-
PDF
- Split View
-
Views
-
Cite
Cite
Stuart J. Connolly, John Eikelboom, Paul Dorian, Stefan H. Hohnloser, Daniel D. Gretler, Uma Sinha, Michael D. Ezekowitz, Betrixaban compared with warfarin in patients with atrial fibrillation: results of a phase 2, randomized, dose-ranging study (Explore-Xa), European Heart Journal, Volume 34, Issue 20, 21 May 2013, Pages 1498–1505, https://doi.org/10.1093/eurheartj/eht039
Close - Share Icon Share
Abstract
Patients with atrial fibrillation (AF) are at increased risk of stroke. Betrixaban is a novel oral factor Xa inhibitor administered once daily, mostly excreted unchanged in the bile and with low (17%) renal excretion.
Patients with AF and more than one risk factor for stroke were randomized to one of three blinded doses of betrixaban (40, 60, or 80 mg once daily) or unblinded warfarin, adjusted to an international normalized ratio of 2.0–3.0. The primary outcome was major or clinically relevant non-major bleeding. The mean follow-up was 147 days. Among 508 patients randomized, the mean CHADS2 score was 2.2; 87% of patients had previously received vitamin K antagonist therapy. The time in therapeutic range on warfarin was 63.4%. There were one, five, five, and seven patients with a primary outcome on betrixaban 40, 60, 80 mg daily, or warfarin, respectively. The rate of the primary outcome was lowest on betrixaban 40 mg (hazard ratio compared with warfarin = 0.14, exact stratified log-rank P-value 0.04, unadjusted for multiple testing). Rates of the primary outcome with betrixaban 60 or 80 mg were more similar to those of wafarin. Two ischaemic strokes occurred, one each on betrixaban 60 and 80 mg daily. There were two vascular deaths, one each on betrixaban 40 mg and warfarin. Betrixaban was associated with higher rates of diarrhoea than warfarin.
Betrixaban was well tolerated and had similar or lower rates of bleeding compared with well-controlled warfarin in patients with AF at risk for stroke.
Introduction
Atrial fibrillation (AF) is an arrhythmia that affects an estimated 7 million people in the USA and the European Union with a median age of 75 years.1 The greatest risk associated with AF is ischaemic stroke resulting from embolism of thrombus from the left atrium. Guidelines recommend treatment with a vitamin K antagonist for stroke prevention in patients with AF based on the results of randomized, controlled trials comparing warfarin with placebo or no warfarin.1–6 The use of warfarin is challenging because it has an unpredictable anticoagulant effect, a high propensity for food and drug interactions, and a risk of significant bleeding. Indeed, a recent meta-analysis of contemporary randomized clinical trials comprising over 50 000 patient-years on warfarin revealed major bleeding rates ranging from 1.4 to 3.4% per year, and are likely higher outside the confines of a clinical trial.7 Routine coagulation monitoring is required, which is inconvenient for patients and costly for the health care system.2
Thrombin plays a key role in arterial and venous thromboses by activating platelets and facilitating the conversion of fibrinogen to fibrin. Recently, dabigatran, a direct thrombin inhibitor, has been shown to be superior to warfarin for the prevention of stroke with improved ease of use; and is available in many countries.8 Factor Xa is also an attractive target for anticoagulant therapy as it is the enzyme responsible for the conversion of prothrombin to thrombin. Rivaroxaban and apixaban, factor Xa inhibitors, have been shown to be effective for stroke prevention in AF.9–11
Betrixaban is an oral, once-daily, direct factor Xa inhibitor. It is rapidly absorbed with mean peak concentrations occurring 3 to 4 h after oral administration, a pharmacodynamic half-life of 20 h and a low peak-to-trough concentration ratio over each 24 h dosing period. Oral bioavailability of an 80 mg dose is 34% and protein binding is ∼60%. Betrixaban is mostly excreted unchanged in bile, with low (17%) renal excretion. When administered immediately after a high-fat, high-calorie breakfast, Cmax and AUC were reduced by ∼50% compared with the fasting state. Betrixaban is a substrate for efflux proteins, including P-glycoprotein; however, it is not a substrate for major CYP enzymes.
Betrixaban has been studied in 215 patients in a phase II venous thrombo-embolism (VTE) prevention study (EXPERT) in patients undergoing total knee replacement.12 Doses of 15 and 40 mg administered twice daily were compared with enoxaparin 30 mg twice daily for 10–14 days. Similar efficacy and safety were observed in all three groups, with a favourable safety profile. The present study was designed to assess the safety and tolerability of betrixaban at three different doses given orally once daily, compared with dose-adjusted [inter-quartile range (INR) 2.0–3.0] warfarin in patients with AF at risk of stroke.
Methods
Patients with new or existing non-valvular AF were recruited from outpatient clinics. They were eligible for enrolment if they fulfilled the following criteria: male or female aged ≥18 years, in AF or atrial flutter at the time of enrolment or documented within the previous year by Holter, ECG, rhythm strip, pacemaker, or other intracardiac recording, and with one or more risk factor(s) for stroke, resulting in an indication for anticoagulation with a vitamin K antagonist. Subjects on warfarin were required to have an INR ≤2.2 at the time of randomization. Major exclusion criteria included: body weight of <40 kg, need for either haemodialysis or peritoneal dialysis, AF due to reversible causes, active bleeding; history of congenital or acquired bleeding disorders or vascular malformation; history of intracranial, retroperitoneal, or intraocular bleeding within the last 6 months; high risk of bleeding for other reasons including from significant liver disease; conditions other than AF that required chronic anticoagulation; persistent uncontrolled hypertension. Patients on verapamil were excluded pending a formal drug interaction study. Patients with renal insufficiency were not excluded unless they were on dialysis.
