P<0.001 vs. aspirin alone). Approximately 38% of the overall COMPASS population had DM, and the proportional benefit–risk profile of the aspirin/rivaroxaban combination over aspirin alone was similar in both populations.343
Of potential major importance was the finding that in patients with lower extremity artery disease (LEAD), adverse limb events plus major amputations were reduced by 46% (see section 10.2.3). Of the patients enrolled in the COMPASS trial, 24 824 were specifically diagnosed with stable CAD (CCS).
7.1.3.6.4 Other anticoagulant strategies
A variety of antiplatelet and antithrombotic strategies have been used in patients with ACS undergoing PCI. These include glycoprotein IIb/IIIa inhibitors, unfractionated heparin, and bivalirudin. The indications for their use are discussed in the 2018 ESC/European Association for Cardio-Thoracic Surgery (EACTS) Guidelines on myocardial revascularization.344
Recommendations for the management of patients with diabetes and acute or chronic coronary syndromes
 |
 |
Recommendations on glucose targets are outlined in section 6.2.1. Recommendations on glucose-lowering drugs for DM are outlined in section 7.1.2.
ACEI = angiotensin-converting enzyme inhibitor; ACS = acute coronary syndromes; ARB = angiotensin receptor blocker; b.i.d. = twice daily (bis in die); CABG = coronary artery bypass graft; CAD = coronary artery disease; CCS = chronic coronary syndromes; CV = cardiovascular; DAPT = dual antiplatelet therapy; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; PCI = percutaneous coronary intervention.
Class of recommendation.
Level of evidence.
Full-dose clopidogrel or reduced-dose ticagrelor (60 mg b.i.d.).
Prior history of intracerebral haemorrhage or ischaemic stroke, history of other intracranial pathology, recent gastrointestinal bleeding or anaemia due to possible gastrointestinal blood loss, other gastrointestinal pathology associated with increased bleeding risk, liver failure, bleeding diathesis or coagulopathy, extreme old age or frailty, or renal failure requiring dialysis or with eGFR <15 mL/min/1.73 m2.
Recommendations for the management of patients with diabetes and acute or chronic coronary syndromes
 |
 |
Recommendations on glucose targets are outlined in section 6.2.1. Recommendations on glucose-lowering drugs for DM are outlined in section 7.1.2.
ACEI = angiotensin-converting enzyme inhibitor; ACS = acute coronary syndromes; ARB = angiotensin receptor blocker; b.i.d. = twice daily (bis in die); CABG = coronary artery bypass graft; CAD = coronary artery disease; CCS = chronic coronary syndromes; CV = cardiovascular; DAPT = dual antiplatelet therapy; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; PCI = percutaneous coronary intervention.
Class of recommendation.
Level of evidence.
Full-dose clopidogrel or reduced-dose ticagrelor (60 mg b.i.d.).
Prior history of intracerebral haemorrhage or ischaemic stroke, history of other intracranial pathology, recent gastrointestinal bleeding or anaemia due to possible gastrointestinal blood loss, other gastrointestinal pathology associated with increased bleeding risk, liver failure, bleeding diathesis or coagulopathy, extreme old age or frailty, or renal failure requiring dialysis or with eGFR <15 mL/min/1.73 m2.
7.2 Revascularization
The anatomical pattern of CAD in patients with DM influences prognosis and the response to revascularization. Angiographic studies have shown that patients with DM are more likely to have left main CAD and multivessel CAD, and that coronary pathology is more frequently diffuse and involves the small vessels.357 In addition, DM frequently has comorbidities, such as CKD, cerebrovascular disease, and LEAD, which adversely affect outcomes after coronary revascularization. The indications for myocardial revascularization, for both symptomatic and prognostic reasons, are the same in patients with and without DM, and have been summarized in the 2018 ESC/EACTS Guidelines on myocardial revascularization.344 In the BARI 2D trial, patients with DM and stable CAD were randomized to optimal medical treatment alone or to revascularization (either PCI or CABG) plus optimal medical treatment.358 After 5 years, no significant differences were noted in the combined endpoint of death, MI, or stroke between groups. Paralleling the observation in non-DM, the negative impact of incomplete revascularization has also been documented in patients with DM.359 In the setting of chronic HF of ischaemic origin, only one RCT (involving 1212 patients) has compared revascularization (with CABG) plus optimal medical management vs. optimal medical management alone in patients with LVEF ≤35%, and found a significant survival benefit in patients allocated to revascularization at a mean follow-up of 9.8 years.360 The benefit observed among patients with DM was of the same degree, but did not reach statistical significance. In non-ST-segment elevation ACS, a meta-analysis of nine RCTs including 9904 patients suggested a similar benefit at 12 months in terms of death, non-fatal MI, or hospitalization for an ACS from an early invasive strategy compared with a conservative strategy in patients with and without DM.361 Yet, because of higher baseline risk, the absolute risk reduction was more pronounced in those with DM. A recent meta-analysis of data from individual patients (n=5324) suggested that at a median follow-up of 6 months, an early invasive strategy compared with a delayed strategy was associated with reduced mortality in patients with DM (HR 0.67, 95% CI 0.45–0.99) in the absence of a reduction in recurrent MI.362
7.2.1 Percutaneous coronary intervention vs. coronary artery bypass graft surgery
DM should be considered as a distinct disease entity that is critical for the selection of myocardial revascularization strategies in multivessel disease.
Three RCTs have compared the two revascularization modalities in patients with DM, mostly in the setting of stable multivessel CAD using mainly first-generation drug-eluting stents (DES), but one of them was prematurely terminated and underpowered.363 In the Coronary Artery Revascularization in Diabetes (CARDia) trial, 510 patients with multivessel or complex single-vessel CAD were randomized to CABG or PCI, with a bare-metal stent (BMS) or a first-generation DES.364 There were no differences between the groups for the primary endpoint of 1 year death, MI, or stroke, but this trial was also underpowered. Repeat revascularization occurred more frequently with PCI (P < 0.001). The Future Revascularization Evaluation in Patients with Diabetes Mellitus (FREEDOM) trial randomized 1900 patients with multivessel CAD, but no left main stenosis, to elective CABG or PCI with a first-generation DES.365 The primary endpoint of all-cause death, non-fatal MI, or stroke at 5 years occurred in 26.6% of patients in the PCI group and in 18.7% patients in the CABG group (P=0.005). The incidences of death (16.3 vs. 10.9%; P=0.049) and MI (13.9 vs. 6.0%; P < 0.001) were higher in the PCI group, while the incidence of stroke was lower (2.4 vs. 5.2%; P=0.03). While patients on insulin had higher event rates, no significant interaction for the primary endpoint was observed between insulin status and treatment effect.366 In addition, no interaction was observed between treatment effect and degree of coronary complexity, as assessed by the Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) score.
In the DM subgroup (n=452) enrolled in the SYNTAX trial, there were no differences between PCI with a first-generation DES and CABG in the composite endpoint of death, stroke, or MI at 5 years. However, the 5 year rates of major adverse CV and cerebrovascular events (MACCE) (PCI 46.5% vs. CABG 29.0%; P < 0.001), and the need for repeat revascularization (HR 2.75; P < 0.001) were higher in the PCI group.367
Overall, the meta-analysis of 3052 patients with DM randomized to PCI with mainly first-generation DES vs. CABG reported a higher risk of death or MI with PCI (relative risk 1.51; P=0.01), while the risk of stroke was lower (relative risk 0.59; P=0.01).368 A sensitivity analysis showed that the superiority of CABG over PCI in terms of MACCE was more pronounced with complex CAD (high SYNTAX score). The most recent meta-analysis of 11 RCTs, involving 11 518 patients allocated to PCI with stents (BMS or DES) or CABG, showed that 5 year all-cause mortality was 11.2% after PCI and 9.2% after CABG (HR 1.20, 95% CI 1.06–1.37; P=0.0038).369 Among patients with DM (38% of the cohort), the corresponding mortality rates were 15.7 and 10.1% (HR 1.44, 95% CI 1.20–1.74; P=0.0001), respectively, while no difference was observed among patients without DM (Pinteraction=0.0077). These findings support a benefit for patients with DM from surgery compared with PCI.
With respect to newer-generation DES, a meta-analysis of RCTs including 8095 patients with DM showed a significant reduction in MI, stent thrombosis, and MACE in patients allocated to newer-generation everolimus-eluting stents compared with those receiving a first-generation DES.370 However, in the subset of patients with DM (n=363) enrolled in the Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients with Multivessel Coronary Artery Disease (BEST) study, the rate of the primary endpoint of death, MI, or target vessel revascularization (TVR) at 2 years was significantly higher in the PCI than the CABG arm (19.2 vs. 9.1%; P=0.007).371 Finally, among the 505 patients with DM in the Evaluation of XIENCE versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL) trial, the primary endpoint of death, MI, or stroke at 3 years occurred in 21.2% of patients in the PCI arm and 19.4% in the CABG arm (HR 1.04, 95% CI 0.70–1.55).372 It remains to be determined whether the use of newer-generation DES will, at least in part, reduce the gap in outcomes favouring CABG in patients with DM and multivessel CAD, and whether the extended follow-up in the EXCEL trial will again show no statistically significant differences between PCI and CABG for left main disease. In non-ST-segment elevation ACS, limited data are available comparing PCI and CABG. In a registry of 2947 patients with DM and stabilized ACS, CABG was compared with PCI with DES.373 The primary outcome measure of the study was a composite of death, MI, and non-fatal stroke. The benefit of CABG over PCI was significant at 30 days (HR 0.49, 95% CI 0.34–0.71) and at a median follow-up of 3.3 years (HR 0.67, 95% CI 0.55–0.81). A recent observational study investigated outcomes with PCI or CABG for multivessel CAD and LV dysfunction in 1738 propensity-matched patients with DM. CABG compared with PCI was associated with significantly lower risks of MACE and mortality at a mean follow-up of 5.5 years.374 The survival advantage of CABG was observed in patients with LVEF 35–49% as well as in those with LVEF <35%.360,374,375
The best surgical coronary revascularization strategy and graft selection in patients with DM is still subject to debate. The superior graft patency of the internal mammary artery, and its impact on survival when grafted to the left anterior descending (LAD) coronary artery, would make the use of bilateral internal mammary arteries the most logical and beneficial strategy.376 However, the superiority of bilateral internal mammary artery (BIMA) grafting over a single internal mammary artery (SIMA) in terms of mortality has been confirmed only by observational studies and respective meta-analysis.377 Factors not related to graft patency, such as the patient’s general status and other unmeasured confounders, may have accounted for the survival benefit of BIMA grafting in the observational series.378 The Arterial Revascularization Trial (ART) compared BIMA with SIMA and additional veins in 1554 patients, and at 10 years showed no significant differences in the rate of death or the composite outcome of death, MI, or stroke.379,380 The radial artery may be the preferred second graft in view of better long-term patency of the radial artery compared with the saphenous vein, but further studies are needed381 (see the 2018 ESC/EACTS Guidelines on myocardial revascularization for further information344).
