-
PDF
- Split View
-
Views
-
Cite
Cite
Jeffrey S. Borer, Michael Böhm, Ian Ford, Michel Komajda, Luigi Tavazzi, Jose Lopez Sendon, Marco Alings, Esteban Lopez-de-Sa, Karl Swedberg, on behalf of the SHIFT Investigators, Effect of ivabradine on recurrent hospitalization for worsening heart failure in patients with chronic systolic heart failure: the SHIFT Study, European Heart Journal, Volume 33, Issue 22, November 2012, Pages 2813–2820, https://doi.org/10.1093/eurheartj/ehs259
Close - Share Icon Share
Abstract
We explored the effect of treatment with ivabradine, a pure heart rate-slowing agent, on recurrent hospitalizations for worsening heart failure (HF) in the SHIFT trial.
SHIFT was a double-blind clinical trial in which 6505 patients with moderate-to-severe HF and left ventricular systolic dysfunction, all of whom had been hospitalized for HF during the preceding year, were randomized to ivabradine or to placebo on a background of guideline-recommended HF therapy (including maximized β-blockade). In total, 1186 patients experienced at least one additional HF hospitalization during the study, 472 suffered at least two, and 218 suffered at least 3. Patients with additional HF hospitalizations had more severe disease than those without. Ivabradine was associated with fewer total HF hospitalizations [902 vs. 1211 events with placebo; incidence rate ratio, 0.75, 95% confidence interval (CI), 0.65–0.87, P = 0.0002] during the 22.9-month median follow-up. Ivabradine-treated patients evidenced lower risk for a second or third additional HF hospitalization [hazard ratio (HR): 0.66, 95% CI, 0.55–0.79, P < 0.001 and HR: 0.71, 95% CI, 0.54–0.93, P = 0.012, respectively]. Similar observations were made for all-cause and cardiovascular hospitalizations.
Treatment with ivabradine, on a background of guidelines-based HF therapy, is associated with a substantial reduction in the likelihood of recurrent hospitalizations for worsening HF. This benefit can be expected to improve the quality of life and to substantially reduce health-care costs.
See page 2764 for the editorial comment on this article (doi:10.1093/eurheartj/ehs277)
Introduction
Despite current intensive multidrug therapy, persons with heart failure (HF) are frequently admitted to hospital because of exacerbation of their symptoms and, once admitted, are often readmitted.1 The reported 3-month to 1-year readmission rate has varied between 30 and 50%.2–4 Indeed, worsening HF is the most common cause of hospitalization in patients with HF and, when recurrent, presages death.5–7 Heart failure accounts for between 1 and 2% of the total health-care expenditure and the total economic burden of HF is increasing;8 the greatest portion is attributable to HF-related hospitalizations, accounting for two-thirds of the costs.9 Thus, maximal benefit to society from HF therapy also requires the maintenance of benefits after initial hospitalization.10 However, most evaluations of HF therapy involve time-to-first event analyses and do not consider the impact of therapy after the initial event.
In SHIFT (Systolic Heart failure treatment with the If inhibitor ivabradine Trial),11 heart rate reduction with ivabradine was associated with an 18% reduction in the primary composite endpoint of time-to-first event of cardiovascular death or hospitalization for worsening HF (P < 0.0001 vs. placebo). First hospitalization for worsening HF was reduced by 26% (P < 0.0001), as was HF death, also by 26% (P = 0.014).11 The effect of continued treatment on subsequent HF hospitalizations was not analysed. In this post hoc analysis, we have explored the effect of continued treatment with ivabradine on recurrent hospitalizations for worsening HF.
Methods
Study design
As previously reported,11,12 SHIFT was a randomized, double-blind, placebo-controlled, parallel-group clinical trial in patients in sinus rhythm with moderate-to-severe HF and left ventricular systolic dysfunction. In total, 6505 patients in 37 countries (677 medical centres) were randomly allocated to either placebo or ivabradine (beginning with 5 mg b.i.d., which could be titrated to 7.5 or 2.5 mg b.i.d., or stopped, depending on heart rate and tolerability). Briefly, study subjects were men or women aged ≥18 years with a stable symptomatic chronic HF of ≥4-week duration with a left ventricular ejection fraction of ≤35%, who had been hospitalized for worsening HF within the previous 12 months, and who were in sinus rhythm with a resting heart rate of ≥70 b.p.m. (by 12-lead electrocardiogram on two consecutive visits). At randomization and throughout the study, participants were expected to receive evidence-based medication for HF at recommended doses if tolerated according to guidelines in force when the study was set up.13 When a participant was not specifically prescribed a β-blocker or was not on the guideline-recommended target dose, the investigator was required to provide a specific reason in a dedicated case report form.
The primary study endpoint was the composite of cardiovascular death or hospitalization for worsening HF. Secondary endpoints included the individual components of the primary endpoint, HF deaths, all-cause hospitalizations, and combinations of these with and without hospitalization for non-fatal myocardial infarction. After an initial non-fatal endpoint such as hospitalization, study medication and follow-up were continued until conclusion of study. Thus, additional hospitalizations, or supervening deaths, were recorded. All hospitalizations and deaths were adjudicated by an endpoint validation committee according to predefined criteria.11
We analysed hospitalizations in randomized patients who had experienced at least one HF hospitalization during the study; thus, these patients had been hospitalized for worsening HF at least twice given the inclusion criterion of at least one hospitalization for worsening HF in the 12 months prior to study entry. We also assessed the relevant data in patients who suffered at least a second HF hospitalization during the study and in those who suffered at least a third HF hospitalization during the study.
Statistical methods
Baseline characteristics are presented as numbers and percentages for categorical variables and means (±SD) for continuous variables. Baseline characteristics were compared according to the number of hospitalizations for worsening HF during the study (none, one, two, or three or more) in pooled treatment groups, using a Kruskal–Wallis test for continuous variables and a χ2 test for categorical variables. A similar comparison between the ivabradine and placebo groups was carried out in the subgroup of patients with at least one hospitalization for worsening HF during the study.