Outcomes
The primary outcome was the time to occurrence of major or clinically relevant non–major (CRNM) bleeding. The secondary outcomes were any bleeding (including major, CRNM, and any other reported bleeding) and the time to occurrence of death, ischaemic or non–ischaemic stroke, MI, or other systemic embolism. An independent adjudicator, blinded to treatment groups, adjudicated all major bleeds, CRNM bleeds, strokes, MI, other systemic embolism, and deaths.
Major bleeding was defined as clinically overt bleeding that was associated with a reduction in haemoglobin of ≥20 g/L or transfusion of ≥2 units of blood or packed cells, or symptomatic bleeding in a critical area or organ: intraocular, intracranial, intraspinal, retroperitoneal, intra-articular, pericardial, or intramuscular with compartment syndrome or a fatal outcome. Clinically relevant non-major bleeding was defined as overt bleeding not meeting the criteria for major bleeding but associated with medical intervention, unscheduled contact (visit or telephone call) with a physician, (temporary) cessation of study treatment, or discomfort for the patient such as pain or impairment of activities of daily life. All other overt bleeding episodes were classified as minimal bleeding events, and were not adjudicated. Diagnosis of stroke required acute onset of a focal neurological deficit of presumed vascular origin that lasted for ≥24 h or resulted in death. A stroke was categorized as ischaemic, haemorrhagic, or unknown, based on CT or MR scanning or autopsy. Deaths were classified as vascular (including bleeding), non-vascular, due to other specific causes (e.g. malignancy), or unknown. Vascular death was considered to occur when there was no obvious non-vascular event to explain death, such as cancer, trauma, or respiratory failure. Sudden or un-witnessed deaths were considered vascular.
Safety monitoring
A data safety monitoring committee was appointed to independently review all safety and efficacy data at regular intervals during the study and make recommendations, as appropriate. No formal analyses of efficacy were performed.
Randomization and follow-up
Patients were randomly assigned (1:1:1:1 allocation) to betrixaban 40, 60, 80 mg orally, once daily, or warfarin (target INR of 2.0–3.0). Assignment to betrixaban or warfarin was not blinded, but the betrixaban dose was double-blinded. Subjects assigned to betrixaban were instructed to take the drug at bedtime, preferably at least 2 h after the evening meal. A dynamic randomization was used to assign and balance patients by country, concurrent aspirin use, and antecedent warfarin. Aspirin (up to 162 mg daily), clopidogrel, ticlopidine, and any concomitant medications or treatments deemed necessary by the investigator to provide adequate supportive care were allowed during the study. Verapamil use was not allowed, as a drug interaction study had not been performed. The study was conducted according to ethical standards, local laws, and GCPs in the USA, Canada, and Germany. Subjects were assessed at screening, and at weeks 0, 1, 2, 4, 8, and 12 and then every 2 months thereafter for a maximum of 1 year.
Laboratory analyses
Study measurements included plasma betrixaban concentrations, D-dimer concentrations, and thrombin generation (TG). Inhibition of tissue factor-initiated TG was measured as previously described.12 D-dimer levels were assessed in both warfarin naive (13%) and experienced patients (87%) at baseline and at 12 weeks.
Statistical analyses
Under the assumptions that the event rate for the composite of major and CRNM bleeding would be 5.7% in the warfarin group at 3 months, the enrolment period would be 8 months, and the total study duration would be 11 months, enrolment of 500 patients was planned to provide 80% power to detect a hazard ratio (HR) of 2.27 in the primary endpoint for an individual betrixaban dose vs. warfarin using a two-sided log-rank test at the 0.05 significance level. Continuous parameters were summarized by n (number of non-missing observations), mean, standard deviation, median, minimum, maximum, and the first and third quartiles. Categorical parameters were summarized by count and per cent. Time in therapeutic range (TTR) of the INR was calculated by linear interpolation.13 Survival estimates were computed using the Kaplan–Meier method. Differences in the primary and other outcomes, between treatment groups, were formally tested using individual exact log-rank tests and stratified by country and concurrent aspirin use. Secondary analyses used Cox proportional hazards models. There was no adjustment for testing multiplicity. Significance testing was two-sided. Adverse event rates were compared using Fisher's exact tests. Statistical analyses were performed on an intent-to-treat population basis, using the SAS® software version 9.1.3 or StatXact® PROCS for SAS version 8.
Results
Patient disposition
There were 561 patients screened, and 508 were randomized to the four treatment groups (Figure 1). All patients received at least one dose of study medication and the rate of premature withdrawal from study medication was similar between betrixaban 40, 60, and 80 mg per day and warfarin (8.7, 9.4, 8.7, 6.3%, respectively). There were 113, 119, 117, and 113 patients who completed a minimum follow-up of 90 days for betrixaban 40, 60, and 80 mg per day and warfarin, respectively. The maximum follow-up duration was 329 days and the median follow-up was 150 days. There were 127 patients enrolled from Canada, 369 from the USA, and 12 from Germany.
