-
PDF
- Split View
-
Views
-
Cite
Cite
Young Joon Hong, Myung Ho Jeong, Yun Ha Choi, Jum Suk Ko, Min Goo Lee, Won Yu Kang, Shin Eun Lee, Soo Hyun Kim, Keun Ho Park, Doo Sun Sim, Nam Sik Yoon, Hyun Ju Youn, Kye Hun Kim, Hyung Wook Park, Ju Han Kim, Youngkeun Ahn, Jeong Gwan Cho, Jong Chun Park, Jung Chaee Kang, Impact of plaque components on no-reflow phenomenon after stent deployment in patients with acute coronary syndrome: a virtual histology-intravascular ultrasound analysis, European Heart Journal, Volume 32, Issue 16, August 2011, Pages 2059–2066, https://doi.org/10.1093/eurheartj/ehp034
Close - Share Icon Share
Abstract
We used virtual histology-intravascular ultrasound (VH-IVUS) to evaluate the relation between coronary plaque characteristics and no-reflow in acute coronary syndrome (ACS) patients.
A total of 190 consecutive ACS patients were imaged using VH-IVUS and analysed retrospectively. Angiographic no-reflow was defined as TIMI flow grade 0, 1, and 2 after stenting. Virtual histology-intravascular ultrasound classified the colour-coded tissue into four major components: fibrotic, fibro-fatty, dense calcium, and necrotic core (NC). Thin-cap fibroatheroma (TCFA) was defined as focal, NC-rich (≥10% of the cross-sectional area) plaques being in contact with the lumen in a plaque burden ≥40%. Of the 190 patients studied at pre-stenting, no-reflow was observed in 24 patients (12.6%) at post-stenting. The absolute and %NC areas at the minimum lumen sites (1.6 ± 1.2 vs. 0.9 ± 0.8 mm2, P < 0.001, and 24.5 ± 14.3 vs. 16.1 ± 10.6%, P = 0.001, respectively) and the absolute and %NC volumes (30 ± 24 vs. 16 ± 17 mm3, P = 0.001, and 22 ± 11 vs. 14 ± 8%, P < 0.001, respectively) were significantly greater, and the presence of at least one TCFA and multiple TCFAs within culprit lesions (71 vs. 36%, P = 0.001, and 38 vs. 15%, P = 0.005, respectively) was significantly more common in the no-reflow group compared with the normal-reflow group. In the multivariable analysis, %NC volume was the only independent predictor of no-reflow (odds ratio = 1.126; 95% CI 1.045–1.214, P = 0.002).
In ACS patients, post-stenting no-reflow is associated with plaque components defined by VH-IVUS analysis with larger NC and more TCFAs.
Introduction
Percutaneous coronary intervention (PCI) is the most common strategy for treating acute coronary syndrome (ACS).1–3 However, PCI fails to achieve Thrombolysis In Myocardial Infarction (TIMI)-3 flow in 12–30% of cases, mainly because of the no-reflow phenomenon.4,5 Several grey-scale intravascular ultrasound (IVUS) studies have demonstrated several predictors of no-reflow phenomenon in ACS patients.6–10
Grey-scale IVUS is widely used to assess coronary artery morphometry. However, this conventional IVUS has significant limitations in assessing plaque composition.11,12 Spectral analysis of IVUS radiofrequency data [virtual histology (VH)-IVUS] can provide quantitative information on plaque composition; it has been validated in study of explanted human coronary segments [in that study, necrotic core (NC) was described as necrotic calcification and then extrapolated to NC].13 Virtual histology-intravascular ultrasound characterizes atherosclerotic plaque as fibrotic (FT), fibro-fatty (FF), dense calcium (DC), and NC.13,14 Several VH-IVUS studies have demonstrated the coronary plaque components in ACS patients.14–16 However, so far, few data are available and there are controversies in the association between plaque components and no-reflow after PCI in ACS patients.17–21 Therefore, the purpose of the present study was to investigate the relation between pre-procedural plaque components assessed by VH-IVUS and no-reflow after stent deployment in ACS patients.
Methods
This study was a retrospective, single-centre study. A total of 2450 ACS patients were admitted to our institute from February 2006 to January 2008. Of these, VH-IVUS was performed in 345 patients. We excluded 45 patients who did not undergo PCI and 110 patients who underwent pre-stenting balloon angioplasty. Finally, we identified 190 ACS patients who underwent pre-PCI VH-IVUS and stent implantation of native, de novo coronary lesion. The presence of unstable angina was determined by chest pain within the preceding 72 h with or without ST-T wave changes or positive cardiac biochemical markers. The presence of ST-segment elevation myocardial infarction was determined by >30 min of continuous chest pain, a new ST-segment elevation ≥2 mm on at least two contiguous electrocardiographic leads, and creatine kinase-MB more than three times normal. The presence of non-ST-segment elevation myocardial infarction was diagnosed by chest pain and a positive cardiac biochemical marker without new ST-segment elevation. We excluded patients with subacute or late stent thrombosis, totally occluded lesions, restenosis after stenting, coronary artery bypass graft failure, factors associated with increased risk of bleeding, severe heart failure or cardiogenic shock, important systemic disease, or serum creatinine ≥2.5 mg/dL, and patients in whom adequate IVUS images could not be obtained. All patients were treated with stent implantation: 168 patients with drug-eluting stents and 22 patients with bare-metal stents. The protocol was approved by the institutional review board. Hospital records of patients were reviewed to obtain information on clinical demographics.
Peripheral blood samples were obtained before IVUS study using direct venipuncture. The blood samples were centrifuged, and serum was removed and stored at −70°C until the assay could be performed. Absolute creatine kinase-MB levels were determined by radioimmunoassay (Dade Behring Inc., Miami, FL, USA). Cardiac-specific troponin I levels were measured using paramagnetic particles and a chemiluminescent immunoenzymatic assay (Beckman, Coulter Inc., Fullerton, CA, USA). The serum levels of total cholesterol, triglyceride, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol were measured by standard enzymatic methods. High-sensitivity C-reactive protein was analysed turbidimetrically with sheep antibodies against human C-reactive protein; this has been validated against the Dade–Behring method.22 Serum N-terminal pro-B-type natriuretic peptide was measured using an electrochemiluminescence sandwich immunoassay method with an Elecsys 2010 analyzer (Roche Diagnostics, Mannheim, Germany).
No-reflow was defined as post-PCI TIMI grade 0, 1, or 2 flow in the absence of mechanical obstruction.5,8 Normal-reflow was defined as TIMI grade 3 flow. On this basis, patients were divided into two groups: a no-reflow group (n = 24) and a normal-reflow group (n = 166). If TIMI flow post-PCI was 0, 1, or 2 in the absence of angiographic stenosis, repeated IVUS was performed to exclude the possibility of mechanical vessel obstruction.