Because this study is a post hoc analysis of SHIFT data, the statistical methods employed also were selected post hoc, though they are standard for analyses of this type. The incidence rate ratio (IRR) for hospitalization events in the ivabradine group vs. the placebo group was estimated from a Poisson regression14 (with correction of over-dispersion), censoring follow-up at death or the end of study, whichever came first, and adjusted for pre-specified baseline prognostic factors [β-blocker intake (which was, in fact, a stratification factor for randomization, as well), New York Heart Association (NYHA) functional class, ischaemic cause of HF, age, systolic blood pressure, heart rate, left ventricular ejection fraction (LVEF), and creatinine clearance]. The cumulative incidence rate of hospitalizations for HF, plotted by treatment group, was calculated using the Nelson–Aalen's estimator, which corrects for the competing risk of death.15
In view of the recent approval of ivabradine for HF by the European Medicines Agency specifically for patients with a heart rate of ≥75 b.p.m. (even though the SHIFT inclusion criterion was ≥70 b.p.m.), we also performed the same analyses in the subpopulation that entered SHIFT with a heart rate of ≥75 b.p.m. (4150 patients in the whole study).16 In addition, we explored the effect of ivabradine on the total number of repeated all-cause and cardiovascular hospitalizations, including hospitalization for causes other than worsening HF.
The effect of ivabradine on repeated hospitalizations for worsening HF was explored using two time-to-event approaches, as follows: Patient follow-ups were censored at the time of death or at the end of the study. Both approaches were adjusted for prognostic factors.
A total-time approach was used for all randomized patients, considering times from randomization to the onset of first, second, third, and each subsequent hospitalization using a Wei, Lin, and Weissfeld model, employing robust sandwich estimators for standard errors.17 This model preserves randomization when comparing treatment groups and enables analysis of the cumulative effect of ivabradine vs. placebo on hospitalizations from randomization (i.e. the effect on second hospitalization includes the effect on the first, and the effect on third hospitalization includes the effects on the first and second). The corresponding cumulative hazard ratios (HRs), 95% confidence intervals (CIs), and P-values comparing treatment groups on the first, second, and third hospitalizations are presented.
A gap-time approach was used for patients with at least one hospitalization during the study. This approach considers the time from the onset of the first post-randomization hospitalization until the onset of the second using a Cox proportional hazards model and enables a non-randomized comparison of the time to the second event between the treatment groups. The corresponding HR, 95% CI, and P-value are provided.
Total days alive out of hospital (considering hospitalization from any cause) was calculated as the potential follow-up time (randomization date to trial closure, 31 March 2010)11 minus the number of days in hospital minus the number of days dead, as described elsewhere.18 This parameter was compared between treatment groups by analysis of covariance, adjusted for potential follow-up, to produce an estimate of the mean difference between treatment groups, as well as associated 95% CI and P-values.
All tests were two-sided with a P-value of <0.05 considered significant. The sponsor was responsible for data management and data analyses. Methodology and results were reviewed by the statistician co-author (I.F.). SAS (version 9.1) and R 2.14.0 were used for analyses.
Results
Patients with one (n = 714), two (n = 254), and three or more (n = 218) hospitalizations for worsening HF during the study had more risk markers at the baseline (e.g. greater age, diabetes, renal dysfunction, and prior stroke) than those with no hospitalization for worsening HF (n = 5319; Table 1). They were also more likely to have severe disease (e.g. 62–66% of hospitalized patients were in NYHA class III or IV vs. 49% for patients with no HF hospitalization during the study). Patients with one or more post-randomization HF hospitalization had higher resting heart rate, lower systolic and diastolic blood pressures, lower left ventricular ejection fraction, higher use of mineralocorticoid receptor antagonists and diuretics, and fewer prescriptions of β-blockers at randomization than those without a HF hospitalization after randomization. The baseline characteristics for SHIFT patients with at least one HF hospitalization during the study were generally similar in the placebo and ivabradine groups (Table 2).
Baseline characteristics of patients according to the number of hospitalizations for worsening heart failure during the trial
| . | Number of hospitalizations for worsening HF during trial . | P-valuea . | |||
