-
PDF
- Split View
-
Views
-
Cite
Cite
Till Keller, Claudia Martina Messow, Edith Lubos, Viviane Nicaud, Philipp S. Wild, Hans J. Rupprecht, Christoph Bickel, Stergios Tzikas, Dirk Peetz, Karl J. Lackner, Laurence Tiret, Thomas F. Münzel, Stefan Blankenberg, Renate B. Schnabel, Cystatin C and cardiovascular mortality in patients with coronary artery disease and normal or mildly reduced kidney function: results from the AtheroGene study, European Heart Journal, Volume 30, Issue 3, February 2009, Pages 314–320, https://doi.org/10.1093/eurheartj/ehn598
Close - Share Icon Share
Abstract
Chronic kidney disease is associated with increased risk of cardiovascular disease. Cystatin C is a promising marker to reliably mirror renal function. The role of cystatin C in patients with coronary artery disease (CAD) and normal or mildly reduced kidney function is the subject of current investigation.
In 2162 patients, over the whole spectrum of CAD, baseline cystatin C concentrations were measured. Patients with an estimated glomerular filtration rate of ≤60 mL/min per 1.73 m2 (n = 295) were excluded. In patients with complete follow-up information (n = 1827), 66 cardiovascular deaths were registered during a median follow-up of 3.65 years. Logarithmically transformed, standardized cystatin C was associated with cardiovascular death [hazard ratio: 1.94, 95% confidence interval (CI): 1.59–2.37, P < 0.001]. A potential threshold effect was observed; patients in the upper quartile had a 3.87-fold (95% CI: 2.33–6.42; P < 0.001) risk of mortality compared with the pooled lower quartiles. This risk association remained robust after adjustment for potential confounders including classical risk factors and N-terminal pro B-type natriuretic peptide. Serum creatinine was not associated with the outcome in this group of patients with normal renal function.
Results of this prospective study show that cystatin C is a potent predictor of cardiovascular mortality beyond classical risk factors in patients with CAD and normal or mildly reduced kidney function.
Introduction
Renal insufficiency is a major risk factor for cardiovascular disease and death.1–4 Even less severe kidney dysfunction is associated with an increase in cardiovascular risk in the community.5,6 In patients suffering from cardiovascular disease, concomitant chronic kidney disease is a common condition7,8 and substantially increases morbidity and mortality as an independent risk factor.9,10 Recently, serum creatinine-based formulas to assess renal function have been challenged by cystatin C measurements which found cystatin C being potentially superior to assess renal function.11,12 Cystatin C, a cysteine proteinase inhibitor, is an endogenous marker produced at a constant rate not relevantly excreted in the urine. As an indicator of renal function, cystatin C captures risk of cardiovascular events and mortality in ambulatory individuals with coronary artery disease (CAD) better than routine measures of renal function.13,14 Similar findings were reported in non-ST-elevation acute coronary syndrome patients with the highest risk observed in patients with cystatin C concentrations >1.25 mg/L.15 In addition, cystatin determination seems to be more sensitive to small changes in a glomerular filtration rate (GFR) and might be a superior indicator in subjects with mild renal impairment which is not detected by the creatinine measurement.16 In acute heart failure patients, cystatin C is an independent predictor of 1 year mortality, despite normal creatinine values.17
The value of cystatin C measurement in patients with manifest CAD and normal to mild renal impairment is less well defined. The primary aim of the current investigation was to examine the value of cystatin C as a predictor of cardiovascular mortality in a prospective cohort of CAD patients with an estimated GFR (eGFR) >60 mL/min per 1.73 m2. We assessed the prognostic value of cystatin C when taking into account classical cardiovascular risk factors and additionally, a strong risk predictor in cardiovascular disease, N-terminal pro B-type natriuretic peptide (Nt-proBNP).18,19
Methods
Study participants
Between June 1999 and February 2004, patients who presented with chest pain at the Department of Medicine II of the Johannes Gutenberg-University Mainz or the Bundeswehrzentralkrankenhaus Koblenz and who had at least one stenosis >30% diagnosed in a major coronary artery were enrolled in the AtheroGene study registry (n = 3800). The concept of the study and details on enrolment criteria have been outlined previously.20 The cohort comprised consecutively enrolled individuals with stable CAD or acute coronary syndrome. Exclusion criteria were evidence of haemodynamically significant valvular heart disease, surgery or trauma within the previous month, known cardiomyopathy, known cancer, febrile conditions, or use of oral anticoagulant therapy within the previous 4 weeks. In addition, patients with a serum creatinine >2.5 mg/dL on the admission laboratory test were excluded because the conditions mentioned are related to decreased cardiovascular survival independent of CAD. All exclusion criteria together were the reason for non-eligibility in about 5% of consecutive patients. Only 4% of patients declined the invitation to participate. After enrolment, most patients remained in the study after initial assessment (>98%). Patients who were on antihypertensive treatment or who had blood pressure measurements above 140/90 mm Hg were considered to have hypertension. Patients were classified as currently smoking, as having smoked in the past (if they had stopped more than 4 weeks and less than 40 years earlier), or as never having smoked (if they had never smoked or had stopped 40 or more years earlier). Baseline blood specimen and coronary angiography results were available in all subjects.