Coronary angiogram was analysed with validated QCA system (Phillips H5000 or Allura DCI program, Philips Medical Systems, Eindhoven, The Netherlands). With the outer diameter of the contrast-filled catheter as the calibration standard, the minimal lumen diameter, reference diameter, and lesion length were measured in diastolic frames from orthogonal projections. Perfusion was evaluated according to TIMI criteria.23
All pre-PCI VH-IVUS examinations were performed after intracoronary administration of 300 µg nitroglycerin. A 20-MHz, 2.9F IVUS imaging catheter (Eagle Eye, Volcano Corp, Rancho Cordova, CA, USA) was advanced >10 mm beyond the lesion; and automated pullback was performed to a point >10 mm proximal to the lesion at a speed of 0.5 mm/s. Quantitative volumetric grey-scale and VH-IVUS analyses were performed across the entire lesion segment, and cross-sectional analysis was performed at the minimum lumen sites and at the largest NC sites. Grey-scale IVUS analysis was performed according to the American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies.24 External elastic membrane (EEM) and lumen cross-sectional areas (CSA) were measured. Plaque plus media (P&M) CSA was calculated as EEM minus lumen CSA; and plaque burden was calculated as P&M divided by EEM CSA. Proximal and distal references were the single slices with the largest lumen and smallest plaque CSAs within 10 mm proximally and distally, but before any large side branch. Remodelling index was the ratio of lesion site EEM CSA divided by the average of the proximal and distal reference EEM CSA. Post-PCI, we measured the minimum stent CSA. Stent expansion was calculated as minimum stent CSA divided by mean reference lumen CSA. Virtual histology-intravascular ultrasound analysis classified the colour-coded tissue into four major components: green (FT); yellow-green (FF); white (DC); and red (NC).13,14 Virtual histology-intravascular ultrasound analysis was reported in absolute amounts and as a percentage of plaque area or volume. Thin-cap fibroatheroma (TCFA) was defined as a NC ≥10% of plaque area in at least three consecutive frames without overlying fibrous tissue in the presence of ≥40% plaque burden.14
The statistical Package for Social Sciences (SPSS) for Windows, version 15.0 (Chicago, IL, USA) was used for all analyses. Continuous variables were presented as the mean value ± 1 SD; comparisons were conducted by student’s t-test or the Wilcoxon rank-sum test if normality assumption was violated. Discrete variables were presented as percentages and frequencies; comparisons were conducted by χ2 statistics or Fisher’s exact test as appropriate. Multivariable analysis was performed to identify independent predictors of no-reflow phenomenon. Univariable analyses were first conducted to identify potential risk factors for no-reflow. The likelihood ratio test was used, and the variables with a P-value of <0.2 were included in the multivariable model. Finally, a stepdown logistic regression was performed. The least significant variable was dropped at each step until only covariates with a P-value <0.05 remained. A P-value <0.05 was considered statistically significant.
Results
Of the 190 patients studied at pre-stenting, no-reflow was observed in 24 patients (12.6%) at post-stenting. The baseline characteristics are summarized in Table 1. There were no significant differences in age, gender, clinical presentations, risk factors for coronary artery disease, ejection fraction, serum creatinine levels, and lipid profiles. There were no significant differences in the use of glycoprotein IIb/IIIa inhibitors and pre-PCI clopidogrel loading. Creatine kinase-MB and cardiac-specific troponin-I levels tended to be higher in the no-reflow group compared with the normal-reflow group. High-sensitivity C-reactive protein and N-terminal pro-B type natriuretic peptide levels were significantly higher in the no-reflow group compared with the normal-reflow group. In-hospital mortality tended to be higher in the no-reflow group compared with the normal-reflow group [4/24 (17%) vs. 9/166 (5%), P = 0.064].
| . | No-reflow (n = 24) . | Normal-reflow (n = 166) . | P-value . |
|---|---|---|---|
| Age (years) | 60.1 ± 14.4 | 60.5 ± 12.2 | 0.9 |
| Male gender (%) | 14 (58) | 109 (66) | 0.5 |
| Clinical presentation | 0.6 | ||
| Unstable angina (%) | 13 (54) | 98 (59) | |
| Non-ST-segment elevation MI (%) | 6 (25) | 28 (17) | |
| ST-segment elevation MI (%) | 5 (21) | 40 (24) | |
| Diabetes mellitus (%) | 6 (25) | 32 (19) | 0.5 |
| Hypertension (%) | 17 (71) | 87 (52) | 0.090 |
| Smoking (%) | 13 (54) | 82 (49) | 0.7 |
| Prior MI (%) | 1 (4) | 5 (3) | 0.8 |
| Ejection fraction (%) | 62 ± 9 | 62 ± 9 | 1.0 |
| Glycoprotein IIb/IIIa inhibitor use (%) | 7 (29) | 39 (23) | 0.5 |
| Pre-PCI clopidogrel loading (%) | 23 (96) | 160 (96) | 1.0 |
| White blood cells (103/mm3) | 8.9 ± 3.3 | 8.5 ± 2.9 | 0.5 |
| Haemoglobin (g/dL) | 13.9 ± 1.6 | 14.3 ± 1.6 | 0.3 |
| Platelet count (103/mm3) | 238 ± 97 | 221 ± 66 | 0.4 |
| Creatine kinase-MB (U/L) | 22.8 ± 59.6 | 12.7 ± 14.6 | 0.14 |
| Cardiac-specific troponin-I (ng/mL) | 13.0 ± 61.1 | 4.0 ± 6.5 | 0.13 |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.6 |
| Glucose (mg/dL | 160 ± 54 | 140 ± 41 | 0.15 |