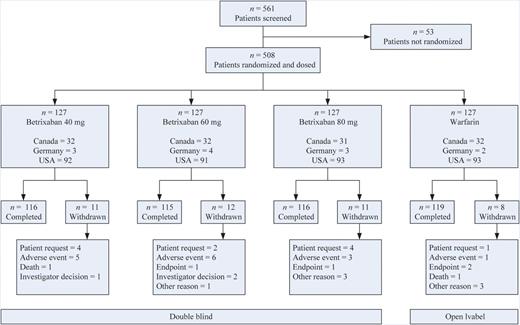
Baseline characteristics and compliance
Baseline patient risk factors were well balanced (Table 1). The mean age of patients was 73 years, 33.5% were female, and most patients were Caucasian. The overall mean weight was 90.9 kg. Only 13% of patients had never previously received a vitamin K antagonist. Baseline estimated calculated glomerular filtration rate (GFR) was >70 mL/min in 53.5% of patients, and 8.1% of patients had a GFR <40 mL/min. The mean CHADS2 score was 2.2.
| Demographic or baseline measure . | Betrixaban 40 mg (n = 127) . | Betrixaban 60 mg (n = 127) . | Betrixaban 80 mg (n = 127) . | Warfarin (n = 127) . | Overall (n = 508) . |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean (SD) | 73.3 (8.50) | 73.8 (8.35) | 72.0 (7.65) | 72.7 (8.75) | 73.0 (8.32) |
| Age category [n (%)] | |||||
| ≥75 years | 63 (49.6) | 66 (52.0) | 51 (40.2) | 60 (47.2) | 240 (47.2) |
| Gender/race [n (%)] | |||||
| Female | 48 (37.8) | 46 (36.2) | 38 (29.9) | 38 (29.9) | 170 (33.5) |
| White | 125 (98.4) | 123 (96.9) | 123 (96.9) | 126 (99.2) | 497 (97.8) |
| Other | 2 (1.6) | 4 (3.1) | 4 (3.1) | 1 (0.8) | 11 (2.2) |
| Weight (kg) | |||||
| Mean (SD) | 89.58 (21.409) | 89.14 (23.438) | 91.25 (20.690) | 93.73 (24.337) | 90.93 (22.523) |
| Weight category [n (%)] | |||||
| >90 kg | 53 (41.7) | 54 (42.5) | 65 (51.2) | 62 (48.8) | 234 (46.1) |
| Prior use of vitamin K antagonist [n (%)] | |||||
| No | 15 (11.8) | 17 (13.4) | 16 (12.6) | 18 (14.2) | 66 (13.0) |
| Concomitant antiplatelet therapies [n (%)] | |||||
| At least one antiplatelet therapy | 53 (41.7) | 49 (38.6) | 51 (40.2) | 52 (40.9) | 205 (40.4) |
| Exactly one antiplatelet therapy | 49 (38.6) | 47 (37.0) | 46 (36.2) | 49 (38.6) | 191 (37.6) |
| Two or more antiplatelet therapies | 4 (3.1) | 2 (1.6) | 5 (3.9) | 3 (2.4) | 14 (2.8) |
| GFR level (Cockcroft–Gault) [n (%)] | |||||
| <40 mL/min | 14 (11.0) | 14 (11.0) | 7 (5.5) | 6 (4.7) | 41 (8.1) |
| 40–70 mL/min | 47 (37.0) | 50 (39.4) | 50 (39.4) | 48 (37.8) | 195 (38.4) |
| >70 mL/min | 66 (52.0) | 63 (49.6) | 70 (55.1) | 73 (57.5) | 272 (53.5) |
| Classification of AF [n (%)] | |||||
| Permanent | 49 (38.6) | 34 (26.8) | 35 (27.6) | 52 (40.9) | 170 (33.5) |
| Persistent | 28 (22.0) | 25 (19.7) | 26 (20.5) | 26 (20.5) | 105 (20.7) |
| Paroxysmal | 50 (39.4) | 68 (53.5) | 66 (52.0) | 49 (38.6) | 233 (45.9) |
| CHADS2 scorea [n (%)] | |||||
| 0–1 | 28 (22.0) | 36 (28.3) | 43 (33.9) | 37 (29.1) | 144 (28.3) |
| 2 | 52 (40.9) | 45 (35.4) | 55 (43.3) | 42 (33.1) | 194 (38.2) |
| 3–6 | 47 (37.0) | 46 (36.2) | 29 (22.8) | 48 (37.8) | 170 (33.5) |
| Demographic or baseline measure . | Betrixaban 40 mg (n = 127) . | Betrixaban 60 mg (n = 127) . | Betrixaban 80 mg (n = 127) . | Warfarin (n = 127) . | Overall (n = 508) . |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean (SD) | 73.3 (8.50) | 73.8 (8.35) | 72.0 (7.65) | 72.7 (8.75) | 73.0 (8.32) |
| Age category [n (%)] | |||||
| ≥75 years | 63 (49.6) | 66 (52.0) | 51 (40.2) | 60 (47.2) | 240 (47.2) |
| Gender/race [n (%)] | |||||
| Female | 48 (37.8) | 46 (36.2) | 38 (29.9) | 38 (29.9) | 170 (33.5) |
| White | 125 (98.4) | 123 (96.9) | 123 (96.9) | 126 (99.2) | 497 (97.8) |
| Other | 2 (1.6) | 4 (3.1) | 4 (3.1) | 1 (0.8) | 11 (2.2) |
| Weight (kg) | |||||
| Mean (SD) | 89.58 (21.409) | 89.14 (23.438) | 91.25 (20.690) | 93.73 (24.337) | 90.93 (22.523) |
| Weight category [n (%)] | |||||