The patients were followed for a median of 3.65 years (maximum 6.9 years). Follow-up information was obtained on death from cardiovascular causes (n = 66) and death from non-cardiovascular causes (n = 40). Information on the cause of death was obtained from death certificates and verified by hospital or general practitioner charts.
Participation was restricted to German nationality and European descent. The study was approved by the local Ethics Committee. Participation was voluntary, and each subject gave written informed consent.
Cystatin C concentrations were successfully measured in 2162 consecutive patients. Glomerular filtration rate was estimated using the modification of diet in renal disease (MDRD) formula. Patients with an eGFR of 60 mL/min per 1.73 m2 or less were excluded from statistical analyses (n = 295). Follow-up information was missing for 40 subjects. Thus 1827 patients were included in the analyses.
Laboratory methods
Blood was drawn in the cath lab under standardized conditions before coronary angiography was performed. Patients with stable angina underwent venipuncture after a 12 h fast. Samples were immediately processed and stored at −80°C until analysis.
Plasma cystatin C was analysed by immunonephelometry using a Behring Nephelometer II (Dade-Behring, Inc.). Coefficients of variation have been reported to be ≤1.8% in the concentration range between 0.87 and 4.63 mg/L. Recovery is up to 101.3%.18
Serum Nt-proBNP was determined using an electrochemiluminescence sandwich immunoassay (ECLIA, Roche Diagnostics, Mannheim, Germany) on an Elecsys System 2010. Intra- and interassay precisions for the luminescence-sandwich-immunoassay are 0.8–3.0 and 2.2–5.8%, respectively. The linear range of detection of this assay is 5–35 000 pg/mL, cross-reactivity with brain natriuretic peptide, atrial natriuretic peptide is ≤0.001%. C-reactive protein was determined by a highly sensitive, latex particle-enhanced immunoassay (detection range of 0–20 mg/L, Roche Diagnostics). Lipid serum levels and creatinine were measured immediately by standardized routine methods. For conversion of cholesterol concentrations into millimoles per litre, they need to be multiplied by 0.02586.
Statistical analysis
Continuous variables are presented as mean ± standard deviation (Table 1). Cystatin C, serum creatinine, Nt-proBNP, and C-reactive protein are provided as median, 25th/75th tile, and log-transformed for testing because of positive skewness. For discrete variables, absolute and relative frequencies per category are given. Partial Pearson's correlation coefficients between cystatin C and continuous variables with potential relations to cystatin C concentrations were calculated, adjusted for age and sex. A general linear model with cystatin C as the dependent variable was computed, including age, sex, body mass index (BMI), diabetes, smoking (ex-smoker and current smoker by reference to never smoker), hypertension, low-density lipoprotein (LDL)–high-density lipoprotein (HDL) ratio, medication with angiotensin-converting enzyme-inhibitors and statins, C-reactive protein, Nt-proBNP, and creatinine. Association between risk factors and cardiovascular death during follow-up was tested by a χ2 test, for categorical variables, or by an analysis of variance, for continuous traits. Sex-specific quartiles of cystatin C and creatinine were created. Kaplan–Meier curves for cardiovascular death according to quartiles of cystatin C are shown. When considering cystatin C as a continuous variable (log-transformed and standardized) in primary analyses, the relationship with risk was linear. In exploratory analyses, the hazard ratios for cardiovascular death in each quartile of cystatin C by reference to the lowest one, then in the last quartile of cystatin C by reference to the three lower pooled, were calculated using a Cox competing risk survival model where the primary outcome was cardiovascular death and death from non-cardiovascular cause was considered as a competing outcome. We fitted first a model including age and sex, then a model adjusting for classical risk factors (age, sex, BMI, hypertension, diabetes mellitus, smoking status, LDL/HDL ratio, creatinine, and C-reactive protein). A third model was calculated with the addition of Nt-proBNP to the second model. A similar analysis was run on quartiles of creatinine.