|---|---|---|---|---|---|
| None (n = 5319) . | One (n = 714) . | Two (n = 254) . | Three or more (n = 218) . | ||
| Demographic characteristics | |||||
| Age (years) | 60.0 ± 11.3 | 62.3 ± 11.1 | 61.8 ± 12.5 | 62.4 ± 11.7 | <0.0001 |
| Male | 4069 (77%) | 529 (74%) | 195 (77%) | 177 (81%) | 0.18 |
| Current smoker | 927 (17%) | 116 (16%) | 43 (17%) | 32 (15%) | 0.17 |
| BMI (kg/m²) | 28.0 ± 5.0 | 27.8 ± 5.4 | 27.9 ± 5.1 | 27.8 ± 5.3 | 0.29 |
| Cardiac parameters | |||||
| Heart rate (b.p.m.) | 79.3 ± 9.2 | 82.2 ± 11.3 | 83.4 ± 11.7 | 82.2 ± 10.1 | <0.0001 |
| SBP (mmHg) | 122.3 ± 15.7 | 119.8 ± 16.4 | 118.1 ± 16.9 | 117.6 ± 17.4 | <0.0001 |
| DBP (mmHg) | 76.0 ± 9.4 | 75.0 ± 10.0 | 73.4 ± 9.7 | 73.3 ± 9.4 | <0.0001 |
| LVEF (%) | 29.3 ± 5.0 | 27.6 ± 5.3 | 27.8 ± 5.3 | 27.1 ± 5.9 | <0.0001 |
| NYHA class II | 2724 (51%) | 274 (38%) | 96 (38%) | 75 (34%) | <0.0001 |
| NYHA class III | 2516 (47%) | 422 (59%) | 150 (59%) | 135 (62%) | |
| NYHA class IV | 77 (2%) | 18 (3%) | 8 (3%) | 8 (4%) | |
| eGFR (mL/min/1.73 m²) | 75.8 ± 22.7 | 70.4 ± 22.5 | 69.4 ± 22.7 | 68.0 ± 27.8 | <0.0001 |
| Medical history | |||||
| Duration of HF (years) | 3.3 ± 4.1 | 4.2 ± 4.5 | 4.3 ± 4.7 | 4.6 ± 4.7 | <0.0001 |
| Ischaemic cause of HF | 3605 (68%) | 503 (70%) | 171 (67%) | 139 (64%) | 0.27 |
| Myocardial infarction | 2986 (56%) | 423 (59%) | 142 (56%) | 115 (53%) | 0.30 |
| Hypertension | 3545 (67%) | 478 (67%) | 158 (62%) | 133 (61%) | 0.17 |
| Diabetes | 1552 (29%) | 251 (35%) | 88 (35%) | 88 (40%) | <0.0001 |
| Stroke | 398 (7%) | 66 (9%) | 30 (12%) | 29 (13%) | 0.0008 |
| History of atrial fibrillation and/or flutter | 389 (7%) | 83 (12%) | 24 (9%) | 26 (12%) | <0.0001 |
| CAD | 3863 (73%) | 536 (75%) | 182 (72%) | 151 (69%) | 0.33 |
| Treatment at randomization | |||||
| β-Blockers | 4797 (90%) | 633 (89%) | 203 (80%) | 187 (86%) | <0.0001 |
| ACE-inhibitor | 4216 (79%) | 535 (75%) | 193 (76%) | 172 (79%) | 0.043 |
| ARB | 741 (14%) | 111 (16%) | 41 (16%) | 34 (16%) | 0.48 |
| ACE-inhibitor and/or ARB | 4858 (91%) | 635 (89%) | 228 (90%) | 202 (93%) | 0.13 |
| Mineralocorticoid receptor antagonists | 3098 (58%) | 494 (69%) | 170 (67%) | 160 (73%) | <0.0001 |
| Diuretics | 4335 (82%) | 643 (90%) | 229 (90%) | 207 (95%) | <0.0001 |
| Digitalis | 1039 (20%) | 215 (30%) | 85 (33%) | 77 (35%) | <0.0001 |
| . | Number of hospitalizations for worsening HF during trial . | P-valuea . | |||
|---|---|---|---|---|---|
| None (n = 5319) . | One (n = 714) . | Two (n = 254) . | Three or more (n = 218) . | ||
| Demographic characteristics | |||||
| Age (years) | 60.0 ± 11.3 | 62.3 ± 11.1 | 61.8 ± 12.5 | 62.4 ± 11.7 | <0.0001 |
| Male | 4069 (77%) | 529 (74%) | 195 (77%) | 177 (81%) | 0.18 |
| Current smoker | 927 (17%) | 116 (16%) | 43 (17%) | 32 (15%) | 0.17 |
| BMI (kg/m²) | 28.0 ± 5.0 | 27.8 ± 5.4 | 27.9 ± 5.1 | 27.8 ± 5.3 | 0.29 |
| Cardiac parameters | |||||
| Heart rate (b.p.m.) | 79.3 ± 9.2 | 82.2 ± 11.3 | 83.4 ± 11.7 | 82.2 ± 10.1 | <0.0001 |
| SBP (mmHg) | 122.3 ± 15.7 | 119.8 ± 16.4 | 118.1 ± 16.9 | 117.6 ± 17.4 | <0.0001 |
| DBP (mmHg) | 76.0 ± 9.4 | 75.0 ± 10.0 | 73.4 ± 9.7 | 73.3 ± 9.4 | <0.0001 |
| LVEF (%) | 29.3 ± 5.0 | 27.6 ± 5.3 | 27.8 ± 5.3 | 27.1 ± 5.9 | <0.0001 |
| NYHA class II | 2724 (51%) | 274 (38%) | 96 (38%) | 75 (34%) | <0.0001 |
| NYHA class III | 2516 (47%) | 422 (59%) | 150 (59%) | 135 (62%) | |
| NYHA class IV | 77 (2%) | 18 (3%) | 8 (3%) | 8 (4%) | |
| eGFR (mL/min/1.73 m²) | 75.8 ± 22.7 | 70.4 ± 22.5 | 69.4 ± 22.7 | 68.0 ± 27.8 | <0.0001 |
| Medical history | |||||
| Duration of HF (years) | 3.3 ± 4.1 | 4.2 ± 4.5 | 4.3 ± 4.7 | 4.6 ± 4.7 | <0.0001 |
| Ischaemic cause of HF | 3605 (68%) | 503 (70%) | 171 (67%) | 139 (64%) | 0.27 |
| Myocardial infarction | 2986 (56%) | 423 (59%) | 142 (56%) | 115 (53%) | 0.30 |
| Hypertension | 3545 (67%) | 478 (67%) | 158 (62%) | 133 (61%) | 0.17 |
| Diabetes | 1552 (29%) | 251 (35%) | 88 (35%) | 88 (40%) | <0.0001 |
| Stroke | 398 (7%) | 66 (9%) | 30 (12%) | 29 (13%) | 0.0008 |
| History of atrial fibrillation and/or flutter | 389 (7%) | 83 (12%) | 24 (9%) | 26 (12%) | <0.0001 |
| CAD | 3863 (73%) | 536 (75%) | 182 (72%) | 151 (69%) | 0.33 |
| Treatment at randomization | |||||
| β-Blockers | 4797 (90%) | 633 (89%) | 203 (80%) | 187 (86%) | <0.0001 |
| ACE-inhibitor | 4216 (79%) | 535 (75%) | 193 (76%) | 172 (79%) | 0.043 |
| ARB | 741 (14%) | 111 (16%) | 41 (16%) | 34 (16%) | 0.48 |
| ACE-inhibitor and/or ARB | 4858 (91%) | 635 (89%) | 228 (90%) | 202 (93%) | 0.13 |
| Mineralocorticoid receptor antagonists | 3098 (58%) | 494 (69%) | 170 (67%) | 160 (73%) | <0.0001 |
| Diuretics | 4335 (82%) | 643 (90%) | 229 (90%) | 207 (95%) | <0.0001 |
| Digitalis | 1039 (20%) | 215 (30%) | 85 (33%) | 77 (35%) | <0.0001 |
Values are n (%) or means ± SD. ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate (Modification in Diet in Renal Disease Formula); NYHA, New York Heart Association.
aThe Kruskal–Wallis test for continuous variables or the χ2 test for categorical variables.
