-
PDF
- Split View
-
Views
-
Cite
Cite
Gilbert Habib, Paola Anna Erba, Bernard Iung, Erwan Donal, Bernard Cosyns, Cécile Laroche, Bogdan A Popescu, Bernard Prendergast, Pilar Tornos, Anita Sadeghpour, Leopold Oliver, Jolanta-Justina Vaskelyte, Rouguiatou Sow, Olivier Axler, Aldo P Maggioni, Patrizio Lancellotti, EURO-ENDO Investigators , Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study, European Heart Journal, Volume 40, Issue 39, 14 October 2019, Pages 3222–3232, https://doi.org/10.1093/eurheartj/ehz620
Close - Share Icon Share
Abstract
The EURO-ENDO registry aimed to study the management and outcomes of patients with infective endocarditis (IE).
Prospective cohort of 3116 adult patients (2470 from Europe, 646 from non-ESC countries), admitted to 156 hospitals in 40 countries between January 2016 and March 2018 with a diagnosis of IE based on ESC 2015 diagnostic criteria. Clinical, biological, microbiological, and imaging [echocardiography, computed tomography (CT) scan, 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT)] data were collected. Infective endocarditis was native (NVE) in 1764 (56.6%) patients, prosthetic (PVIE) in 939 (30.1%), and device-related (CDRIE) in 308 (9.9%). Infective endocarditis was community-acquired in 2046 (65.66%) patients. Microorganisms involved were staphylococci in 1085 (44.1%) patients, oral streptococci in 304 (12.3%), enterococci in 390 (15.8%), and Streptococcus gallolyticus in 162 (6.6%). 18F-fluorodeoxyglucose positron emission tomography/computed tomography was performed in 518 (16.6%) patients and presented with cardiac uptake (major criterion) in 222 (42.9%) patients, with a better sensitivity in PVIE (66.8%) than in NVE (28.0%) and CDRIE (16.3%). Embolic events occurred in 20.6% of patients, and were significantly associated with tricuspid or pulmonary IE, presence of a vegetation and Staphylococcus aureus IE. According to ESC guidelines, cardiac surgery was indicated in 2160 (69.3%) patients, but finally performed in only 1596 (73.9%) of them. In-hospital death occurred in 532 (17.1%) patients and was more frequent in PVIE. Independent predictors of mortality were Charlson index, creatinine > 2 mg/dL, congestive heart failure, vegetation length > 10 mm, cerebral complications, abscess, and failure to undertake surgery when indicated.
Infective endocarditis is still a life-threatening disease with frequent lethal outcome despite profound changes in its clinical, microbiological, imaging, and therapeutic profiles.
Introduction
Infective endocarditis (IE) is a severe disease that is associated with high morbidity and mortality1–5 and whose incidence and severity remain unchanged (or even increased) despite improvements in diagnostic and therapeutic strategies. Reasons for this persistently poor prognosis are numerous and include an increasing proportion of older patients with more severe disease, changing epidemiological profiles and greater numbers of patients with prosthetic valve or device-related infection.5 , 6
There were several motivations to create an ESC-EORP European Endocarditis Registry:
The epidemiological profile of IE has changed in recent decades, with important differences between countries and increasing numbers of staphylococcal and nosocomial cases.1 , 6 While the European Society of Cardiology (ESC) Euro Heart Survey on valvular heart disease performed in 2001 provided useful information regarding the management of IE across Europe at that time,7 no attempt has been made to update and implement the results of the Euro Heart Survey in the contemporary era. There is, therefore, a need for a comprehensive and dedicated IE survey.
New strategies have been developed to improve diagnosis and prognosis. The ESC Guidelines on the management of IE were published in 2015 and provided new insights into the diagnostic and therapeutic management of these patients.8 However, their implementation in real-world clinical practice has never been studied.
Although echocardiography is the principal diagnostic imaging technique used in IE9, other non-invasive imaging techniques have received increasing attention, including multislice computed tomography (MSCT), magnetic resonance imaging, and nuclear imaging (18F-fluorodeoxyglucose positron emission tomography/computed tomography, and leucocyte scintigraphy).10–12 However, their availability and application in different countries are unknown.
Early surgery is recommended in patients with complicated IE but its impact on prognosis is still debated.13–15 With the present IE registry, it will be possible to assess whether the implementation of guidelines and increased use of early surgery are associated with a reduced in-hospital and 1-year mortality.
The aim of the ESC-EORP European Endocarditis Registry (EURO-ENDO) is to undertake a contemporary international investigation of the care and outcomes of IE in Europe. The primary objective is to evaluate the outcome of patients diagnosed with IE. The secondary objectives are to assess the current clinical, epidemiological, microbiological, therapeutic, and prognostic characteristics of IE in Europe, to assess the current practices of imaging in IE in Europe and in affiliated countries, to assess the degree of implementation of the ESC guidelines in practice, and to compare these current data with those obtained in the Euro Heart Survey. The additional input of several non-European countries which accepted to participate in the registry will also enable comparison of IE in Europe and other international regions.
Methods
Study design and data collection
The ESC-EORP EURO-ENDO registry is a prospective multicentre observational study of patients presenting with definite or possible IE to hospitals in Europe and ESC-affiliated/non-affiliated countries. The detailed methodology of EURO-ENDO has already been reported.16 Briefly, from 1 January 2016 to 31 March 2018, centres were asked to include consecutive patients aged greater than 18 years who presented with IE during a 1-year period. Participating centres were identified by the European Association of Cardiovascular Imaging as either high level IE centres: high volume of treated patients (≥20 patients per year) with expertise in IE diagnosis, management, imaging and surgical therapy or low-volume centres (<20 patients per year), without surgical facilities16. Inclusion criteria were a diagnosis of definite IE (or possible IE considered and treated as IE) based on the ESC 2015 IE diagnostic criteria.8 After informed consent, data were collected at inclusion and during hospitalization, including demographics, patient history, Charlson index, taking into account age and several comorbidities,17 clinical, biological, microbiological, and echocardiographic findings, use of other imaging techniques (CT scan, 18F-FDG PET/CT, leucocyte scintigraphy), medical therapy, complications (embolic event, infectious, and haemodynamic complications), theoretical indications for surgery, and in-hospital mortality.16
Data management and statistical analysis
All data were collected by the collecting officers or investigators at the participating centres and included in an electronic case report form (CRF) for on-line data entry. All patients enrolled with possible or definite IE were included in the analyses. Univariable analysis was applied to both continuous and categorical variables. Continuous variables were reported as mean ± SD or as median and interquartile range (IQR). Among-group comparisons were made using a non-parametric test (Kruskal–Wallis test). Categorical variables were reported as counts and percentages. Among-group 2 × 2 comparisons were made using a χ2 test or Fisher’s exact test if any expected cell count was <5. In other cases, the Monte-Carlo estimate of the exact P-value was used. Plots of the Kaplan–Meier curves for time to all causes of deaths were performed. The Kaplan–Meier curve of time to death were also adjusted for the covariates from the Cox proportional hazard model.
Pairwise correlations between all candidate variables (variables with P < 0.10 in univariate) within the model and variables considered of relevant clinical interest were tested before proceeding to the multivariable model. In case of correlation, some criteria were not taken into account. A backward multivariable Cox regression analysis was performed to identify the independent predictors of in-hospital all-cause mortality (only the first month of survival was taken into account). Significance levels of 0.05 were required to allow a variable to stay (SLSTAY = 0.05) within the model. Some measures of model fit were considered: concordance and the Goodness of fit test proposed by May and Hosmer.18 In addition, the proportional hazard ratios assumptions were verified graphically and with the Schoenfeld residuals test. A backward multivariable logistic regression analysis was performed to identify the independent predictors of indication of cardiac surgery. Significance levels of 0.05 were required to allow a variable to stay within the model. All analyses were performed using SAS statistical software version 9.4 (SAS Institute, Inc., Cary, NC, USA) and R version 3.5.1.
Results
A total of 156 centres from 40 countries included 3116 cases of IE (Supplementary material online, Table S1), representing a mean of 20.19 patients per centre per year. Among the 156 active centres, 120 (76.9%) were from ESC-affiliated countries and 36 (23.1%) were outside Europe, including 79.5% high-volume centres and 20.5% low-volume centres16. Low-volume centres included 238 (7.6%) patients, high-volume centres 2878 (92.4%) patients, without difference between European and non-European countries. Patients were enrolled from the cardiology ward in 48.9% of cases, the ICC/CCU in 15.0%, the general internal medicine ward in 8.8%, the echocardiography laboratory in 7.8%, the cardiac surgical ward in 10.1%, the infectious diseases ward in 9.3%, and the electrophysiology ward in 0.1%. Infective endocarditis was definite in 2610 (83.8%) patients and possible in 506 (16.2%).
Patient demographics and characteristics
The main demographic and characteristics of patients are displayed in Table 1. Among the 3116 patients, 1764 (56.6%) had native valve IE (NVE), 939 (30.1%) prosthetic IE (PVIE), and 308 (9.9%) intracardiac device-related IE (CDRIE), with similar proportions observed within and outside Europe. Infective endocarditis complicated congenital heart disease in 365 (11.7%) patients. The 105 remaining patients not categorized as PVIE, NVE, or CDRIE corresponded either to another location or to an undetermined location of the infective process. These patients were included in total analysis but not in groups comparisons analysis. Mean age was 59.25 ± 18.03 years (46.3% ≥ 65 years and 12.0% ≥ 80 years) and was higher in European than in non-European countries (60.97 ± 17.36 vs. 52.66 ± 19.01, P < 0.0001). There was a history of intravenous drug use in 212 (6.9%) patients. Infective endocarditis was healthcare associated in 1027 (32.96%) patients (nosocomial in 60.8% of them, non-nosocomial in 39.2%), community-acquired in 2046 (65.66%) patients, and undetermined in 1.38% of patients, without significant difference between European and non-European countries.