Baseline characteristics of the study cohort for individuals with and without cardiovascular death and relation of clinical variables and biomarkers to the outcome
| Characteristic . | No cardiovascular death (n = 1761) . | Cardiovascular death (n = 66) . | P-value . |
|---|---|---|---|
| Age (years) | 60.6 (9.8) | 64.7 (9.8) | <0.001 |
| Male sex | 1429 (81.2) | 55 (83.3) | 0.66 |
| Classical risk factors | |||
| Body mass index (kg/m2) | 27.8 (4.0) | 27.6 (3.9) | 0.73 |
| Diabetes | 337 (19.1) | 20 (30.3) | 0.02 |
| Smoking status | |||
| Never | 601 (34.1) | 17 (25.8) | 0.35 |
| Past | 805 (45.7) | 33 (50.0) | |
| Present | 355 (20.2) | 16 (24.2) | |
| Hypertension | 1326 (75.3) | 53 (80.3) | 0.35 |
| Family history | 699 (39.7) | 21 (31.8) | 0.42 |
| Number of diseased vessels | |||
| 1 | 520 (29.6) | 7 (10.6) | 0.002 |
| 2 | 540 (30.7) | 22 (33.3) | |
| 3 | 700 (39.8) | 37 (56.1) | |
| LDL/HDL cholesterol | 2.63 (1.01) | 2.81 (0.94) | 0.17 |
| Cardiac medication | |||
| Angiotensin-converting enzyme-inhibitors | 883 (50.2) | 33 (50.0) | 0.98 |
| Statins | 905 (51.4) | 31 (47.0) | 0.48 |
| β-Blockers | 1145 (65.1) | 36 (54.6) | 0.08 |
| Antiplatelet therapya | |||
| None | 227 (14.3) | 13 (21.3) | |
| Aspirin only | 1124 (70.9) | 43 (70.5) | 0.16 |
| Platelet antagonist | 233 (14.7) | 5 (8.2) | |
| Biomarkers | |||
| Serum creatinine (mg/dL) | 0.92 (0.16) | 0.95 (0.17) | 0.14 |
| Cystatin C (mg/L)b | 0.79 (0.70–0.90) | 0.94 (0.79–1.08) | <0.001 |
| Nt-proBNP (pg/mL)b | 194 (87–544) | 977 (284–2416) | <0.001 |
| C-reactive protein (mg/L)b | 2.22 (0.96–5.92) | 4.35 (1.93–13.60) | <0.001 |
| Characteristic . | No cardiovascular death (n = 1761) . | Cardiovascular death (n = 66) . | P-value . |
|---|---|---|---|
| Age (years) | 60.6 (9.8) | 64.7 (9.8) | <0.001 |
| Male sex | 1429 (81.2) | 55 (83.3) | 0.66 |
| Classical risk factors | |||
| Body mass index (kg/m2) | 27.8 (4.0) | 27.6 (3.9) | 0.73 |
| Diabetes | 337 (19.1) | 20 (30.3) | 0.02 |
| Smoking status | |||
| Never | 601 (34.1) | 17 (25.8) | 0.35 |
| Past | 805 (45.7) | 33 (50.0) | |
| Present | 355 (20.2) | 16 (24.2) | |
| Hypertension | 1326 (75.3) | 53 (80.3) | 0.35 |
| Family history | 699 (39.7) | 21 (31.8) | 0.42 |
| Number of diseased vessels | |||
| 1 | 520 (29.6) | 7 (10.6) | 0.002 |
| 2 | 540 (30.7) | 22 (33.3) | |
| 3 | 700 (39.8) | 37 (56.1) | |
| LDL/HDL cholesterol | 2.63 (1.01) | 2.81 (0.94) | 0.17 |
| Cardiac medication | |||
| Angiotensin-converting enzyme-inhibitors | 883 (50.2) | 33 (50.0) | 0.98 |
| Statins | 905 (51.4) | 31 (47.0) | 0.48 |
| β-Blockers | 1145 (65.1) | 36 (54.6) | 0.08 |
| Antiplatelet therapya | |||
| None | 227 (14.3) | 13 (21.3) | |
| Aspirin only | 1124 (70.9) | 43 (70.5) | 0.16 |
| Platelet antagonist | 233 (14.7) | 5 (8.2) | |
| Biomarkers | |||
| Serum creatinine (mg/dL) | 0.92 (0.16) | 0.95 (0.17) | 0.14 |
| Cystatin C (mg/L)b | 0.79 (0.70–0.90) | 0.94 (0.79–1.08) | <0.001 |
| Nt-proBNP (pg/mL)b | 194 (87–544) | 977 (284–2416) | <0.001 |
| C-reactive protein (mg/L)b | 2.22 (0.96–5.92) | 4.35 (1.93–13.60) | <0.001 |
Data presented are the number (percentage) of patients, mean (standard deviation) or median (25th/75th tile) for cystatin C, Nt-proBNP, and C-reactive protein. LDL, low-density lipoprotein; HDL, high-density lipoprotein; Nt-proBNP, N-terminal pro B-type natriuretic peptide. P-values refer to the relation of the variable to survival.
aAntiplatelet therapy could be verified in only 1605 subjects.
bMedian and interquartile range are presented for these variables and tests are performed on log-transformed values.