The appropriate revascularization modality in patients with DM and multivessel disease should be discussed by the Heart Team, taking into consideration individual cardiac and extracardiac characteristics, as well as preferences of the well-informed patient. Overall, current evidence indicates that in stable patients with coronary anatomy suitable for both procedures and low predicted surgical mortality, CABG is superior to PCI in reducing the composite risk of death, MI, or stroke, as well as death. However, in patients with DM with low complexity of coronary anatomy (SYNTAX score ≤22), PCI has achieved similar outcomes to CABG with respect to death and the composite of death, MI, or stroke. Therefore, PCI may represent an alternative to CABG for low complexity of the coronary anatomy, while CABG is recommended for intermediate-to-high anatomical complexity (SYNTAX score >22).
7.2.2 Adjunctive pharmacotherapy
As a general rule, adjunctive pharmacotherapy in the setting of myocardial revascularization does not differ between DM and non-DM (see section 7.1.3.6 for antithrombotic therapy and section 7.1.2 for glucose lowering). There are insufficient data to support the practice of stopping metformin 24–48 h before angiography or PCI, as the risk of lactic acidosis is negligible. In patients with CKD, metformin should be stopped before the procedure. Renal function should be carefully monitored after PCI in all patients with baseline renal impairment or on metformin. If renal function deteriorates in patients on metformin undergoing coronary angiography/PCI, metformin should be withheld for 48 h or until renal function has returned to its initial level.
Recommendations for coronary revascularization in patients with diabetes
 |
 |
For details see 2018 ESC/EACTS Guidelines on myocardial revascularization.344
CABG = coronary artery bypass graft; CCS = chronic coronary syndromes; DES = drug-eluting stent; DM = diabetes mellitus; EACTS = European Association for Cardio-Thoracic Surgery; ESC = European Society of Cardiology; LAD = left anterior descending coronary artery; PCI = percutaneous coronary intervention.
Class of recommendation.
Level of evidence.
Recommendations for coronary revascularization in patients with diabetes
 |
 |
For details see 2018 ESC/EACTS Guidelines on myocardial revascularization.344
CABG = coronary artery bypass graft; CCS = chronic coronary syndromes; DES = drug-eluting stent; DM = diabetes mellitus; EACTS = European Association for Cardio-Thoracic Surgery; ESC = European Society of Cardiology; LAD = left anterior descending coronary artery; PCI = percutaneous coronary intervention.
Class of recommendation.
Level of evidence.
Recommendations for the type of revascularization in patients with diabetes with stable coronary artery disease, suitable coronary anatomy for both procedures, and low predicted surgical mortality
 |
 |
CABG = coronary artery bypass graft; CAD = coronary artery disease; DM = diabetes mellitus; LAD = left anterior descending coronary artery; PCI = percutaneous coronary intervention; SYNTAX = Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery.
Class of recommendation.
Level of evidence.
SYNTAX score calculation: http://www.syntaxscore.com.
Recommendations for the type of revascularization in patients with diabetes with stable coronary artery disease, suitable coronary anatomy for both procedures, and low predicted surgical mortality
 |
 |
CABG = coronary artery bypass graft; CAD = coronary artery disease; DM = diabetes mellitus; LAD = left anterior descending coronary artery; PCI = percutaneous coronary intervention; SYNTAX = Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery.
Class of recommendation.
Level of evidence.
SYNTAX score calculation: http://www.syntaxscore.com.
Gaps in the evidence
The pathophysiological mechanisms underlying the development of CAD and the worse prognosis in patients with DM need to be further elucidated.
The effects of secondary preventive measures in patients with CAD and DM are mainly based on subgroup analyses of trials enrolling patients with and without DM.
Studies comparing different antithrombotic strategies in patients with DM and CAD are lacking.
Optimal glycaemic control for the outcomes of ACS and stable CAD, as well as after coronary revascularization, remain to be established.
Mechanisms of CV event reduction by the newer therapies need to be determined.
The role of hypoglycaemia in the occurrence of CV events/mortality needs to be established.
Following revascularization, the rate of adverse events remains higher in patients with vs. without DM; specific preventive therapies should be investigated.
Although newer-generation DES have improved outcomes in patients with DM, RCTs are needed to determine whether they can reduce the gap in outcomes between CABG and PCI.
8 Heart failure and diabetes
Key messages
Patients with pre-DM and DM are at increased risk of developing HF.
Patients with DM are at greater risk of HF with reduced ejection fraction (HFrEF) or HF with preserved ejection fraction (HFpEF); conversely, HF increases the risk of DM.
The coexistence of DM and HF imparts a higher risk of HF hospitalization, all-cause death, and CV death.
Guideline-based medical and device therapies are equally effective in patients with and without DM; as renal dysfunction and hyperkalaemia are more prevalent in patients with DM, dose adjustments of some HF drugs (e.g. RAAS blockers) are advised.
First-line treatment of DM in HF should include metformin and SGLT2 inhibitors; conversely, saxagliptin, pioglitazone, and rosiglitazone are not recommended for patients with DM and HF.
DM is an important risk factor for HF.405–407 In trials of glucose-lowering medications, HF was seen in 4–30% of participants.292,299,306,408 Unrecognized HF may also be frequent in patients DM: observational data indicate that HF is present in 28% of patients (∼25% HFrEF and ∼75% HFpEF).409 Patients with DM free of HF at baseline are ∼2–5 times more likely to develop HF.410,411 The risk of HF is also increased in those with HbA1c levels in the pre-DM range (≥5.5–6.4%), who have a 20 − 40% higher risk of HF.412 HF itself is associated with a greater prevalence of DM and other dysglycaemic states, and is considered a risk factor for the development of DM, most likely related to an insulin-resistant state.413–416 Available data indicate that the prevalence of DM in HF is similar, irrespective of LVEF category [HFpEF, HF with mid-range ejection fraction (HFmrEF) and HFrEF (see Table 11 below)].417–419 Indeed, ∼30–40% of patients with HF have been reported to have pre-DM or DM in trials of HFrEF345,420,421 and HFpEF.422–425 Findings from a large pan-European registry indicated that ∼36% of outpatients with stable HF had DM,426 while in patients hospitalized for acute HF, DM was present in ≤50%.427 Importantly, patients with HF without DM are at increased risk of DM,413,428 and the risk is aggravated by the severity of HF and the use of loop diuretics.428
| HFpEF | HFmrEF | HFrEF | |
| Criterion 1 | Symptoms and/or signsa | Symptoms and/or signsa | Symptoms and/or signsa |
| Criterion 2 | LVEF ≥50% | LVEF 40–49% | LVEF <40% |
| Criterion 3 | 1. Elevated natriuretic peptidesb | 1. Elevated natriuretic peptidesb | None |
| 2. At least one additional criterion: | 2. At least one additional criterion: | ||
| a) structural heart disease (i.e. LVH and/or LAE) | a) structural heart disease (i.e. LVH and/or LAE) | ||
| b) diastolic dysfunctionc | b) diastolic dysfunctionc |
| HFpEF | HFmrEF | HFrEF | |
| Criterion 1 | Symptoms and/or signsa | Symptoms and/or signsa | Symptoms and/or signsa |
| Criterion 2 | LVEF ≥50% | LVEF 40–49% | LVEF <40% |
| Criterion 3 | 1. Elevated natriuretic peptidesb | 1. Elevated natriuretic peptidesb | None |
| 2. At least one additional criterion: | 2. At least one additional criterion: | ||
| a) structural heart disease (i.e. LVH and/or LAE) | a) structural heart disease (i.e. LVH and/or LAE) | ||
| b) diastolic dysfunctionc | b) diastolic dysfunctionc |
HF = heart failure; HFmrEF = heart failure with mid-range ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LAE = left atrial enlargement; LVEF = left ventricular ejection fraction; LVH = left ventricular hypertrophy; NT-proBNP = N-terminal pro-B-type natriuretic peptide.
Signs may not be present at an early stage or in patients receiving diuretics.
Elevation of B-type natriuretic peptide ≥35 pg/mL and/or NT-proBNP ≥125 pg/mL.
For example, E/e′ ≥13, and a mean e’ septal and lateral wall <9 cm/s on echocardiography.
| HFpEF | HFmrEF | HFrEF | |
| Criterion 1 | Symptoms and/or signsa | Symptoms and/or signsa | Symptoms and/or signsa |
| Criterion 2 | LVEF ≥50% | LVEF 40–49% | LVEF <40% |
| Criterion 3 | 1. Elevated natriuretic peptidesb | 1. Elevated natriuretic peptidesb | None |
| 2. At least one additional criterion: | 2. At least one additional criterion: | ||
| a) structural heart disease (i.e. LVH and/or LAE) | a) structural heart disease (i.e. LVH and/or LAE) | ||
| b) diastolic dysfunctionc | b) diastolic dysfunctionc |
| HFpEF | HFmrEF | HFrEF | |
| Criterion 1 | Symptoms and/or signsa | Symptoms and/or signsa | Symptoms and/or signsa |
| Criterion 2 | LVEF ≥50% | LVEF 40–49% | LVEF <40% |
| Criterion 3 | 1. Elevated natriuretic peptidesb | 1. Elevated natriuretic peptidesb | None |
| 2. At least one additional criterion: | 2. At least one additional criterion: | ||
| a) structural heart disease (i.e. LVH and/or LAE) | a) structural heart disease (i.e. LVH and/or LAE) | ||
| b) diastolic dysfunctionc | b) diastolic dysfunctionc |
HF = heart failure; HFmrEF = heart failure with mid-range ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LAE = left atrial enlargement; LVEF = left ventricular ejection fraction; LVH = left ventricular hypertrophy; NT-proBNP = N-terminal pro-B-type natriuretic peptide.