| >90 kg | 53 (41.7) | 54 (42.5) | 65 (51.2) | 62 (48.8) | 234 (46.1) |
| Prior use of vitamin K antagonist [n (%)] | |||||
| No | 15 (11.8) | 17 (13.4) | 16 (12.6) | 18 (14.2) | 66 (13.0) |
| Concomitant antiplatelet therapies [n (%)] | |||||
| At least one antiplatelet therapy | 53 (41.7) | 49 (38.6) | 51 (40.2) | 52 (40.9) | 205 (40.4) |
| Exactly one antiplatelet therapy | 49 (38.6) | 47 (37.0) | 46 (36.2) | 49 (38.6) | 191 (37.6) |
| Two or more antiplatelet therapies | 4 (3.1) | 2 (1.6) | 5 (3.9) | 3 (2.4) | 14 (2.8) |
| GFR level (Cockcroft–Gault) [n (%)] | |||||
| <40 mL/min | 14 (11.0) | 14 (11.0) | 7 (5.5) | 6 (4.7) | 41 (8.1) |
| 40–70 mL/min | 47 (37.0) | 50 (39.4) | 50 (39.4) | 48 (37.8) | 195 (38.4) |
| >70 mL/min | 66 (52.0) | 63 (49.6) | 70 (55.1) | 73 (57.5) | 272 (53.5) |
| Classification of AF [n (%)] | |||||
| Permanent | 49 (38.6) | 34 (26.8) | 35 (27.6) | 52 (40.9) | 170 (33.5) |
| Persistent | 28 (22.0) | 25 (19.7) | 26 (20.5) | 26 (20.5) | 105 (20.7) |
| Paroxysmal | 50 (39.4) | 68 (53.5) | 66 (52.0) | 49 (38.6) | 233 (45.9) |
| CHADS2 scorea [n (%)] | |||||
| 0–1 | 28 (22.0) | 36 (28.3) | 43 (33.9) | 37 (29.1) | 144 (28.3) |
| 2 | 52 (40.9) | 45 (35.4) | 55 (43.3) | 42 (33.1) | 194 (38.2) |
| 3–6 | 47 (37.0) | 46 (36.2) | 29 (22.8) | 48 (37.8) | 170 (33.5) |
AF, atrial fibrillation; GFR, glomerular filtration rate.
aThe CHADS2 score is used to predict the risk of stroke in AF. It ranges from 0 to 6. One point is given for each of congestive heart failure, hypertension, age ≥75, and diabetes mellitus. Two points are given for either stroke or transient ischaemic attack. The CHADS2 score was computed regardless of timing of component events and diagnoses
| Demographic or baseline measure . | Betrixaban 40 mg (n = 127) . | Betrixaban 60 mg (n = 127) . | Betrixaban 80 mg (n = 127) . | Warfarin (n = 127) . | Overall (n = 508) . |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean (SD) | 73.3 (8.50) | 73.8 (8.35) | 72.0 (7.65) | 72.7 (8.75) | 73.0 (8.32) |
| Age category [n (%)] | |||||
| ≥75 years | 63 (49.6) | 66 (52.0) | 51 (40.2) | 60 (47.2) | 240 (47.2) |
| Gender/race [n (%)] | |||||
| Female | 48 (37.8) | 46 (36.2) | 38 (29.9) | 38 (29.9) | 170 (33.5) |
| White | 125 (98.4) | 123 (96.9) | 123 (96.9) | 126 (99.2) | 497 (97.8) |
| Other | 2 (1.6) | 4 (3.1) | 4 (3.1) | 1 (0.8) | 11 (2.2) |
| Weight (kg) | |||||
| Mean (SD) | 89.58 (21.409) | 89.14 (23.438) | 91.25 (20.690) | 93.73 (24.337) | 90.93 (22.523) |
| Weight category [n (%)] | |||||
| >90 kg | 53 (41.7) | 54 (42.5) | 65 (51.2) | 62 (48.8) | 234 (46.1) |
| Prior use of vitamin K antagonist [n (%)] | |||||
| No | 15 (11.8) | 17 (13.4) | 16 (12.6) | 18 (14.2) | 66 (13.0) |
| Concomitant antiplatelet therapies [n (%)] | |||||
| At least one antiplatelet therapy | 53 (41.7) | 49 (38.6) | 51 (40.2) | 52 (40.9) | 205 (40.4) |
| Exactly one antiplatelet therapy | 49 (38.6) | 47 (37.0) | 46 (36.2) | 49 (38.6) | 191 (37.6) |
| Two or more antiplatelet therapies | 4 (3.1) | 2 (1.6) | 5 (3.9) | 3 (2.4) | 14 (2.8) |
| GFR level (Cockcroft–Gault) [n (%)] | |||||
| <40 mL/min | 14 (11.0) | 14 (11.0) | 7 (5.5) | 6 (4.7) | 41 (8.1) |
| 40–70 mL/min | 47 (37.0) | 50 (39.4) | 50 (39.4) | 48 (37.8) | 195 (38.4) |
| >70 mL/min | 66 (52.0) | 63 (49.6) | 70 (55.1) | 73 (57.5) | 272 (53.5) |
| Classification of AF [n (%)] | |||||
| Permanent | 49 (38.6) | 34 (26.8) | 35 (27.6) | 52 (40.9) | 170 (33.5) |