Baseline characteristics of the study cohort for individuals with and without cardiovascular death and relation of clinical variables and biomarkers to the outcome
| Characteristic . | No cardiovascular death (n = 1761) . | Cardiovascular death (n = 66) . | P-value . |
|---|---|---|---|
| Age (years) | 60.6 (9.8) | 64.7 (9.8) | <0.001 |
| Male sex | 1429 (81.2) | 55 (83.3) | 0.66 |
| Classical risk factors | |||
| Body mass index (kg/m2) | 27.8 (4.0) | 27.6 (3.9) | 0.73 |
| Diabetes | 337 (19.1) | 20 (30.3) | 0.02 |
| Smoking status | |||
| Never | 601 (34.1) | 17 (25.8) | 0.35 |
| Past | 805 (45.7) | 33 (50.0) | |
| Present | 355 (20.2) | 16 (24.2) | |
| Hypertension | 1326 (75.3) | 53 (80.3) | 0.35 |
| Family history | 699 (39.7) | 21 (31.8) | 0.42 |
| Number of diseased vessels | |||
| 1 | 520 (29.6) | 7 (10.6) | 0.002 |
| 2 | 540 (30.7) | 22 (33.3) | |
| 3 | 700 (39.8) | 37 (56.1) | |
| LDL/HDL cholesterol | 2.63 (1.01) | 2.81 (0.94) | 0.17 |
| Cardiac medication | |||
| Angiotensin-converting enzyme-inhibitors | 883 (50.2) | 33 (50.0) | 0.98 |
| Statins | 905 (51.4) | 31 (47.0) | 0.48 |
| β-Blockers | 1145 (65.1) | 36 (54.6) | 0.08 |
| Antiplatelet therapya | |||
| None | 227 (14.3) | 13 (21.3) | |
| Aspirin only | 1124 (70.9) | 43 (70.5) | 0.16 |
| Platelet antagonist | 233 (14.7) | 5 (8.2) | |
| Biomarkers | |||
| Serum creatinine (mg/dL) | 0.92 (0.16) | 0.95 (0.17) | 0.14 |
| Cystatin C (mg/L)b | 0.79 (0.70–0.90) | 0.94 (0.79–1.08) | <0.001 |
| Nt-proBNP (pg/mL)b | 194 (87–544) | 977 (284–2416) | <0.001 |
| C-reactive protein (mg/L)b | 2.22 (0.96–5.92) | 4.35 (1.93–13.60) | <0.001 |
| Characteristic . | No cardiovascular death (n = 1761) . | Cardiovascular death (n = 66) . | P-value . |
|---|---|---|---|
| Age (years) | 60.6 (9.8) | 64.7 (9.8) | <0.001 |
| Male sex | 1429 (81.2) | 55 (83.3) | 0.66 |
| Classical risk factors | |||
| Body mass index (kg/m2) | 27.8 (4.0) | 27.6 (3.9) | 0.73 |
| Diabetes | 337 (19.1) | 20 (30.3) | 0.02 |
| Smoking status | |||
| Never | 601 (34.1) | 17 (25.8) | 0.35 |
| Past | 805 (45.7) | 33 (50.0) | |
| Present | 355 (20.2) | 16 (24.2) | |
| Hypertension | 1326 (75.3) | 53 (80.3) | 0.35 |
| Family history | 699 (39.7) | 21 (31.8) | 0.42 |
| Number of diseased vessels | |||
| 1 | 520 (29.6) | 7 (10.6) | 0.002 |
| 2 | 540 (30.7) | 22 (33.3) | |
| 3 | 700 (39.8) | 37 (56.1) | |
| LDL/HDL cholesterol | 2.63 (1.01) | 2.81 (0.94) | 0.17 |
| Cardiac medication | |||
| Angiotensin-converting enzyme-inhibitors | 883 (50.2) | 33 (50.0) | 0.98 |
| Statins | 905 (51.4) | 31 (47.0) | 0.48 |
| β-Blockers | 1145 (65.1) | 36 (54.6) | 0.08 |
| Antiplatelet therapya | |||
| None | 227 (14.3) | 13 (21.3) | |
| Aspirin only | 1124 (70.9) | 43 (70.5) | 0.16 |
| Platelet antagonist | 233 (14.7) | 5 (8.2) | |
| Biomarkers | |||
| Serum creatinine (mg/dL) | 0.92 (0.16) | 0.95 (0.17) | 0.14 |
| Cystatin C (mg/L)b | 0.79 (0.70–0.90) | 0.94 (0.79–1.08) | <0.001 |
| Nt-proBNP (pg/mL)b | 194 (87–544) | 977 (284–2416) | <0.001 |
| C-reactive protein (mg/L)b | 2.22 (0.96–5.92) | 4.35 (1.93–13.60) | <0.001 |
Data presented are the number (percentage) of patients, mean (standard deviation) or median (25th/75th tile) for cystatin C, Nt-proBNP, and C-reactive protein. LDL, low-density lipoprotein; HDL, high-density lipoprotein; Nt-proBNP, N-terminal pro B-type natriuretic peptide. P-values refer to the relation of the variable to survival.