Baseline characteristics of patients according to the number of hospitalizations for worsening heart failure during the trial
| . | Number of hospitalizations for worsening HF during trial . | P-valuea . | |||
|---|---|---|---|---|---|
| None (n = 5319) . | One (n = 714) . | Two (n = 254) . | Three or more (n = 218) . | ||
| Demographic characteristics | |||||
| Age (years) | 60.0 ± 11.3 | 62.3 ± 11.1 | 61.8 ± 12.5 | 62.4 ± 11.7 | <0.0001 |
| Male | 4069 (77%) | 529 (74%) | 195 (77%) | 177 (81%) | 0.18 |
| Current smoker | 927 (17%) | 116 (16%) | 43 (17%) | 32 (15%) | 0.17 |
| BMI (kg/m²) | 28.0 ± 5.0 | 27.8 ± 5.4 | 27.9 ± 5.1 | 27.8 ± 5.3 | 0.29 |
| Cardiac parameters | |||||
| Heart rate (b.p.m.) | 79.3 ± 9.2 | 82.2 ± 11.3 | 83.4 ± 11.7 | 82.2 ± 10.1 | <0.0001 |
| SBP (mmHg) | 122.3 ± 15.7 | 119.8 ± 16.4 | 118.1 ± 16.9 | 117.6 ± 17.4 | <0.0001 |
| DBP (mmHg) | 76.0 ± 9.4 | 75.0 ± 10.0 | 73.4 ± 9.7 | 73.3 ± 9.4 | <0.0001 |
| LVEF (%) | 29.3 ± 5.0 | 27.6 ± 5.3 | 27.8 ± 5.3 | 27.1 ± 5.9 | <0.0001 |
| NYHA class II | 2724 (51%) | 274 (38%) | 96 (38%) | 75 (34%) | <0.0001 |
| NYHA class III | 2516 (47%) | 422 (59%) | 150 (59%) | 135 (62%) | |
| NYHA class IV | 77 (2%) | 18 (3%) | 8 (3%) | 8 (4%) | |
| eGFR (mL/min/1.73 m²) | 75.8 ± 22.7 | 70.4 ± 22.5 | 69.4 ± 22.7 | 68.0 ± 27.8 | <0.0001 |
| Medical history | |||||
| Duration of HF (years) | 3.3 ± 4.1 | 4.2 ± 4.5 | 4.3 ± 4.7 | 4.6 ± 4.7 | <0.0001 |
| Ischaemic cause of HF | 3605 (68%) | 503 (70%) | 171 (67%) | 139 (64%) | 0.27 |
| Myocardial infarction | 2986 (56%) | 423 (59%) | 142 (56%) | 115 (53%) | 0.30 |
| Hypertension | 3545 (67%) | 478 (67%) | 158 (62%) | 133 (61%) | 0.17 |
| Diabetes | 1552 (29%) | 251 (35%) | 88 (35%) | 88 (40%) | <0.0001 |
| Stroke | 398 (7%) | 66 (9%) | 30 (12%) | 29 (13%) | 0.0008 |
| History of atrial fibrillation and/or flutter | 389 (7%) | 83 (12%) | 24 (9%) | 26 (12%) | <0.0001 |
| CAD | 3863 (73%) | 536 (75%) | 182 (72%) | 151 (69%) | 0.33 |
| Treatment at randomization | |||||
| β-Blockers | 4797 (90%) | 633 (89%) | 203 (80%) | 187 (86%) | <0.0001 |
| ACE-inhibitor | 4216 (79%) | 535 (75%) | 193 (76%) | 172 (79%) | 0.043 |
| ARB | 741 (14%) | 111 (16%) | 41 (16%) | 34 (16%) | 0.48 |
| ACE-inhibitor and/or ARB | 4858 (91%) | 635 (89%) | 228 (90%) | 202 (93%) | 0.13 |
| Mineralocorticoid receptor antagonists | 3098 (58%) | 494 (69%) | 170 (67%) | 160 (73%) | <0.0001 |
| Diuretics | 4335 (82%) | 643 (90%) | 229 (90%) | 207 (95%) | <0.0001 |
| Digitalis | 1039 (20%) | 215 (30%) | 85 (33%) | 77 (35%) | <0.0001 |
| . | Number of hospitalizations for worsening HF during trial . | P-valuea . | |||
|---|---|---|---|---|---|
| None (n = 5319) . | One (n = 714) . | Two (n = 254) . | Three or more (n = 218) . | ||
| Demographic characteristics | |||||
| Age (years) | 60.0 ± 11.3 | 62.3 ± 11.1 | 61.8 ± 12.5 | 62.4 ± 11.7 | <0.0001 |
| Male | 4069 (77%) | 529 (74%) | 195 (77%) | 177 (81%) | 0.18 |
| Current smoker | 927 (17%) | 116 (16%) | 43 (17%) | 32 (15%) | 0.17 |
| BMI (kg/m²) | 28.0 ± 5.0 | 27.8 ± 5.4 | 27.9 ± 5.1 | 27.8 ± 5.3 | 0.29 |
| Cardiac parameters | |||||
| Heart rate (b.p.m.) | 79.3 ± 9.2 | 82.2 ± 11.3 | 83.4 ± 11.7 | 82.2 ± 10.1 | <0.0001 |
| SBP (mmHg) | 122.3 ± 15.7 | 119.8 ± 16.4 | 118.1 ± 16.9 | 117.6 ± 17.4 | <0.0001 |
| DBP (mmHg) | 76.0 ± 9.4 | 75.0 ± 10.0 | 73.4 ± 9.7 | 73.3 ± 9.4 | <0.0001 |
| LVEF (%) | 29.3 ± 5.0 | 27.6 ± 5.3 | 27.8 ± 5.3 | 27.1 ± 5.9 | <0.0001 |
| NYHA class II | 2724 (51%) | 274 (38%) | 96 (38%) | 75 (34%) | <0.0001 |
| NYHA class III | 2516 (47%) | 422 (59%) | 150 (59%) | 135 (62%) | |
| NYHA class IV | 77 (2%) | 18 (3%) | 8 (3%) | 8 (4%) | |
| eGFR (mL/min/1.73 m²) | 75.8 ± 22.7 | 70.4 ± 22.5 | 69.4 ± 22.7 | 68.0 ± 27.8 | <0.0001 |
| Medical history | |||||
| Duration of HF (years) | 3.3 ± 4.1 | 4.2 ± 4.5 | 4.3 ± 4.7 | 4.6 ± 4.7 | <0.0001 |
| Ischaemic cause of HF | 3605 (68%) | 503 (70%) | 171 (67%) | 139 (64%) | 0.27 |
| Myocardial infarction | 2986 (56%) | 423 (59%) | 142 (56%) | 115 (53%) | 0.30 |
| Hypertension | 3545 (67%) | 478 (67%) | 158 (62%) | 133 (61%) | 0.17 |
| Diabetes | 1552 (29%) | 251 (35%) | 88 (35%) | 88 (40%) | <0.0001 |
| Stroke | 398 (7%) | 66 (9%) | 30 (12%) | 29 (13%) | 0.0008 |
| History of atrial fibrillation and/or flutter | 389 (7%) | 83 (12%) | 24 (9%) | 26 (12%) | <0.0001 |
| CAD | 3863 (73%) | 536 (75%) | 182 (72%) | 151 (69%) | 0.33 |
| Treatment at randomization | |||||
| β-Blockers | 4797 (90%) | 633 (89%) | 203 (80%) | 187 (86%) | <0.0001 |
| ACE-inhibitor | 4216 (79%) | 535 (75%) | 193 (76%) | 172 (79%) | 0.043 |
| ARB | 741 (14%) | 111 (16%) | 41 (16%) | 34 (16%) | 0.48 |
| ACE-inhibitor and/or ARB | 4858 (91%) | 635 (89%) | 228 (90%) | 202 (93%) | 0.13 |
| Mineralocorticoid receptor antagonists | 3098 (58%) | 494 (69%) | 170 (67%) | 160 (73%) | <0.0001 |
| Diuretics | 4335 (82%) | 643 (90%) | 229 (90%) | 207 (95%) | <0.0001 |
| Digitalis | 1039 (20%) | 215 (30%) | 85 (33%) | 77 (35%) | <0.0001 |
Values are n (%) or means ± SD. ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate (Modification in Diet in Renal Disease Formula); NYHA, New York Heart Association.
aThe Kruskal–Wallis test for continuous variables or the χ2 test for categorical variables.