| Persistent | 28 (22.0) | 25 (19.7) | 26 (20.5) | 26 (20.5) | 105 (20.7) |
| Paroxysmal | 50 (39.4) | 68 (53.5) | 66 (52.0) | 49 (38.6) | 233 (45.9) |
| CHADS2 scorea [n (%)] | |||||
| 0–1 | 28 (22.0) | 36 (28.3) | 43 (33.9) | 37 (29.1) | 144 (28.3) |
| 2 | 52 (40.9) | 45 (35.4) | 55 (43.3) | 42 (33.1) | 194 (38.2) |
| 3–6 | 47 (37.0) | 46 (36.2) | 29 (22.8) | 48 (37.8) | 170 (33.5) |
| Demographic or baseline measure . | Betrixaban 40 mg (n = 127) . | Betrixaban 60 mg (n = 127) . | Betrixaban 80 mg (n = 127) . | Warfarin (n = 127) . | Overall (n = 508) . |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean (SD) | 73.3 (8.50) | 73.8 (8.35) | 72.0 (7.65) | 72.7 (8.75) | 73.0 (8.32) |
| Age category [n (%)] | |||||
| ≥75 years | 63 (49.6) | 66 (52.0) | 51 (40.2) | 60 (47.2) | 240 (47.2) |
| Gender/race [n (%)] | |||||
| Female | 48 (37.8) | 46 (36.2) | 38 (29.9) | 38 (29.9) | 170 (33.5) |
| White | 125 (98.4) | 123 (96.9) | 123 (96.9) | 126 (99.2) | 497 (97.8) |
| Other | 2 (1.6) | 4 (3.1) | 4 (3.1) | 1 (0.8) | 11 (2.2) |
| Weight (kg) | |||||
| Mean (SD) | 89.58 (21.409) | 89.14 (23.438) | 91.25 (20.690) | 93.73 (24.337) | 90.93 (22.523) |
| Weight category [n (%)] | |||||
| >90 kg | 53 (41.7) | 54 (42.5) | 65 (51.2) | 62 (48.8) | 234 (46.1) |
| Prior use of vitamin K antagonist [n (%)] | |||||
| No | 15 (11.8) | 17 (13.4) | 16 (12.6) | 18 (14.2) | 66 (13.0) |
| Concomitant antiplatelet therapies [n (%)] | |||||
| At least one antiplatelet therapy | 53 (41.7) | 49 (38.6) | 51 (40.2) | 52 (40.9) | 205 (40.4) |
| Exactly one antiplatelet therapy | 49 (38.6) | 47 (37.0) | 46 (36.2) | 49 (38.6) | 191 (37.6) |
| Two or more antiplatelet therapies | 4 (3.1) | 2 (1.6) | 5 (3.9) | 3 (2.4) | 14 (2.8) |
| GFR level (Cockcroft–Gault) [n (%)] | |||||
| <40 mL/min | 14 (11.0) | 14 (11.0) | 7 (5.5) | 6 (4.7) | 41 (8.1) |
| 40–70 mL/min | 47 (37.0) | 50 (39.4) | 50 (39.4) | 48 (37.8) | 195 (38.4) |
| >70 mL/min | 66 (52.0) | 63 (49.6) | 70 (55.1) | 73 (57.5) | 272 (53.5) |
| Classification of AF [n (%)] | |||||
| Permanent | 49 (38.6) | 34 (26.8) | 35 (27.6) | 52 (40.9) | 170 (33.5) |
| Persistent | 28 (22.0) | 25 (19.7) | 26 (20.5) | 26 (20.5) | 105 (20.7) |
| Paroxysmal | 50 (39.4) | 68 (53.5) | 66 (52.0) | 49 (38.6) | 233 (45.9) |
| CHADS2 scorea [n (%)] | |||||
| 0–1 | 28 (22.0) | 36 (28.3) | 43 (33.9) | 37 (29.1) | 144 (28.3) |
| 2 | 52 (40.9) | 45 (35.4) | 55 (43.3) | 42 (33.1) | 194 (38.2) |
| 3–6 | 47 (37.0) | 46 (36.2) | 29 (22.8) | 48 (37.8) | 170 (33.5) |
AF, atrial fibrillation; GFR, glomerular filtration rate.
aThe CHADS2 score is used to predict the risk of stroke in AF. It ranges from 0 to 6. One point is given for each of congestive heart failure, hypertension, age ≥75, and diabetes mellitus. Two points are given for either stroke or transient ischaemic attack. The CHADS2 score was computed regardless of timing of component events and diagnoses
Concomitant antiplatelet therapy was used in 42, 39, 40, 41% of patients in the betrixaban 40, 60, 80 mg, and warfarin groups, respectively. The mean TTR for patients receiving warfarin was 63.4%. The mean compliance with betrixaban therapy (based on pill counts at follow-up visits) was 96.6, 96.5, and 96.0% on betrixaban 40, 60, and 80 mg per day, respectively.
Bleeding outcomes
The numbers of patients with a major or CRNM bleed were one, five, five, and seven on betrixaban 40, 60, 80 mg, and warfarin, respectively (Table 2).