aAntiplatelet therapy could be verified in only 1605 subjects.
bMedian and interquartile range are presented for these variables and tests are performed on log-transformed values.
All analyses were carried out using SAS v9.1 or R2.5.1.21 As P-values are not adjusted for multiple testing, they have to be considered as descriptive.
Results
The mean age of the study cohort was 61 years, 81.2% were of male gender. A summary of the baseline characteristics of the study population is given in Table 1 for patients who died of cardiovascular causes (n = 66) during follow-up and those who did not (n = 1761, including 40 deaths from other causes). Patients with cardiovascular death, compared with others, were slightly older and more often diagnosed with diabetes. The number of diseased vessels was higher in the subgroup which died of cardiovascular causes. With regard to biomarkers, cystatin C, C-reactive protein, and Nt-proBNP were higher in those with cardiovascular death. The survival analyses showed that age, diabetes, number of diseased vessels, cystatin C, Nt-proBNP, and C-reactive protein were all associated with cardiovascular death. Creatinine was not significantly associated with the outcome.
Cystatin C showed a moderate to strong correlation with creatinine (partial Pearson's correlation coefficient: 0.32) and age (partial Pearson's correlation coefficient: 0.37) (Table 2). The biomarkers Nt-proBNP and C-reactive protein were positively related to cystatin C measurements (r = 0.21 and 0.15, respectively, P < 0.0001).
Partial Pearson's correlation coefficients between cystatin C concentration and other variables, adjusted on age and sex
| . | Cystatin C . | eGFR . | Creatinine . | Age . | BMI . | LDL/HDL ratio . | Nt-proBNP . | C-reactive protein . |
|---|---|---|---|---|---|---|---|---|
| Cystatin C | 1 | −0.32, <0.001 | 0.33, <0.001 | 0.37, <0.001 | 0.15, <0.001 | 0.04, 0.12 | 0.21, <0.001 | 0.15, <0.001 |
| eGFR | 1 | −0.99, <0.001 | −0.32, <0.001 | −0.07, 0.002 | 0.00, 0.86 | −0.08, <0.001 | −0.01, 0.80 | |
| Creatinine | 1 | 0.15, <0.001 | 0.06, 0.01 | 0.00, 0.85 | 0.09, <0.001 | 0.01, 0.71 | ||
| Age | 1 | −0.06, 0.007 | −0.18, <0.001 | 0.26, <0.001 | 0.01, 0.59 | |||
| BMI | 1 | 0.08, <0.001 | −0.06, 0.01 | 0.14, <0.001 | ||||
| LDL/HDL ratio | 1 | 0.09, <0.001 | 0.19, <0.001 | |||||
| Nt-proBNP | 1 | 0.41, <0.001 |
| . | Cystatin C . | eGFR . | Creatinine . | Age . | BMI . | LDL/HDL ratio . | Nt-proBNP . | C-reactive protein . |
|---|---|---|---|---|---|---|---|---|
| Cystatin C | 1 | −0.32, <0.001 | 0.33, <0.001 | 0.37, <0.001 | 0.15, <0.001 | 0.04, 0.12 | 0.21, <0.001 | 0.15, <0.001 |
| eGFR | 1 | −0.99, <0.001 | −0.32, <0.001 | −0.07, 0.002 | 0.00, 0.86 | −0.08, <0.001 | −0.01, 0.80 | |
| Creatinine | 1 | 0.15, <0.001 | 0.06, 0.01 | 0.00, 0.85 | 0.09, <0.001 | 0.01, 0.71 | ||
| Age | 1 | −0.06, 0.007 | −0.18, <0.001 | 0.26, <0.001 | 0.01, 0.59 | |||
| BMI | 1 | 0.08, <0.001 | −0.06, 0.01 | 0.14, <0.001 | ||||
| LDL/HDL ratio | 1 | 0.09, <0.001 | 0.19, <0.001 | |||||
| Nt-proBNP | 1 | 0.41, <0.001 |
Provided are partial Pearson's correlation coefficients (top) and respective P-values (bottom). eGFR, estimated glomerular filtration rate; BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; Nt-proBNP, N-terminal pro B-type natriuretic peptide.