Baseline characteristics of patients who were hospitalized at least once for worsening heart failure during the trial
| . | Patients with at least one hospitalization due to worsening heart failure during the study (n = 1186) . | P-value* . | |
|---|---|---|---|
| Ivabradine (n = 514) . | Placebo (n = 672) . | ||
| Demographic characteristics | |||
| Age (years) | 63.3 ± 10.8 | 61.4 ± 12.0 | 0.0071 |
| Male | 397 (77%) | 504 (75%) | 0.37 |
| Current smoker | 69 (13%) | 122 (18%) | 0.023 |
| Body mass index (kg/m²) | 28.0 ± 5.26 | 27.7 ± 5.4 | 0.38 |
| Cardiac parameters | |||
| Heart rate (b.p.m.) | 81.8 ± 11.1 | 82.9 ± 11.2 | 0.024 |
| Systolic blood pressure (mmHg) | 119.1 ± 17.3 | 118.9 ± 16.3 | 0.98 |
| Diastolic blood pressure (mmHg) | 74.0 ± 9.8 | 74.5 ± 9.9 | 0.73 |
| Left ventricular ejection fraction (%) | 27.4 ± 5.5 | 27.7 ± 5.4 | 0.46 |
| NYHA class II | 188 (37%) | 257 (38%) | 0.66 |
| NYHA class III | 313 (61%) | 394 (59%) | |
| NYHA class IV | 13 (3%) | 21 (3%) | |
| eGFR (mL/min/1.73 m²) | 68.9 ± 22.3 | 70.4 ± 24.5 | 0.40 |
| Medical history | |||
| Duration of heart failure (years) | 4.3 ± 4.5 | 4.3 ± 4.6 | 0.82 |
| Ischaemic cause of heart failure | 369 (72%) | 444 (66%) | 0.036 |
| Myocardial infarction | 311 (61%) | 369 (55%) | 0.054 |
| Hypertension | 331 (64%) | 438 (65%) | 0.78 |
| Diabetes | 181 (35%) | 246 (37%) | 0.62 |
| Stroke | 43 (8%) | 82 (12%) | 0.033 |
| History of atrial fibrillation and/or flutter | 64 (12%) | 69 (10%) | 0.24 |
| Coronary artery disease | 394 (77%) | 475 (71%) | 0.021 |
| Treatment at randomization | |||
| β-Blockers | 449 (87%) | 574 (85%) | 0.34 |
| ACE-inhibitor | 389 (76%) | 511 (76%) | 0.89 |
| ARB | 80 (16%) | 106 (16%) | 0.92 |
| ACE-inhibitor and/or ARB | 460 (89%) | 605 (90%) | 0.76 |
| Mineralocorticoid receptor antagonists | 372 (72%) | 452 (67%) | 0.058 |
| Diuretics | 478 (93%) | 601 (89%) | 0.034 |
| Digitalis | 176 (34%) | 201 (30%) | 0.11 |
| . | Patients with at least one hospitalization due to worsening heart failure during the study (n = 1186) . | P-value* . | |
|---|---|---|---|
| Ivabradine (n = 514) . | Placebo (n = 672) . | ||
| Demographic characteristics | |||
| Age (years) | 63.3 ± 10.8 | 61.4 ± 12.0 | 0.0071 |
| Male | 397 (77%) | 504 (75%) | 0.37 |
| Current smoker | 69 (13%) | 122 (18%) | 0.023 |
| Body mass index (kg/m²) | 28.0 ± 5.26 | 27.7 ± 5.4 | 0.38 |
| Cardiac parameters | |||
| Heart rate (b.p.m.) | 81.8 ± 11.1 | 82.9 ± 11.2 | 0.024 |
| Systolic blood pressure (mmHg) | 119.1 ± 17.3 | 118.9 ± 16.3 | 0.98 |
| Diastolic blood pressure (mmHg) | 74.0 ± 9.8 | 74.5 ± 9.9 | 0.73 |
| Left ventricular ejection fraction (%) | 27.4 ± 5.5 | 27.7 ± 5.4 | 0.46 |
| NYHA class II | 188 (37%) | 257 (38%) | 0.66 |
| NYHA class III | 313 (61%) | 394 (59%) | |
| NYHA class IV | 13 (3%) | 21 (3%) | |
| eGFR (mL/min/1.73 m²) | 68.9 ± 22.3 | 70.4 ± 24.5 | 0.40 |
| Medical history | |||
| Duration of heart failure (years) | 4.3 ± 4.5 | 4.3 ± 4.6 | 0.82 |
| Ischaemic cause of heart failure | 369 (72%) | 444 (66%) | 0.036 |
| Myocardial infarction | 311 (61%) | 369 (55%) | 0.054 |
| Hypertension | 331 (64%) | 438 (65%) | 0.78 |
| Diabetes | 181 (35%) | 246 (37%) | 0.62 |
| Stroke | 43 (8%) | 82 (12%) | 0.033 |
| History of atrial fibrillation and/or flutter | 64 (12%) | 69 (10%) | 0.24 |
| Coronary artery disease | 394 (77%) | 475 (71%) | 0.021 |
| Treatment at randomization | |||
| β-Blockers | 449 (87%) | 574 (85%) | 0.34 |
| ACE-inhibitor | 389 (76%) | 511 (76%) | 0.89 |
| ARB | 80 (16%) | 106 (16%) | 0.92 |
| ACE-inhibitor and/or ARB | 460 (89%) | 605 (90%) | 0.76 |
| Mineralocorticoid receptor antagonists | 372 (72%) | 452 (67%) | 0.058 |
| Diuretics | 478 (93%) | 601 (89%) | 0.034 |
| Digitalis | 176 (34%) | 201 (30%) | 0.11 |
Values are n (%) or means ± SD. *P-values comparing patients in the ivabradine and placebo groups (the Kruskal–Wallis test for continuous variables or the χ2 test for categorical variables). ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate (Modification in Diet in Renal Disease Formula); NYHA, New York Heart Association.