| Outcome . | Betrixaban 40 mg (n = 127), events . | Betrixaban 60 mg (n = 127), events . | Betrixaban 80 mg (n = 127), events . | Warfarin (n = 127), events . | Betrixaban 40 mg vs. warfarin [HR (95% confidence interval)] . | Betrixaban 60 mg vs. warfarin [HR (95% confidence interval)] . | Betrixaban 80 mg vs. warfarin [HR (95% confidence interval)] . |
|---|---|---|---|---|---|---|---|
| Major or CRNM bleeding | 1 | 5 | 5 | 7 | 0.140 (0.017–1.135) | 0.711 (0.225–2.243) | 0.755 (0.239–2.389) |
| Major bleeding | 0 | 0 | 3 | 5 | NA (NA) | NA (NA) | 0.609 (0.145–2.557) |
| CRNM bleeding | 1 | 5 | 2 | 4 | 0.264 (0.030–2.364) | 1.257 (0.337–4.684) | 0.538 (0.098–2.937) |
| Minimal bleeding | 22 | 28 | 23 | 36 | 0.572 (0.336–0.974) | 0.752 (0.458–1.235) | 0.584 (0.346–0.986) |
| Any bleeding | 22 | 32 | 24 | 40 | 0.508 (0.301–0.856) | 0.767 (0.481–1.224) | 0.551 (0.332–0.914) |
| Stroke | 0 | 1 (ischaemic ) | 1 (ischaemic ) | 0 | – | – | – |
| Death | 1 (vascular) | 0 | 0 | 1 (vascular) | – | – | – |
| Outcome . | Betrixaban 40 mg (n = 127), events . | Betrixaban 60 mg (n = 127), events . | Betrixaban 80 mg (n = 127), events . | Warfarin (n = 127), events . | Betrixaban 40 mg vs. warfarin [HR (95% confidence interval)] . | Betrixaban 60 mg vs. warfarin [HR (95% confidence interval)] . | Betrixaban 80 mg vs. warfarin [HR (95% confidence interval)] . |
|---|---|---|---|---|---|---|---|
| Major or CRNM bleeding | 1 | 5 | 5 | 7 | 0.140 (0.017–1.135) | 0.711 (0.225–2.243) | 0.755 (0.239–2.389) |
| Major bleeding | 0 | 0 | 3 | 5 | NA (NA) | NA (NA) | 0.609 (0.145–2.557) |
| CRNM bleeding | 1 | 5 | 2 | 4 | 0.264 (0.030–2.364) | 1.257 (0.337–4.684) | 0.538 (0.098–2.937) |
| Minimal bleeding | 22 | 28 | 23 | 36 | 0.572 (0.336–0.974) | 0.752 (0.458–1.235) | 0.584 (0.346–0.986) |
| Any bleeding | 22 | 32 | 24 | 40 | 0.508 (0.301–0.856) | 0.767 (0.481–1.224) | 0.551 (0.332–0.914) |
| Stroke | 0 | 1 (ischaemic ) | 1 (ischaemic ) | 0 | – | – | – |
| Death | 1 (vascular) | 0 | 0 | 1 (vascular) | – | – | – |
HR and 95% confidence interval values are from a secondary analysis using a Cox proportional hazards model with treatment, country, and concurrent aspirin use effects.
CRNM, clinically relevant non-major; rate per year, rate per 100 patient years of follow-up; n, number of patients randomized; HR, hazard ratio.
| Outcome . | Betrixaban 40 mg (n = 127), events . | Betrixaban 60 mg (n = 127), events . | Betrixaban 80 mg (n = 127), events . | Warfarin (n = 127), events . | Betrixaban 40 mg vs. warfarin [HR (95% confidence interval)] . | Betrixaban 60 mg vs. warfarin [HR (95% confidence interval)] . | Betrixaban 80 mg vs. warfarin [HR (95% confidence interval)] . |
|---|---|---|---|---|---|---|---|
| Major or CRNM bleeding | 1 | 5 | 5 | 7 | 0.140 (0.017–1.135) | 0.711 (0.225–2.243) | 0.755 (0.239–2.389) |
| Major bleeding | 0 | 0 | 3 | 5 | NA (NA) | NA (NA) | 0.609 (0.145–2.557) |
| CRNM bleeding | 1 | 5 | 2 | 4 | 0.264 (0.030–2.364) | 1.257 (0.337–4.684) | 0.538 (0.098–2.937) |
| Minimal bleeding | 22 | 28 | 23 | 36 | 0.572 (0.336–0.974) | 0.752 (0.458–1.235) | 0.584 (0.346–0.986) |
| Any bleeding | 22 | 32 | 24 | 40 | 0.508 (0.301–0.856) | 0.767 (0.481–1.224) | 0.551 (0.332–0.914) |
| Stroke | 0 | 1 (ischaemic ) | 1 (ischaemic ) | 0 | – | – | – |
| Death | 1 (vascular) | 0 | 0 | 1 (vascular) | – | – | – |
| Outcome . | Betrixaban 40 mg (n = 127), events . | Betrixaban 60 mg (n = 127), events . | Betrixaban 80 mg (n = 127), events . | Warfarin (n = 127), events . | Betrixaban 40 mg vs. warfarin [HR (95% confidence interval)] . | Betrixaban 60 mg vs. warfarin [HR (95% confidence interval)] . | Betrixaban 80 mg vs. warfarin [HR (95% confidence interval)] . |
|---|---|---|---|---|---|---|---|
| Major or CRNM bleeding | 1 | 5 | 5 | 7 | 0.140 (0.017–1.135) | 0.711 (0.225–2.243) | 0.755 (0.239–2.389) |
| Major bleeding | 0 | 0 | 3 | 5 | NA (NA) | NA (NA) | 0.609 (0.145–2.557) |
| CRNM bleeding | 1 | 5 | 2 | 4 | 0.264 (0.030–2.364) | 1.257 (0.337–4.684) | 0.538 (0.098–2.937) |
| Minimal bleeding | 22 | 28 | 23 | 36 | 0.572 (0.336–0.974) | 0.752 (0.458–1.235) | 0.584 (0.346–0.986) |
| Any bleeding | 22 | 32 | 24 | 40 | 0.508 (0.301–0.856) | 0.767 (0.481–1.224) | 0.551 (0.332–0.914) |
| Stroke | 0 | 1 (ischaemic ) | 1 (ischaemic ) | 0 | – | – | – |
| Death | 1 (vascular) | 0 | 0 | 1 (vascular) | – | – | – |
HR and 95% confidence interval values are from a secondary analysis using a Cox proportional hazards model with treatment, country, and concurrent aspirin use effects.
CRNM, clinically relevant non-major; rate per year, rate per 100 patient years of follow-up; n, number of patients randomized; HR, hazard ratio.