Signs may not be present at an early stage or in patients receiving diuretics.
Elevation of B-type natriuretic peptide ≥35 pg/mL and/or NT-proBNP ≥125 pg/mL.
For example, E/e′ ≥13, and a mean e’ septal and lateral wall <9 cm/s on echocardiography.
8.1 Prognostic implications of diabetes mellitus in heart failure
A significant association exists between DM and adverse outcomes in HF, with the strongest predictive value of DM for outcomes seen in patients with HFrEF.421,423,426,429–432 CV mortality, including death caused by worsening HF, is also ∼50–90% higher in patients with HF and DM, regardless of HF phenotype.421,432–434 Two trials have shown that pre-DM and undiagnosed DM in patients with HF are associated with a higher risk of death, and adverse clinical outcomes.421,431,435 In addition, in patients with worsening HFrEF, newly diagnosed pre-DM was independently associated with a higher long-term risk of all-cause and CV death, which underlies the importance of screening for pre-DM in this population.436 In acute HF, DM increases the risks of in-hospital death,427 1 year all-cause death,437 and 1 year HF rehospitalization.427
8.2 Mechanisms of left ventricular dysfunction in diabetes mellitus
Major causes of HF in patients with DM are CAD, CKD (see section 11), hypertension, and direct effects of insulin resistance/hyperglycaemia on the myocardium.438 CAD is often accelerated, severe, diffuse, and silent, and increases the risk of MI and ischaemic myocardial dysfunction.411,439–441 Hypertension control is associated with a lower risk of HF development.439 Observational data have also identified LEAD, a longer duration of DM, ageing, increased body mass index, and CKD as predictors of HF in patients with DM.411,439–441 Complex pathophysiological mechanisms may be responsible for the development of myocardial dysfunction, even in the absence of CAD or hypertension.442 The existence of diabetic cardiomyopathy has not been confirmed.438,443 The body of evidence for diabetic cardiomyopathy mostly comes from experimental and smaller observational studies.438,444–448
8.3 Phenotypes of left ventricular dysfunction in diabetes mellitus
LV dysfunction in patients with DM may present as HFpEF, HFmrEF, or HFrEF (Table 11). LV diastolic dysfunction is frequent in both pre-DM and overt DM, and severity correlates with insulin resistance and the degree of glucose dysregulation.449–453 DM and HFpEF are frequently seen together in older, hypertensive, and female patients with DM.454
8.4 Treatment of heart failure in diabetes mellitus
Treatment of HF encompasses pharmacological and device therapies with confirmed benefits in RCTs, in which ∼30–40% of patients had DM. Treatment effects are consistent with and without DM, with the exception of aliskiren, which is not recommended in patients with DM due to the risk of serious adverse events.455,456
8.4.1 Renin-angiotensin-aldosterone system and neprilysin inhibitors
ACEIs and ARBs have similar treatment effects in patients with HFrEF, with and without DM.457,–462 RAAS blockers should be started at a low dose and up-titrated to the maximally tolerated dose.459,463 There is evidence for a positive effect of ACEIs and ARBs on the prevention of DM.464 MRAs reduce death and HF hospitalization in HFrEF.465,466 As RAAS blockers increase the risk of worsening renal function and hyperkalaemia in patients with DM, routine surveillance of serum creatinine and potassium levels is advised.467–470 The angiotensin receptor neprilysin inhibitor sacubitril/valsartan has shown superior efficacy to enalapril in the reduction of CV death and HF hospitalization in patients with HFrEF. However, the treatment effect was less pronounced in patients with baseline DM.421 The beneficial effect of sacubitril/valsartan over enalapril is consistent across the spectrum of baseline HbA1c.421,471 Sacubitril/valsartan therapy has also resulted in a greater reduction in HbA1c levels and a lower rate of insulin initiation over 3 year follow-up compared with enalapril in patients with DM.472
8.4.2 Beta-blockers
Beta-blockers are effective at reducing all-cause death and hospitalization for HFrEF in patients with DM.473,–476 Treatment benefits strongly support beta-blocker use in patients with HFrEF and DM.
8.4.3 Ivabradine
Ivabradine improves the treatment of HFrEF in sinus rhythm, particularly with regard to the reduction of HF hospitalization and the improvement of LV function.335
8.4.4 Digoxin
Digoxin may reduce the risk of HF hospitalization in HFrEF treated with ACEIs.477
8.4.5 Diuretics
Despite a lack of evidence for the efficacy of either thiazide or loop diuretics in the reduction of CV outcomes in patients with HF, diuretics prevent and treat symptoms and signs of fluid congestion in patients with HF.478
8.4.6 Device therapy and surgery
Device therapies [implantable cardioverter defibrillator (ICD), cardiac resynchronization therapy (CRT), and CRT with an implantable defibrillator (CRT-D)] have similar efficacies and risks in patients with and without DM.479–481 These therapies should be considered according to treatment guidelines in the general population. In a clinical trial of CABG in HFrEF and two- or three-vessel CAD, there was no difference in the efficacy of surgical revascularization with or without DM.482 Heart transplantation could be considered in end-stage HF, but a large, prospective study of transplanted patients indicated a decreased likelihood of 10 year survival of patients with DM.483
8.5 Effect of oral glucose-lowering agents on heart failure
8.5.1 Metformin
Metformin is safe at all stages of HF with preserved or stable moderately reduced renal function (i.e. eGFR >30 mL/min), and results in a lower risk of death and HF hospitalization compared with insulin and sulfonylureas.484,485 Concerns regarding lactic acidosis have not been substantiated.486
8.5.2 Sulfonylureas
Data on the effects of sulfonylureas on HF are inconsistent. A signal of an adverse safety profile showed an ∼20–60% higher death rate and an ∼20–30% increased risk of HF compared with metformin.487,488 Addition of a sulfonylurea to metformin was associated with a higher risk of adverse events and death, compared with the combination of metformin and a DPP4 inhibitor.489 However, in the UKPDS, NAVIGATOR, and ADOPT studies, there was no increased HF signal.145,278,490
8.5.3 Thiazolidinediones
Thiazolidinediones are not recommended in patients with DM and symptomatic HF.279,,491–494
8.5.4 Dipeptidyl peptidase-4 inhibitors
Saxagliptin significantly increased the risk of HF hospitalization291 and is not recommended in patients with DM with HF. Alogliptin was associated with a non-significant trend towards HF hospitalization.292 Sitagliptin and linagliptin had a neutral effect.293,294 Vildagliptin had no significant effect of LVEF but led to an increase in LV volumes.495
8.5.5 Glucagon-like peptide-1 receptor agonists
All GLP1-RAs had a neutral effect on the risk of HF hospitalization in their placebo-controlled RCTs, suggesting that they should be considered in patients with DM and HF.272,–274
8.5.6 Sodium-glucose co-transporter 2 inhibitors
Empagliflozin reduced the risk of HF hospitalization by 35% in patients with and without previous HF, while patients hospitalized for HF were at a lower risk of death.306 Canagliflozin also significantly reduced the risk of HF hospitalization by 32%.496 Dapagliflozin significantly reduced the combined endpoint of CV death and HF hospitalization, a result driven mainly by lower rates of HF hospitalization.311 SGLT2 inhibitors are recommended for patients with DM at high risk of HF. See also section 7.1.2.2.3.
Recommendations for the treatment of heart failure in patients with diabetes
 |
 |
ACEIs = angiotensin-converting enzyme inhibitors; ARBs = angiotensin receptor blockers; b.p.m. = beats per minute; CABG = coronary artery bypass graft; CAD = coronary artery disease; CRT = cardiac resynchronization therapy; CRT-D = cardiac resynchronization therapy with implantable defibrillator; DM = diabetes mellitus; HF = heart failure; HFmrEF = heart failure with mid-range ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter defibrillator; LAD = left anterior descending coronary artery; MRAs = mineralocorticoid receptor antagonists
Class of recommendation.
Level of evidence.
Recommendations for the treatment of heart failure in patients with diabetes
 |
 |
ACEIs = angiotensin-converting enzyme inhibitors; ARBs = angiotensin receptor blockers; b.p.m. = beats per minute; CABG = coronary artery bypass graft; CAD = coronary artery disease; CRT = cardiac resynchronization therapy; CRT-D = cardiac resynchronization therapy with implantable defibrillator; DM = diabetes mellitus; HF = heart failure; HFmrEF = heart failure with mid-range ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter defibrillator; LAD = left anterior descending coronary artery; MRAs = mineralocorticoid receptor antagonists
Class of recommendation.
Level of evidence.
Recommendations for the treatment of patients with diabetes to reduce heart failure risk
 |
 |
DM = diabetes mellitus; DPP4 = dipeptidyl peptidase-4; eGFR = estimated glomerular filtration rate; GLP1-RA = glucagon-like peptide-1 receptor agonist; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; SGLT2 = sodium-glucose co-transporter type 2; T2DM = type 2 diabetes mellitus.
Class of recommendation.
Level of evidence.
Recommendations for the treatment of patients with diabetes to reduce heart failure risk
 |
 |
DM = diabetes mellitus; DPP4 = dipeptidyl peptidase-4; eGFR = estimated glomerular filtration rate; GLP1-RA = glucagon-like peptide-1 receptor agonist; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; SGLT2 = sodium-glucose co-transporter type 2; T2DM = type 2 diabetes mellitus.