Partial Pearson's correlation coefficients between cystatin C concentration and other variables, adjusted on age and sex
| . | Cystatin C . | eGFR . | Creatinine . | Age . | BMI . | LDL/HDL ratio . | Nt-proBNP . | C-reactive protein . |
|---|---|---|---|---|---|---|---|---|
| Cystatin C | 1 | −0.32, <0.001 | 0.33, <0.001 | 0.37, <0.001 | 0.15, <0.001 | 0.04, 0.12 | 0.21, <0.001 | 0.15, <0.001 |
| eGFR | 1 | −0.99, <0.001 | −0.32, <0.001 | −0.07, 0.002 | 0.00, 0.86 | −0.08, <0.001 | −0.01, 0.80 | |
| Creatinine | 1 | 0.15, <0.001 | 0.06, 0.01 | 0.00, 0.85 | 0.09, <0.001 | 0.01, 0.71 | ||
| Age | 1 | −0.06, 0.007 | −0.18, <0.001 | 0.26, <0.001 | 0.01, 0.59 | |||
| BMI | 1 | 0.08, <0.001 | −0.06, 0.01 | 0.14, <0.001 | ||||
| LDL/HDL ratio | 1 | 0.09, <0.001 | 0.19, <0.001 | |||||
| Nt-proBNP | 1 | 0.41, <0.001 |
| . | Cystatin C . | eGFR . | Creatinine . | Age . | BMI . | LDL/HDL ratio . | Nt-proBNP . | C-reactive protein . |
|---|---|---|---|---|---|---|---|---|
| Cystatin C | 1 | −0.32, <0.001 | 0.33, <0.001 | 0.37, <0.001 | 0.15, <0.001 | 0.04, 0.12 | 0.21, <0.001 | 0.15, <0.001 |
| eGFR | 1 | −0.99, <0.001 | −0.32, <0.001 | −0.07, 0.002 | 0.00, 0.86 | −0.08, <0.001 | −0.01, 0.80 | |
| Creatinine | 1 | 0.15, <0.001 | 0.06, 0.01 | 0.00, 0.85 | 0.09, <0.001 | 0.01, 0.71 | ||
| Age | 1 | −0.06, 0.007 | −0.18, <0.001 | 0.26, <0.001 | 0.01, 0.59 | |||
| BMI | 1 | 0.08, <0.001 | −0.06, 0.01 | 0.14, <0.001 | ||||
| LDL/HDL ratio | 1 | 0.09, <0.001 | 0.19, <0.001 | |||||
| Nt-proBNP | 1 | 0.41, <0.001 |
Provided are partial Pearson's correlation coefficients (top) and respective P-values (bottom). eGFR, estimated glomerular filtration rate; BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; Nt-proBNP, N-terminal pro B-type natriuretic peptide.
Linear regression analysis revealed the classical risk factors age, sex, BMI, current smoking, C-reactive protein, Nt-proBNP, and creatinine as predictors of cystatin C (see Supplementary material online, Table S1). Overall, the model consisting of classical risk factors, creatinine, and novel biomarkers explained 29% of the variability in cystatin C concentrations. As cystatin C varies over a small range of values, regression coefficients are relatively small.
Kaplan–Meier curves revealed a potential threshold effect for cystatin concentrations in the upper quartile (Figure 1). For logarithmically transformed, standardized cystatin C, however, the association with risk was linear. One unit increase in cystatin C was associated with a 1.94-fold [95% confidence interval (CI): 1.59–2.37, P < 0.001] risk of mortality (Table 3). In addition, we calculated hazard ratios for cardiovascular death-associated cystatin C concentrations in the upper quartile compared with the pooled lower quartiles. Individuals in the highest quartile had a 3.87-fold (95% CI: 2.33–6.42, P < 0.001) increased risk of cardiovascular mortality. This association was slightly attenuated in the models adjusting for classical cardiovascular risk factors and Nt-proBNP. By comparison, the fully adjusted hazard ratio of death from non-cardiovascular causes in the highest quartile of cystatin C was 1.86 (95% CI: 0.90–3.81, P = 0.09).