| . | Total (n = 3116) . | Prosthesis+Repair (n = 939) . | Native (n = 1764) . | PM/ICD (n = 308) . | P-value . |
|---|---|---|---|---|---|
| Demography | |||||
| Age (years) | |||||
| N | 3116 | 939 | 1764 | 308 | |
| Mean ± SD | 59.25 ± 18.03 | 63.36 ± 16.81 | 55.61 ± 18.45 | 66.77 ± 14.11 | <0.0001 |
| Median (IQR) | 63.0 (46.0–73.0) | 67.0 (54.0–75.0) | 58.0 (41.0–70.0) | 69.0 (60.0–76.0) | <0.0001 |
| Age ≥ 65 years | 1443/3116 (46.3%) | 538/939 (57.3%) | 662/1764 (37.5%) | 194/308 (63.0%) | <0.0001 |
| Age ≥ 80 years | 375/3116 (12.0%) | 141/939 (15.0%) | 163/1764 (9.2%) | 56/308 (18.2%) | <0.0001 |
| Females (%) | 969/3116 (31.1%) | 292/939 (31.1%) | 553/1764 (31.3%) | 86/308 (27.9%) | 0.4901 |
| History of cardiovascular diseases | |||||
| Heart failure | 662/2840 (23.3%) | 271/856 (31.7%) | 238/1620 (14.7%) | 123/270 (45.6%) | <0.0001 |
| Congenital disease | 365/3114 (11.7%) | 130/938 (13.9%) | 197/1763 (11.2%) | 18/308 (5.8%) | 0.0001 |
| Ischaemic heart disease | 622/2897 (21.5%) | 266/881 (30.2%) | 207/1637 (12.6%) | 128/284 (45.1%) | <0.0001 |
| Atrial fibrillation | 767/2918 (26.3%) | 365/891 (41.0%) | 240/1634 (14.7%) | 133/294 (45.2%) | <0.0001 |
| Hypertrophic cardiomyopathy | 63/2840 (2.2%) | 20/856 (2.3%) | 28/1620 (1.7%) | 11/270 (4.1%) | 0.0498 |
| Known valve murmur | 972/2840 (34.2%) | 455/856 (53.2%) | 427/1620 (26.4%) | 55/270 (20.4%) | <0.0001 |
| Previous endocarditis (%) | 274/3116 (8.8%) | 170/939 (18.1%) | 67/1764 (3.8%) | 17/308 (5.5%) | <0.0001 |
| Device therapy | |||||
| Pacemaker | 325/3116 (10.4%) | 97/939 (10.3%) | 53/1764 (3.0%) | 161/308 (52.3%) | |
| ICD (defibrillator) | 125/3116 (4.0%) | 18/939 (1.9%) | 15/1764 (0.9%) | 89/308 (28.9%) | |
| CRT-D (with ICD) | 72/3116 (2.3%) | 13/939 (1.4%) | 3/1764 (0.2%) | 47/308 (15.3%) | |
| CRT-P (pacing only) | 15/3116 (0.5%) | 7/939 (0.7%) | 1/1764 (0.1%) | 6/308 (1.9%) | |
| Risk factors | |||||
| Previous stroke/TIA | 340/2860 (11.9%) | 132/867 (15.2%) | 160/1626 (9.8%) | 34/273 (12.5%) | 0.0003 |
| Arterial hypertension | 1502/3111 (48.3%) | 531/938 (56.6%) | 726/1762 (41.2%) | 194/306 (63.4%) | <0.0001 |
| COPD/asthma | 318/3111 (10.2%) | 98/937 (10.5%) | 160/1762 (9.1%) | 49/307 (16.0%) | 0.0011 |
| Chronic renal failure | 553/3113 (17.8%) | 191/938 (20.4%) | 255/1762 (14.5%) | 83/308 (26.9%) | <0.0001 |
| Dialysis | 163/3113 (5.2%) | 28/938 (3.0%) | 107/1762 (6.1%) | 18/308 (5.8%) | <0.0001 |
| HIV | 31/3038 (1.0%) | 3/916 (0.3%) | 24/1726 (1.4%) | 2/294 (0.7%) | 0.0212 |
| Hypo/hyperthyroidism | 226/2820 (8.0%) | 93/852 (10.9%) | 98/1606 (6.1%) | 24/270 (8.9%) | <0.0001 |
| Chronic autoimmune disease | 109/3103 (3.5%) | 18/937 (1.9%) | 74/1753 (4.2%) | 12/308 (3.9%) | 0.0030 |
| Cancer | 361/3088 (11.7%) | 107/930 (11.5%) | 210/1746 (12.0%) | 31/308 (10.1%) | 0.6230 |
| Current pregnancy | 8/3092 (0.3%) | 2/932 (0.2%) | 6/1749 (0.3%) | 0/307 (0.0%) | 0.7716 |
| Smoking | 759/2938 (25.8%) | 166/880 (18.9%) | 507/1666 (30.4%) | 68/296 (23.0%) | <0.0001 |
| Intravenous drug dependency | 212/3067 (6.9%) | 20/930 (2.2%) | 184/1729 (10.6%) | 3/305 (1.0%) | <0.0001 |
| Alcohol abuse | 228/3003 (7.6%) | 44/910 (4.8%) | 166/1691 (9.8%) | 12/301 (4.0%) | <0.0001 |
| Immunosuppressive treatment | 104/2840 (3.7%) | 21/856 (2.5%) | 71/1620 (4.4%) | 8/270 (3.0%) | 0.0450 |
| Long corticotherapy | 127/2840 (4.5%) | 28/856 (3.3%) | 84/1620 (5.2%) | 13/270 (4.8%) | 0.0871 |
| Intravenous catheter | 250/3104 (8.1%) | 63/934 (6.7%) | 150/1760 (8.5%) | 24/306 (7.8%) | 0.2672 |
| . | Total (n = 3116) . | Prosthesis+Repair (n = 939) . | Native (n = 1764) . | PM/ICD (n = 308) . | P-value . |
|---|---|---|---|---|---|
| Demography | |||||
| Age (years) | |||||
| N | 3116 | 939 | 1764 | 308 | |
| Mean ± SD | 59.25 ± 18.03 | 63.36 ± 16.81 | 55.61 ± 18.45 | 66.77 ± 14.11 | <0.0001 |
| Median (IQR) | 63.0 (46.0–73.0) | 67.0 (54.0–75.0) | 58.0 (41.0–70.0) | 69.0 (60.0–76.0) | <0.0001 |
| Age ≥ 65 years | 1443/3116 (46.3%) | 538/939 (57.3%) | 662/1764 (37.5%) | 194/308 (63.0%) | <0.0001 |
| Age ≥ 80 years | 375/3116 (12.0%) | 141/939 (15.0%) | 163/1764 (9.2%) | 56/308 (18.2%) | <0.0001 |
| Females (%) | 969/3116 (31.1%) | 292/939 (31.1%) | 553/1764 (31.3%) | 86/308 (27.9%) | 0.4901 |
| History of cardiovascular diseases | |||||
| Heart failure | 662/2840 (23.3%) | 271/856 (31.7%) | 238/1620 (14.7%) | 123/270 (45.6%) | <0.0001 |
| Congenital disease | 365/3114 (11.7%) | 130/938 (13.9%) | 197/1763 (11.2%) | 18/308 (5.8%) | 0.0001 |
| Ischaemic heart disease | 622/2897 (21.5%) | 266/881 (30.2%) | 207/1637 (12.6%) | 128/284 (45.1%) | <0.0001 |
| Atrial fibrillation | 767/2918 (26.3%) | 365/891 (41.0%) | 240/1634 (14.7%) | 133/294 (45.2%) | <0.0001 |
| Hypertrophic cardiomyopathy | 63/2840 (2.2%) | 20/856 (2.3%) | 28/1620 (1.7%) | 11/270 (4.1%) | 0.0498 |
| Known valve murmur | 972/2840 (34.2%) | 455/856 (53.2%) | 427/1620 (26.4%) | 55/270 (20.4%) | <0.0001 |
| Previous endocarditis (%) | 274/3116 (8.8%) | 170/939 (18.1%) | 67/1764 (3.8%) | 17/308 (5.5%) | <0.0001 |
| Device therapy | |||||
| Pacemaker | 325/3116 (10.4%) | 97/939 (10.3%) | 53/1764 (3.0%) | 161/308 (52.3%) | |
| ICD (defibrillator) | 125/3116 (4.0%) | 18/939 (1.9%) | 15/1764 (0.9%) | 89/308 (28.9%) | |
| CRT-D (with ICD) | 72/3116 (2.3%) | 13/939 (1.4%) | 3/1764 (0.2%) | 47/308 (15.3%) | |
| CRT-P (pacing only) | 15/3116 (0.5%) | 7/939 (0.7%) | 1/1764 (0.1%) | 6/308 (1.9%) | |
| Risk factors | |||||
| Previous stroke/TIA | 340/2860 (11.9%) | 132/867 (15.2%) | 160/1626 (9.8%) | 34/273 (12.5%) | 0.0003 |
| Arterial hypertension | 1502/3111 (48.3%) | 531/938 (56.6%) | 726/1762 (41.2%) | 194/306 (63.4%) | <0.0001 |
| COPD/asthma | 318/3111 (10.2%) | 98/937 (10.5%) | 160/1762 (9.1%) | 49/307 (16.0%) | 0.0011 |
| Chronic renal failure | 553/3113 (17.8%) | 191/938 (20.4%) | 255/1762 (14.5%) | 83/308 (26.9%) | <0.0001 |
| Dialysis | 163/3113 (5.2%) | 28/938 (3.0%) | 107/1762 (6.1%) | 18/308 (5.8%) | <0.0001 |
| HIV | 31/3038 (1.0%) | 3/916 (0.3%) | 24/1726 (1.4%) | 2/294 (0.7%) | 0.0212 |
| Hypo/hyperthyroidism | 226/2820 (8.0%) | 93/852 (10.9%) | 98/1606 (6.1%) | 24/270 (8.9%) | <0.0001 |
| Chronic autoimmune disease | 109/3103 (3.5%) | 18/937 (1.9%) | 74/1753 (4.2%) | 12/308 (3.9%) | 0.0030 |
| Cancer | 361/3088 (11.7%) | 107/930 (11.5%) | 210/1746 (12.0%) | 31/308 (10.1%) | 0.6230 |
| Current pregnancy | 8/3092 (0.3%) | 2/932 (0.2%) | 6/1749 (0.3%) | 0/307 (0.0%) | 0.7716 |
| Smoking | 759/2938 (25.8%) | 166/880 (18.9%) | 507/1666 (30.4%) | 68/296 (23.0%) | <0.0001 |
| Intravenous drug dependency | 212/3067 (6.9%) | 20/930 (2.2%) | 184/1729 (10.6%) | 3/305 (1.0%) | <0.0001 |
| Alcohol abuse | 228/3003 (7.6%) | 44/910 (4.8%) | 166/1691 (9.8%) | 12/301 (4.0%) | <0.0001 |
| Immunosuppressive treatment | 104/2840 (3.7%) | 21/856 (2.5%) | 71/1620 (4.4%) | 8/270 (3.0%) | 0.0450 |
| Long corticotherapy | 127/2840 (4.5%) | 28/856 (3.3%) | 84/1620 (5.2%) | 13/270 (4.8%) | 0.0871 |
| Intravenous catheter | 250/3104 (8.1%) | 63/934 (6.7%) | 150/1760 (8.5%) | 24/306 (7.8%) | 0.2672 |
COPD, chronic obstructive pulmonary disease; ICD, intracardiac defibrillator; PM, pacemaker; TIA: transient ischaemic attack.