| Total cholesterol (mg/dl) | 200 ± 56 | 187 ± 48 | 0.4 |
| Triglyceride (mg/dL) | 141 ± 69 | 116 ± 63 | 0.17 |
| LDL-cholesterol (mg/dL) | 133 ± 47 | 122 ± 41 | 0.4 |
| HDL-cholesterol (mg/dL) | 48 ± 9 | 46 ± 12 | 0.6 |
| hs-CRP (mg/dL) | 1.8 ± 0.8 | 0.8 ± 0.3 | 0.021 |
| NT-pro-BNP (pg/mL) | 526 ± 533 | 237 ± 293 | 0.029 |
| . | No-reflow (n = 24) . | Normal-reflow (n = 166) . | P-value . |
|---|---|---|---|
| Age (years) | 60.1 ± 14.4 | 60.5 ± 12.2 | 0.9 |
| Male gender (%) | 14 (58) | 109 (66) | 0.5 |
| Clinical presentation | 0.6 | ||
| Unstable angina (%) | 13 (54) | 98 (59) | |
| Non-ST-segment elevation MI (%) | 6 (25) | 28 (17) | |
| ST-segment elevation MI (%) | 5 (21) | 40 (24) | |
| Diabetes mellitus (%) | 6 (25) | 32 (19) | 0.5 |
| Hypertension (%) | 17 (71) | 87 (52) | 0.090 |
| Smoking (%) | 13 (54) | 82 (49) | 0.7 |
| Prior MI (%) | 1 (4) | 5 (3) | 0.8 |
| Ejection fraction (%) | 62 ± 9 | 62 ± 9 | 1.0 |
| Glycoprotein IIb/IIIa inhibitor use (%) | 7 (29) | 39 (23) | 0.5 |
| Pre-PCI clopidogrel loading (%) | 23 (96) | 160 (96) | 1.0 |
| White blood cells (103/mm3) | 8.9 ± 3.3 | 8.5 ± 2.9 | 0.5 |
| Haemoglobin (g/dL) | 13.9 ± 1.6 | 14.3 ± 1.6 | 0.3 |
| Platelet count (103/mm3) | 238 ± 97 | 221 ± 66 | 0.4 |
| Creatine kinase-MB (U/L) | 22.8 ± 59.6 | 12.7 ± 14.6 | 0.14 |
| Cardiac-specific troponin-I (ng/mL) | 13.0 ± 61.1 | 4.0 ± 6.5 | 0.13 |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.6 |
| Glucose (mg/dL | 160 ± 54 | 140 ± 41 | 0.15 |
| Total cholesterol (mg/dl) | 200 ± 56 | 187 ± 48 | 0.4 |
| Triglyceride (mg/dL) | 141 ± 69 | 116 ± 63 | 0.17 |
| LDL-cholesterol (mg/dL) | 133 ± 47 | 122 ± 41 | 0.4 |
| HDL-cholesterol (mg/dL) | 48 ± 9 | 46 ± 12 | 0.6 |
| hs-CRP (mg/dL) | 1.8 ± 0.8 | 0.8 ± 0.3 | 0.021 |
| NT-pro-BNP (pg/mL) | 526 ± 533 | 237 ± 293 | 0.029 |
| . | No-reflow (n = 24) . | Normal-reflow (n = 166) . | P-value . |
|---|---|---|---|
| Age (years) | 60.1 ± 14.4 | 60.5 ± 12.2 | 0.9 |
| Male gender (%) | 14 (58) | 109 (66) | 0.5 |
| Clinical presentation | 0.6 | ||
| Unstable angina (%) | 13 (54) | 98 (59) | |
| Non-ST-segment elevation MI (%) | 6 (25) | 28 (17) | |
| ST-segment elevation MI (%) | 5 (21) | 40 (24) | |
| Diabetes mellitus (%) | 6 (25) | 32 (19) | 0.5 |
| Hypertension (%) | 17 (71) | 87 (52) | 0.090 |
| Smoking (%) | 13 (54) | 82 (49) | 0.7 |
| Prior MI (%) | 1 (4) | 5 (3) | 0.8 |
| Ejection fraction (%) | 62 ± 9 | 62 ± 9 | 1.0 |
| Glycoprotein IIb/IIIa inhibitor use (%) | 7 (29) | 39 (23) | 0.5 |
| Pre-PCI clopidogrel loading (%) | 23 (96) | 160 (96) | 1.0 |
| White blood cells (103/mm3) | 8.9 ± 3.3 | 8.5 ± 2.9 | 0.5 |
| Haemoglobin (g/dL) | 13.9 ± 1.6 | 14.3 ± 1.6 | 0.3 |
| Platelet count (103/mm3) | 238 ± 97 | 221 ± 66 | 0.4 |
| Creatine kinase-MB (U/L) | 22.8 ± 59.6 | 12.7 ± 14.6 | 0.14 |
| Cardiac-specific troponin-I (ng/mL) | 13.0 ± 61.1 | 4.0 ± 6.5 | 0.13 |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.6 |
| Glucose (mg/dL | 160 ± 54 | 140 ± 41 | 0.15 |
| Total cholesterol (mg/dl) | 200 ± 56 | 187 ± 48 | 0.4 |
| Triglyceride (mg/dL) | 141 ± 69 | 116 ± 63 | 0.17 |
| LDL-cholesterol (mg/dL) | 133 ± 47 | 122 ± 41 | 0.4 |
| HDL-cholesterol (mg/dL) | 48 ± 9 | 46 ± 12 | 0.6 |
| hs-CRP (mg/dL) | 1.8 ± 0.8 | 0.8 ± 0.3 | 0.021 |
| NT-pro-BNP (pg/mL) | 526 ± 533 | 237 ± 293 | 0.029 |
| . | No-reflow (n = 24) . | Normal-reflow (n = 166) . | P-value . |
|---|---|---|---|
| Age (years) | 60.1 ± 14.4 | 60.5 ± 12.2 | 0.9 |
| Male gender (%) | 14 (58) | 109 (66) | 0.5 |
| Clinical presentation | 0.6 | ||
| Unstable angina (%) | 13 (54) | 98 (59) | |
| Non-ST-segment elevation MI (%) | 6 (25) | 28 (17) | |
| ST-segment elevation MI (%) | 5 (21) | 40 (24) | |
| Diabetes mellitus (%) | 6 (25) | 32 (19) | 0.5 |
| Hypertension (%) | 17 (71) | 87 (52) | 0.090 |
| Smoking (%) | 13 (54) | 82 (49) | 0.7 |
| Prior MI (%) | 1 (4) | 5 (3) | 0.8 |
| Ejection fraction (%) | 62 ± 9 | 62 ± 9 | 1.0 |
| Glycoprotein IIb/IIIa inhibitor use (%) | 7 (29) | 39 (23) | 0.5 |
| Pre-PCI clopidogrel loading (%) | 23 (96) | 160 (96) | 1.0 |
| White blood cells (103/mm3) | 8.9 ± 3.3 | 8.5 ± 2.9 | 0.5 |
| Haemoglobin (g/dL) | 13.9 ± 1.6 | 14.3 ± 1.6 | 0.3 |
| Platelet count (103/mm3) | 238 ± 97 | 221 ± 66 | 0.4 |
| Creatine kinase-MB (U/L) | 22.8 ± 59.6 | 12.7 ± 14.6 | 0.14 |
| Cardiac-specific troponin-I (ng/mL) | 13.0 ± 61.1 | 4.0 ± 6.5 | 0.13 |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.6 |
| Glucose (mg/dL | 160 ± 54 | 140 ± 41 | 0.15 |
| Total cholesterol (mg/dl) | 200 ± 56 | 187 ± 48 | 0.4 |
| Triglyceride (mg/dL) | 141 ± 69 | 116 ± 63 | 0.17 |
| LDL-cholesterol (mg/dL) | 133 ± 47 | 122 ± 41 | 0.4 |
| HDL-cholesterol (mg/dL) | 48 ± 9 | 46 ± 12 | 0.6 |
| hs-CRP (mg/dL) | 1.8 ± 0.8 | 0.8 ± 0.3 | 0.021 |
| NT-pro-BNP (pg/mL) | 526 ± 533 | 237 ± 293 | 0.029 |
Coronary angiographic findings are summarized in Table 2. There were no significant differences in culprit lesions, diseased vessel number, American College of Cardiology/American Heart Association lesion type, TIMI flow grade, stent diameter and length, inflation pressure, angiographic reference diameter, and minimal lumen diameter. However, the angiographic lesion length and stent length were significantly longer in the no-reflow group compared with the normal-reflow group.