Baseline characteristics of patients who were hospitalized at least once for worsening heart failure during the trial
| . | Patients with at least one hospitalization due to worsening heart failure during the study (n = 1186) . | P-value* . | |
|---|---|---|---|
| Ivabradine (n = 514) . | Placebo (n = 672) . | ||
| Demographic characteristics | |||
| Age (years) | 63.3 ± 10.8 | 61.4 ± 12.0 | 0.0071 |
| Male | 397 (77%) | 504 (75%) | 0.37 |
| Current smoker | 69 (13%) | 122 (18%) | 0.023 |
| Body mass index (kg/m²) | 28.0 ± 5.26 | 27.7 ± 5.4 | 0.38 |
| Cardiac parameters | |||
| Heart rate (b.p.m.) | 81.8 ± 11.1 | 82.9 ± 11.2 | 0.024 |
| Systolic blood pressure (mmHg) | 119.1 ± 17.3 | 118.9 ± 16.3 | 0.98 |
| Diastolic blood pressure (mmHg) | 74.0 ± 9.8 | 74.5 ± 9.9 | 0.73 |
| Left ventricular ejection fraction (%) | 27.4 ± 5.5 | 27.7 ± 5.4 | 0.46 |
| NYHA class II | 188 (37%) | 257 (38%) | 0.66 |
| NYHA class III | 313 (61%) | 394 (59%) | |
| NYHA class IV | 13 (3%) | 21 (3%) | |
| eGFR (mL/min/1.73 m²) | 68.9 ± 22.3 | 70.4 ± 24.5 | 0.40 |
| Medical history | |||
| Duration of heart failure (years) | 4.3 ± 4.5 | 4.3 ± 4.6 | 0.82 |
| Ischaemic cause of heart failure | 369 (72%) | 444 (66%) | 0.036 |
| Myocardial infarction | 311 (61%) | 369 (55%) | 0.054 |
| Hypertension | 331 (64%) | 438 (65%) | 0.78 |
| Diabetes | 181 (35%) | 246 (37%) | 0.62 |
| Stroke | 43 (8%) | 82 (12%) | 0.033 |
| History of atrial fibrillation and/or flutter | 64 (12%) | 69 (10%) | 0.24 |
| Coronary artery disease | 394 (77%) | 475 (71%) | 0.021 |
| Treatment at randomization | |||
| β-Blockers | 449 (87%) | 574 (85%) | 0.34 |
| ACE-inhibitor | 389 (76%) | 511 (76%) | 0.89 |
| ARB | 80 (16%) | 106 (16%) | 0.92 |
| ACE-inhibitor and/or ARB | 460 (89%) | 605 (90%) | 0.76 |
| Mineralocorticoid receptor antagonists | 372 (72%) | 452 (67%) | 0.058 |
| Diuretics | 478 (93%) | 601 (89%) | 0.034 |
| Digitalis | 176 (34%) | 201 (30%) | 0.11 |
| . | Patients with at least one hospitalization due to worsening heart failure during the study (n = 1186) . | P-value* . | |
|---|---|---|---|
| Ivabradine (n = 514) . | Placebo (n = 672) . | ||
| Demographic characteristics | |||
| Age (years) | 63.3 ± 10.8 | 61.4 ± 12.0 | 0.0071 |
| Male | 397 (77%) | 504 (75%) | 0.37 |
| Current smoker | 69 (13%) | 122 (18%) | 0.023 |
| Body mass index (kg/m²) | 28.0 ± 5.26 | 27.7 ± 5.4 | 0.38 |
| Cardiac parameters | |||
| Heart rate (b.p.m.) | 81.8 ± 11.1 | 82.9 ± 11.2 | 0.024 |
| Systolic blood pressure (mmHg) | 119.1 ± 17.3 | 118.9 ± 16.3 | 0.98 |
| Diastolic blood pressure (mmHg) | 74.0 ± 9.8 | 74.5 ± 9.9 | 0.73 |
| Left ventricular ejection fraction (%) | 27.4 ± 5.5 | 27.7 ± 5.4 | 0.46 |
| NYHA class II | 188 (37%) | 257 (38%) | 0.66 |
| NYHA class III | 313 (61%) | 394 (59%) | |
| NYHA class IV | 13 (3%) | 21 (3%) | |
| eGFR (mL/min/1.73 m²) | 68.9 ± 22.3 | 70.4 ± 24.5 | 0.40 |
| Medical history | |||
| Duration of heart failure (years) | 4.3 ± 4.5 | 4.3 ± 4.6 | 0.82 |
| Ischaemic cause of heart failure | 369 (72%) | 444 (66%) | 0.036 |
| Myocardial infarction | 311 (61%) | 369 (55%) | 0.054 |
| Hypertension | 331 (64%) | 438 (65%) | 0.78 |
| Diabetes | 181 (35%) | 246 (37%) | 0.62 |
| Stroke | 43 (8%) | 82 (12%) | 0.033 |
| History of atrial fibrillation and/or flutter | 64 (12%) | 69 (10%) | 0.24 |
| Coronary artery disease | 394 (77%) | 475 (71%) | 0.021 |
| Treatment at randomization | |||
| β-Blockers | 449 (87%) | 574 (85%) | 0.34 |
| ACE-inhibitor | 389 (76%) | 511 (76%) | 0.89 |
| ARB | 80 (16%) | 106 (16%) | 0.92 |
| ACE-inhibitor and/or ARB | 460 (89%) | 605 (90%) | 0.76 |
| Mineralocorticoid receptor antagonists | 372 (72%) | 452 (67%) | 0.058 |
| Diuretics | 478 (93%) | 601 (89%) | 0.034 |
| Digitalis | 176 (34%) | 201 (30%) | 0.11 |
Values are n (%) or means ± SD. *P-values comparing patients in the ivabradine and placebo groups (the Kruskal–Wallis test for continuous variables or the χ2 test for categorical variables). ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate (Modification in Diet in Renal Disease Formula); NYHA, New York Heart Association.
In total, 1186 of the 6505 randomized patients experienced at least one HF hospitalization during the study. Of these 1186 patients, 472 suffered at least a second HF hospitalization and 218 experienced at least a third. Hospitalization for any cause occurred in 2587 patients after randomization, 1328 patients had at least two all-cause hospitalizations, and 718 patients had three or more.
When compared with the effect of placebo, ivabradine was associated with fewer total hospitalizations for worsening HF (902 events with ivabradine vs. 1211 events with placebo, IRR = 0.75, 95% CI, 0.65–0.87, P = 0.0002) during a median follow-up of 22.9 months (Figure 1). Similar results for HF hospitalizations were seen in the higher risk subgroup of patients with a heart rate of ≥75 b.p.m. (n = 4150) (IRR = 0.73, 95% CI, 0.61–0.87, P = 0.0006). [The remaining group, with a heart rate 70–74 b.p.m., had directionally and qualitatively similar results to the ≥75 b.p.m. group; though the difference did not reach statistical significance in the lower heart rate group, there was no significant interaction (P = 0.069) for the groups with the lower and higher heart rates for the hospitalization outcome.]