Class of recommendation.
Level of evidence.
Gaps in the evidence
Studies are needed to better understand the bidirectional relationship between DM and HF, including the pathophysiology of diabetic cardiomyopathy.
Considering the divergent evidence for an association between DPP4 inhibitors and HF risk, research is needed to further clarify this association.
How do SGLT2 inhibitors improve HF outcomes?
Research is needed to confirm whether SGLT2 inhibitors lower the risk of HF in non-DM (HF and pre-DM).
Does the combination of a SGLT2 inhibitor and sacubitril/valsartan lead to excessive diuresis/hypotension?
Future research should address the risks of polypharmacy, in terms of adherence, adverse reactions, and interactions, especially among vulnerable patients with HF and DM, such as those who are elderly and/or frail with multiple comorbidities.
9 Arrhythmias: atrial fibrillation, ventricular arrhythmias, and sudden cardiac death
Key messages
Atrial fibrillation (AF) is common in patients with DM, and increases mortality and morbidity.
Screening for AF should be recommended for patients with DM aged >65 years by pulse palpation or wearable devices. AF should always be confirmed by ECG.
Anticoagulation is recommended in all patients with DM and AF.
Sudden cardiac death is more common in patients with DM, especially in women.
In HF patients with DM, QRS duration and LVEF should be measured regularly to determine eligibility for CRT ± ICD.
9.1 Atrial fibrillation
A recent study reported that DM is an independent risk factor for AF, especially in young patients.501 Several factors, such as autonomic, electromechanical, and structural remodelling, and glycaemic fluctuations, seem to be implicated in AF pathophysiology in the setting of DM.502 Atrial premature beats are also common in patients with DM and may predispose to the development of AF. Patients with DM have an increased risk of acute HF at the time of new-onset AF, as a result of loss of atrial kick and impaired LV filling.427
When DM and AF coexist, there is a substantially higher risk of all-cause death, CV death, stroke, and HF.502 These findings suggest that AF identifies subjects with DM who are likely to obtain greater benefits from aggressive management of CVRFs. Because AF is asymptomatic or mildly symptomatic in a substantial proportion of patients, screening for AF can be recommended in patients with DM and AF must be confirmed by 12 lead ECG, Holter recordings, or event recorders demonstrating a duration of >30 s.
9.1.1 Diabetes and risk of stroke in atrial fibrillation
DM increases the risk of stroke in paroxysmal or permanent AF.503 Current Guidelines recommend that oral anticoagulant therapy, with non-vitamin K antagonist (VKA) oral anticoagulants (NOACs; dabigatran, apixaban, rivaroxaban, or edoxaban) or VKAs, should be considered.503 Kidney function should be carefully evaluated in patients with DM when prescribing a NOAC to avoid over-dosage due to reduced drug elimination.503
9.2 Ventricular arrhythmias and sudden cardiac death
9.2.1 Ventricular premature beats and paroxysmal ventricular tachycardia
Palpitations, premature ventricular beats, and non-sustained ventricular tachycardia (VT) are common in patients with DM. Diagnostic workup and treatment of ventricular arrhythmias does not differ between DM and non-DM patients.504 In patients with DM with frequent symptomatic premature ventricular beats or episodes of non-sustained VT, the presence of underlying structural heart disease should be examined by exercise ECG, echocardiography, coronary angiography, or magnetic resonance imaging. The risk of cardiac events is usually dictated by underlying heart disease rather than ectopic beats. In highly symptomatic patients with premature ventricular beats or non-sustained VT, beta-blockers, calcium antagonists, class Ic drugs (flecainide or propafenone), or catheter ablation (in cases of an absence of structural heart disease) can be used to suppress arrhythmias.505
9.2.2 Sustained ventricular arrhythmias
The diagnosis and treatment of sustained VT, or resuscitated ventricular fibrillation, is similar for patients with or without DM.504 Diagnosis of underlying structural heart disease with imaging techniques and coronary angiography is usually needed, if no obvious trigger factors such as electrolyte imbalance or acute infarction can be identified. Most patients with sustained VT or aborted cardiac arrest without a diagnosed trigger need an ICD to prevent sudden death.504,506
9.2.3 Sudden cardiac death in diabetes
Epidemiological studies have shown that patients with DM or pre-DM are at increased risk of sudden cardiac death.507–509 Women at all ages have a lower risk for sudden cardiac death than men, but in the presence of DM the risk of sudden cardiac death in both men and women is quadrupled.510 In the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) study programme, DM was an independent predictor of mortality, including sudden cardiac death, in HF irrespective of LVEF.432 In post-MI patients, the incidence of sudden cardiac death was higher in those with DM.511 The incidence of sudden cardiac death was substantially increased in patients with DM with an LVEF <35%.511 After acute MI, LVEF should be measured in patients irrespective of DM to identify candidates for ICD implantation. In HF patients with DM, the QRS width and LVEF should be determined to identify candidates for CRT ± ICD.505 In HF patients with HFrEF, beta-blockers, RAAS blockers (including sacubitril/valsartan), and MRAs are recommended to reduce the risk of sudden cardiac death.
The causes underlying increased vulnerability to electrical instability in patients with DM are unclear and are likely to involve several factors. Simultaneous glucose and ambulatory ECG monitoring has shown that bradycardia, and atrial and ventricular ectopic beats, are more common during nocturnal hypoglycaemia in patients with DM.512 This observation suggests a possible mechanism for increased death rates (dead-in-bed syndrome) during intensive glycaemic control.
Nephropathy, autonomic neuropathy, prolonged QTc interval, hypoglycaemia, and comorbidities related to DM are thought to increase the risk of sudden cardiac death. On the basis of the available evidence, it seems that glucose intolerance, even in pre-DM, is associated with the progressive development of a variety of abnormalities that adversely affect survival and predispose to sudden arrhythmic death. Apart from the measurement of LVEF, identification of independent predictors in patients with DM has not progressed to a point where it is possible to devise risk stratification for prevention.
Recommendations for the management of arrhythmias in patients with diabetes
 |
 |
AF = atrial fibrillation; CHA2DS2-VASc = Congestive heart failure, Hypertension, Age ≥75 years (Doubled), Diabetes mellitus, Stroke or transient ischaemic attack (Doubled), Vascular disease, Age 65–74 years, Sex category; DM = diabetes mellitus; ECG = electrocardiogram; HAS-BLED = Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (>65 years), Drugs/alcohol concomitantly; HF = heart failure; ICD = implantable cardioverter defibrillator; LVEF = left ventricular ejection fraction; MI = myocardial infarction; NOAC = non-vitamin K antagonist oral anticoagulant; VKA = vitamin K antagonist; VT = ventricular tachycardia.
Class of recommendation.
Level of evidence.
Recommendations for the management of arrhythmias in patients with diabetes
 |
 |
AF = atrial fibrillation; CHA2DS2-VASc = Congestive heart failure, Hypertension, Age ≥75 years (Doubled), Diabetes mellitus, Stroke or transient ischaemic attack (Doubled), Vascular disease, Age 65–74 years, Sex category; DM = diabetes mellitus; ECG = electrocardiogram; HAS-BLED = Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (>65 years), Drugs/alcohol concomitantly; HF = heart failure; ICD = implantable cardioverter defibrillator; LVEF = left ventricular ejection fraction; MI = myocardial infarction; NOAC = non-vitamin K antagonist oral anticoagulant; VKA = vitamin K antagonist; VT = ventricular tachycardia.
Class of recommendation.
Level of evidence.
Gaps in the evidence
The role of novel wearable gadgets is not well established in the home-based diagnosis of AF and should be tested in well-designed clinical trials.
The roles of several non-invasive risk markers of sudden cardiac death—such as heart rate variability, QTc interval, albuminuria, hypoglycaemia, etc.—are not sufficiently well established to be used in clinical decision-making for the prevention of sudden unexpected death.
The impact of novel antidiabetic drugs on sudden cardiac death is not known.
Prophylactic ICD therapy in patients with DM is not well established.
10 Aortic and peripheral arterial diseases
Key messages
LEAD is a common complication of DM, with increasing prevalence with duration and/or the coexistence of other CVD risk factors.
At any stage of LEAD, the coexistence of DM is associated with poorer prognosis.
Patients with DM are at higher risk of chronic limb-threatening ischaemia (CLTI) as the first clinical manifestation of LEAD, supporting regular screening with ABI measurement for early diagnosis.
The management of, and indications for, different treatment strategies are similar in patients with LEAD with or without DM, although the options for revascularization may be poorer because of diffuse and distal lesions.
The management of carotid artery disease is similar in DM and non-DM patients.
10.1 Aortic disease
Several studies have shown a decreased risk of abdominal aortic aneurysm in patients with DM, the reasons for which are unexplained.519 In turn, short- and long-term outcomes after abdominal aortic aneurysm repair are poorer in patients with DM.520 However, in the absence of any specific study on abdominal aortic aneurysm screening and management in patients with DM, the recommendations on population screening for abdominal aortic aneurysm, as proposed in the 2014 Guidelines on the diagnosis and treatment of aortic diseases,521 remain valid in patients with DM.
10.2 Lower extremity arterial disease
According to the 2017 ESC Guidelines on the diagnosis and treatment of PADs,522 this term includes conditions affecting all arteries, except for the aorta, and the coronary and the intracranial arteries.