| . | No-reflow (n = 24) . | Normal-reflow (n = 166) . | P-value . |
|---|---|---|---|
| Culprit lesion (%) | 0.6 | ||
| Left main | 0 (0) | 2 (1) | |
| Left anterior descending | 8 (33) | 59 (36) | |
| Left circumflex | 5 (21) | 50 (30) | |
| Right | 11 (46) | 55 (33) | |
| Diseased vessel number (%) | 0.5 | ||
| 1 | 6 (25) | 53 (32) | |
| 2 | 13 (54) | 88 (50) | |
| 3 | 5 (21) | 25 (18) | |
| ACC/AHA lesion type (%) | 0.097 | ||
| B1 | 8 (33) | 73 (44) | |
| B2 | 12 (50) | 70 (42) | |
| C | 4 (17) | 23 (14) | |
| TIMI flow grade (%) | 0.5 | ||
| 0 | 0 (0) | 0 (0) | |
| 1 | 0 (0) | 0 (0) | |
| 2 | 7 (29) | 37 (22) | |
| 3 | 17 (71) | 129 (78) | |
| Stent type (%) | 0.9 | ||
| Drug-eluting stents | 21 (88) | 147 (89) | |
| Bare-metal stents | 3 (12) | 19 (11) | |
| Stent diameter (mm) | 3.25 ± 0.68 | 3.18 ± 0.56 | 0.16 |
| Stent length (mm) | 25.8 ± 10.3 | 22.1 ± 9.4 | 0.036 |
| Inflation pressure (atm) | 13.6 ± 3.6 | 13.0 ± 2.9 | 0.3 |
| Post-PCI TIMI flow grade (%) | <0.001 | ||
| 0 | 3 (13) | 0 (0) | |
| 1 | 4 (17) | 0 (0) | |
| 2 | 17 (80) | 0 (0) | |
| 3 | 0 (0) | 166 (100) | |
| Reference diameter (mm) | 3.29 ± 0.87 | 3.22 ± 0.75 | 0.3 |
| Pre-MLD (mm) | 0.62 ± 0.31 | 0.65 ± 0.51 | 0.5 |
| Lesion length (mm) | 18 ± 12 | 14 ± 8 | 0.023 |
| Post-MLD (mm) | 3.20 ± 0.62 | 3.15 ± 0.58 | 0.11 |
| . | No-reflow (n = 24) . | Normal-reflow (n = 166) . | P-value . |
|---|---|---|---|
| Culprit lesion (%) | 0.6 | ||
| Left main | 0 (0) | 2 (1) | |
| Left anterior descending | 8 (33) | 59 (36) | |
| Left circumflex | 5 (21) | 50 (30) | |
| Right | 11 (46) | 55 (33) | |
| Diseased vessel number (%) | 0.5 | ||
| 1 | 6 (25) | 53 (32) | |
| 2 | 13 (54) | 88 (50) | |
| 3 | 5 (21) | 25 (18) | |
| ACC/AHA lesion type (%) | 0.097 | ||
| B1 | 8 (33) | 73 (44) | |
| B2 | 12 (50) | 70 (42) | |
| C | 4 (17) | 23 (14) | |
| TIMI flow grade (%) | 0.5 | ||
| 0 | 0 (0) | 0 (0) | |
| 1 | 0 (0) | 0 (0) | |
| 2 | 7 (29) | 37 (22) | |
| 3 | 17 (71) | 129 (78) | |
| Stent type (%) | 0.9 | ||
| Drug-eluting stents | 21 (88) | 147 (89) | |
| Bare-metal stents | 3 (12) | 19 (11) | |
| Stent diameter (mm) | 3.25 ± 0.68 | 3.18 ± 0.56 | 0.16 |
| Stent length (mm) | 25.8 ± 10.3 | 22.1 ± 9.4 | 0.036 |
| Inflation pressure (atm) | 13.6 ± 3.6 | 13.0 ± 2.9 | 0.3 |
| Post-PCI TIMI flow grade (%) | <0.001 | ||
| 0 | 3 (13) | 0 (0) | |
| 1 | 4 (17) | 0 (0) | |
| 2 | 17 (80) | 0 (0) | |
| 3 | 0 (0) | 166 (100) | |
| Reference diameter (mm) | 3.29 ± 0.87 | 3.22 ± 0.75 | 0.3 |
| Pre-MLD (mm) | 0.62 ± 0.31 | 0.65 ± 0.51 | 0.5 |
| Lesion length (mm) | 18 ± 12 | 14 ± 8 | 0.023 |
| Post-MLD (mm) | 3.20 ± 0.62 | 3.15 ± 0.58 | 0.11 |
| . | No-reflow (n = 24) . | Normal-reflow (n = 166) . | P-value . |
|---|---|---|---|
| Culprit lesion (%) | 0.6 | ||
| Left main | 0 (0) | 2 (1) | |
| Left anterior descending | 8 (33) | 59 (36) | |
| Left circumflex | 5 (21) | 50 (30) | |
| Right | 11 (46) | 55 (33) | |
| Diseased vessel number (%) | 0.5 | ||
| 1 | 6 (25) | 53 (32) | |
| 2 | 13 (54) | 88 (50) | |
| 3 | 5 (21) | 25 (18) | |
| ACC/AHA lesion type (%) | 0.097 | ||
| B1 | 8 (33) | 73 (44) | |
| B2 | 12 (50) | 70 (42) | |
| C | 4 (17) | 23 (14) | |
| TIMI flow grade (%) | 0.5 | ||
| 0 | 0 (0) | 0 (0) | |
| 1 | 0 (0) | 0 (0) | |
| 2 | 7 (29) | 37 (22) | |
| 3 | 17 (71) | 129 (78) | |
| Stent type (%) | 0.9 | ||
| Drug-eluting stents | 21 (88) | 147 (89) | |
| Bare-metal stents | 3 (12) | 19 (11) | |
| Stent diameter (mm) | 3.25 ± 0.68 | 3.18 ± 0.56 | 0.16 |
| Stent length (mm) | 25.8 ± 10.3 | 22.1 ± 9.4 | 0.036 |
| Inflation pressure (atm) | 13.6 ± 3.6 | 13.0 ± 2.9 | 0.3 |
| Post-PCI TIMI flow grade (%) | <0.001 | ||
| 0 | 3 (13) | 0 (0) | |
| 1 | 4 (17) | 0 (0) | |
| 2 | 17 (80) | 0 (0) | |
| 3 | 0 (0) | 166 (100) | |
| Reference diameter (mm) | 3.29 ± 0.87 | 3.22 ± 0.75 | 0.3 |
| Pre-MLD (mm) | 0.62 ± 0.31 | 0.65 ± 0.51 | 0.5 |
| Lesion length (mm) | 18 ± 12 | 14 ± 8 | 0.023 |
| Post-MLD (mm) | 3.20 ± 0.62 | 3.15 ± 0.58 | 0.11 |
| . | No-reflow (n = 24) . | Normal-reflow (n = 166) . | P-value . |
|---|---|---|---|
| Culprit lesion (%) | 0.6 | ||
| Left main | 0 (0) | 2 (1) | |
| Left anterior descending | 8 (33) | 59 (36) | |
| Left circumflex | 5 (21) | 50 (30) | |
| Right | 11 (46) | 55 (33) | |
| Diseased vessel number (%) | 0.5 | ||
| 1 | 6 (25) | 53 (32) | |
| 2 | 13 (54) | 88 (50) | |
| 3 | 5 (21) | 25 (18) | |
| ACC/AHA lesion type (%) | 0.097 | ||