| . | Total (n = 3116) . | Prosthesis+Repair (n = 939) . | Native (n = 1764) . | PM/ICD (n = 308) . | P-value . |
|---|---|---|---|---|---|
| Demography | |||||
| Age (years) | |||||
| N | 3116 | 939 | 1764 | 308 | |
| Mean ± SD | 59.25 ± 18.03 | 63.36 ± 16.81 | 55.61 ± 18.45 | 66.77 ± 14.11 | <0.0001 |
| Median (IQR) | 63.0 (46.0–73.0) | 67.0 (54.0–75.0) | 58.0 (41.0–70.0) | 69.0 (60.0–76.0) | <0.0001 |
| Age ≥ 65 years | 1443/3116 (46.3%) | 538/939 (57.3%) | 662/1764 (37.5%) | 194/308 (63.0%) | <0.0001 |
| Age ≥ 80 years | 375/3116 (12.0%) | 141/939 (15.0%) | 163/1764 (9.2%) | 56/308 (18.2%) | <0.0001 |
| Females (%) | 969/3116 (31.1%) | 292/939 (31.1%) | 553/1764 (31.3%) | 86/308 (27.9%) | 0.4901 |
| History of cardiovascular diseases | |||||
| Heart failure | 662/2840 (23.3%) | 271/856 (31.7%) | 238/1620 (14.7%) | 123/270 (45.6%) | <0.0001 |
| Congenital disease | 365/3114 (11.7%) | 130/938 (13.9%) | 197/1763 (11.2%) | 18/308 (5.8%) | 0.0001 |
| Ischaemic heart disease | 622/2897 (21.5%) | 266/881 (30.2%) | 207/1637 (12.6%) | 128/284 (45.1%) | <0.0001 |
| Atrial fibrillation | 767/2918 (26.3%) | 365/891 (41.0%) | 240/1634 (14.7%) | 133/294 (45.2%) | <0.0001 |
| Hypertrophic cardiomyopathy | 63/2840 (2.2%) | 20/856 (2.3%) | 28/1620 (1.7%) | 11/270 (4.1%) | 0.0498 |
| Known valve murmur | 972/2840 (34.2%) | 455/856 (53.2%) | 427/1620 (26.4%) | 55/270 (20.4%) | <0.0001 |
| Previous endocarditis (%) | 274/3116 (8.8%) | 170/939 (18.1%) | 67/1764 (3.8%) | 17/308 (5.5%) | <0.0001 |
| Device therapy | |||||
| Pacemaker | 325/3116 (10.4%) | 97/939 (10.3%) | 53/1764 (3.0%) | 161/308 (52.3%) | |
| ICD (defibrillator) | 125/3116 (4.0%) | 18/939 (1.9%) | 15/1764 (0.9%) | 89/308 (28.9%) | |
| CRT-D (with ICD) | 72/3116 (2.3%) | 13/939 (1.4%) | 3/1764 (0.2%) | 47/308 (15.3%) | |
| CRT-P (pacing only) | 15/3116 (0.5%) | 7/939 (0.7%) | 1/1764 (0.1%) | 6/308 (1.9%) | |
| Risk factors | |||||
| Previous stroke/TIA | 340/2860 (11.9%) | 132/867 (15.2%) | 160/1626 (9.8%) | 34/273 (12.5%) | 0.0003 |
| Arterial hypertension | 1502/3111 (48.3%) | 531/938 (56.6%) | 726/1762 (41.2%) | 194/306 (63.4%) | <0.0001 |
| COPD/asthma | 318/3111 (10.2%) | 98/937 (10.5%) | 160/1762 (9.1%) | 49/307 (16.0%) | 0.0011 |
| Chronic renal failure | 553/3113 (17.8%) | 191/938 (20.4%) | 255/1762 (14.5%) | 83/308 (26.9%) | <0.0001 |
| Dialysis | 163/3113 (5.2%) | 28/938 (3.0%) | 107/1762 (6.1%) | 18/308 (5.8%) | <0.0001 |
| HIV | 31/3038 (1.0%) | 3/916 (0.3%) | 24/1726 (1.4%) | 2/294 (0.7%) | 0.0212 |
| Hypo/hyperthyroidism | 226/2820 (8.0%) | 93/852 (10.9%) | 98/1606 (6.1%) | 24/270 (8.9%) | <0.0001 |
| Chronic autoimmune disease | 109/3103 (3.5%) | 18/937 (1.9%) | 74/1753 (4.2%) | 12/308 (3.9%) | 0.0030 |
| Cancer | 361/3088 (11.7%) | 107/930 (11.5%) | 210/1746 (12.0%) | 31/308 (10.1%) | 0.6230 |
| Current pregnancy | 8/3092 (0.3%) | 2/932 (0.2%) | 6/1749 (0.3%) | 0/307 (0.0%) | 0.7716 |
| Smoking | 759/2938 (25.8%) | 166/880 (18.9%) | 507/1666 (30.4%) | 68/296 (23.0%) | <0.0001 |
| Intravenous drug dependency | 212/3067 (6.9%) | 20/930 (2.2%) | 184/1729 (10.6%) | 3/305 (1.0%) | <0.0001 |
| Alcohol abuse | 228/3003 (7.6%) | 44/910 (4.8%) | 166/1691 (9.8%) | 12/301 (4.0%) | <0.0001 |
| Immunosuppressive treatment | 104/2840 (3.7%) | 21/856 (2.5%) | 71/1620 (4.4%) | 8/270 (3.0%) | 0.0450 |
| Long corticotherapy | 127/2840 (4.5%) | 28/856 (3.3%) | 84/1620 (5.2%) | 13/270 (4.8%) | 0.0871 |
| Intravenous catheter | 250/3104 (8.1%) | 63/934 (6.7%) | 150/1760 (8.5%) | 24/306 (7.8%) | 0.2672 |
| . | Total (n = 3116) . | Prosthesis+Repair (n = 939) . | Native (n = 1764) . | PM/ICD (n = 308) . | P-value . |
|---|---|---|---|---|---|
| Demography | |||||
| Age (years) | |||||
| N | 3116 | 939 | 1764 | 308 | |
| Mean ± SD | 59.25 ± 18.03 | 63.36 ± 16.81 | 55.61 ± 18.45 | 66.77 ± 14.11 | <0.0001 |
| Median (IQR) | 63.0 (46.0–73.0) | 67.0 (54.0–75.0) | 58.0 (41.0–70.0) | 69.0 (60.0–76.0) | <0.0001 |
| Age ≥ 65 years | 1443/3116 (46.3%) | 538/939 (57.3%) | 662/1764 (37.5%) | 194/308 (63.0%) | <0.0001 |
| Age ≥ 80 years | 375/3116 (12.0%) | 141/939 (15.0%) | 163/1764 (9.2%) | 56/308 (18.2%) | <0.0001 |
| Females (%) | 969/3116 (31.1%) | 292/939 (31.1%) | 553/1764 (31.3%) | 86/308 (27.9%) | 0.4901 |
| History of cardiovascular diseases | |||||
| Heart failure | 662/2840 (23.3%) | 271/856 (31.7%) | 238/1620 (14.7%) | 123/270 (45.6%) | <0.0001 |
| Congenital disease | 365/3114 (11.7%) | 130/938 (13.9%) | 197/1763 (11.2%) | 18/308 (5.8%) | 0.0001 |
| Ischaemic heart disease | 622/2897 (21.5%) | 266/881 (30.2%) | 207/1637 (12.6%) | 128/284 (45.1%) | <0.0001 |
| Atrial fibrillation | 767/2918 (26.3%) | 365/891 (41.0%) | 240/1634 (14.7%) | 133/294 (45.2%) | <0.0001 |
| Hypertrophic cardiomyopathy | 63/2840 (2.2%) | 20/856 (2.3%) | 28/1620 (1.7%) | 11/270 (4.1%) | 0.0498 |
| Known valve murmur | 972/2840 (34.2%) | 455/856 (53.2%) | 427/1620 (26.4%) | 55/270 (20.4%) | <0.0001 |
| Previous endocarditis (%) | 274/3116 (8.8%) | 170/939 (18.1%) | 67/1764 (3.8%) | 17/308 (5.5%) | <0.0001 |
| Device therapy | |||||
| Pacemaker | 325/3116 (10.4%) | 97/939 (10.3%) | 53/1764 (3.0%) | 161/308 (52.3%) | |
| ICD (defibrillator) | 125/3116 (4.0%) | 18/939 (1.9%) | 15/1764 (0.9%) | 89/308 (28.9%) | |
| CRT-D (with ICD) | 72/3116 (2.3%) | 13/939 (1.4%) | 3/1764 (0.2%) | 47/308 (15.3%) | |
| CRT-P (pacing only) | 15/3116 (0.5%) | 7/939 (0.7%) | 1/1764 (0.1%) | 6/308 (1.9%) | |
| Risk factors | |||||
| Previous stroke/TIA | 340/2860 (11.9%) | 132/867 (15.2%) | 160/1626 (9.8%) | 34/273 (12.5%) | 0.0003 |
| Arterial hypertension | 1502/3111 (48.3%) | 531/938 (56.6%) | 726/1762 (41.2%) | 194/306 (63.4%) | <0.0001 |
| COPD/asthma | 318/3111 (10.2%) | 98/937 (10.5%) | 160/1762 (9.1%) | 49/307 (16.0%) | 0.0011 |
| Chronic renal failure | 553/3113 (17.8%) | 191/938 (20.4%) | 255/1762 (14.5%) | 83/308 (26.9%) | <0.0001 |
| Dialysis | 163/3113 (5.2%) | 28/938 (3.0%) | 107/1762 (6.1%) | 18/308 (5.8%) | <0.0001 |
| HIV | 31/3038 (1.0%) | 3/916 (0.3%) | 24/1726 (1.4%) | 2/294 (0.7%) | 0.0212 |
| Hypo/hyperthyroidism | 226/2820 (8.0%) | 93/852 (10.9%) | 98/1606 (6.1%) | 24/270 (8.9%) | <0.0001 |
| Chronic autoimmune disease | 109/3103 (3.5%) | 18/937 (1.9%) | 74/1753 (4.2%) | 12/308 (3.9%) | 0.0030 |
| Cancer | 361/3088 (11.7%) | 107/930 (11.5%) | 210/1746 (12.0%) | 31/308 (10.1%) | 0.6230 |
| Current pregnancy | 8/3092 (0.3%) | 2/932 (0.2%) | 6/1749 (0.3%) | 0/307 (0.0%) | 0.7716 |
| Smoking | 759/2938 (25.8%) | 166/880 (18.9%) | 507/1666 (30.4%) | 68/296 (23.0%) | <0.0001 |
| Intravenous drug dependency | 212/3067 (6.9%) | 20/930 (2.2%) | 184/1729 (10.6%) | 3/305 (1.0%) | <0.0001 |
| Alcohol abuse | 228/3003 (7.6%) | 44/910 (4.8%) | 166/1691 (9.8%) | 12/301 (4.0%) | <0.0001 |
| Immunosuppressive treatment | 104/2840 (3.7%) | 21/856 (2.5%) | 71/1620 (4.4%) | 8/270 (3.0%) | 0.0450 |
| Long corticotherapy | 127/2840 (4.5%) | 28/856 (3.3%) | 84/1620 (5.2%) | 13/270 (4.8%) | 0.0871 |
| Intravenous catheter | 250/3104 (8.1%) | 63/934 (6.7%) | 150/1760 (8.5%) | 24/306 (7.8%) | 0.2672 |
COPD, chronic obstructive pulmonary disease; ICD, intracardiac defibrillator; PM, pacemaker; TIA: transient ischaemic attack.
The most frequent preceding non-cardiac interventions performed within the last 6 months were dental procedure (7.9%), gastrointestinal intervention (3.4%), colonoscopy (3.3%), and urogenital intervention (2.8%). The portal of entry was dental in 9.8%, digestive in 6.3%, and genitourinary in 4.5%. The location of IE was aortic in 49.5% patients, mitral in 42.0%, tricuspid in 11.4%, and pulmonary in 2.4%. Infective endocarditis affected two or more valves in 556 (18.2%) patients. The median time since first medical consultation was 4.0 (0.0–15.0) days and higher in NVE than PVIE (P < 0.0001).
Clinical, electrocardiographic, and biological features
The main clinical and biological characteristics of patients are displayed in Supplementary material online, Tables S2 and S3. Fever (77.7%) and congestive heart failure (27.2%) were the most frequent symptoms. Symptomatic embolic events were present on admission in 25.3% of patients. Conduction abnormalities were observed on admission in 11.5% of cases, including first-degree AV block in 8.1% of cases, and third-degree AV block in 2.8%. New AV block under therapy occurred in 4.6% of cases. Blood cultures were positive for IE in 2461/3116 (79.0%) patients. The most frequent microorganisms identified were staphylococci in 1085 (44.1%) patients, oral streptococci in 304 (12.4%), enterococci in 390 (15.8%), and Streptococcus gallolyticus in 162 (6.6%). Coxiella burnetii IE was observed in 26 (0.8%) patients. The only difference between European and non-European countries was a higher frequency of Methi-S Staphylococcus aureus in European countries (25.7 vs. 17.7%, P = 0.0002).
Imaging
Among the 3116 patients, echocardiography was performed in 3111 (99.8%), transthoracic echocardiography (TTE) in 2793 (89.8%), and transoesophageal echocardiography (TOE) in 1808 (58.1%), with TOE being more frequently used in suspected PVIE (P < 0.0001). 18F-fluorodeoxyglucose positron emission tomography/computed tomography was performed in 518 (16.6%), leucocyte scintigraphy in 38 (1.2%), and multislice CT in 1656 (53.1%) patients (Figure 1). 18F-fluorodeoxyglucose positron emission tomography/computed tomography was more frequently used in PVIE (25.0%) and CDRIE (26.0%) than in NVE (9.5%) (P < 0.0001).
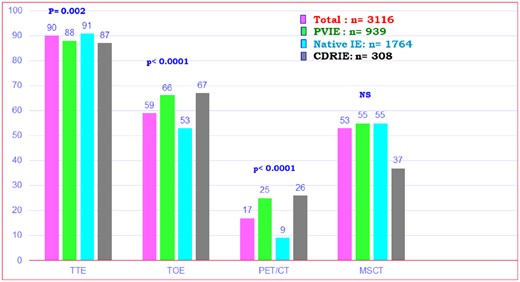
Imaging studies performed in the overall population and three subgroups. MSCT, multislice computed tomography; PET-CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography; TOE, transoesophageal echocardiography; TTE, transthoracic echocardiography.
Use of different imaging techniques differed between countries (Supplementary material online, Table S4). For instance, although echocardiography was used similarly between countries, 18F-FDG PET CT was more frequently used in Western Europe (33.9%), but very rarely in South America (2.1%), North Africa (0%), and Asia (0.5 to 8.5%).
A major imaging criterion was found by echocardiography in 2776 (89.1%) patients, including vegetation in 72.7% of patients, abscess or false aneurysm in 13.9%, and new prosthetic dehiscence in 3.4%. Abscesses were more frequently observed in PVIE than in other forms of IE (Figure 2).
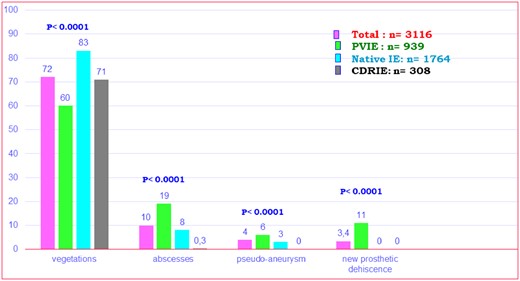
Frequency of major echocardiographic criteria observed in the overall population and three subgroups.
Positron emission tomography/computed tomography equipment was available in 71 (70.3%) centres in ESC-affiliated countries and in 18 (56.3%) centres elsewhere and was ultimately used in 16.6% of patients (25.0% in suspected PVIE). 18F-fluorodeoxyglucose positron emission tomography/computed tomography was performed at a mean of 8 (IQR 4.0–15.0) days after inclusion, including 46.7% during the first week, 27.2% during the second week, and 26.1% thereafter. 18F-fluorodeoxyglucose positron emission tomography/computed tomography presented with any positivity in 361 (69.7%) patients, 74.9% in PVIE, 62.5% in NVE, and 77.5% in CDRIE (P = 0.018). Considering only cardiac uptake (major criterion), 18F-FDG PET/CT was positive in 222 (42.9%) patients, with a better sensitivity in PVIE (66.8%) than in NVE (28.0%) and CDRIE (16.3%). Extra-cardiac uptake was observed in 201 (38.8%) patients, including 34.5% in PVIE, 42.3% in NVE, and 43.8% in CDRIE, respectively, the most frequent uptake being in the liver in 5.0% of cases, lung in 27.1%, spleen in 19.6%, spine in 21.8%, and bowel in 18.9%.