10.2.1 Epidemiology and natural history
LEAD is a frequent vascular complication of DM, with one-third of patients hospitalized for LEAD having DM.523 Prolonged DM duration, suboptimal glycaemic control, the coexistence of other CVRFs, and/or other end-organ damage (e.g. proteinuria) increase LEAD prevalence.523 LEAD in pre-DM is infrequent in the absence of other risk factors.524 In patients with DM, LEAD more frequently affects arteries below the knee; as a consequence, the revascularization options, as well as their chances of success, are reduced.523 In patients with DM, LEAD is often diagnosed at a later stage (e.g. a non-healing ulcer), because of concomitant neuropathy with decreased pain sensitivity. All of these factors increase the risk of limb infection.525
Clinically, patients with DM often have atypical forms of pain on exertion that do not meet the typical criteria for intermittent claudication.526 CLTI is the clinical presentation of advanced disease, characterized by ischaemic rest pain, but which may be absent in patients with DM. About 50–70% of all patients with CLTI have DM. The 2017 ESC Guidelines on the diagnosis and treatment of PADs proposed the Wound, Ischemia, and foot Infection (WIfI) classification to stratify amputation risk and the potential benefits of revascularization (Table 12).522
Assessment of the risk of amputation: the Wound, Ischaemia, and foot Infection classification522
 |
 |
ABI = ankle–brachial index; DM = diabetes mellitus; fI = foot Infection, H = high risk, L = low risk, M = moderate risk; PAD = peripheral arterial disease; TcPO2 = transcutaneous oxygen pressure; VL = very low risk, W = wound; WIfI = Wound, Ischaemia, and foot Infection.
Assessment of the risk of amputation: the Wound, Ischaemia, and foot Infection classification522
 |
 |
ABI = ankle–brachial index; DM = diabetes mellitus; fI = foot Infection, H = high risk, L = low risk, M = moderate risk; PAD = peripheral arterial disease; TcPO2 = transcutaneous oxygen pressure; VL = very low risk, W = wound; WIfI = Wound, Ischaemia, and foot Infection.
10.2.2 Screening and diagnosis
Screening and early diagnosis are of major importance in patients with DM. Clinical evaluation includes medical history, symptom assessment, and examination for neuropathy on a yearly basis. The ABI is the current method for LEAD screening. An ABI <0.90 is diagnostic for LEAD, with 80% sensitivity and 95% specificity in all populations.523 However, the accuracy of ABI is lower in patients with DM (see below).527 Beyond LEAD, an ABI <0.90 (or >1.40) is associated with an increased risk of death and CV events (Figure 5).528
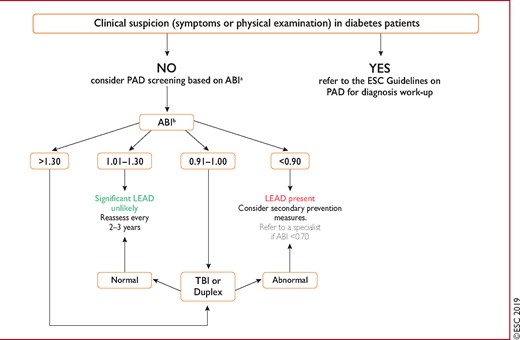
Screening for lower extremity artery disease in patients with diabetes. ABI = ankle–brachial index; DM = diabetes mellitus; ESC = European Society of Cardiology; LEAD = lower extremity artery disease; PAD = peripheral arterial disease; TBI = toe–brachial index. aABI-based screening should be performed once when DM is diagnosed, and then after 10 years of DM if the results from the initial examination were normal (can be considered after 5 years of diagnosis if other risk factors such as smoking exist). Patients should be assessed every year for symptoms and pulses should be checked. ABI-based screening is proposed in the absence of any clinical suspicion of PAD. bIn case of borderline results (e.g. 0.89), repeat the measurement and average the results to increase accuracy. If TBI is available, this can be done in conjunction with the ABI.
If symptoms suggest LEAD but the ABI result is normal, sensitivity can be improved by post-exercise ABI or the toe–brachial index (TBI) at rest.522,529 With intermittent claudication, the treadmill test is helpful for the assessment of walking distance. An ABI >1.40 is mostly related to medial calcinosis but is associated with LEAD in 50% of cases.530 Other tests are useful for the diagnosis of LEAD in the presence of medial calcinosis, including Doppler waveform analysis of the ankle arteries or the TBI, which may be helpful because medial calcinosis barely affects digital arteries. A TBI <0.70 is diagnostic for LEAD.529
The value of duplex as first-line imaging for confirmation of LEAD,522 CT angiography and/or magnetic resonance imaging in planned revascularization, and other more detailed imaging tests are fully described in 2017 ESC Guidelines on the diagnosis and treatment of PADs.522
10.2.3 Management of lower extremity artery disease in diabetes mellitus
The medical management of LEAD in patients with DM is not significantly different from that recommended for patients with CVD in general (see sections 5 and 6). The main COMPASS trial results reported the benefit of (i) rivaroxaban 2.5 mg b.i.d. plus aspirin 100 mg o.d. against (ii) rivaroxaban 5 mg b.i.d. or (iii) aspirin 100 mg o.d. in 27 395 patients with stable atherosclerotic vascular disease, indicating a significant reduction in the primary outcome of CV death, stroke, or MI, which led to early termination of the trial.342 In a substudy of 7240 patients with CAD or LEAD with a mean follow-up of 23 months (44% with DM), major adverse limb events including amputation were significantly decreased with combination therapy (HR 0.54; P=0.0037).531 These benefits were observed at the cost of major bleeding risk (HR 1.61; P=0.0089). The significant reduction in major adverse limb events in this COMPASS substudy raises the possibility of a novel therapeutic regimen in high-risk vascular patients to ameliorate the complications of LEAD.532,533
Patients with intermittent claudication should take part in exercise training programmes (>30–45 min, at least three times per week) as regular intensive exercise improves walking distance, although with less pronounced benefits in patients with DM.534
In patients with CLTI, strict glycaemic control is associated with improved limb outcomes.535,536 However, revascularization must be attempted when possible, and amputation only considered when revascularization options fail.522 Revascularization should also be considered in severe/disabling claudication. With respect to the revascularization modality of choice, we refer the reader to dedicated Guidelines.522 There has not been a specific trial on revascularization strategies in patients with DM; however, a review of 56 studies including patients with DM suggested higher limb salvage rates after revascularization (78–85% at 1 year) compared with conservative management.537
10.3 Carotid artery disease
Thromboembolism from a carotid artery stenosis is the mechanism underlying 10–15% of all strokes. In brief, carotid artery disease must be rapidly ruled out in all patients presenting with transient ischaemic attack or stroke. In patients with DM without a history of cerebrovascular disease, there is no evidence that carotid screening improves outcomes and systematic screening is not recommended.
Asymptomatic carotid disease is frequently treated conservatively, and the patient is followed-up with duplex ultrasound. Carotid revascularization should be considered in asymptomatic patients in the presence of one or more indicators of increased stroke risk (previous transient ischaemic attack/stroke, ipsilateral silent infarction, stenosis progression, or high-risk plaques), and if the estimated peri-operative stroke or death rate is <3% and the patient’s life expectancy is >5 years.522
In symptomatic patients, carotid revascularization is indicated if the stenosis is >70%, and should be considered if the stenosis is >50%, assuming that the estimated peri-operative stroke or death rate is <6%.522
RCTs comparing carotid endarterectomy with carotid artery stenting in the peri-procedural period have shown an excess of minor strokes with carotid artery stenting, and more episodes of myocardial ischaemia and cranial nerve palsies with endarterectomy. Post-operatively, both treatments offer similar protection from recurrent stroke and have similar rates of repeat interventions.538 Carotid endarterectomy remains the standard of care, while stenting may be considered as an alternative in patients at high risk of endarterectomy.522
With respect to the impact of DM on carotid revascularization, a meta-analysis of 14 observational studies involving 16 264 patients showed that those with DM had a higher risk of peri-operative stroke and death.539 Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) was the only trial comparing carotid endarterectomy and carotid artery stenting to enrol enough patients with DM (n=759) for subgroup analysis. Although restenosis rates were low at 2 years after carotid stenting (6.0%) and carotid endarterectomy (6.3%), DM was a predictor of restenosis with both techniques.540
Recommendations for the diagnosis and management of peripheral arterial disease in patients with diabetes
 |
 |
ABI = ankle–brachial index; b.i.d. = twice daily (bis in die); CLTI = chronic limb-threatening ischaemia; CT = computed tomography; CV = cardiovascular; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; LDL-C = low-density lipoprotein cholesterol; LEAD = lower extremity artery disease; o.d. = once daily (omni die); PAD = peripheral arterial disease; TBI = toe–brachial index; WIfI = Wound, Ischaemia, and foot Infection.
Class of recommendation.
Level of evidence.
Including a diabetologist and a vascular specialist.
See Table 7.
See Table 12.
High bleeding risk is defined as history of intracerebral haemorrhage or ischaemic stroke, history of other intracranial pathology, recent gastrointestinal bleeding or anaemia due to possible gastrointestinal blood loss, other gastrointestinal pathology associated with increased bleeding risk, liver failure, bleeding diathesis or coagulopathy, extreme old age or frailty, or renal failure requiring dialysis or with eGFR <15 mL/min/1.73 m2.
Recommendations for the diagnosis and management of peripheral arterial disease in patients with diabetes
 |
 |
ABI = ankle–brachial index; b.i.d. = twice daily (bis in die); CLTI = chronic limb-threatening ischaemia; CT = computed tomography; CV = cardiovascular; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; LDL-C = low-density lipoprotein cholesterol; LEAD = lower extremity artery disease; o.d. = once daily (omni die); PAD = peripheral arterial disease; TBI = toe–brachial index; WIfI = Wound, Ischaemia, and foot Infection.
Class of recommendation.
Level of evidence.
Including a diabetologist and a vascular specialist.
See Table 7.
See Table 12.
High bleeding risk is defined as history of intracerebral haemorrhage or ischaemic stroke, history of other intracranial pathology, recent gastrointestinal bleeding or anaemia due to possible gastrointestinal blood loss, other gastrointestinal pathology associated with increased bleeding risk, liver failure, bleeding diathesis or coagulopathy, extreme old age or frailty, or renal failure requiring dialysis or with eGFR <15 mL/min/1.73 m2.
Gaps in the evidence
The regularity and mode of vascular screening in patients with DM have not been adequately assessed.
The use of antithrombotic therapies at different clinical stages has been poorly addressed.
Specific trials are needed to help clinicians to choose different pharmacological strategies according to the presence of PAD.