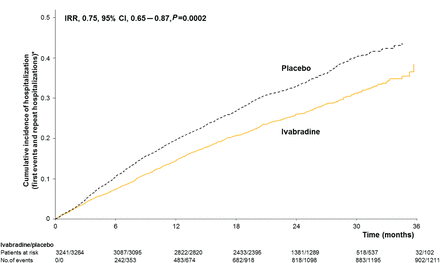
Cumulative incidence of hospitalizations for worsening heart failure (mean number of events per patient) during the study. IRR, incidence rate ratio. CI, confidence interval. *Estimate of rate of hospitalizations over time (corrected for the competing risk of death).
Hospitalizations for any cause (2661 vs. 3110 events, IRR = 0.85, 95% CI, 0.78–0.94, P = 0.001) and cardiovascular hospitalizations (1909 vs. 2272 events, IRR = 0.84, 95% CI, 0.76–0.94, P = 0.002) were also less frequent with ivabradine than with placebo. Importantly, hospitalizations for causes other than worsening HF (1759 events with ivabradine vs. 1899 events with placebo, IRR = 0.92, 95% CI, 0.83–1.02, P = 0.12) were not increased by ivabradine. Hospitalizations (HF and all cause) are expressed per patient for all patients and for patients actually hospitalized in Table 3.
Hospitalizations expressed per patient for all patients and for patients actually hospitalized during the trial
| . | Ivabradine (n = 3241) . | Placebo (n = 3264) . | P-value . |
|---|---|---|---|
| Hospitalizations for worsening heart failure (number of patients) | |||
| No hospitalization | 2727 (84%) | 2592 (79%) | |
| 1 hospitalization | 325 (10%) | 389 (12%) | |
| 2 hospitalizations | 99 (3%) | 155 (5%) | |
| ≥3 hospitalizations | 90 (3%) | 128 (4%) | |
| Hospitalizations for worsening heart failure (number of events) | |||
| Total number of hospitalization events | 902 | 1211 | 0.0002 |
| Number of events per patient | |||
| Whole population | 0.3 | 0.4 | |
| Patients with ≥1 hospitalization | 1.8 | 1.8 | |
| Hospitalizations for any cause (number of patients) | |||
| No hospitalization | 2010 (62%) | 1908 (58%) | |
| 1 hospitalization | 613 (19%) | 646 (20%) | |
| 2 hospitalizations | 297 (9%) | 313 (10%) | |
| ≥3 hospitalizations | 321 (10%) | 397 (12%) | |
| Hospitalizations for any cause (number of events) | |||
| Total number of hospitalization events | 2661 | 3110 | 0.001 |
| Number of events per patient | |||
| Whole population | 0.8 | 1.0 | |
| Patients with ≥1 hospitalization | 2.2 | 2.3 | |
| . | Ivabradine (n = 3241) . | Placebo (n = 3264) . | P-value . |
|---|---|---|---|
| Hospitalizations for worsening heart failure (number of patients) | |||
| No hospitalization | 2727 (84%) | 2592 (79%) | |
| 1 hospitalization | 325 (10%) | 389 (12%) | |
| 2 hospitalizations | 99 (3%) | 155 (5%) | |
| ≥3 hospitalizations | 90 (3%) | 128 (4%) | |
| Hospitalizations for worsening heart failure (number of events) | |||
| Total number of hospitalization events | 902 | 1211 | 0.0002 |
| Number of events per patient | |||
| Whole population | 0.3 | 0.4 | |
| Patients with ≥1 hospitalization | 1.8 | 1.8 | |
| Hospitalizations for any cause (number of patients) | |||
| No hospitalization | 2010 (62%) | 1908 (58%) | |
| 1 hospitalization | 613 (19%) | 646 (20%) | |
| 2 hospitalizations | 297 (9%) | 313 (10%) | |
| ≥3 hospitalizations | 321 (10%) | 397 (12%) | |
| Hospitalizations for any cause (number of events) | |||
| Total number of hospitalization events | 2661 | 3110 | 0.001 |
| Number of events per patient | |||
| Whole population | 0.8 | 1.0 | |
| Patients with ≥1 hospitalization | 2.2 | 2.3 | |
P-values comparing patients in the ivabradine and placebo groups (Poisson's regression model).
Hospitalizations expressed per patient for all patients and for patients actually hospitalized during the trial
| . | Ivabradine (n = 3241) . | Placebo (n = 3264) . | P-value . |
|---|---|---|---|
| Hospitalizations for worsening heart failure (number of patients) | |||
| No hospitalization | 2727 (84%) | 2592 (79%) | |
| 1 hospitalization | 325 (10%) | 389 (12%) | |
| 2 hospitalizations | 99 (3%) | 155 (5%) | |
| ≥3 hospitalizations | 90 (3%) | 128 (4%) | |
| Hospitalizations for worsening heart failure (number of events) | |||
| Total number of hospitalization events | 902 | 1211 | 0.0002 |
| Number of events per patient | |||
| Whole population | 0.3 | 0.4 | |
| Patients with ≥1 hospitalization | 1.8 | 1.8 | |
| Hospitalizations for any cause (number of patients) | |||
| No hospitalization | 2010 (62%) | 1908 (58%) | |
| 1 hospitalization | 613 (19%) | 646 (20%) | |
| 2 hospitalizations | 297 (9%) | 313 (10%) | |
| ≥3 hospitalizations | 321 (10%) | 397 (12%) | |
| Hospitalizations for any cause (number of events) | |||
| Total number of hospitalization events | 2661 | 3110 | 0.001 |
| Number of events per patient | |||
| Whole population | 0.8 | 1.0 | |
| Patients with ≥1 hospitalization | 2.2 | 2.3 | |
| . | Ivabradine (n = 3241) . | Placebo (n = 3264) . | P-value . |
|---|---|---|---|
| Hospitalizations for worsening heart failure (number of patients) | |||
| No hospitalization | 2727 (84%) | 2592 (79%) | |
| 1 hospitalization | 325 (10%) | 389 (12%) | |
| 2 hospitalizations | 99 (3%) | 155 (5%) | |
| ≥3 hospitalizations | 90 (3%) | 128 (4%) | |
| Hospitalizations for worsening heart failure (number of events) | |||
| Total number of hospitalization events | 902 | 1211 | 0.0002 |
| Number of events per patient | |||
| Whole population | 0.3 | 0.4 | |
| Patients with ≥1 hospitalization | 1.8 | 1.8 | |
| Hospitalizations for any cause (number of patients) | |||
| No hospitalization | 2010 (62%) | 1908 (58%) | |
| 1 hospitalization | 613 (19%) | 646 (20%) | |
| 2 hospitalizations | 297 (9%) | 313 (10%) | |
| ≥3 hospitalizations | 321 (10%) | 397 (12%) | |
| Hospitalizations for any cause (number of events) | |||
| Total number of hospitalization events | 2661 | 3110 | 0.001 |
| Number of events per patient | |||
| Whole population | 0.8 | 1.0 | |
| Patients with ≥1 hospitalization | 2.2 | 2.3 | |
P-values comparing patients in the ivabradine and placebo groups (Poisson's regression model).