Cardiac CT was performed in 300 (9.6%) patients, including 11.4% of PVIE, 9.9% of NVE, and 4.2% of CDRIE. Valvular lesions were observed in 179 (59.7%) patients, 52 (48.6%) of PVIE, and 119 (68.4%) of NVE (P = 0.01). Perivalvular extension was observed in 101 (33.7%) patients, in 53 (49.5%) of PVIE, and 45 (25.9%) of NVE (P < 0.0001). Extra-cardiac CT was performed in 1656 patients and revealed extra-cardiac lesions in 798 (48.2%).
No positive imaging criterion was observed in only 280/3116 (9.0%) patients.
In-hospital follow-up under treatment
Main events occurring during hospitalization are summarized in Supplementary material online, Table S5.
Embolic events were the most frequent complication, observed in 20.6% of patients, followed by acute renal failure (17.7%) and heart failure (14.1%). Embolic events under therapy were more frequent in patients with definite (22.7%) than in patients with possible (9.5%) IE (P < 0.0001) and was similar between European and non-European countries (21.1% vs. 18.4%, P = ns). Factors associated with new embolic events under therapy by univariate analysis are age, previous pulmonary embolism, heart failure, statins, previous VKA therapy, aortic, tricuspid IE or CDRIE, pulmonary endocarditis, intracardiac defibrillator (ICD)/pacemaker (PM) endocarditis, vegetation presence and size, positive blood cultures, other germs, and S. aureus infection. No relationship was observed between this time delay and the risk of embolism (P = ns). By multivariable analysis, tricuspid (OR = 1.78, 95% CI [1.38–2.29], P < 0.0001) or pulmonary (OR =2.31, 95% CI [1.42–3.74], P = 0.0007) or ICD/PM (OR = 0.61, 95% CI [0.44–0.84], P = 0.0028) endocarditis, presence of a vegetation (OR = 1.31, 95% CI [1.06–1.62], P = 0.0138), Methi-S S. aureus endocarditis (OR =1.41, 95% CI [1.13–1.75], P = 0.0024) and Methi-R S. aureus endocarditis (OR =1.77, 95% CI [1.26–2.50], P = 0.0011) were independently associated with new embolic events under therapy.
Surgery was performed during hospitalization in 1596 (51.2%) patients. Following ESC guidelines, theoretical indication for cardiac surgery was present in 2160 (69.3%) patients. Among them, surgery was finally performed in 46.1% of PVIE, 53.7% of NVE, and surgical or percutaneous extraction in 65.3% of CDRIE. Surgery was performed on an emergency basis in 6.7% of operated patients, urgently in 24.8%, beyond the 1st week in 32%, and as an elective procedure in 36.5%. Cardiac surgery was less frequently performed in possible (64.7%) than in definite (75.3%) IE (P < 0.0001). Indications for surgery were haemodynamic in 46.3% of cases, embolic in 32.1%, and infectious in 64.2%. Among factors associated with more frequent cardiac surgery by multivariable analysis, the most powerful were congestive heart failure (OR = 2.27 [1.57–3.28], P < 0.0001), vegetation length > 15 mm (OR = 2.33 [1.75–3.10], P < 0.0001), cerebral complication (OR= 1.69 [1.13–2.53], P = 0.0105), abscess (OR = 4.18 [2.59–6.76], P < 0.0001), and management in an European country (OR 1.45 [1.15–1.82], P = 0.0016). Conversely, high Charlson index (OR =0.94 [0.91–0.97], P = 0.0005), and female gender (OR =0.73 [0.59–0.90], P = 0.0028) were associated with lower surgical use.
Reasons for absence of surgery when indicated and types of surgery performed are summarized in Supplementary material online, Table S6. Comparison of the reasons for absence of surgery when indicated between European and non-European countries found more frequent patient refusal (30.4% vs. 16.5%, P = 0.0018) and more frequent neurological complication (17.4% vs. 10.0%, P = 0.038) in non-European countries.
In-hospital death occurred in 532 (17.1%) patients and was higher in PVIE than in other groups (Table 2) but was similar between European and non-European countries (16.8% vs. 18.1%, P = ns). Causes of in-hospital death are reported in Table 2. By univariate Cox regression analysis (Table 3), type of endocarditis, age, Charlson index, creatinine > 2 mg/dL, congestive heart failure, S. aureus infection, vegetation length, cerebral complications, nosocomial source of infection, and perivalvular complications were significantly associated with in-hospital mortality. Predictors of mortality by multivariable analysis (Table 4) were Charlson index, creatinine > 2 mg/dL, congestive heart failure, vegetation length > 10 mm, cerebral complications, abscess, and failure to undertake surgery despite a guideline recommended indication. In-hospital mortality was not significantly different between NVE, PVIE, and CDRIE (Figure 3A) but was higher in patients in whom surgery was indicated but not performed (Figure 3B). Similar results were obtained when the Kaplan–Meier curve of time to death were adjusted for the covariates from the Cox proportional hazard model (Take home figure).

(A) The Kaplan–Meier curves for all-cause mortality according to type of endocarditis. (B) Cumulative survival in three subgroups according to the presence or absence of an indication of surgery and whether surgery was performed or not.
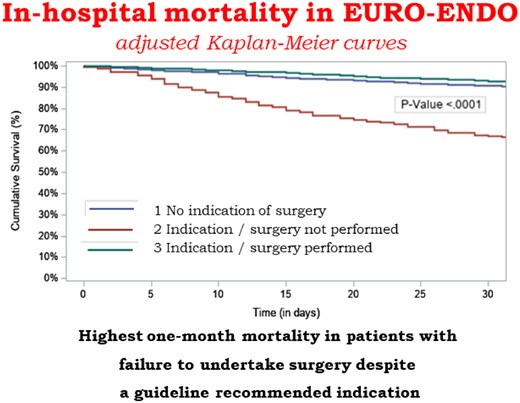
Prognosis of infective endocarditis is dismal when cardiac surgery is indicated but not performed (adjusted Kaplan–Meier curve).
| . | Total (n = 3116) . | Prosthesis+Repair (n = 939) . | Native (n =1764) . | PM/ICD (n = 308) . | P-value . |
|---|---|---|---|---|---|
| Death | 532/3116 (17.1%) | 187/939 (19.9%) | 286/1764 (16.2%) | 47/308 (15.3%) | 0.038 |
| Cause of death | |||||
| Cardiovascular | 151/531 (28.4%) | 65/186 (34.9%) | 76/286 (26.6%) | 8/47 (17.0%) | |
| Non-cardiovascular | 156/531 (29.4%) | 47/186 (25.3%) | 85/286 (29.7%) | 18/47 (38.3%) | |
| Cardiovascular + non-cardiovascular | 194/531 (36.5%) | 60/186 (32.3%) | 110/286 (38.5%) | 21/47 (44.7%) | |
| Unknown | 30/531 (5.6%) | 14/186 (7.5%) | 15/286 (5.2%) | 0/47 (0.0%) | |
| If cardiovascular | |||||
| Heart failure | 245/345 (71.0%) | 94/125 (75.2%) | 133/186 (71.5%) | 16/29 (55.2%) | |
| Arrhythmia | 42/345 (12.2%) | 9/125 (7.2%) | 23/186 (12.4%) | 9/29 (31.0%) | |
| Cardiac perforation/tamponade | 11/345 (3.2%) | 1/125 (0.8%) | 9/186 (4.8%) | 1/29 (3.4%) | |
| Acute MI | 8/345 (2.3%) | 5/125 (4.0%) | 3/186 (1.6%) | 0/29 (0.0%) | |
| Cerebral embolism | 41/345 (11.9%) | 11/125 (8.8%) | 26/186 (14.0%) | 1/29 (3.4%) | |
| Pulmonary embolism | 13/345 (3.8%) | 3/125 (2.4%) | 8/186 (4.3%) | 2/29 (6.9%) | |
| Peripheral embolism | 3/345 (0.9%) | 0/125 (0.0%) | 3/186 (1.6%) | 0/29 (0.0%) | |
| Other cardiovascular | 40/345 (11.6%) | 16/125 (12.8%) | 22/186 (11.8%) | 2/29 (6.9%) | |
| If non-cardiovascular | |||||
| Neoplasia | 12/350 (3.4%) | 2/107 (1.9%) | 8/195 (4.1%) | 1/39 (2.6%) | |
| Sepsis | 269/350 (76.9%) | 80/107 (74.8%) | 152/195 (77.9%) | 31/39 (79.5%) | |
| Other | 70/350 (20.0%) | 26/107 (24.3%) | 37/195 (19.0%) | 4/39 (10.3%) | |
| If surgery performed | |||||
| Post-cardiac surgery | 170/532 (32.0%) | 74/187 (39.6%) | 79/286 (27.6%) | 16/47 (34.0%) | |
| Post-non-cardiac surgery | 16/532 (3.0%) | 1/187 (0.5%) | 6/286 (2.1%) | 9/47 (19.1%) |
| . | Total (n = 3116) . | Prosthesis+Repair (n = 939) . | Native (n =1764) . | PM/ICD (n = 308) . | P-value . |
|---|---|---|---|---|---|
| Death | 532/3116 (17.1%) | 187/939 (19.9%) | 286/1764 (16.2%) | 47/308 (15.3%) | 0.038 |
| Cause of death | |||||
| Cardiovascular | 151/531 (28.4%) | 65/186 (34.9%) | 76/286 (26.6%) | 8/47 (17.0%) | |
| Non-cardiovascular | 156/531 (29.4%) | 47/186 (25.3%) | 85/286 (29.7%) | 18/47 (38.3%) | |
| Cardiovascular + non-cardiovascular | 194/531 (36.5%) | 60/186 (32.3%) | 110/286 (38.5%) | 21/47 (44.7%) | |
| Unknown | 30/531 (5.6%) | 14/186 (7.5%) | 15/286 (5.2%) | 0/47 (0.0%) | |
| If cardiovascular | |||||
| Heart failure | 245/345 (71.0%) | 94/125 (75.2%) | 133/186 (71.5%) | 16/29 (55.2%) | |
| Arrhythmia | 42/345 (12.2%) | 9/125 (7.2%) | 23/186 (12.4%) | 9/29 (31.0%) | |
| Cardiac perforation/tamponade | 11/345 (3.2%) | 1/125 (0.8%) | 9/186 (4.8%) | 1/29 (3.4%) | |
| Acute MI | 8/345 (2.3%) | 5/125 (4.0%) | 3/186 (1.6%) | 0/29 (0.0%) | |
| Cerebral embolism | 41/345 (11.9%) | 11/125 (8.8%) | 26/186 (14.0%) | 1/29 (3.4%) | |
| Pulmonary embolism | 13/345 (3.8%) | 3/125 (2.4%) | 8/186 (4.3%) | 2/29 (6.9%) | |
| Peripheral embolism | 3/345 (0.9%) | 0/125 (0.0%) | 3/186 (1.6%) | 0/29 (0.0%) | |
| Other cardiovascular | 40/345 (11.6%) | 16/125 (12.8%) | 22/186 (11.8%) | 2/29 (6.9%) | |
| If non-cardiovascular | |||||
| Neoplasia | 12/350 (3.4%) | 2/107 (1.9%) | 8/195 (4.1%) | 1/39 (2.6%) | |
| Sepsis | 269/350 (76.9%) | 80/107 (74.8%) | 152/195 (77.9%) | 31/39 (79.5%) | |
| Other | 70/350 (20.0%) | 26/107 (24.3%) | 37/195 (19.0%) | 4/39 (10.3%) | |
| If surgery performed | |||||
| Post-cardiac surgery | 170/532 (32.0%) | 74/187 (39.6%) | 79/286 (27.6%) | 16/47 (34.0%) | |
| Post-non-cardiac surgery | 16/532 (3.0%) | 1/187 (0.5%) | 6/286 (2.1%) | 9/47 (19.1%) |
ICD, intracardiac defibrillator; PM, pacemaker.