| B1 | 8 (33) | 73 (44) | |
| B2 | 12 (50) | 70 (42) | |
| C | 4 (17) | 23 (14) | |
| TIMI flow grade (%) | 0.5 | ||
| 0 | 0 (0) | 0 (0) | |
| 1 | 0 (0) | 0 (0) | |
| 2 | 7 (29) | 37 (22) | |
| 3 | 17 (71) | 129 (78) | |
| Stent type (%) | 0.9 | ||
| Drug-eluting stents | 21 (88) | 147 (89) | |
| Bare-metal stents | 3 (12) | 19 (11) | |
| Stent diameter (mm) | 3.25 ± 0.68 | 3.18 ± 0.56 | 0.16 |
| Stent length (mm) | 25.8 ± 10.3 | 22.1 ± 9.4 | 0.036 |
| Inflation pressure (atm) | 13.6 ± 3.6 | 13.0 ± 2.9 | 0.3 |
| Post-PCI TIMI flow grade (%) | <0.001 | ||
| 0 | 3 (13) | 0 (0) | |
| 1 | 4 (17) | 0 (0) | |
| 2 | 17 (80) | 0 (0) | |
| 3 | 0 (0) | 166 (100) | |
| Reference diameter (mm) | 3.29 ± 0.87 | 3.22 ± 0.75 | 0.3 |
| Pre-MLD (mm) | 0.62 ± 0.31 | 0.65 ± 0.51 | 0.5 |
| Lesion length (mm) | 18 ± 12 | 14 ± 8 | 0.023 |
| Post-MLD (mm) | 3.20 ± 0.62 | 3.15 ± 0.58 | 0.11 |
Grey-scale IVUS findings are summarized in Table 3. At the minimum lumen sites, the lumen CSA was significantly smaller in the no-reflow group compared with the normal-reflow group; in contrast, the EEM and P&M CSAs and plaque burden were significantly greater in the no-reflow group compared with the normal-reflow group. Intravascular ultrasound lesion length was significantly longer in the no-reflow group compared with the normal-reflow group. There were strong trends towards greater remodelling index, greater minimum stent CSA, and greater percent stent expansion. There were no significant differences in the IVUS parameters at the proximal and distal reference sites between both groups.
| . | No-reflow (n = 24) . | Normal-reflow (n = 166) . | P-value . |
|---|---|---|---|
| Proximal reference | |||
| EEM CSA (mm2) | 15.8 ± 7.8 | 15.6 ± 5.8 | 0.4 |
| Lumen CSA (mm2) | 9.8 ± 4.5 | 9.8 ± 3.5 | 1.0 |
| P&M CSA (mm2) | 6.0 ± 3.2 | 5.8 ± 3.2 | 0.4 |
| Plaque burden (%) | 38 ± 9 | 37 ± 9 | 0.6 |
| Minimum lumen site | |||
| EEM CSA (mm2) | 16.2 ± 6.0 | 15.6 ± 5.8 | 0.042 |
| Lumen CSA (mm2) | 3.4 ± 2.4 | 4.0 ± 2.1 | 0.021 |
| P&M CSA (mm2) | 12.8 ± 4.8 | 11.6 ± 4.3 | 0.013 |
| Plaque burden (%) | 79 ± 12 | 74 ± 16 | 0.008 |
| IVUS lesion length (mm) | 22 ± 13 | 18 ± 12 | 0.020 |
| Distal reference | |||
| EEM CSA (mm2) | 15.2 ± 5.7 | 15.0 ± 5.0 | 0.4 |
| Lumen CSA (mm2) | 9.3 ± 3.6 | 9.6 ± 3.1 | 0.2 |
| P&M CSA (mm2) | 5.9 ± 2.8 | 5.4 ± 2.7 | 0.14 |
| Plaque burden (%) | 39 ± 13 | 36 ± 12 | 0.16 |
| Remodelling index | 1.05 ± 0.38 | 1.02 ± 0.23 | 0.068 |
| Minimum stent CSA (mm2) | 8.8 ± 3.4 | 8.4 ± 2.9 | 0.087 |
| Stent expansion (%) | 92 ± 19 | 87 ± 17 | 0.076 |
| . | No-reflow (n = 24) . | Normal-reflow (n = 166) . | P-value . |
|---|---|---|---|
| Proximal reference | |||
| EEM CSA (mm2) | 15.8 ± 7.8 | 15.6 ± 5.8 | 0.4 |
| Lumen CSA (mm2) | 9.8 ± 4.5 | 9.8 ± 3.5 | 1.0 |
| P&M CSA (mm2) | 6.0 ± 3.2 | 5.8 ± 3.2 | 0.4 |
| Plaque burden (%) | 38 ± 9 | 37 ± 9 | 0.6 |
| Minimum lumen site | |||
| EEM CSA (mm2) | 16.2 ± 6.0 | 15.6 ± 5.8 | 0.042 |
| Lumen CSA (mm2) | 3.4 ± 2.4 | 4.0 ± 2.1 | 0.021 |
| P&M CSA (mm2) | 12.8 ± 4.8 | 11.6 ± 4.3 | 0.013 |
| Plaque burden (%) | 79 ± 12 | 74 ± 16 | 0.008 |
| IVUS lesion length (mm) | 22 ± 13 | 18 ± 12 | 0.020 |
| Distal reference | |||
| EEM CSA (mm2) | 15.2 ± 5.7 | 15.0 ± 5.0 | 0.4 |
| Lumen CSA (mm2) | 9.3 ± 3.6 | 9.6 ± 3.1 | 0.2 |
| P&M CSA (mm2) | 5.9 ± 2.8 | 5.4 ± 2.7 | 0.14 |
| Plaque burden (%) | 39 ± 13 | 36 ± 12 | 0.16 |
| Remodelling index | 1.05 ± 0.38 | 1.02 ± 0.23 | 0.068 |
| Minimum stent CSA (mm2) | 8.8 ± 3.4 | 8.4 ± 2.9 | 0.087 |
| Stent expansion (%) | 92 ± 19 | 87 ± 17 | 0.076 |
| . | No-reflow (n = 24) . | Normal-reflow (n = 166) . | P-value . |
|---|---|---|---|
| Proximal reference | |||
| EEM CSA (mm2) | 15.8 ± 7.8 | 15.6 ± 5.8 | 0.4 |
| Lumen CSA (mm2) | 9.8 ± 4.5 | 9.8 ± 3.5 | 1.0 |
| P&M CSA (mm2) | 6.0 ± 3.2 | 5.8 ± 3.2 | 0.4 |
| Plaque burden (%) | 38 ± 9 | 37 ± 9 | 0.6 |
| Minimum lumen site | |||
| EEM CSA (mm2) | 16.2 ± 6.0 | 15.6 ± 5.8 | 0.042 |
| Lumen CSA (mm2) | 3.4 ± 2.4 | 4.0 ± 2.1 | 0.021 |
| P&M CSA (mm2) | 12.8 ± 4.8 | 11.6 ± 4.3 | 0.013 |
| Plaque burden (%) | 79 ± 12 | 74 ± 16 | 0.008 |
| IVUS lesion length (mm) | 22 ± 13 | 18 ± 12 | 0.020 |
| Distal reference | |||
| EEM CSA (mm2) | 15.2 ± 5.7 | 15.0 ± 5.0 | 0.4 |
| Lumen CSA (mm2) | 9.3 ± 3.6 | 9.6 ± 3.1 | 0.2 |
| P&M CSA (mm2) | 5.9 ± 2.8 | 5.4 ± 2.7 | 0.14 |
| Plaque burden (%) | 39 ± 13 | 36 ± 12 | 0.16 |