11. Chronic kidney disease in diabetes
Key messages
CKD is associated with a high prevalence of CVD and should be considered in the highest risk group for risk factor management.
Screening for kidney disease in patients with DM requires serum creatinine measurement to enable the calculation of eGFR and urine tests of albumin excretion.
Optimizing glycaemic and BP control may slow decline in kidney function.
ACEIs and ARBs are the preferred antihypertensive drugs in patients with albuminuria.
Therapeutic reductions in albuminuria are associated with ‘renoprotection’.
Data from recent CVOTs suggest that SGLT2 inhibitors and GLP1-RAs, may confer renoprotection.
In the CREDENCE trial, canagliflozin reduced the relative risk of the primary renal outcome by 30% compared with placebo.
CKD developing in the context of DM is a major health issue, which is associated with the highest risk of CVD23 and should therefore be managed accordingly. CKD is defined as a reduction in eGFR to <60 mL/min/1.73m2 and/or persistent proteinuria (e.g. urinary albumin:creatinine ratio >3 mg/mmol), sustained over ≥90 days. The most widely used classified system, developed by Kidney Disease: Improving Global Outcomes, stratifies patients according to both their eGFR (‘G’ stage) and their urinary albumin excretion (‘A’ stage) in a two-dimensional manner (Table 13).543 Monitoring of DM should include the assessment of kidney function by both blood and urine testing to determine the eGFR and albumin:creatinine ratio, respectively. Approximately 30% of patients with T1DM and 40% with T2DM will develop CKD.544 A decline in eGFR makes glycaemic control more challenging and increases the risks of drug-induced adverse events such as hypoglycaemia.545
Chronic kidney disease classification by estimated glomerular filtration rate and albuminuria543
 |
 |
Green = low risk; yellow = medium risk; orange = high risk; red = very high risk.
CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate.
Chronic kidney disease classification by estimated glomerular filtration rate and albuminuria543
 |
 |
Green = low risk; yellow = medium risk; orange = high risk; red = very high risk.
CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate.
11.1 Management
11.1.1 Glycaemic control
Improving glycaemia may reduce the risk of progression of nephropathy,546 but is more complex in diabetic kidney disease because a fall in eGFR restricts the use of several oral glucose-lowering drugs.545 For example, although metformin is useful and possibly beneficial in stage 1 − 3 CKD, an observational study from Taiwan reported a 35% increase in death in metformin users with stage 5 CKD, a finding that was not replicated with other glucose-lowering agents. Metformin should therefore be used with caution as the eGFR drops towards 30 mL/min/1.73m2. Accumulation of renally excreted sulfonylureas may increase the likelihood of hypoglycaemia.547 As kidney function deteriorates, the use of insulin in place of oral regimens is likely to assist in achieving better glycaemic control, particularly as patients near renal replacement therapy. The GLP1-RAs liraglutide, dulaglutide, and semaglutide can even be administered with an eGFR >15 mL/min/1.73 m2.
11.1.2 New approaches to nephroprotection
Data on composite kidney endpoints from recent CVOTs suggest that some of the newer oral antihyperglycaemic drugs have beneficial renal effects. Nephroprotection has been observed in two GLP1-RA (liraglutide176 and semaglutide299) and three SGLT2 inhibitor (empagliflozin,548 canagliflozin,309 dapagliflozin311) CVOTs. These trials did not include patients with advanced CKD and nephroprotection was not the adjudicated primary outcome. In response to these preliminary findings, several studies have been initiated to investigate renal outcomes [DAPA-CKD (clinicaltrialts.gov ID: NCT03036150), EMPA-Kidney,549 and CREDENCE550]. The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation trial313 assigned patients with T2DM and eGFR 30 to <90 mL/min/1.73m2 (urinary albumin:creatinine ratio 33.9–565 mg/mmol) to either canagliflozin 100 mg/day or placebo. The trial was stopped prematurely by the safety committee after an interim analysis demonstrated superiority. A total of 4401 patients were followed for 2.6 years and the relative risk of the primary outcome (a composite of end-stage renal disease, a doubling of serum creatinine levels, or renal or CV death) was reduced by 30% (43.2 vs. 61.2/1000 patient-years, P=0.00001). Secondary outcomes, including the composite of CV death or hospitalization for HF; the composite of CV death, MI, or stroke; and the analysis of hospitalization for HF alone, all demonstrated significant benefits with canagliflozin. These findings in a high-risk population of patients with T2DM and renal impairment validate the secondary outcome observations in the CVOTs, and confirm the importance of SGLT2 inhibitors in managing DM, CKD, and associated CVD. The CREDENCE trial also demonstrated that the SGLT2 inhibitor canagliflozin may be used with benefit down to an eGFR of 30 mL/min/1.73m2.
Recommendations for the prevention and management of chronic kidney disease in patients with diabetes
 |
 |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BP = blood pressure; CKD = chronic kidney disease; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; GLP1-RA = glucagon-like peptide-1 receptor agonist; HbA1c = haemoglobin A1c; LVH = left ventricular hypertrophy; RAAS = renin–angiotensin–aldosterone system; SBP = systolic blood pressure; SGLT2 = sodium-glucose co-transporter 2.
Class of recommendation.
Level of evidence.
Recommendations for the prevention and management of chronic kidney disease in patients with diabetes
 |
 |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BP = blood pressure; CKD = chronic kidney disease; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; GLP1-RA = glucagon-like peptide-1 receptor agonist; HbA1c = haemoglobin A1c; LVH = left ventricular hypertrophy; RAAS = renin–angiotensin–aldosterone system; SBP = systolic blood pressure; SGLT2 = sodium-glucose co-transporter 2.
Class of recommendation.
Level of evidence.
Gaps in the evidence
Lack of renal primary outcome trials with GLP1-RAs in patients with DM.
Whether the nephroprotection shown in the CREDENCE trial is a class effect of SGLT2 inhibition or specific to canagliflozin remains to be determined.
12 Patient-centred care
Key message
Group-based structured education programmes improve disease knowledge, glycaemic control, disease management, and empowerment in patients with DM.
12.1 General aspects
Supporting patients in achieving and sustaining lifestyle changes on an individualized basis, using defined therapeutic goals, continues to be a challenge.551 For instance, 33–49% of patients with DM fail to meet targets for glycaemic, cholesterol, or BP control, and even fewer meet targets for all three measures.552 Whereas a wide range of studies have documented the effects of self-management education and support programmes in patients with DM on DM outcomes, and in patients with CVD delivered separately, the evidence underpinning the best approach to deliver educational or self-management interventions targeted at both DM and CVD is limited. A patient-centred approach is considered an important way to help strengthen patients’ capabilities for self-managing their conditions,553 and should also be the basis of healthcare professional−patient interactions in patients with DM and CVD.
Patient-centred care is an approach that facilitates shared control and decision-making between patient and provider. It emphasizes a focus on the whole person and their experiences of illness within social contexts, rather than a single disease or organ system, and it develops a therapeutic alliance between patient and provider.554 It is also a care strategy that is respectful and responsive to individual patient preferences, needs, and values,555 and it places the patient as an ‘active drug’ at the centre of care, working in collaboration with healthcare professionals. Different approaches on how to integrate patient-centred care in clinical practice exist. One such approach comprises six interactive components, including validating the patients’ experiences, considering the broader context in which the illness is experienced, working towards mutual understandings between healthcare professionals and patients, engaging in health promotion, taking a partnership approach to the healthcare professional−patient relationship, and being realistic about goals.556 In addition, patients with low socio-economic status are more likely to have DM557 and CVD.558 Limited health literacy is a major barrier to disease prevention, disease management, and positive outcomes. Attention to health literacy skills in healthcare provider−patient interactions are thus important in patients with DM and CVD.559
The effects of education and self-management strategies have been evaluated on both DM outcomes and CVD risk factors. A systematic review including patients with DM found that group-based, structured education programmes resulted in clinically relevant improvements in glycaemic control, DM knowledge, triglyceride levels, BP, medication reduction, and self-management for 12–14 months. Benefits for 2–4 years, including decreased DM-related retinopathy, were apparent when group classes were provided on an annual basis.560 A systematic review with meta-analysis showed that group-based, structured DM self-management patient education programmes reduced HbA1c, FPG, and body weight, and improved DM knowledge, self-management skills, and empowerment.561 Another study compared the effectiveness of group-based structured interventions with individual structured interventions or usual care for patients with DM. Outcomes favoured reductions in HbA1c for group-based structured education programmes compared with controls.562 Studies of self-management education programmes indicate that they are cost-effective in the long-term.563
Empowerment strategies including individual consultations, phone calls, web-based sessions, and the use of a booklet were evaluated across 11 studies. Outcomes included HbA1c levels, self-efficacy, levels of DM knowledge, and quality of life. In addition, some of the studies assessed secondary outcomes in the form of CVD risk factors. These studies were carried out in both T1DM and T2DM patients, in primary and secondary care. Improvements with individual empowerment strategies were shown in self-efficacy, levels of DM knowledge, and quality of life. However, no statistically significant improvement was found for HbA1c levels.564
Patients with pre-DM benefit from structured empowerment interventions and lifestyle education to reduce progression to DM,565–567 and beneficial effects on CVD risk factors, such as BP and total cholesterol, have been reported.82,568 The Diabetes Prevention Program provides the strongest evidence for DM prevention in individuals with pre-DM.569
In patients with DM after an ACS, four RCTs included in a systematic review evaluated the effectiveness of structured self-management interventions plus an intensified comprehensive cardiac rehabilitation programme. The review concluded that there is currently no evidence to support the effectiveness of combined interventions in promoting self-management behaviour with regard to clinical, psychological, or behavioural outcomes.570 In patients undergoing PCI, a retrospective study found that patients with DM benefited from cardiac rehabilitation, with regard to all-cause death, to a similar degree as those without DM.571 However, several studies have also indicated that cardiac rehabilitation uptake is low in patients with DM.571,572
Recommendations for patient-centred care of patients with diabetes
 |
 |
DM = diabetes mellitus.