| . | Total (n = 3116) . | Prosthesis+Repair (n = 939) . | Native (n =1764) . | PM/ICD (n = 308) . | P-value . |
|---|---|---|---|---|---|
| Death | 532/3116 (17.1%) | 187/939 (19.9%) | 286/1764 (16.2%) | 47/308 (15.3%) | 0.038 |
| Cause of death | |||||
| Cardiovascular | 151/531 (28.4%) | 65/186 (34.9%) | 76/286 (26.6%) | 8/47 (17.0%) | |
| Non-cardiovascular | 156/531 (29.4%) | 47/186 (25.3%) | 85/286 (29.7%) | 18/47 (38.3%) | |
| Cardiovascular + non-cardiovascular | 194/531 (36.5%) | 60/186 (32.3%) | 110/286 (38.5%) | 21/47 (44.7%) | |
| Unknown | 30/531 (5.6%) | 14/186 (7.5%) | 15/286 (5.2%) | 0/47 (0.0%) | |
| If cardiovascular | |||||
| Heart failure | 245/345 (71.0%) | 94/125 (75.2%) | 133/186 (71.5%) | 16/29 (55.2%) | |
| Arrhythmia | 42/345 (12.2%) | 9/125 (7.2%) | 23/186 (12.4%) | 9/29 (31.0%) | |
| Cardiac perforation/tamponade | 11/345 (3.2%) | 1/125 (0.8%) | 9/186 (4.8%) | 1/29 (3.4%) | |
| Acute MI | 8/345 (2.3%) | 5/125 (4.0%) | 3/186 (1.6%) | 0/29 (0.0%) | |
| Cerebral embolism | 41/345 (11.9%) | 11/125 (8.8%) | 26/186 (14.0%) | 1/29 (3.4%) | |
| Pulmonary embolism | 13/345 (3.8%) | 3/125 (2.4%) | 8/186 (4.3%) | 2/29 (6.9%) | |
| Peripheral embolism | 3/345 (0.9%) | 0/125 (0.0%) | 3/186 (1.6%) | 0/29 (0.0%) | |
| Other cardiovascular | 40/345 (11.6%) | 16/125 (12.8%) | 22/186 (11.8%) | 2/29 (6.9%) | |
| If non-cardiovascular | |||||
| Neoplasia | 12/350 (3.4%) | 2/107 (1.9%) | 8/195 (4.1%) | 1/39 (2.6%) | |
| Sepsis | 269/350 (76.9%) | 80/107 (74.8%) | 152/195 (77.9%) | 31/39 (79.5%) | |
| Other | 70/350 (20.0%) | 26/107 (24.3%) | 37/195 (19.0%) | 4/39 (10.3%) | |
| If surgery performed | |||||
| Post-cardiac surgery | 170/532 (32.0%) | 74/187 (39.6%) | 79/286 (27.6%) | 16/47 (34.0%) | |
| Post-non-cardiac surgery | 16/532 (3.0%) | 1/187 (0.5%) | 6/286 (2.1%) | 9/47 (19.1%) |
| . | Total (n = 3116) . | Prosthesis+Repair (n = 939) . | Native (n =1764) . | PM/ICD (n = 308) . | P-value . |
|---|---|---|---|---|---|
| Death | 532/3116 (17.1%) | 187/939 (19.9%) | 286/1764 (16.2%) | 47/308 (15.3%) | 0.038 |
| Cause of death | |||||
| Cardiovascular | 151/531 (28.4%) | 65/186 (34.9%) | 76/286 (26.6%) | 8/47 (17.0%) | |
| Non-cardiovascular | 156/531 (29.4%) | 47/186 (25.3%) | 85/286 (29.7%) | 18/47 (38.3%) | |
| Cardiovascular + non-cardiovascular | 194/531 (36.5%) | 60/186 (32.3%) | 110/286 (38.5%) | 21/47 (44.7%) | |
| Unknown | 30/531 (5.6%) | 14/186 (7.5%) | 15/286 (5.2%) | 0/47 (0.0%) | |
| If cardiovascular | |||||
| Heart failure | 245/345 (71.0%) | 94/125 (75.2%) | 133/186 (71.5%) | 16/29 (55.2%) | |
| Arrhythmia | 42/345 (12.2%) | 9/125 (7.2%) | 23/186 (12.4%) | 9/29 (31.0%) | |
| Cardiac perforation/tamponade | 11/345 (3.2%) | 1/125 (0.8%) | 9/186 (4.8%) | 1/29 (3.4%) | |
| Acute MI | 8/345 (2.3%) | 5/125 (4.0%) | 3/186 (1.6%) | 0/29 (0.0%) | |
| Cerebral embolism | 41/345 (11.9%) | 11/125 (8.8%) | 26/186 (14.0%) | 1/29 (3.4%) | |
| Pulmonary embolism | 13/345 (3.8%) | 3/125 (2.4%) | 8/186 (4.3%) | 2/29 (6.9%) | |
| Peripheral embolism | 3/345 (0.9%) | 0/125 (0.0%) | 3/186 (1.6%) | 0/29 (0.0%) | |
| Other cardiovascular | 40/345 (11.6%) | 16/125 (12.8%) | 22/186 (11.8%) | 2/29 (6.9%) | |
| If non-cardiovascular | |||||
| Neoplasia | 12/350 (3.4%) | 2/107 (1.9%) | 8/195 (4.1%) | 1/39 (2.6%) | |
| Sepsis | 269/350 (76.9%) | 80/107 (74.8%) | 152/195 (77.9%) | 31/39 (79.5%) | |
| Other | 70/350 (20.0%) | 26/107 (24.3%) | 37/195 (19.0%) | 4/39 (10.3%) | |
| If surgery performed | |||||
| Post-cardiac surgery | 170/532 (32.0%) | 74/187 (39.6%) | 79/286 (27.6%) | 16/47 (34.0%) | |
| Post-non-cardiac surgery | 16/532 (3.0%) | 1/187 (0.5%) | 6/286 (2.1%) | 9/47 (19.1%) |
ICD, intracardiac defibrillator; PM, pacemaker.
Univariate Cox regression analysis for all causes of death at discharge (1-month period)
| . | Effect* . | Hazard ratio . | 95% CI . | P-value* . |
|---|---|---|---|---|
| Type of endocarditis | PM/ICD | 0.80 | 0.53–1.20 | 0.08 |
| Prosthesis+Repair | 1.21 | 0.97–1.52 | ||
| Age (per 10 years) | 1.10 | 1.04–1.18 | 0.002 | |
| Gender | Female | 1.15 | 0.92–1.44 | 0.216 |
| Charlson index | 1.14 | 1.11–1.17 | <0.0001 | |
| Creatinine >2 mg/dL | 2.53 | 2.01–3.19 | <0.0001 | |
| Staphylococcus aureus | 1.36 | 1.04–1.74 | 0.008 | |
| Congestive heart failure | 2.90 | 2.31–3.64 | <0.0001 | |
| Vegetation length > 10 mm | 2.44 | 1.93–3.08 | <0.0001 | |
| Cerebral complication | 2.62 | 2.02–3.41 | <0.0001 | |
| Abscess | 1.71 | 1.29–2.27 | 0.0002 | |
| ESC countries | 1.12 | 0.86–1.46 | 0.42 | |
| Source of infection | Non-nosocomial | 1.35 | 0.95–1.93 | 0.0007 |
| Nosocomial | 1.68 | 1.27–2.21 | ||
| Indication—surgery performed | Indication—not performed | 4.02 | 3.06–5.27 | <0.0001 |
| Indication—performed | 0.72 | 0.54–0.97 | ||
| Type of centres | High-volume centres | 1.13 | 0.74–1.71 | 0.57 |
| Transferred from another hospital | 0.75 | 0.60–0.95 | 0.02 |
| . | Effect* . | Hazard ratio . | 95% CI . | P-value* . |
|---|---|---|---|---|
| Type of endocarditis | PM/ICD | 0.80 | 0.53–1.20 | 0.08 |
| Prosthesis+Repair | 1.21 | 0.97–1.52 | ||
| Age (per 10 years) | 1.10 | 1.04–1.18 | 0.002 | |
| Gender | Female | 1.15 | 0.92–1.44 | 0.216 |
| Charlson index | 1.14 | 1.11–1.17 | <0.0001 | |
| Creatinine >2 mg/dL | 2.53 | 2.01–3.19 | <0.0001 | |
| Staphylococcus aureus | 1.36 | 1.04–1.74 | 0.008 | |
| Congestive heart failure | 2.90 | 2.31–3.64 | <0.0001 | |
| Vegetation length > 10 mm | 2.44 | 1.93–3.08 | <0.0001 | |
| Cerebral complication | 2.62 | 2.02–3.41 | <0.0001 | |
| Abscess | 1.71 | 1.29–2.27 | 0.0002 | |
| ESC countries | 1.12 | 0.86–1.46 | 0.42 | |
| Source of infection | Non-nosocomial | 1.35 | 0.95–1.93 | 0.0007 |
| Nosocomial | 1.68 | 1.27–2.21 | ||
| Indication—surgery performed | Indication—not performed | 4.02 | 3.06–5.27 | <0.0001 |
| Indication—performed | 0.72 | 0.54–0.97 | ||
| Type of centres | High-volume centres | 1.13 | 0.74–1.71 | 0.57 |
| Transferred from another hospital | 0.75 | 0.60–0.95 | 0.02 |
P-value corresponds to the results of Wald test. For Region: the reference is North America; For type of endocarditis: Native; For indication—Surgery performed: No indication; For countries: Non-ESC; For type of centres: Low level IE; For source of infection: Community.
ICD, intracardiac defibrillator; PM, pacemaker.
Univariate Cox regression analysis for all causes of death at discharge (1-month period)
| . | Effect* . | Hazard ratio . | 95% CI . | P-value* . |
|---|---|---|---|---|
| Type of endocarditis | PM/ICD | 0.80 | 0.53–1.20 | 0.08 |
| Prosthesis+Repair | 1.21 | 0.97–1.52 | ||
| Age (per 10 years) | 1.10 | 1.04–1.18 | 0.002 | |
| Gender | Female | 1.15 | 0.92–1.44 | 0.216 |
| Charlson index | 1.14 | 1.11–1.17 | <0.0001 | |
| Creatinine >2 mg/dL | 2.53 | 2.01–3.19 | <0.0001 | |
| Staphylococcus aureus | 1.36 | 1.04–1.74 | 0.008 | |
| Congestive heart failure | 2.90 | 2.31–3.64 | <0.0001 | |
| Vegetation length > 10 mm | 2.44 | 1.93–3.08 | <0.0001 | |
| Cerebral complication | 2.62 | 2.02–3.41 | <0.0001 | |
| Abscess | 1.71 | 1.29–2.27 | 0.0002 | |
| ESC countries | 1.12 | 0.86–1.46 | 0.42 | |
| Source of infection | Non-nosocomial | 1.35 | 0.95–1.93 | 0.0007 |
| Nosocomial | 1.68 | 1.27–2.21 | ||
| Indication—surgery performed | Indication—not performed | 4.02 | 3.06–5.27 | <0.0001 |
| Indication—performed | 0.72 | 0.54–0.97 | ||
| Type of centres | High-volume centres | 1.13 | 0.74–1.71 | 0.57 |
| Transferred from another hospital | 0.75 | 0.60–0.95 | 0.02 |
| . | Effect* . | Hazard ratio . | 95% CI . | P-value* . |
|---|---|---|---|---|
| Type of endocarditis | PM/ICD | 0.80 | 0.53–1.20 | 0.08 |
| Prosthesis+Repair | 1.21 | 0.97–1.52 | ||
| Age (per 10 years) | 1.10 | 1.04–1.18 | 0.002 | |
| Gender | Female | 1.15 | 0.92–1.44 | 0.216 |
| Charlson index | 1.14 | 1.11–1.17 | <0.0001 | |
| Creatinine >2 mg/dL | 2.53 | 2.01–3.19 | <0.0001 | |
| Staphylococcus aureus | 1.36 | 1.04–1.74 | 0.008 | |
| Congestive heart failure | 2.90 | 2.31–3.64 | <0.0001 | |
| Vegetation length > 10 mm | 2.44 | 1.93–3.08 | <0.0001 | |
| Cerebral complication | 2.62 | 2.02–3.41 | <0.0001 | |
| Abscess | 1.71 | 1.29–2.27 | 0.0002 | |
| ESC countries | 1.12 | 0.86–1.46 | 0.42 | |
| Source of infection | Non-nosocomial | 1.35 | 0.95–1.93 | 0.0007 |
| Nosocomial | 1.68 | 1.27–2.21 | ||
| Indication—surgery performed | Indication—not performed | 4.02 | 3.06–5.27 | <0.0001 |
| Indication—performed | 0.72 | 0.54–0.97 | ||
| Type of centres | High-volume centres | 1.13 | 0.74–1.71 | 0.57 |
| Transferred from another hospital | 0.75 | 0.60–0.95 | 0.02 |
P-value corresponds to the results of Wald test. For Region: the reference is North America; For type of endocarditis: Native; For indication—Surgery performed: No indication; For countries: Non-ESC; For type of centres: Low level IE; For source of infection: Community.