| Remodelling index | 1.05 ± 0.38 | 1.02 ± 0.23 | 0.068 |
| Minimum stent CSA (mm2) | 8.8 ± 3.4 | 8.4 ± 2.9 | 0.087 |
| Stent expansion (%) | 92 ± 19 | 87 ± 17 | 0.076 |
| . | No-reflow (n = 24) . | Normal-reflow (n = 166) . | P-value . |
|---|---|---|---|
| Proximal reference | |||
| EEM CSA (mm2) | 15.8 ± 7.8 | 15.6 ± 5.8 | 0.4 |
| Lumen CSA (mm2) | 9.8 ± 4.5 | 9.8 ± 3.5 | 1.0 |
| P&M CSA (mm2) | 6.0 ± 3.2 | 5.8 ± 3.2 | 0.4 |
| Plaque burden (%) | 38 ± 9 | 37 ± 9 | 0.6 |
| Minimum lumen site | |||
| EEM CSA (mm2) | 16.2 ± 6.0 | 15.6 ± 5.8 | 0.042 |
| Lumen CSA (mm2) | 3.4 ± 2.4 | 4.0 ± 2.1 | 0.021 |
| P&M CSA (mm2) | 12.8 ± 4.8 | 11.6 ± 4.3 | 0.013 |
| Plaque burden (%) | 79 ± 12 | 74 ± 16 | 0.008 |
| IVUS lesion length (mm) | 22 ± 13 | 18 ± 12 | 0.020 |
| Distal reference | |||
| EEM CSA (mm2) | 15.2 ± 5.7 | 15.0 ± 5.0 | 0.4 |
| Lumen CSA (mm2) | 9.3 ± 3.6 | 9.6 ± 3.1 | 0.2 |
| P&M CSA (mm2) | 5.9 ± 2.8 | 5.4 ± 2.7 | 0.14 |
| Plaque burden (%) | 39 ± 13 | 36 ± 12 | 0.16 |
| Remodelling index | 1.05 ± 0.38 | 1.02 ± 0.23 | 0.068 |
| Minimum stent CSA (mm2) | 8.8 ± 3.4 | 8.4 ± 2.9 | 0.087 |
| Stent expansion (%) | 92 ± 19 | 87 ± 17 | 0.076 |
At the minimum lumen sites, the absolute and %NC areas and absolute DC area were significantly greater in the no-reflow group compared with the normal-reflow group; conversely %FF area was significantly smaller in the no-reflow group compared with the normal-reflow group (Figure 1). At the largest NC sites, the absolute and %NC areas and absolute DC area were significantly greater in the no-reflow group compared with the normal-reflow group; conversely %FT and FF areas were significantly smaller in the no-reflow group compared with the normal-reflow group (Figure 2). The absolute and %NC and DC volumes were significantly greater in the no-reflow group compared with the normal-reflow group; conversely %FT and FF volumes were significantly smaller in the no-reflow group compared with the normal-reflow group (Figure 3). At the proximal reference sites, the %NC and FT areas were significantly greater in the no-reflow group compared with the normal-reflow group; conversely %FF area was significantly smaller in the no-reflow group compared with the normal-reflow group (Figure 4A). At the distal reference sites, the %NC and DC areas were significantly greater in the no-reflow group compared with the normal-reflow group (Figure 4B). The presence of at least one TCFA and multiple TCFAs within culprit lesions were more common in the no-reflow group than in the normal-reflow group (Figure 5).
![The absolute (A) and relative (B) plaque components at the minimum lumen site. Virtual histology-intravascular ultrasound analysis classified the colour-coded tissue into four major components: green [fibrotic (FT)]; yellow-green [fibro-fatty (FF)]; white [dense calcium (DC); and red [necrotic core (NC)]. At the minimum lumen site, the absolute and relative necrotic core areas and the absolute dense calcium area were significantly greater in the no-reflow group than in the normal-reflow group; conversely the relative fibro-fatty area was significantly smaller in the no-reflow group than in the normal-reflow group.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/eurheartj/32/16/10.1093/eurheartj/ehp034/2/m_ehp03401.gif?Expires=1748008394&Signature=LAEW2MFU46GI6GWi-pY0D5slcTGCepwrQSNSMbXiyGqo928xi3reQpr1AO9YlLYPyWEywLiWrh1Ef8LFFWTUSNklw1fW3qYk~ZAkHHp7WOtMI1OsYx~lIpR9dF3Bd7CrHj-QfRGfd25v625e1xIQ2y5-CLVm6YHzi7ruuPuDkrnKlj4ZKplUodpq45Np8BQ-yxXG69422CihS6zYztiaR6d7cGNJdXh-v4rnWX5fLzKEecCHwM2H3OxDxY9n680uGWUHSNuKCuGV0utdFWVMZ76dEPbVmQwM~9txti9Hvf6vzes38WJd7cdprJIKAKpU-h19vCzWy0RFf9ankLBK-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
The absolute (A) and relative (B) plaque components at the minimum lumen site. Virtual histology-intravascular ultrasound analysis classified the colour-coded tissue into four major components: green [fibrotic (FT)]; yellow-green [fibro-fatty (FF)]; white [dense calcium (DC); and red [necrotic core (NC)]. At the minimum lumen site, the absolute and relative necrotic core areas and the absolute dense calcium area were significantly greater in the no-reflow group than in the normal-reflow group; conversely the relative fibro-fatty area was significantly smaller in the no-reflow group than in the normal-reflow group.