Class of recommendation.
Level of evidence.
Recommendations for patient-centred care of patients with diabetes
 |
 |
DM = diabetes mellitus.
Class of recommendation.
Level of evidence.
Gaps in the evidence
Further research is required to determine the effects of group- and individual-based structured patient education programmes on CVD risk factors.
The effects of patient-centred interventions on micro- and macrovascular complications are unknown.
More research is needed to develop robust combined self-management interventions, including cost-effectiveness evaluations of joint DM and CVD interventions; future studies should compare different modes delivering individual empowerment strategies.
In patients with CVD and concomitant DM, barriers to cardiac rehabilitation should be explored, and future prospective studies should investigate the benefit of cardiac rehabilitation programmes.
Uptake of empowerment programmes in different ethnic groups requires evaluation.
Possible differences between men and women with regards to optimal delivery of patient-centred care, structured education, and self-management programmes should be explored.
13 ‘What to do’ and ‘what not to do’ messages from the Guidelines
 |
 |
 |
 |
 |
 |
 |
 |
ABI = ankle–brachial index; ACEI = angiotensin-converting enzyme inhibitor; ACS = acute coronary syndromes; AF = atrial fibrillation; ARB = angiotensin receptor blocker; BP = blood pressure; CABG = coronary artery bypass graft; CAD = coronary artery disease; CCS = chronic coronary syndromes; CHA2DS2-VASc = Congestive heart failure, Hypertension, Age ≥75 years (Doubled), Diabetes mellitus, Stroke or transient ischaemic attack (Doubled), Vascular disease, Age 65–74 years, Sex category; CKD = chronic kidney disease; CLTI = chronic limb-threatening ischaemia; CRT = cardiac resynchronization therapy; CRT-D = cardiac resynchronization therapy with implantable defibrillator; CT = computed tomography; CV = cardiovascular; CVD = cardiovascular disease; DAPT = dual antiplatelet therapy; DBP = diastolic blood pressure; DES = drug-eluting stent; DM = diabetes mellitus; DPP4 = dipeptidyl peptidase-4; EAS = European Atherosclerosis Society; ECG = electrocardiogram; eGFR = estimated glomerular filtration rate; ESC = European Society of Cardiology; FPG = fasting plasma glucose; GLP1-RA = glucagon-like peptide-1 receptor agonist; HAS-BLED = Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (>65 years), Drugs/alcohol concomitantly; HbA1c = haemoglobin A1c; HDL-C = high-density lipoprotein cholesterol; HR = heart failure; HFmrEF = heart failure with mid-range ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter defibrillator; IGT = impaired glucose tolerance; LAD = left anterior descending coronary artery; LDL-C = low-density lipoprotein cholesterol; LEAD = lower extremity artery disease; LV = left ventricular; LVEF = left ventricular ejection fraction; LVH = left ventricular hypertrophy; MI = myocardial infarction; MRAs = mineralocorticoid receptor antagonists; NOAC = non-vitamin K antagonist oral anticoagulant; OGTT = oral glucose tolerance test; PAD = peripheral arterial disease; PCI = percutaneous coronary intervention; PCSK9 = proprotein convertase subtilisin/kexin type 9; RAAS = renin–angiotensin–aldosterone system; SBP = systolic blood pressure; SGLT2 = sodium-glucose co-transporter 2; T2DM = type 2 diabetes mellitus; TBI = toe–brachial index; VKA = vitamin K antagonist; VT = ventricular tachycardia; WIfI = Wound, Ischaemia, and foot Infection.
Class of recommendation.
Level of evidence.
A commonly stated goal for obese patients with DM is to lose around 5% of baseline weight.
It is recommended that all individuals reduce the amount of sedentary time by breaking up periods of sedentary activity with moderate-to-vigorous physical activity in bouts of ≥10 min (broadly equivalent to 1000 steps).
See Table 7.
See the 2019 ESC/EAS Guidelines for the management of dyslipidaemias for non-HDL-C and apolipoprotein B targets.
Including a diabetologist and a vascular specialist.
See Table 12.
 |
 |
 |
 |
 |
 |
 |
 |
ABI = ankle–brachial index; ACEI = angiotensin-converting enzyme inhibitor; ACS = acute coronary syndromes; AF = atrial fibrillation; ARB = angiotensin receptor blocker; BP = blood pressure; CABG = coronary artery bypass graft; CAD = coronary artery disease; CCS = chronic coronary syndromes; CHA2DS2-VASc = Congestive heart failure, Hypertension, Age ≥75 years (Doubled), Diabetes mellitus, Stroke or transient ischaemic attack (Doubled), Vascular disease, Age 65–74 years, Sex category; CKD = chronic kidney disease; CLTI = chronic limb-threatening ischaemia; CRT = cardiac resynchronization therapy; CRT-D = cardiac resynchronization therapy with implantable defibrillator; CT = computed tomography; CV = cardiovascular; CVD = cardiovascular disease; DAPT = dual antiplatelet therapy; DBP = diastolic blood pressure; DES = drug-eluting stent; DM = diabetes mellitus; DPP4 = dipeptidyl peptidase-4; EAS = European Atherosclerosis Society; ECG = electrocardiogram; eGFR = estimated glomerular filtration rate; ESC = European Society of Cardiology; FPG = fasting plasma glucose; GLP1-RA = glucagon-like peptide-1 receptor agonist; HAS-BLED = Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (>65 years), Drugs/alcohol concomitantly; HbA1c = haemoglobin A1c; HDL-C = high-density lipoprotein cholesterol; HR = heart failure; HFmrEF = heart failure with mid-range ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter defibrillator; IGT = impaired glucose tolerance; LAD = left anterior descending coronary artery; LDL-C = low-density lipoprotein cholesterol; LEAD = lower extremity artery disease; LV = left ventricular; LVEF = left ventricular ejection fraction; LVH = left ventricular hypertrophy; MI = myocardial infarction; MRAs = mineralocorticoid receptor antagonists; NOAC = non-vitamin K antagonist oral anticoagulant; OGTT = oral glucose tolerance test; PAD = peripheral arterial disease; PCI = percutaneous coronary intervention; PCSK9 = proprotein convertase subtilisin/kexin type 9; RAAS = renin–angiotensin–aldosterone system; SBP = systolic blood pressure; SGLT2 = sodium-glucose co-transporter 2; T2DM = type 2 diabetes mellitus; TBI = toe–brachial index; VKA = vitamin K antagonist; VT = ventricular tachycardia; WIfI = Wound, Ischaemia, and foot Infection.
Class of recommendation.
Level of evidence.
A commonly stated goal for obese patients with DM is to lose around 5% of baseline weight.
It is recommended that all individuals reduce the amount of sedentary time by breaking up periods of sedentary activity with moderate-to-vigorous physical activity in bouts of ≥10 min (broadly equivalent to 1000 steps).
See Table 7.
See the 2019 ESC/EAS Guidelines for the management of dyslipidaemias for non-HDL-C and apolipoprotein B targets.
Including a diabetologist and a vascular specialist.
See Table 12.
The disclosure forms of all experts involved in the development of these Guidelines are available on the ESC website www.escardio.org/guidelines
Authors/Task Force Member Affiliations: listed in the Appendix.
ESC Committee for Practice Guidelines (CPG) and National Cardiac Societies document reviewers: listed in the Appendix.
ESC entities having participated in the development of this document:
Associations: Acute Cardiovascular Care Association (ACCA), Association of Cardiovascular Nursing & Allied Professions (ACNAP), European Association of Cardiovascular Imaging (EACVI), European Association of Preventive Cardiology (EAPC), European Association of Percutaneous Cardiovascular Interventions (EAPCI), European Heart Rhythm Association (EHRA), Heart Failure Association (HFA).
Councils: Council on Cardiovascular Primary Care, Council on Hypertension.
Working Groups: Aorta and Peripheral Vascular Diseases, Cardiovascular Surgery, Thrombosis.
The content of these ESC Guidelines has been published for personal and educational use only. No commercial use is authorized. No part of the Guidelines may be translated or reproduced in any form without written permission from the ESC. Permission can be obtained upon submission of a written request to Oxford University Press, the publisher of the European Heart Journal and the party authorized to handle such permissions on behalf of the ESC ([email protected]).
Disclaimer: The Guidelines represent the views of the ESC and were produced after careful consideration of the scientific and medical knowledge, and the evidence available at the time of their publication. The ESC and EASD are not responsible in the event of any contradiction, discrepancy, and/or ambiguity between the Guidelines and any other official recommendations or guidelines issued by the relevant public health authorities, in particular in relation to good use of healthcare or therapeutic strategies. Health professionals are encouraged to take the Guidelines fully into account when exercising their clinical judgment, as well as in the determination and the implementation of preventive, diagnostic, or therapeutic medical strategies; however, the Guidelines do not override, in any way whatsoever, the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient’s health condition and in consultation with that patient and, where appropriate and/or necessary, the patient’s caregiver. Nor do the Guidelines exempt health professionals from taking into full and careful consideration the relevant official updated recommendations or guidelines issued by the competent public health authorities, in order to manage each patient’s case in light of the scientifically accepted data pursuant to their respective ethical and professional obligations. It is also the health professional’s responsibility to verify the applicable rules and regulations relating to drugs and medical devices at the time of prescription.