ICD, intracardiac defibrillator; PM, pacemaker.
Multivariable Cox regression analysis for all causes of death at discharge (1-month period)
| . | Hazard ratio . | 95% CI . | P-value* . |
|---|---|---|---|
| Charlson index | 1.07 | [1.04–1.11] | <0.0001 |
| Creatinine >2 mg/dL | 1.58 | [1.19–2.11] | <0.0017 |
| Congestive heart failure | 2.09 | [1.58–2.77] | <0.0001 |
| Vegetation length > 10 mm | 2.12 | [1.64–2.73] | <0.0001 |
| Cerebral complication | 2.21 | [1.61–3.04] | <0.0001 |
| Abscess | 1.50 | [1.07–2.10] | 0.0186 |
| Indication—surgery not performed | 2.84 | [2.00–4.03] | <0.001 |
| Indication—surgery performed | 0.63 | [0.43–0.92] | 0.0169 |
| . | Hazard ratio . | 95% CI . | P-value* . |
|---|---|---|---|
| Charlson index | 1.07 | [1.04–1.11] | <0.0001 |
| Creatinine >2 mg/dL | 1.58 | [1.19–2.11] | <0.0017 |
| Congestive heart failure | 2.09 | [1.58–2.77] | <0.0001 |
| Vegetation length > 10 mm | 2.12 | [1.64–2.73] | <0.0001 |
| Cerebral complication | 2.21 | [1.61–3.04] | <0.0001 |
| Abscess | 1.50 | [1.07–2.10] | 0.0186 |
| Indication—surgery not performed | 2.84 | [2.00–4.03] | <0.001 |
| Indication—surgery performed | 0.63 | [0.43–0.92] | 0.0169 |
Goodness of fit test: P = 0.18. Concordance = 0.77—Global Schoenfeld residual test P = 0.12.
P-value corresponds to the results of Wald test. For indication—surgery performed or not, the reference is no indication.
Multivariable Cox regression analysis for all causes of death at discharge (1-month period)
| . | Hazard ratio . | 95% CI . | P-value* . |
|---|---|---|---|
| Charlson index | 1.07 | [1.04–1.11] | <0.0001 |
| Creatinine >2 mg/dL | 1.58 | [1.19–2.11] | <0.0017 |
| Congestive heart failure | 2.09 | [1.58–2.77] | <0.0001 |
| Vegetation length > 10 mm | 2.12 | [1.64–2.73] | <0.0001 |
| Cerebral complication | 2.21 | [1.61–3.04] | <0.0001 |
| Abscess | 1.50 | [1.07–2.10] | 0.0186 |
| Indication—surgery not performed | 2.84 | [2.00–4.03] | <0.001 |
| Indication—surgery performed | 0.63 | [0.43–0.92] | 0.0169 |
| . | Hazard ratio . | 95% CI . | P-value* . |
|---|---|---|---|
| Charlson index | 1.07 | [1.04–1.11] | <0.0001 |
| Creatinine >2 mg/dL | 1.58 | [1.19–2.11] | <0.0017 |
| Congestive heart failure | 2.09 | [1.58–2.77] | <0.0001 |
| Vegetation length > 10 mm | 2.12 | [1.64–2.73] | <0.0001 |
| Cerebral complication | 2.21 | [1.61–3.04] | <0.0001 |
| Abscess | 1.50 | [1.07–2.10] | 0.0186 |
| Indication—surgery not performed | 2.84 | [2.00–4.03] | <0.001 |
| Indication—surgery performed | 0.63 | [0.43–0.92] | 0.0169 |
Goodness of fit test: P = 0.18. Concordance = 0.77—Global Schoenfeld residual test P = 0.12.
P-value corresponds to the results of Wald test. For indication—surgery performed or not, the reference is no indication.
Discussion
The EURO-ENDO registry provides a unique opportunity to assess the current characteristics of IE in Europe, including clinical presentation, microbiology, complications, management, and prognosis. Since several countries beyond Europe also participated, it will also enable comparison of the characteristics of IE according to geographical and socioeconomic factors. Finally, although 20 times larger, it will allow historical comparison with the previous EuroHeart survey7 which enrolled patients over a 4-month period in 2001.
The following main messages arise from the analysis of EURO ENDO:
IE more frequently affects men around 60 years of age.
PVIE, CDRIE, nosocomial, staphylococcal, and enterococcal endocarditis are more frequent.
Oral streptococcal endocarditis is less frequent, and its frequency has not increased since implementation of the 2009 and 2015 recommendations restricting indications for antibiotic prophylaxis.
New imaging techniques (18F-FDG PET/CT) have emerged and are used in several countries worldwide.
Mechanical valve replacement is decreasing, and mitral valve repair is still underused in IE.
The prognosis of IE is still unacceptably poor and more aggressive management of this deadly disease remains necessary.
Current clinical and microbiological characteristics of IE
EURO-ENDO confirms the increasing age of the population with IE. This in agreement with the progressive increase in age observed in three 1-year population-based surveys conducted in 1991, 1999, and 2008 in three French regions,5 , 6 in which total IE incidence remained stable over time, but mean age progressively increased from 57.9 ± 16.6 years in 1991 to 61.6 ± 16.3 years in 2008.
The frequency of prosthetic IE is also increasing, accounting for 30% of cases in EURO-ENDO. In comparison, PVIE represented only 26% of cases in the EuroHeart survey,7 25% in the 2008 French registry,5 and 21% in the International Collaboration on Endocarditis-Prospective Cohort Study reported in 2009.1 In this latter series, the frequency of CDRIE was also much lower (7% of IE) than in the EURO-ENDO registry, confirming the increasing proportion of both PVIE and CDRIE as compared with NVE.
The low frequency of dental portal of entry in EUROENDO, combined with the low frequency of oral streptococci [lower than in the EuroHeart Survey (15%),7 the 2008 French registry (20.6%),5 and the International Collaboration on Endocarditis-Prospective Cohort Study (17%)1], is reassuring in the wake of the 2009 and 2015 ESC guidelines, which recommended restricting the use of antibiotic prophylaxis to high risk populations undergoing at-risk dental procedures.8 Conversely, the high frequency of enterococci observed in EURO-ENDO represents a significant change compared with the 8% and 10% frequency observed in the 2002 French survey5 and 2009 International Collaboration on Endocarditis-Prospective Cohort Study,1 respectively. The burden of enterococcal IE may relate to increasing age.19 Finally, the number of culture-negative IE observed in EURO-ENDO (21%) was higher than those previously reported [14% and 11% frequency observed in the 2002 French survey5 and 2009 International Collaboration on Endocarditis-Prospective Cohort Study1].
Imaging
EURO-ENDO reveals a transformation in the use of imaging techniques since publication of the 2015 ESC guidelines.8 Echocardiography remains the most frequently used technique. Although the respective diagnostic values of TTE and TOE were not compared in EURO-ENDO, the observed more frequent use of TOE in suspected PVIE is in agreement with current guidelines for the use of echocardiography in IE.9 Surprisingly, TOE was performed in only 58.1% of patients, which may be considered a low proportion compared with current guidelines.8 However, a wide range of TOE use was observed among countries and regions (Supplementary material online, Table S4).
18F-fluorodeoxyglucose positron emission tomography/computed tomography and multislice CT are more frequently used, particularly in the field of suspected PVIE. Interestingly, there was an important gap in EURO-ENDO between the high availability of PET/CT and its relatively limited use in clinical practice. This underuse might have several explanations, but the most important are probably the variable availability of PET/CT for cardiac studies, the technique being preferentially used for the follow-up of patients with cancer in many centres, and its limitations in haemodynamically unstable patients.8 , 10–12
18F-fluorodeoxyglucose positron emission tomography/computed tomography has recently been found to be useful for the diagnosis of prosthetic valve IE,10–12 supporting the implementation of abnormal FDG uptake as a novel major criterion for IE in the 2015 ESC guidelines,8 despite inherent limitations.20 In EURO-ENDO, 18F-FDG PET/CT demonstrated high sensitivity in PVIE (63%) compared with NVE (28%) and CDRIE (16.2%). This confirms that 18F-FDG PET/CT should be used in suspected PVIE (particularly when the diagnosis is uncertain) and that additional studies are needed to define the best indications for this test in NVE and CDRIE. The additional diagnostic value of 18F-FDG PET/CT and cardiac CT in suspected PVIE validates use of the ESC criteria rather than the Duke criteria21 in this setting.
Although MSCT was used in more than half of patients in EURO-ENDO, cardiac CT was performed in only 10%, demonstrating clear underuse of this technique, particularly for the diagnosis of perivalvular involvement in PVIE.8 , 11
Management and outcome of infective endocarditis in EUROENDO registry
Use of surgery during hospitalization in EURO-ENDO was similar to the patterns observed in the Euro Heart Survey,7 2008 French Registry,5 and 2009 International Collaboration on Endocarditis-Prospective Cohort Study.1 Approximately 50% of patients with IE underwent surgery during hospitalization (both in NVE and PVIE) and the principal indications for surgery were haemodynamic, embolic, and infectious. Surprisingly the most frequent indication in EURO-ENDO was infectious, probably related to the high proportion of PVIE and perivalvular lesions. Other important observations related to the choice of surgical technique. Bioprosthetic aortic valves were used in most cases (58.3%) in EURO-ENDO whereas mechanical valves were preferred at the time of the EuroHeart Survey (74%). In contrast, the application of mitral valve repair techniques was relatively disappointing (25.1%) in EURO-ENDO and similar to that reported in the Euro Heart Survey. One explanation is that significant delay before surgery in EURO-ENDO resulted in valve destruction and difficulty in performing valve repair.11 Indeed, one of the key reasons for withholding appropriate surgery in EURO-ENDO was death before surgery could be performed (22.5% of cases). This unacceptable feature underlines the need for early discussion with surgeons within the IE team, as recommended by ESC guidelines.8
EURO-ENDO confirms the high risk of embolic events in IE. Embolic events occur in up to 40% of patients with IE (with a high burden of cerebral manifestations22 , 23) and are associated with increased morbidity and mortality.23 The risk of new embolic events (i.e. after initiation of antibiotic therapy) is only 6–21%,21 yet embolism was already present on admission in 25.2% patients in the EURO-ENDO registry and the rate of new embolic events occurring during hospitalization was very high (20.5%). In comparison, embolic events were observed in 23% in the ICE cohort,1 and embolism was the reason for surgery in 18% of the Euro Heart survey.7 Factors associated with embolic events were similar to previous studies, with a major role of staphylococcal infection, vegetation presence, and size. More surprising was the fact that tricuspid, pulmonary, and aortic IE were associated with the highest risk of embolism.
In-hospital mortality rate is associated with known risk factors, including PVIE, age, comorbidity, S. aureus infection, congestive heart failure, cerebral complications, perivalvular lesions, and vegetation length. The major cause of death was heart failure. Interestingly, mortality was particularly high in EURO-ENDO when surgery was indicated but not performed, emphasizing the role of an aggressive surgical strategy in these patients. Although the exact reason to contra-indicate surgery in 58.2% of patients with a theoretical surgical indication is unknown, it is probably related to a combination of several parameters (age, frailty, left ventricular dysfunction, and multiple surgeries).
The 17.1% mortality observed in EURO-ENDO is consistent with the 18% mortality observed in the ICE cohort1 but higher than the 12.6% mortality observed in the Euro Heart survey7 (although this latter series included only 159 patients).
Study limitations
EURO-ENDO represents a unique evaluation of the current features and treatment of IE.
Nevertheless, the study has inherent limitations and is unlikely to be a true population-based sample. For instance, we cannot be sure that all centres really included all their patients consecutively and prospectively, since the study was based on the volunteer participation of each centre. Like other voluntary registries, it is an observational study of patients from centres with a special interest in IE, most centres being tertiary referral centres with cardiac surgical programmes. These limitations were counterbalanced by the high number of enrolled patients, the quality of CRF completion, and representation of a wide range of both university and non-academic hospitals in many countries around the world. Another limitation of the EURO-ENDO registry is that information concerning the number of patients transferred from the centres without cardiac surgery to centres with cardiac surgery was missing, as well as the number of centres having an ‘endocarditis team’.8 Both might have influenced the outcome of the patients but could not be included in the multivariate analysis of predictors of death, since they were not collected in the CRF. Finally, long-term follow-up, including events occurring during the first year will be assessed in future ancillary studies of EURO-ENDO.