![The absolute (A) and relative (B) plaque components at the largest necrotic core site. Virtual histology-intravascular ultrasound analysis classified the colour-coded tissue into four major components: green [fibrotic (FT)]; yellow-green [fibro-fatty (FF)]; white [dense calcium (DC); and red [necrotic core (NC)]. At the largest necrotic core site, the absolute and relative necrotic core areas and absolute dense calcium area were significantly greater in the no-reflow group than in the normal-reflow group; conversely relative fibrotic and fibro-fatty areas were significantly smaller in the no-reflow group than in the normal-reflow group.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/eurheartj/32/16/10.1093/eurheartj/ehp034/2/m_ehp03402.gif?Expires=1748008394&Signature=4lYojLzZHdVqYNTd0FYvUjX6--DzzIL2HGxQ50xM1YEzs7XE5hdOIIaLjG23Y1R9GHp6fyKzmME8fsYWp3uQmQloWTgHc3fX2vqLFbwC4JLvWJkPAHhuCASu8dsLxgkkz-rapS9VjXdjMBWQbI6yWnxNk-ubzAJLK8VzX0cNLjSK3ycY79UvzN6OPa4rTWMenr9GPahmE7~Ih5OOPBrMOYR0cCRi0ib5A19mqXNitfBKyPC0fNsdzwe~PNkxrt~KhxNDATZnBt-JCDnWQO71kLJ6cQjVmXHu3kYaJTIIU5Qq~POtXi0fVNUsOaT0uA2b3-PH55uyVQ5ZxWWEazMn3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
The absolute (A) and relative (B) plaque components at the largest necrotic core site. Virtual histology-intravascular ultrasound analysis classified the colour-coded tissue into four major components: green [fibrotic (FT)]; yellow-green [fibro-fatty (FF)]; white [dense calcium (DC); and red [necrotic core (NC)]. At the largest necrotic core site, the absolute and relative necrotic core areas and absolute dense calcium area were significantly greater in the no-reflow group than in the normal-reflow group; conversely relative fibrotic and fibro-fatty areas were significantly smaller in the no-reflow group than in the normal-reflow group.
![The volumetric absolute (A) and relative (B) plaque components. Virtual histology-intravascular ultrasound analysis classified the colour-coded tissue into four major components: green [fibrotic (FT)]; yellow-green [fibro-fatty (FF)]; white [dense calcium (DC); and red [necrotic core (NC)]. The absolute and relative necrotic core and dense calcium volumes were significantly greater in the no-reflow group than in the normal-reflow group; conversely relative fibrotic and fibro-fatty volumes were significantly smaller in the no-reflow group than in the normal-reflow group.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/eurheartj/32/16/10.1093/eurheartj/ehp034/2/m_ehp03403.gif?Expires=1748008394&Signature=I1VHQFfyxgwNaKwErxJ6IidXf1T4QpiGEg6bWS8o592B5bfzs4oeKQLRq6hN27YzGImZ0Xm5U6wth6ZXNikiq07PTlDIEGfzIWlR-vAu1QByOFnDq7lGKrl4knimBeUMPAteJybs8kG1GWA7DI5GrwO-6XM~9r3OA7pwlwi8WhlkB~0tmepjUEV-TW0tm8GJRn5FQ-1-yiIW8XoqHTaixSB9pDOkYDIrPo9s2lqsHdF-XKL9q6WAQ6z7IVAxGmRoslqekKEm4UL--KWX4YMxNQ-qznkkEHrP-s-oJJj3lS0SveOP2zXPwuYb8VN-MjLCKIFtOzV70koTYj1LHAmzuw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
The volumetric absolute (A) and relative (B) plaque components. Virtual histology-intravascular ultrasound analysis classified the colour-coded tissue into four major components: green [fibrotic (FT)]; yellow-green [fibro-fatty (FF)]; white [dense calcium (DC); and red [necrotic core (NC)]. The absolute and relative necrotic core and dense calcium volumes were significantly greater in the no-reflow group than in the normal-reflow group; conversely relative fibrotic and fibro-fatty volumes were significantly smaller in the no-reflow group than in the normal-reflow group.
![The relative plaque components at the proximal (A) and distal (B) reference sites. Virtual histology-intravascular ultrasound analysis classified the colour-coded tissue into four major components: green [fibrotic (FT)]; yellow-green [fibro-fatty (FF)]; white [dense calcium (DC); and red [necrotic core (NC)]. At the proximal reference sites, the relative necrotic core and fibrotic areas were significantly greater in the no-reflow group than in the normal-reflow group; conversely relative fibro-fatty area was significantly smaller in the no-reflow group than in the normal-reflow group. At the distal reference sites, the relative necrotic core and dense calcium areas were significantly greater in the no-reflow group than in the normal-reflow group.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/eurheartj/32/16/10.1093/eurheartj/ehp034/2/m_ehp03404.gif?Expires=1748008394&Signature=X1B--~feZVBz4z3M0VrbPvksdoNajf-g5VWKZEwAy07JUSMA5mxYkATsMOoLn33KCRUeRXEzfxOabF0AcgW~u8HUO3utHcldRN~zjaaLhngFurZYzUfHV79lq0LW2V0qRqbJjlkaRVkHmmJHxRV~Mcraoa-1-2KhqowsqscG2aSphEKnpmsVScXLWGEriSvYPzS4h6a4wKI9O2ZH2iVbmZGurvEvetV8nHSkDoRM~LVGsq~DIhC1zzN5Led~Nenzz1GOgm1wSzM3WYuDqEtUU4lOS6VEU0W1ZSGA7pL8hH4EAsd~1bShqi4YgplL87f3pr34MMyV07-wZCsbHDbc9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
The relative plaque components at the proximal (A) and distal (B) reference sites. Virtual histology-intravascular ultrasound analysis classified the colour-coded tissue into four major components: green [fibrotic (FT)]; yellow-green [fibro-fatty (FF)]; white [dense calcium (DC); and red [necrotic core (NC)]. At the proximal reference sites, the relative necrotic core and fibrotic areas were significantly greater in the no-reflow group than in the normal-reflow group; conversely relative fibro-fatty area was significantly smaller in the no-reflow group than in the normal-reflow group. At the distal reference sites, the relative necrotic core and dense calcium areas were significantly greater in the no-reflow group than in the normal-reflow group.