Using the total time (cumulative) approach, over about 2 years of follow-up, ivabradine-treated patients were at significantly lower risk for suffering a second hospitalization for worsening HF than were patients receiving placebo (Figure 2). The risk for suffering a third hospitalization for worsening HF was also significantly reduced by ivabradine (Figure 2).
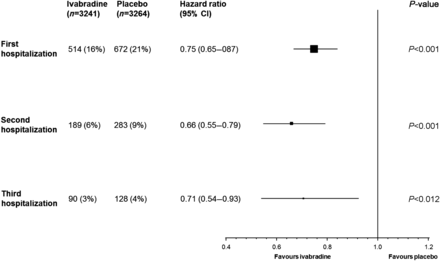
Estimate of treatment effect on recurrence of hospitalizations for worsening heart failure (total-time approach). The approach allows for a cumulative effect in which the second hospitalization includes the effect of the first, and the third hospitalization includes the effects of the first and second. All data adjusted for prognostic factors at the baseline (β-blocker, New York Heart Association class, left ventricular ejection fraction, ischaemic cause of heart failure, age, systolic blood pressure, heart rate, and creatinine clearance).
The gap-time approach analysis was performed in the patients with at least one hospitalization for worsening HF over a median follow-up of 21.1 months. The number of patients involved in this analysis provided only modest power to assess the effect and the result did not reach statistical significance (HR = 0.84, 95% CI, 0.69–1.01, P = 0.058), but the nominal effect on risk of second hospitalization for worsening HF with ivabradine compared with placebo was consistent with that found with the total-time approach. In addition, treatment with ivabradine was associated with more days alive out of hospital than with placebo (estimate, 13.00, 95% CI, 3.93–22.07, P = 0.005) during the study.
Discussion
Our results show that treatment with ivabradine in patients with chronic HF, who are in sinus rhythm, with a heart rate of ≥70 b.p.m. and are treated with guideline-based background therapy including maximally tolerated β-blockade (though not necessarily reaching guideline-suggested target doses), substantially decreases the risk of clinical deterioration. This benefit is reflected by a reduction in total hospitalizations for worsening HF, in the incidence of recurrent HF hospitalizations, and in an increase in time to first and subsequent hospitalizations. At the same time, no deleterious effects on other causes of hospitalization were observed.
The primary SHIFT analysis demonstrated that treatment with ivabradine is associated with a substantial reduction in first hospitalizations for worsening HF (P < 0.0001), as well as in first hospitalization for any cause (P = 0.003).11 Our supplementary post hoc analysis is consistent with the conclusion that the benefit of ivabradine on HF hospitalization is maintained over several years of therapy and, specifically, mitigates the likelihood of recurrent events.
Chronic HF is common and is associated with frequent exacerbations that often result in hospitalization and death.13 Worsening HF is one of the most common causes of hospitalization in patients with HF and is often recurrent. Even though the rate of hospitalization for worsening HF has declined over several decades, it remains relatively high.19,20 HF hospitalizations are also powerful predictors of subsequent HF mortality.5–7 Thus, a reduction in HF admissions contributes to a reduction in the overall burden of HF on patients and to a reduction in the risk of subsequent hospitalizations and death. For all these reasons, the development of therapeutic strategies that can prevent recurring hospital admissions can provide important clinical benefit. Our results are also consistent with the most recent guidelines from the European Society of Cardiology for the management of HF,10 which recommend ivabradine for the reduction in risk for HF hospitalization.
The reduction in total hospitalizations for worsening HF with ivabradine is consistent with data reported from other clinical trials, including renin–angiotensin system inhibitors and β-blockers.21–25 Randomized cardiac resynchronization therapy trials have also consistently reported a reduction in admissions for worsening HF, including recurrent events.26,27
A reduction in HF hospitalizations has another important benefit that adheres not only to the individual patient, but also to society as a whole. Heart failure hospitalizations account for more than two-thirds of the cost of HF care.8,9 Thus, reducing total burden of hospitalizations, to the extent seen with ivabradine in SHIFT, is likely to importantly reduce the cost of care for patients with HF.
This paper evaluating the effect of continued treatment with ivabradine on recurrent hospitalizations for worsening HF is based on post hoc analysis. The statistical models used have limitations. The Poisson regression approach, although corrected for over-dispersion and baseline covariates, could be affected by the combination of overall unmodelled random subject effects and the within-subject correlations resulting from the increased risk propagating from each HF admission. The total-time approach includes the treatment effect on previous hospitalizations in the evaluation of the effect on a recurrent hospitalization (thus providing an effect that depends on the effect on previous hospitalizations). The gap-time approach evaluates the specific effect on a second hospitalization and is restricted to patients who have had a first event. It does not therefore preserve the randomization planned in the original trial design. Consequently, the gap-time approach was adjusted for all differences in baseline characteristics between the ivabradine and placebo groups and results similar to those of the total-time approach (though not statistically significant) were found (HR = 0.83, 95% CI, 0.69–1.01, P = 0.058). Recurrent event analyses are potentially biased by informative censoring due to different mortality rates in the treatment groups. In SHIFT, there was not a statistically significant difference in mortality in the treatment groups and hence issues associated with this source of bias are likely to be minimal. Finally, data on hospitalization burden may be influenced by differences between health-care systems in different countries.
Conclusion
Our findings support the importance of heart rate reduction with ivabradine, when undertaken on a background of guideline-based therapy, for improving clinical outcomes in HF. Specifically, these results indicate that treatment with ivabradine is associated with a pronounced reduction in the risk of repeated hospitalizations (and, thus, of total burden of hospitalizations) for worsening HF.
Funding
The SHIFT study was funded by Servier, Suresnes, France. Funding to pay the Open Access publication charges for this article was provided by Servier, France.
Conflict of interest: All authors have received fees, research grants or both from Servier.