Summary and conclusion
The ESC EORP EURO-ENDO registry is the most comprehensive observational cohort of patients hospitalized with IE. Its main findings are the high frequency of PVIE, CDRIE, nosocomial, staphylococcal and enterococcal IE, the low frequency of streptococcal IE, the emergence of new imaging techniques, and the persistently high in-hospital mortality.
Although the result of EURO-ENDO should be considered with caution, since important limitations are inherent to such a registry, it provides new insights concerning the contemporary profile of patients admitted to hospital with IE, their investigation, treatment and clinical outcomes, and the influence of the 2015 ESC Clinical Practice Guidelines for the management of IE on clinical practice. Given the paucity of randomized or large-scale observational data in IE, this registry offers a unique perspective on the current care and clinical outcomes of patients with IE across a wide range of countries.
Appendix 1
EORP Oversight Committee : C.P. Gale, GB (Chair); B. Beleslin, RS; A. Budaj, PL; O. Chioncel, RO; N. Dagres, DE; N. Danchin, FR; J. Emberson, GB; D. Erlinge, SE; M. Glikson, IL; A. Gray, GB; M. Kayikcioglu, TR; A.P. Maggioni, IT; V.K. Nagy, HU; A. Nedoshivin, RU; A-S. Petronio, IT; J. Roos-Hesselink, NL; L. Wallentin, SE; U. Zeymer, DE.
Executive Committee: G. Habib, FR (Chair); P. Lancellotti, BE (Chair); B. Cosyns, BE; E. Donal, FR; P. Erba, IT; B. Iung, FR; A.P. Maggioni, IT; B.A. Popescu, RO; B. Prendergast, GB; P. Tornos, ES.
EORP Team: M. Andarala, C. Berle, A. Brunel-Lebecq, E. Fiorucci, C. Laroche, V. Missiamenou, C. Taylor.
National Coordinators: N.N. Ali Tatar-Chentir, DZ; M. Al-Mallah, SA; M. Astrom Aneq, SE; G. Athanassopoulos, GR; L.P. Badano, IT; S. Benyoussef, TN; E. Calderon Aranda, MX; N.M. Cardim, PT; K-L. Chan, CA; B. Cosyns, BE; I. Cruz, PT; T. Edvardsen, NO; G. Goliasch, AT; G. Habib, FR; A. Hagendorff, DE; K. Hristova, BG; B. Iung, FR; O. Kamp, NL; D-H. Kang, KR; W. Kong, SG; S. Matskeplishvili, RU; M. Meshaal, EG; M. Mirocevic, ME; A.N. Neskovic, RS; M. Pazdernik, CZ; E. Plonska-Gosciniak, PL; B.A. Popescu, RO; B. Prendergast, GB; M. Raissouni, MA; R. Ronderos, AR; L.E. Sade, TR; A. Sadeghpour, IR; A. Sambola, ES; S. Sengupta, IN; J. Separovic-Hanzevacki, HR; M. Takeuchi, JP; E. Tucay, PH; A.C. Tude Rodrigues, BR; A. Varga, HU; J. Vaskelyte, LT; K. Yamagata, MT; K. Yiangou, CY; H. Zaky, AE.
Investigators
Argentina: Buenos Aires: I. Granada, M. Mahia, S. Ressi, F. Nacinovich, A. Iribarren, P. Fernandez Oses, G. Avegliano, E. Filipini, Corrientes: R. Obregon, M. Bangher, J. Dho, La Plata: L. Cartasegna, M.L. Plastino, V. Novas, C. Shigel, Florencio Varela: G. Reyes, M. De Santos, N. Gastaldello, M. Granillo Fernandez, M. Potito, G. Streitenberger, P. Velazco, J.H. Casabé, C. Cortes, E. Guevara, F. Salmo, M. Seijo, Austria: Vienna: F. Weidinger, M. Heger, R. Brooks, C. Stöllberger, C-Y. Ho, L. Perschy, L. Puskas, C. Binder, R. Rosenhek, M. Schneider, M-P. Winter, Belgium: Liege: E. Hoffer, M. Melissopoulou, E. Lecoq, D. Legrand, S. Jacquet, M. Massoz, L. Pierard, S. Marchetta, R. Dulgheru, C. D´ Emal, C. Oury, Jette: S. Droogmans, D. Kerkhove, D. Plein, L. Soens, C. Weytjens, A. Motoc, B. Roosens, I. Lemoine, Edegem: I. Rodrigus, B. Paelinck, B. Amsel, Brussels: P. Unger, D. Konopnicki, C. Beauloye, A. Pasquet, J.L. Vanoverschelde , S. Pierard, D. Vancraeynest, F. Sinnaeve, Brazil: Sao Paulo: J.L. Andrade, K. Staszko, Porto Alegre: R. Dos Santos Monteiro, M.H. Miglioranza, D.L. Shuha, Rio de Janeiro: M. Alcantara, V. Cravo, L. Fazzio, A. Felix, M. Iso, C. Musa, A.P. Siciliano, Marilia: F. Villaca Filho, A. Rodrigues, F. Vilela, J. Braga, R. Silva, D. Rodrigues, L. Silva, S. Morhy, C. Fischer, R. Silva, M. Vieira, T. Afonso, Fortaleza: J. Abreu, S.N. Falcao, V.A. Moises, A. Gouvea, F.J. Mancuso, A.C. Souza, C.Y. Silva, G. João, C.S. Abboud, R. Bellio de Mattos Barretto, A. Ramos, R. Arnoni, J.E. Assef, D.J. Della Togna, D. Le Bihan, L. Miglioli, A.P. Romero Oliveira, R. Tadeu Magro Kroll, D. Cortez, Belo Horizonte: C.L. Gelape, M.d.C. Peirira Nunes, T.C. De Abreu Ferrari, Canada: Ottawa: K. Hay , Montreal: V. Le, M. Page, F. Poulin, C. Sauve, K. Serri, C. Mercure, Quebec: J. Beaudoin, P. Pibarot, I.A. Sebag, L.G. Rudski, G. Ricafort, Croatia: Zagreb: B. Barsic, V. Krajinovic, M. Vargovic, D. Lovric, V. Reskovic-Luksic, J. Vincelj, S. Jaksic Jurinjak, Cyprus: Nicosia: V. Yiannikourides, M. Ioannides, C. Pofaides, V. Masoura, Czech Republic: Ostrava-Poruba: J. Pudich, Prague: A. Linhart, M. Siranec, J. Marek, K. Blechova, M. Kamenik, Hradec Kralove: R. Pelouch, Zlin: Z. Coufal, M. Mikulica, M. Griva, E. Jancova, M. Mikulcova, Olomouc: M. Taborsky, J. Precek, M. Jecmenova, J. Latal, Liberec: J. Widimsky, T. Butta, S. Machacek, Pilsen: R. Vancata, Brno: J. Spinar, M. Holicka, Ecuador: Guayaquil: F. Pow Chon Long, N. Anzules, A. Bajana Carpio, G. Largacha, E. Penaherrera, D. Moreira, Egypt: Mansoura: E. Mahfouz, E. Elsafty, A. Soliman, Y. Zayed, J. Aboulenein, Alexandria: M. Abdel-Hay, A. Almaghraby, M. Abdelnaby, M. Ahmed, B. Hammad, Y. Saleh, H. Zahran, O. Elgebaly, Zagazig: A. Saad, M. Ali , A. Zeid, R. El Sharkawy, Cairo: A. Al Kholy, R. Doss, D. Osama, H. Rizk, A. Elmogy, M. Mishriky, France: Kremlin-Bicêtre: P. Assayag, S. El Hatimi, Marseille: S. Hubert, J-P. Casalta, F. Gouriet, F. Arregle, S. Cammilleri, L. Tessonnier, A. Riberi, Saint-Etienne: E. Botelho-Nevers, A. Gagneux-Brunon, R. Pierrard, C. Tulane, S. Campisi, J-F. Fuzellier, M. Detoc, T. Mehalla, Nantes: D. Boutoille, A.S. Lecompte, M. Lefebvre, S. Pattier, O. Al Habash, N. Asseray-Madani, C. Biron, J. Brochard, J. Caillon, C. Cueff, T. Le Tourneau, R. Lecomte, M.M. Magali Michel, J. Orain, S. Delarue, M. Le Bras, Limoges: J-F. Faucher, V. Aboyans, A. Beeharry, H. Durox, M. Lacoste, J. Magne, D. Mohty, A. David, V. Pradel, Thonon-les-Bains: V. Sierra, A. Neykova, B. Bettayeb, S. Elkentaoui, B. Tzvetkov, G. Landry, Reims: C. Strady, K. Ainine, S. Baumard, C. Brasselet, C. Tassigny, V. Valente-Pires, M. Lefranc, Pointe-à-Pitre: B. Hoen, B. Lefevre, E. Curlier, C. Callier, N. Fourcade, Brest: Y. Jobic, S. Ansard, R. Le Berre, F. Le Ven, M-C. Pouliquen, G. Prat, P. Le Roux, Rouen: F. Bouchart, A. Savoure, C. Alarcon, C. Chapuzet, I. Gueit, Amiens: C. Tribouilloy, Y. Bohbot, F. Peugnet , M. Gun, Paris: X. Duval, X. Lescure, E. Ilic-Habensus, Nancy: N. Sadoul, C. Selton-Suty, F. Alla, F. Goehringer, O. Huttin, E. Chevalier, Poitiers: R. Garcia, V. Le Marcis, Rennes: P. Tattevin, E. Flecher, M. Revest, Besançon: C. Chirouze, K. Bouiller, L. Hustache-Mathieu, T. Klopfenstein, J. Moreau, D. Fournier, A-S. Brunel, Créteil: P. Lim, L. Oliver, J. Ternacle, A. Moussafeur, Dijon: P. Chavanet, L. Piroth, A. Salmon-Rousseau, M. Buisson, S. Mahy, C. Martins, S. Gohier, Noumea: O. Axler, F. Baumann, S. Lebras, Germany: Bad Oeynhausen: C. Piper, D. Guckel, J. Börgermann, D. Horstkotte, E. Winkelmann, B. Brockmeier, Leipzig: D. Grey, Bonn: G. Nickenig, R. Schueler, C. Öztürk, E. Stöhr, Bad Nauheim: C. Hamm, T. Walther, R. Brandt, A-C. Frühauf, C.T. Hartung, C. Hellner, C. Wild, Aachen: M. Becker, S. Hamada, W. Kaestner, Berlin: K. Stangl, F. Knebel, G. Baldenhofer, A. Brecht, H. Dreger, C. Isner, F. Pfafflin, M. Stegemann, Ludwigshafen: R. Zahn, B. Fraiture, C. Kilkowski, A-K. Karcher, S. Klinger, H. Tolksdorf, Greece: Athens: D. Tousoulis, C. Aggeli, S. Sideris, E. Venieri, G. Sarri, D. Tsiapras, I. Armenis, A. Koutsiari, G. Floros, C. Grassos, S. Dragasis, L. Rallidis, C. Varlamos, Ioannina: L. Michalis, K. Naka, A. Bechlioulis, A. Kotsia, L. Lakkas, K. Pappas, C. Papadopoulos, S. Kiokas, A. Lioni, S. Misailidou, J. Barbetseas, M. Bonou, C. Kapelios, I. Tomprou, K. Zerva, Voula: A. Manolis, E. Hamodraka, D. Athanasiou, G. Haralambidis, H. Samaras, L. Poulimenos, Hungary: Budapest: A. Nagy, A. Bartykowszki, E. Gara, India: Nagpur: K. Mungulmare, Gurgaon: R. Kasliwal, M. Bansal, S. Ranjan, A. Bhan, Iran: Tehran: M. Kyavar, M. Maleki, F. Noohi Bezanjani, A. Alizadehasl, S. Boudagh, A. Ghavidel, P. Moradnejad, H.R. Pasha, B. Ghadrdoost, Israel: Jerusalem: D. Gilon, J. Strahilevitz, M. Wanounou, S. Israel, Italy: Bari: C. d'Agostino, P. Colonna, L. De Michele, F. Fumarola, M. Stante, Florence: N. Marchionni, V. Scheggi, B. Alterini, S. Del Pace, P. Stefano, C. Sparano, Padova: N. Ruozi, R. Tenaglia, D. Muraru , Grosseto: U. Limbruno, A. Cresti, P. Baratta, M. Solari, Milan: C. Giannattasio, A. Moreo, B. De Chiara, B. Lopez