The rates of the primary outcome were lowest with betrixaban 40 mg [hazard ratio (HR) compared with warfarin = 0.14, exact stratified log-rank P-value unadjusted for multiple testing = 0.04; 95% confidence interval 0.017–1.135]. Betrixaban 60 mg per day and betrixaban 80 mg per day had rates of the primary outcome, which were more similar to warfarin. The outcome of any bleeding event occurred in 22, 32, 24, and 40 patients on betrixaban 40, 60, 80 mg, and warfarin, respectively. With betrixaban 40 mg and betrixaban 80 mg per day, the rates of any bleeding were reduced compared with warfarin [exact stratified log-rank P-values of 0.01 and 0.02 (unadjusted for multiple testing), respectively].
Stroke and death
There was one stroke on betrixaban 60 mg and one on betrixaban 80 mg (both ischaemic) and no strokes on betrixaban 40 mg or warfarin (Table 2). There were two deaths, both vascular, one on betrixaban 40 mg and one on warfarin. There were no occurrences of myocardial infarction, systemic embolic events, or pulmonary embolism during the study.
Adverse events and liver function tests
There were similar rates of serious adverse events in the four treatment arms: 9.4, 9.4, 8.7, 9.4% on betrixaban 40, 60, 80 mg, and warfarin, respectively. There were significantly higher rates of diarrhoea in the pooled betrixaban group vs. warfarin, 6.0 vs. 0.8%, respectively. Rates of premature study discontinuation (for any cause) were higher on betrixaban compared with warfarin, 8.9 vs. 6.3%. Liver function tests were measured in all patients at each visit. The per cent of patients with an elevated alanine aminotransferase greater than three times the upper limit of normal on betrixaban (all doses) and on warfarin were 1.8 vs. 0.8%, respectively. There was no dose-related increase in these numbers with 3 (2.4%), 3 (2.4%), and 1 (0.8%) cases in the 40, 60, and 80 mg betrixaban group, respectively.
Pharmacodynamic data
Figure 2 shows the levels of TG associated with increasing concentrations of betrixaban based on blood sample collection at ∼13 h (median) after dosing (range 2–18 h) for up to 11 occasions throughout the study. The inset shows the TG associated with increasing intensity of anticoagulation with warfarin, as measured by the INR. Levels of TG similar to those seen with therapeutic warfarin (INR 2.0–3.0) are achieved with betrixaban at plasma concentrations between 12 and 30 ng/mL. The geometric mean betrixaban plasma concentrations, at steady state (week 4), were 6, 9.6, and 12 ng/mL for betrixaban 40, 60, and 80 mg, respectively.
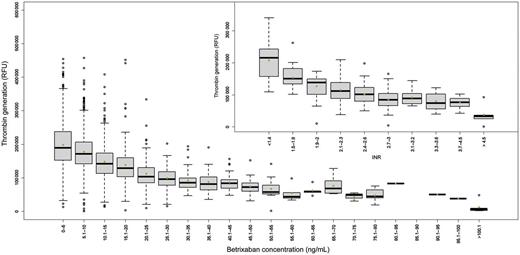
Box and whiskers plot of dose responsive effect of betrixaban on thrombin generation in patient plasma at around a median 13 h (2–18 h) post-dose. Thrombin activity is measured following cleavage of a specific fluorogenic substrate for thrombin. The figure depicts the fluorescence signal vs. the range of betrixaban plasma concentrations. The boxes represent first and third quartile of the population and the whiskers extend to 1.5 inter-quartile range. The inset represents the effect of anticoagulation by warfarin (inter-quartile range) on thrombin generation.
Figure 3 shows the plasma D-dimer concentrations in study patients after 12 weeks of treatment. Because warfarin therapy reduces D-dimer concentration, patients who were warfarin-naïve at baseline are displayed separately from the overall study population. In the warfarin-naïve population, there is a reduction in D-dimer levels on treatment relative to their respective baseline level with betrixaban (all doses combined) (P ≤ 0.001) and with warfarin (P = 0.08). The reductions in the three betrixaban groups are not significantly different from that seen with warfarin (P-values of 0.91, 0.49, and 0.19 for the three comparisons).
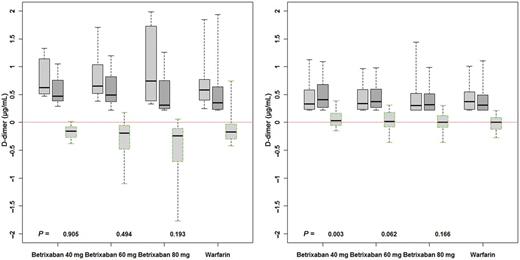
Changes in D-dimer levels in the overall patient population (right panel) and warfarin-naïve patients (left panel) after 12 weeks of treatment. Box and whiskers plots represent levels at baseline, 12 weeks, and changes relative to baseline. Each betrixaban group is compared with the warfarin group; P-values were determined by non-parametric test (Kruskal–Wallis).
Discussion
Betrixaban is a novel once-daily factor Xa inhibitor which has been shown to competitively bind to factor Xa and to inhibit its activity. A dose ranging study in 215 patients at risk for VTE events after orthopedic surgery reported that betrixaban demonstrated clinical antithrombotic activity comparable with that of enoxaparin 30 mg s.c. given every 12 h.12 Patients with AF have an increased risk of stroke, and vitamin K antagonist therapy is recommended in those at moderate or high risk of stroke.
The EXPLORE-Xa study was designed to evaluate the safety and tolerability of betrixaban compared with warfarin in patients with AF who are receiving or are eligible for vitamin K antagonist therapy. Patients enrolled were typical of AF patients who would typically receive warfarin for the prevention of stroke. Unlike other similar studies with novel factor Xa inhibitors, patients with renal insufficiency were allowed to be enrolled unless they were on dialysis. The main findings of the study are the rates of the primary outcome (major or CRNM bleeding) were numerically lower with all three doses of betrixaban compared with warfarin; and betrixaban 40 mg daily has a statistically significant lower rate than with warfarin. These findings suggest that there may be a dose–response relationship for bleeding with betrixaban. Thus, all three doses of betrixaban studied, 40, 60, and 80 mg once daily, appear to have an acceptable safety profile in comparison with warfarin.