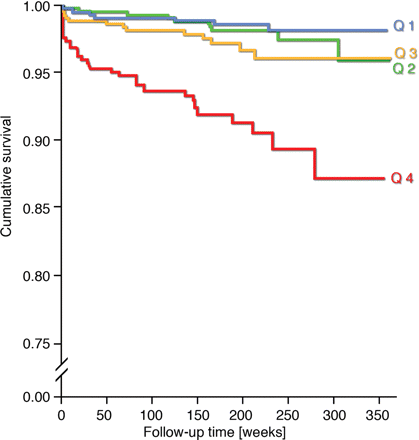
Kaplan–Meier survival curves for quartiles (Q) of cystatin C concentration measured in microgram per litre. Plog rank < 0.001.
| Sex-specific quartiles . | Cardiovascular death (n) . | Hazard ratios (95% CI), P-value . | |||
|---|---|---|---|---|---|
| . | No . | Yes . | Model 1 . | Model 2 . | Model 3 . |
| Associated with quartiles of cystatin C | |||||
| Cystatin C (log-transformed, standardized) | 1816 | 66 | 1.94 (1.59–2.37), <0.001 | 1.78 (1.45–2.19), <0.001 | 1.55 (1.25–1.92), <0.001 |
| Females: 0.42–0.69 | 450 | 7 | Referent | Referent | Referent |
| Males: 0.43–0.70 | |||||
| Females: 0.69–0.79 | 442 | 10 | 1.36 (0.51–3.61), 0.54 | 1.32 (0.49–3.52), 0.58 | 1.29 (0.48–3.46), 0.61 |
| Males: 0.71–0.80 | |||||
| Females: 0.79–0.92 | 442 | 12 | 1.64 (0.63–4.25), 0.31 | 1.59 (0.60–4.21), 0.35 | 1.45 (0.54–3.87), 0.46 |
| Males: 0.80–0.91 | |||||
| Females: 0.93–2.25 | 416 | 37 | 5.26 (2.25–12.30), <0.001 | 4.86 (1.98–11.89), <0.001 | 3.77 (1.50–9.46), 0.005 |
| Males: 0.91–2.48 | |||||
| Fourth quartile vs. all others pooled | 3.87 (2.33–6.42), <0.001 | 3.59 (2.09–6.14), <0.001 | 2.91 (1.67–5.05), <0.001 | ||
| Associated with quartiles of creatinine | |||||
| Creatinine (standardized) | 1816 | 66 | 1.12 (0.85–1.48), 0.43 | 1.12 (0.85–1.48), 0.43 | 1.00 (0.76–1.33), 0.99 |
| Females: 0.38–0.68 | 430 | 12 | Referent | Referent | Referent |
| Males: 0.28–0.84 | |||||
| Females: 0.69–0.77 | 442 | 15 | 1.11 (0.52–2.38), 0.79 | 1.04 (0.48–2.23), 0.92 | 0.99 (0.46–2.14), 0.99 |
| Males: 0.85–0.94 | |||||
| Females: 0.78–0.86 | 463 | 14 | 0.93 (0.43–2.01), 0.85 | 0.90 (0.41–1.95), 0.78 | 0.77 (0.35–1.68), 0.51 |
| Males: 0.95–1.05 | |||||
| Females: 0.87–1.02 | 415 | 25 | 1.67 (0.83–3.34), 0.15 | 1.62 (0.80–3.28), 0.18 | 1.27 (0.62–2.61), 0.51 |
| Males: 1.06–1.32 | |||||
| Sex-specific quartiles . | Cardiovascular death (n) . | Hazard ratios (95% CI), P-value . | |||
|---|---|---|---|---|---|
| . | No . | Yes . | Model 1 . | Model 2 . | Model 3 . |
| Associated with quartiles of cystatin C | |||||
| Cystatin C (log-transformed, standardized) | 1816 | 66 | 1.94 (1.59–2.37), <0.001 | 1.78 (1.45–2.19), <0.001 | 1.55 (1.25–1.92), <0.001 |
| Females: 0.42–0.69 | 450 | 7 | Referent | Referent | Referent |
| Males: 0.43–0.70 | |||||
| Females: 0.69–0.79 | 442 | 10 | 1.36 (0.51–3.61), 0.54 | 1.32 (0.49–3.52), 0.58 | 1.29 (0.48–3.46), 0.61 |
| Males: 0.71–0.80 | |||||
| Females: 0.79–0.92 | 442 | 12 | 1.64 (0.63–4.25), 0.31 | 1.59 (0.60–4.21), 0.35 | 1.45 (0.54–3.87), 0.46 |
| Males: 0.80–0.91 | |||||
| Females: 0.93–2.25 | 416 | 37 | 5.26 (2.25–12.30), <0.001 | 4.86 (1.98–11.89), <0.001 | 3.77 (1.50–9.46), 0.005 |
| Males: 0.91–2.48 | |||||
| Fourth quartile vs. all others pooled | 3.87 (2.33–6.42), <0.001 | 3.59 (2.09–6.14), <0.001 | 2.91 (1.67–5.05), <0.001 | ||
| Associated with quartiles of creatinine | |||||
| Creatinine (standardized) | 1816 | 66 | 1.12 (0.85–1.48), 0.43 | 1.12 (0.85–1.48), 0.43 | 1.00 (0.76–1.33), 0.99 |
| Females: 0.38–0.68 | 430 | 12 | Referent | Referent | Referent |
| Males: 0.28–0.84 | |||||
| Females: 0.69–0.77 | 442 | 15 | 1.11 (0.52–2.38), 0.79 | 1.04 (0.48–2.23), 0.92 | 0.99 (0.46–2.14), 0.99 |
| Males: 0.85–0.94 | |||||
| Females: 0.78–0.86 | 463 | 14 | 0.93 (0.43–2.01), 0.85 | 0.90 (0.41–1.95), 0.78 | 0.77 (0.35–1.68), 0.51 |
| Males: 0.95–1.05 | |||||
| Females: 0.87–1.02 | 415 | 25 | 1.67 (0.83–3.34), 0.15 | 1.62 (0.80–3.28), 0.18 | 1.27 (0.62–2.61), 0.51 |
| Males: 1.06–1.32 | |||||
Model 1: age- and sex-adjusted.