Montero, F. Musca, C.A. Orcese, F. Panzeri, F. Spano, C.F. Russo, O. Alfieri, M. De Bonis, S. Chiappetta, B. Del Forno, M. Ripa, P. Scarpellini, C. Tassan Din, B. Castiglioni , R. Pasciuta, S. Carletti, D. Ferrara, M. Guffanti, G. Iaci, E. Lapenna, T. Nisi, C. Oltolini, E. Busnardo, U. Pajoro, E. Agricola, R. Meneghin, D. Schiavi, Salerno: F. Piscione, R. Citro, R.M. Benvenga, L. Greco, L. Soriente, I. Radano, C. Prota, M. Bellino, D. Di Vece, Genoa: F. Santini, A. Salsano, G.M. Olivieri, Modena: F. Turrini, R. Messora, S. Tondi, A. Olaru, V. Agnoletto, L. Grassi, C. Leonardi, S. Sansoni, Turin: S. Del Ponte, G.M. Actis Dato, A. De Martino, Japan: Nagoya: N. Ohte, S. Kikuchi, K. Wakami, Tsukuba: K. Aonuma, Y. Seo, T. Ishizu, T. Machino-Ohtsuka, M. Yamamoto, N. Iida, H. Nakajima, Tenri: Y. Nakagawa, C. Izumi, M. Amano, M. Miyake, K. Takahashi, Osaka: I. Shiojima, Y. Miyasaka, H. Maeba, Y. Suwa, N. Taniguchi, S. Tsujimoto, Kobe: T. Kitai, M. Ota, Sapporo: S. Yuda, S. Sasaki, Tokyo: N. Hagiwara, K. Yamazaki, K. Ashihara, K. Arai, C. Saitou, S. Saitou, G. Suzuki, Miyazaki: Y. Shibata, N. Watanabe, S. Nishino, K. Ashikaga, N. Kuriyama, K. Mahara, T. Okubo, H. Fujimaki, H. Shitan, H. Yamamoto, K. Abe, M. Terada, S. Takanashi, Tokushima: M. Sata, H. Yamada, K. Kusunose, Y. Saijo, H. Seno, O. Yuichiro, Suita: T. Onishi, F. Sera, S. Nakatani, H. Mizuno, K. Sengoku, Korea, Republic Of: Seoul: S.W. Park, K. Eun Kyoung, L. Ga Yeon, J-w. Hwang, C. Jin-Oh, S-J. Park, L. Sang-Chol, C. Sung-A, S.Y. Jang, R. Heo, S. Lee, J-M. Song, E. Jung, Lithuania: Siauliai: J. Plisiene, A. Dambrauskaite, G. Gruodyte, Kaunas: R. Jonkaitiene, V. Mizariene, J. Atkocaityte, R. Zvirblyte, Luxembourg: Luxembourg: R. Sow, A. Codreanu, T. Staub, C. Michaux, E.C.L. De la Vega, L. Jacobs-Orazi, Malta: Msida: C. Mallia Azzopardi, R.G. Xuereb, T. Piscopo, J. Farrugia, M. Fenech, E. Pllaha, C. Vella, D. Borg, R. Casha, Moldova, Republic Of: Chisinau: L. Grib, E. Raevschi, A. Grejdieru, D. Kravcenco, E. Prisacari, E. Samohvalov, S. Samohvalov, N. Sceglova, E. Panfile, L. Cardaniuc, V. Corcea, A. Feodorovici, V. Gaina, L. Girbu, P. Jimbei, G. Balan, I. Cardaniuc, I. Benesco, V. Marian, N. Sumarga, Montenegro: Podgorica: B. Bozovic, N. Bulatovic, P. Lakovic, L. Music, Netherlands: Rotterdam: R. Budde, A. Wahadat, T. Gamela, Amsterdam: T. Meijers, Groningen: J.P. Van Melle, V.M. Deursen, Maastricht: H.J. Crijns, S.C. Bekkers, E.C. Cheriex, M. Gilbers, B.L. Kietselaer, C. Knackstedt, R. Lorusso, S. Schalla, S.A. Streukens, Utrecht: S. Chamuleau, M-J. Cramer, A. Teske, T. Van der Spoel, A. Wind, J. Lokhorst, O. Liesbek, H. Van Heusden, The Hague: W. Tanis, I. Van der Bilt, J. Vriend, H. De Lange-van Bruggen, E. Karijodikoro, R. Riezebos, E. van Dongen, J. Schoep, V. Stolk, Norway: Oslo: J.T. Offstad, J.O. Beitnes, T. Helle-Valle, H. Skulstad, R. Skardal, Pakistan: Karachi: N. Qamar, S. Furnaz, B. Ahmed, M.H. Butt, M.F. Khanzada, T. Saghir, A. Wahid, Poland: Warsaw: T. Hryniewiecki, P. Szymanski, K. Marzec, M. Misztal-Ogonowska, Wroclaw: W. Kosmala, M. Przewlocka-Kosmala, A. Rojek, K. Woznicka, J. Zachwyc, Bialystok: A. Lisowska, M. Kaminska, Lodz: J.D. Kasprzak, E. Kowalczyk, D.F. Strzecka, P. Wejner-Mik, Portugal: Carnaxide: M. Trabulo, P. Freitas, S. Ranchordas, G. Rodrigues, Guilhufe: P. Pinto, C. Queiros, J. Azevedo, L. Marques, D. Seabra, Lisbon: L. Branco, J. Abreu, M. Cruz, A. Galrinho, R. Moreira, P. Rio, A.T. Timoteo, M. Selas, V. Carmelo, B. Duque Neves, Almada: H. Pereira, A. Guerra, A. Marques, I. Pintassilgo, Romania: Timisoara: M.C. Tomescu, N-M. Trofenciuc, M. Andor, A. Bordejevic, H.S. Branea, F. Caruntu, L.A. Velcean, A. Mavrea, M.F. Onel, T. Parvanescu, D. Pop, A.L. Pop-Moldovan, M.I. Puticiu, L. Cirin, I.M. Citu, C.A. Cotoraci, D. Darabantiu, R. Farcas, I. Marincu, A. Ionac, D. Cozma, C. Mornos, F. Goanta, I. Popescu, Cluj-Napoca: R. Beyer, R. Mada, R. Rancea, R. Tomoaia, H. Rosianu, C. Stanescu, Russian Federation: Moscow: Z. Kobalava, J. Karaulova, E. Kotova, A. Milto, A. Pisaryuk, N. Povalyaev, M. Sorokina, Saudi Arabia: Jeddah: J. Alrahimi, A. Elshiekh, Riyadh: A. Jamiel, A. Ahmed, N. Attia, Serbia: Belgrade: B. Putnikovic, A. Dimic, B. Ivanovic, S. Matic, D. Trifunovic, J. Petrovic, D. Kosevic, I. Stojanovic, I. Petrovic, P. Dabic, P. Milojevic, Sremska Kamenica: I. Srdanovic, S. Susak, L. Velicki, A. Vulin, M. Kovacevic, A. Redzek, M. Stefanovic, Singapore: Singapore: T.C. Yeo, W. KF Kong, K.K. Poh, Spain: Madrid: I. Vilacosta, C. Ferrera, C. Olmos, M. Abd El- Nasser , Vigo - Pontevedra: F. Calvo Iglesias, E. Blanco-Gonzalez, M. Bravo Amaro, E. Lopez-Rodriguez, J. Lugo Adan, A.N. Germinas, P. Pazos-Lopez, M. Pereira Loureiro, M.T. Perez, S. Raposeiras-Roubin, S. Rasheed Yas, M-M. Suarez-Varela, F. Vasallo Vidal, Barcelona: D. Garcia-Dorado, N. Fernandez-Hidalgo, T. Gonzalez-Alujas, J. Lozano, O. Maisterra, N. Pizzi, R. Rios, Badalona: A. Bayes-Genis, L. Pedro Botet, N. Vallejo, C. Llibre, L. Mateu, R. Nunez, D. Quesada, E. Berastegui, Girona: D. Bosch Portell, J. Aboal Vinas, X. Albert Bertran, R. Brugada Tarradellas, P. Loma-Osorio Ricon, C. Tiron de Llano, Valencia: M.A. Arnau, A. Bel, M. Blanes, A. Osa, Cordoba: M. Anguita, F. Carrasco, J.C. Castillo, J.L. Zamorano, J.L. Moya Mur, M. Alvaro, C. Fernandez-Golfin, J.M. Monteagudo, E. Navas Elorza, Santander: M.C. Farinas Alvarez, J. Aguero Balbin, J. Zarauza, J.F. Gutierrez-Diez, C. Arminanzas , F. Arnaiz de las Revillas, A. Arnaiz Garcia, M. Cobo Belaustegui, M. Fernandez Sampedro, M. Gutierrez Cuadra, L. Garcia Cuello, C. Gonzalez Rico, Barakaldo: R. Rodriguez-Alvarez, J. Goikoetxea , M. Montejo , J.M. Miro, M. Almela, J. Ambrosioni, A. Moreno, E. Quintana, E. Sandoval, A. Tellez, J.M. Tolosana, B. Vidal, C. Falces, D. Fuster, C. Garcia-de-la-Maria, M. Hernandez-Meneses, J. Llopis, F. Marco, I. Ruiz-Zamora, Tarragona: A. Bardaji Ruiz, E. Sanz Girgas, G. Garcia-Pardo, M. Guillen Marzo, A. Rodriguez Oviedo, A. Villares Jimenez, Tunisia: Sfax: L. Abid, R. Hammami, S. Kammoun, Tunis: M.S. Mourali, F. Mghaieth Zghal, M. Ben Hlima, S. Boudiche, S. Ouali, La Marsa: L. Zakhama, S. Antit, I. Slama, Turkey: Samsun: O. Gulel, M. Sahin, Ankara: L.E. Sade, E. Karacaglar, Istanbul: S. Kucukoglu, O. Cetinarslan, U.Y. Sinan, U. Canpolat, B. Mutlu, H. Atas, R. Dervishova, C. Ileri, United Arab Emirates: Dubai: J. Alhashmi, J. Tahir, P. Zarger, F. Baslib, United Kingdom: London: S. Woldman, L. Menezes, C. Primus, R. Uppal, I. Bvekerwa, Swindon: B. Chandrasekaran, A. Kopanska, J. Chambers, J. Hancock, J. Klein, R. Rajani, M.P. Ursi, S. Cannata, R. Dworakowski, A. Fife, J. Breeze, M. Browne-Morgan, M. Gunning, S. Streather, United States: Washington: F.M. Asch, M. Zemedkun, Uzbekistan: Tashkent: B. Alyavi, J. Uzokov.
Acknowledgements
EORP Oversight Committee, Registry Executive and Steering Committees. The Data collection was conducted by the EORP department of the ESC: Emanuela Fiorucci, as Project Officer; Viviane Missiamenou, Florian Larras, and Rachid Mir Hassaine, as Data Managers. Statistical analyses were performed by Cécile Laroche. Overall activities were coordinated and supervised by Doctor Aldo P. Maggioni (EORP Scientific Coordinator). Special thanks to the EACVI (European Association of CardioVascular Imaging) and to the ESC Working Group on Valvular Heart Disease for their support.
Funding
Abbott Vascular Int. (2011–21), Amgen Cardiovascular (2009–18), AstraZeneca (2014–21), Bayer AG (2009–18), Boehringer Ingelheim (2009–19), Boston Scientific (2009–12), The Bristol Myers Squibb and Pfizer Alliance (2011–19), Daiichi Sankyo Europe GmbH (2011–20), The Alliance Daiichi Sankyo Europe GmbH and Eli Lilly and Company (2014–17), Edwards (2016–19), Gedeon Richter Plc. (2014–16), Menarini Int. Op. (2009–12), MSD-Merck & Co. (2011–14), Novartis Pharma AG (2014–20), ResMed (2014–16), Sanofi (2009–11), SERVIER (2009–21), and Vifor (2019–22).
Conflict of interest: none declared.
See page 3233 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz694)
References
Author notes
A complete list of the EURO-ENDO Investigators Group and EURO-ENDO National Coordinators is provided in Appendix S1.
- echocardiography
- fluorodeoxyglucose f18
- bacterial endocarditis
- vegetation
- cardiac surgery procedures
- lung
- congestive heart failure
- abscess
- hospital mortality
- prospective studies
- staphylococcus
- surgical procedures, operative
- brain
- diagnosis
- diagnostic imaging
- guidelines
- heart
- mortality
- embolism
- computed tomography/positron emission tomography imaging
- causality
- european society of cardiology