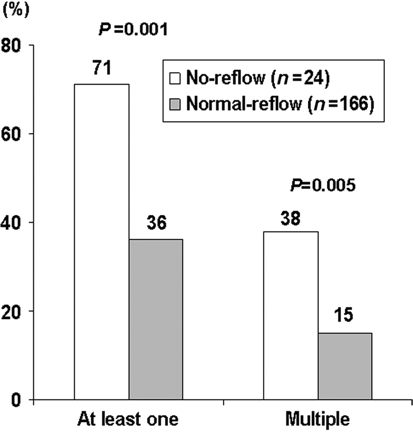
The incidence of thin-cap fibroatheromas. Virtual histology-intravascular ultrasound-detected thin-cap fibroatheroma was defined as a necrotic core ≥10% of plaque area in at least three consecutive frames without overlying fibrous tissue in the presence of ≥40% plaque burden. The presence of at least one thin-cap fibroatheroma and multiple thin-cap fibroatheromas within culprit lesions was more common in the no-reflow group than in the normal-reflow group.
Multivariable analysis was performed to identify independent predictors of no-reflow phenomenon. Univariable analysis was first conducted to identify potential predictors for no-reflow. All variables with P < 0.2 in univariable analysis (hypertension, cardiac specific troponin-I, serum glucose, triglyceride, high-sensitivity C-reactive protein, N-terminal pro-B-type natriuretic peptide, type B2/C lesion, stent diameter, minimum lumen site EEM CSA and plaque burden, IVUS lesion length, stent expansion, and volumetric VH-IVUS parameters: absolute DC and NC volumes, relative FT, FF, DC, and NC volumes) were tested for multivariable analysis. %NC volume was the only independent predictor of no-reflow after stent deployment in ACS patients (odds ratio = 1.126; 95% CI 1.045–1.214, P = 0.002).
Discussion
The present VH-IVUS study demonstrates that ACS patients with no-reflow phenomenon have more NC-containing lesions and more TCFA lesions compared with ACS patients with normal-reflow after stent deployment. Therefore, we should consider intensive use of glycoprotein IIb/IIIa inhibitors and high doses of anti-platelet agents as well as optimal stent implantation to reduce distal embolization and no-reflow phenomenon after stent deployment in ACS patients with greater NC-containing lesions.
No-reflow phenomenon was attributable to the embolization of thrombus and plaque debris that results from mechanical fragmentation of the vulnerable plaque by PCI.6–8,25 Several grey-scale IVUS studies have demonstrated that thrombus formation,6,7 positive remodelling,8,9 greater plaque burden,6,7 and decreased post-PCI plaque volume7,10 were the independent predictors of no-reflow phenomenon in ACS patients.
Virtual histology-intravascular ultrasound has the potential to provide detailed qualitative and quantitative information, and it can identify four specific plaque components. Several studies have demonstrated the coronary plaque components assessed by VH-IVUS in ACS patients.14–16 Rodriguez-Granillo et al.14 reported that VH-IVUS identified TCFA as a more prevalent finding in ACS patients than in stable angina patients. Thin-cap fibroatheroma is the precursor of plaque rupture, which accounts for a majority of coronary thrombi and coronary death.26–29 Hong et al.15 reported that the amounts of NC were larger and the amounts of FT and FF plaque were less in ACS patients compared with stable angina patients. Missel et al.16 reported that the percentage of NC and its ratio to DC in diseased coronary segments are positively associated with a high-risk ACS presentation. Plaque composition plays a role in the plaque disruption and thrombosis, which leads to acute coronary events.26,27,30,31 Lesions with a large lipid core have a higher risk for disruption than sclerotic plaques.31–33
There are controversies in the association between plaque components and no-reflow or slow-flow phenomenon after PCI in ACS patients.17–21 Kawaguchi et al.18 reported that NC volume clearly predicted distal embolization after stent deployment in patients with ST-segment elevation myocardial infarction as compared with FT, FF, DC, and total plaque volumes. Kawamoto et al.19 reported that the NC component identified with VH-IVUS was related to liberation of small embolic particles during coronary stenting, which resulted in the poorer recovery of coronary flow velocity reserve. However, Bae et al.20 reported that patients with slow flow had more FT and FF volumes, not NC volumes, at the time of the primary PCI for acute myocardial infarction. Nakamura et al.21 reported that VH-IVUS showed a trend towards larger percentage of FF plaque volume in the no-reflow group than in the normal-reflow group. In the present study, we demonstrated the NC-rich plaque as assessed by VH-IVUS was independently associated with no-reflow, and NC content through the entire lesion segment (NC volume) was more important than NC content at a single coronary site, such as minimum lumen site or largest NC site. Moreover, the culprit lesion TCFAs were observed more frequently in the no-reflow group than in the normal-reflow group. The NC components contain fragile tissues, such as lipid deposition with foam cells, intramural bleeding, and cholesterol crystals, so they can be easily liberated as small emboli by mechanical fragmentation during coronary stenting. Our results from the present study suggest that NC-rich plaque should be considered as an important predictor of distal embolization and no-reflow during stenting procedures.
Limitations
First, this was a retrospective single-centre study. The results of this study should be verified by further prospective investigation. Secondly, this study was based on a small number of patients with no-reflow, raising the possibility of selection bias. Thirdly, we did not attempt to differentiate between atherosclerotic plaque and thrombus because VH-IVUS could not determine the presence of thrombus. This may obscure the identification of TCFA. Fourth, we excluded the patients with serious conditions such as totally occluded coronary lesions, increased risk of bleeding, severe heart failure, cardiogenic shock, important systemic disease, or renal dysfunction. Thus, the present study might not represent the whole spectrum of ACS patients. Fifth, we have used model-building procedures to assess the prognostic value of the variables of interest on no-reflow. There is an increased probability that chance predictors are included in the final model because of the high amount of testing. And, the strength of the association of true predictors with no-reflow can vary at each step depending on which (true and chance) predictors are included at that step.
Clinical implications
We used VH-IVUS to evaluate the relation between coronary plaque components and no-reflow phenomenon in 190 ACS patients. No-reflow was observed in 24 patients (12.6%) at post-stenting. The absolute and %NC volumes were significantly greater in the no-reflow group compared with the normal-reflow group. The presence of at least one TCFA and multiple TCFAs within culprit lesions was more common in the no-reflow group compared with the normal-reflow group. In the multivariable analysis, %NC volume was the only independent predictor of no-reflow. Virtual histology-intravascular ultrasound analysis demonstrates that post-stenting no-reflow is associated with plaque components defined by VH-IVUS analysis with larger NC and more TCFAs in ACS patients.
Funding
This study was supported by Grants from Cardiovascular Research Foundation Asia and Cardiovascular Research Institute of Chonnam National University, Korea. Funding to pay the Open Access publication charges for this article was provided by the author, Myung Ho Jeong.
Conflict of interest. none declared.