The effectiveness of warfarin and its safety have been shown to be associated with the control of the INR.14 This is often measured by the TTR, which can be calculated by linear interpolation of the INR values over time. The average TTR reported in a recent meta-analysis of surveys of clinical practice in the USA is 55%, with somewhat high levels achieved using anticoagulation clinics.15 In the EXPLORE Xa study, most patients were experienced with warfarin and would therefore be expected to have a somewhat higher TTR than naïve patients. The INR control achieved in this study was good, and was similar to that achieved in recent randomized trials of warfarin therapy in AF patients.8,10,11,16 Therefore, one would have expected a rate of major bleeding on warfarin similar to rates recently reported in these trials. However, the rate of major bleeding on warfarin in this study was 10.3% per year which is unexpectedly higher.8,10,11,16
Rates of ischaemic stroke would be expected to be low on warfarin in patients with a mean CHADS2 score of 2 and with good INR control. There were two ischaemic strokes observed in the study and no systemic embolic events, myocardial infarctions, or pulmonary emboli. One stroke occurred in each of the betrixaban 60 and 80 mg arms; no strokes occurred on betrixaban 40 mg or on warfarin. Given the mean duration of follow-up (0.4 years), risk profile of the patients, and the INR control achieved, one would expect about 0–1 strokes in such well-managed warfarin patients, consistent with the overall stroke rate observed in this study.
The evaluation of the TG data indicates that betrixaban has an anticoagulant effect at the concentrations achieved by the doses used in this study and that this effect is comparable with that of warfarin. This supports the clinical findings and suggests that at the same level of TG as therapeutic warfarin, betrixaban may have a lower risk of bleeding than warfarin. However, this will need to be evaluated in an adequately powered study. Warfarin suppresses D-dimer concentrations.17 Patients enrolled in this study who were treated with a vitamin K antagonist at baseline had lower D-dimer levels than naïve patients. In vitamin K antagonist-naïve patients, the three doses of betrixaban and warfarin reduced D-dimer levels, thus demonstrating anticoagulant activity of the betrixaban doses tested. Of particular importance, the reduction in D-dimer for betrixaban patients on 60 and 80 mg resulted in levels within the 0.5 µg/mL concentration, which has been shown to correlate with a reduction in thrombo-embolic, cardiovascular, and bleeding events.18
Betrixaban was well tolerated in this relatively short-term study. Patients in this study had mostly used warfarin before enrolment, which would be expected to result in a low rate of intolerance to warfarin. Nonetheless, betrixaban was as well tolerated as warfarin with similar rates of serious adverse effects. Betrixaban was associated with reports of gastro-intestinal symptoms in some patients, especially diarrhoea. These symptoms were mild and did not result in discontinuation of therapy.
The main limitation of this study is its small size. Hence, it was statistically underpowered to provide definitive conclusions regarding the safety or efficacy of any particular betrixaban dose vs. warfarin. For example, no statistical adjustment was made for multiple comparisons (each dose vs. warfarin) in the primary endpoint. Nevertheless, this study provides some guidance regarding dose of betrixaban for evaluation in larger clinical trials. The lowest rate of bleeding was seen with betrixaban 40 mg. The pharmacodynamic and kinetic data indicated that all betrixaban doses tested reduce TG and that the 80 mg daily had a similar reduction in TG as would be expected with therapeutic warfarin (INR 2.0–3.0). All three betrixaban doses reduced D-dimer to levels expected to be associated with clinical benefit using warfarin therapy.
In conclusion, betrixaban at doses of 40–80 mg per day was well tolerated in AF patients at risk for stroke with a risk of bleeding that was similar to, or lower than, that of well-managed warfarin. A larger and definitive phase 3 trial of betrixaban is indicated.
Funding
The trial was funded by Portola Pharmaceuticals, South San Francisco, CA, USA.
Conflict of interest: S.J.C.: grant—Boehringer Ingelheim, Bristol Myers Squibb, Bayer; consulting fee or honorarium—Boehringer Ingelheim, Bristol Myers Squibb, Bayer, Portola. J.E.: consulting fee or honorarium—Portola, Bayer, Boehringer Ingelheim, sanofi-aventis, Daiichi Sanyko; consultancy—Bayer, Boehringer Ingelheim; sanofi-aventis, Bristol Myers Squibb; GlaxoSmithKline, McNeil, Daiichi Sankyo; grants/grants pending—Bayer, Boehringer Ingelheim; payment for lectures including service on speakers bureaus—Bayer, Boehringer Ingelheim, Portola; sanofi-aventis, Bristol Myers Squibb; GlaxoSmithKline, McNeil, Daiichi Sankyo. U.S.: employment—Portola Pharmaceuticals; patents (planned, pending or issued)—Portola Pharmaceuticals, Inc.; stock/stock options—Portola Pharmaceuticals, Inc. M.D.E.: consultant and speaker: Boehringer Ingelheim; consultant—ARYx Therapeutics, Pfizer, sanofi-aventis, Bristol Myers Squibb, PORTOLA, Bayer, Diachi Sanko, Medtronics, Eisai, Merck, J&J, Gilead, Janssen Scientific Affairs, Pozen, Inc., Coherex. D.D.G. is an employee of Portola Pharmaceuticals.