14 Appendix
Author/Task Force Member Affiliations:
Victor Aboyans, Department of Cardiology, Dupuytren University Hospital, Limoges, France; Clifford J. Bailey, Life and Health Sciences, Aston University, Birmingham, United Kingdom; Antonio Ceriello, Cardiovascular and Metabolic Diseases, IRCCS MultiMedica, Milan, Italy; Victoria Delgado, Cardiology, Leiden University Medical Center, Leiden, Netherlands; Massimo Federici, Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy; Gerasimos Filippatos, University of Athens, Athens, Greece and University of Cyprus, Nicosia, Cyprus; Diederick E. Grobbee, Julius Center for Health Sciences and Primary Care, University Medical Center, Utrecht, Netherlands; Tina Birgitte Hansen, Department of Cardiology, Zealand University Hospital, Roskilde, Denmark and Department of Regional Health Research, University of Southern Denmark, Odense, Denmark; Heikki V. Huikuri, Internal Medicine, University of Oulu, Oulu, Finland; Isabelle Johansson, Cardiology Unit, Department of Medicine K2, Karolinska Institute and Karolinska University Stockholm, Sweden; Peter Jüni, Applied Health Research Centre, Li Ka Shing Knowledge Institute of St. Michael's Hospital, Toronto, Canada; Maddalena Lettino, Cardiology, Cardiovascular Department, San Gerardo Hospital ASST Monza, Monza, Italy; Nikolaus Marx, Internal Medicine I, RWTH Aachen University, Aachen, Germany; Linda G. Mellbin, Cardiology Unit, Department of Medicine Solna, Karolinska Institute, Stockholm, Sweden; Carl J. Östgren, Department of Medical and Health Sciences, Linkoping University, Linkoping, Sweden; Bianca Rocca, Pharmacology, Catholic University School of Medicine, Rome, Italy; Marco Roffi, Cardiology, University Hospitals, Geneva, Switzerland; Naveed Sattar, Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, United Kingdom; Petar M. Seferović, Heart Failure Center, Belgrade University Medical Center, University of Belgrade, Faculty of Medicine, Belgrade, Serbia; Miguel Sousa-Uva, Cardiac Surgery, Hospital Santa Cruz, Carnaxide, Portugal; Paul Valensi, Endocrinology Diabetology Nutrition, Jean Verdier Hospital, APHP, Paris Nord University, CINFO, CRNH-IdF, Bondy, France; David C. Wheeler, Department of Renal Medicine, University College London, London, United Kingdom.
ESC Committee for Practice Guidelines (CPG): Stephan Windecker (Chairperson) (Switzerland), Victor Aboyans (France), Colin Baigent (UK), Jean-Philippe Collet (France), Veronica Dean (France), Victoria Delgado (Netherlands), Donna Fitzsimons (United Kingdom), Chris P. Gale (United Kingdom), Diederick E. Grobbee (Netherlands), Sigrun Halvorsen (Norway), Gerhard Hindricks (Germany), Bernard Iung (France), Peter Jüni (Canada), Hugo A. Katus (Germany), Ulf Landmesser (Germany), Christophe Leclercq (France), Maddalena Lettino (Italy), Basil S. Lewis (Israel), Bela Merkely (Hungary), Christian Mueller (Switzerland), Steffen E. Petersen (United Kingdom), Anna Sonia Petronio (Italy), Dimitrios J. Richter (Greece), Marco Roffi (Switzerland), Evgeny Shlyakhto (Russian Federation), Iain A. Simpson (United Kingdom), Miguel Sousa-Uva (Portugal), Rhian M. Touyz (United Kingdom).
ESC National Cardiac Societies actively involved in the review process of the 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD.
Armenia: Armenian Cardiologists Association, Parounak H. Zelveian; Austria: Austrian Society of Cardiology, Daniel Scherr; Azerbaijan: Azerbaijan Society of Cardiology, Tofig Jahangirov; Belarus: Belorussian Scientific Society of Cardiologists, Irina Lazareva; Belgium: Belgian Society of Cardiology, Bharati Shivalkar; Bosnia and Herzegovina: Association of Cardiologists of Bosnia and Herzegovina, Nabil Naser; Bulgaria: Bulgarian Society of Cardiology, Ivan Gruev; Croatia: Croatian Cardiac Society, Davor Milicic; Cyprus: Cyprus Society of Cardiology, Petros M. Petrou; Czech Republic: Czech Society of Cardiology, Aleš Linhart; Denmark: Danish Society of Cardiology, Per Hildebrandt; Egypt: Egyptian Society of Cardiology, Hosam Hasan-Ali; Estonia: Estonian Society of Cardiology, Toomas Marandi; Finland: Finnish Cardiac Society, Seppo Lehto; France: French Society of Cardiology, Jacques Mansourati; Georgia: Georgian Society of Cardiology, Ramaz Kurashvili; Greece: Hellenic Society of Cardiology, Gerasimos Siasos; Hungary: Hungarian Society of Cardiology, Csaba Lengyel; Iceland: Icelandic Society of Cardiology, Inga S. Thrainsdottir; Israel: Israel Heart Society, Doron Aronson; Italy: Italian Federation of Cardiology, Andrea Di Lenarda; Kazakhstan: Association of Cardiologists of Kazakhstan, Aigul Raissova; Kosovo (Republic of): Kosovo Society of Cardiology, Pranvera Ibrahimi; Kyrgyzstan: Kyrgyz Society of Cardiology, Saamai Abilova; Latvia: Latvian Society of Cardiology, Karlis Trusinskis; Lebanon: Lebanese Society of Cardiology, Georges Saade; Libya: Libyan Cardiac Society, Hisham Benlamin; Lithuania: Lithuanian Society of Cardiology, Zaneta Petrulioniene; Luxembourg: Luxembourg Society of Cardiology, Cristiana Banu; Malta: Maltese Cardiac Society, Caroline Jane Magri; Moldova (Republic of): Moldavian Society of Cardiology, Lilia David; Montenegro: Montenegro Society of Cardiology, Aneta Boskovic; Morocco: Moroccan Society of Cardiology, Mohamed Alami; Netherlands: Netherlands Society of Cardiology, An Ho Liem; North Macedonia: North Macedonian Society of Cardiology, Marijan Bosevski; Norway: Norwegian Society of Cardiology, Gard Frodahl Tveitevaag Svingen; Poland: Polish Cardiac Society, Marianna Janion; Portugal: Portuguese Society of Cardiology, Cristina Gavina; Romania: Romanian Society of Cardiology, Dragos Vinereanu; Russian Federation: Russian Society of Cardiology, Sergey Nedogoda; San Marino: San Marino Society of Cardiology, Tatiana Mancini; Serbia: Cardiology Society of Serbia, Marina Deljanin Ilic; Slovakia: Slovak Society of Cardiology, Lubomira Fabryova; Slovenia: Slovenian Society of Cardiology, Zlatko Fras; Spain: Spanish Society of Cardiology, Manuel F. Jiménez-Navarro; Sweden: Swedish Society of Cardiology, Anna Norhammar; Switzerland: Swiss Society of Cardiology, Roger Lehmann; Tunisia: Tunisian Society of Cardiology and Cardio-Vascular Surgery, Mohamed Sami Mourali; Turkey: Turkish Society of Cardiology, Dilek Ural; Ukraine: Ukrainian Association of Cardiology, Elena Nesukay; United Kingdom of Great Britain and Northern Ireland: British Cardiovascular Society, Tahseen Ahmad Chowdhury.
15 References
International Diabetes Federation. IDF Diabetes Atlas - 8th Edition. http://diabetesatlas.org/resources/2017-atlas.html (June 14 2019).
World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate and hyperglycaemia. Report of a WHO/IDF consultation. http://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/ (June 14 2019).
World Health Organization. Use of Ggycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: abbreviated report of a WHO consultation. http://www.who.int/diabetes/publications/report-hba1c_2011.pdf (June 14 2019).
American Diabetes Association.
American Diabetes Association.
Emerging Risk Factors Collaboration,
Prospective Studies Collaboration and Asia Pacific Cohort Studies Collaboration.
Emerging Risk Factors Collaboration,
American Diabetes Association.
Diabetes Prevention Program Research Group.
Look AHEAD Research Group,
ORIGIN Trial Investigators,
ASCEND Study Collaborative Group,
GBD 2015 Tobacco Collaborators.
Scottish Intercollegiate Guidelines Network. Risk estimation and the prevention of cardiovascular disease. http://www.sign.ac.uk/sign-149-risk-estimation-and-the-prevention-of-cardiovascular-disease.html (July 3 2018).
Control Group
The DECODE study group on behalf of the European Diabetes Epidemiology Group.
ADVANCE Collaborative Group
ORIGIN Trial Investigators
UK Prospective Diabetes Study (UKPDS) Group.
UK Prospective Diabetes Study (UKPDS) Group.
Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group,
Diabetes Control and Complications Trial Research Group,
ORIGIN Trial Investigators.
DREAM Trial Investigators,
NAVIGATOR Study Group,
Cholesterol Treatment Trialists' (CTT) Collaborators,
ACCORD Study Group,
Cholesterol Treatment Trialists' (CTT) Collaboration,
Cholesterol Treatment Trialists' (CTT) Collaboration,
Cholesterol Treatment Trialists' (CTT) Collaborators,
Antithrombotic Trialists' Collaboration,
ASCEND Study Collaborative Group,
The Task Force on the management of stable coronary artery disease of the European Society of Cardiology,
ACCORD Study Group,
Action to Control Cardiovascular Risk in Diabetes Study Group,
NAVIGATOR Study Group,
ORIGIN Trial Investigators,
ACE Inhibitor Myocardial Infarction Collaborative Group.
European Medicines Agency. Questions and answers on the review of medicines containing trimetazidine (20 mg tablets, 35 mg modified release tablet and 20 mg/ml oral solution). http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Trimetazidine_31/WC500129195.pdf (June 14 2019).
SOLVD Investigators,
Antiplatelet Trialists' Collaboration.
Antithrombotic Trialists' Collaboration,
CAPRIE Steering Committee.
BARI 2D Study Group
Digitalis Investigation Group.
The Diabetes Control and Complications Trial Research Group.
DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators,
American Diabetes Association.
Ankle Brachial Index Collaboration,
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group.
Diabetes Prevention Program Research Group,
Institute of Medicine (US) Committee on Quality of Health Care in America.
Author notes
Clifford J. Bailey, Antonio Ceriello, Massimo Federici, Naveed Sattar, David C. Wheeler, Kåre I. Birkeland, Miles Fisher, Richard I. G. Holt, Philip Home, Frederik Persson, Peter Rossing: representing the EASD.