Model 2: additionally adjusted for risk factors (body mass index, smoking status, diabetes, hypertension, LDL/HDL ratio, and C-reactive protein).
Model 3: additionally adjusted for Nt-proBNP.
| Sex-specific quartiles . | Cardiovascular death (n) . | Hazard ratios (95% CI), P-value . | |||
|---|---|---|---|---|---|
| . | No . | Yes . | Model 1 . | Model 2 . | Model 3 . |
| Associated with quartiles of cystatin C | |||||
| Cystatin C (log-transformed, standardized) | 1816 | 66 | 1.94 (1.59–2.37), <0.001 | 1.78 (1.45–2.19), <0.001 | 1.55 (1.25–1.92), <0.001 |
| Females: 0.42–0.69 | 450 | 7 | Referent | Referent | Referent |
| Males: 0.43–0.70 | |||||
| Females: 0.69–0.79 | 442 | 10 | 1.36 (0.51–3.61), 0.54 | 1.32 (0.49–3.52), 0.58 | 1.29 (0.48–3.46), 0.61 |
| Males: 0.71–0.80 | |||||
| Females: 0.79–0.92 | 442 | 12 | 1.64 (0.63–4.25), 0.31 | 1.59 (0.60–4.21), 0.35 | 1.45 (0.54–3.87), 0.46 |
| Males: 0.80–0.91 | |||||
| Females: 0.93–2.25 | 416 | 37 | 5.26 (2.25–12.30), <0.001 | 4.86 (1.98–11.89), <0.001 | 3.77 (1.50–9.46), 0.005 |
| Males: 0.91–2.48 | |||||
| Fourth quartile vs. all others pooled | 3.87 (2.33–6.42), <0.001 | 3.59 (2.09–6.14), <0.001 | 2.91 (1.67–5.05), <0.001 | ||
| Associated with quartiles of creatinine | |||||
| Creatinine (standardized) | 1816 | 66 | 1.12 (0.85–1.48), 0.43 | 1.12 (0.85–1.48), 0.43 | 1.00 (0.76–1.33), 0.99 |
| Females: 0.38–0.68 | 430 | 12 | Referent | Referent | Referent |
| Males: 0.28–0.84 | |||||
| Females: 0.69–0.77 | 442 | 15 | 1.11 (0.52–2.38), 0.79 | 1.04 (0.48–2.23), 0.92 | 0.99 (0.46–2.14), 0.99 |
| Males: 0.85–0.94 | |||||
| Females: 0.78–0.86 | 463 | 14 | 0.93 (0.43–2.01), 0.85 | 0.90 (0.41–1.95), 0.78 | 0.77 (0.35–1.68), 0.51 |
| Males: 0.95–1.05 | |||||
| Females: 0.87–1.02 | 415 | 25 | 1.67 (0.83–3.34), 0.15 | 1.62 (0.80–3.28), 0.18 | 1.27 (0.62–2.61), 0.51 |
| Males: 1.06–1.32 | |||||
| Sex-specific quartiles . | Cardiovascular death (n) . | Hazard ratios (95% CI), P-value . | |||
|---|---|---|---|---|---|
| . | No . | Yes . | Model 1 . | Model 2 . | Model 3 . |
| Associated with quartiles of cystatin C | |||||
| Cystatin C (log-transformed, standardized) | 1816 | 66 | 1.94 (1.59–2.37), <0.001 | 1.78 (1.45–2.19), <0.001 | 1.55 (1.25–1.92), <0.001 |
| Females: 0.42–0.69 | 450 | 7 | Referent | Referent | Referent |
| Males: 0.43–0.70 | |||||
| Females: 0.69–0.79 | 442 | 10 | 1.36 (0.51–3.61), 0.54 | 1.32 (0.49–3.52), 0.58 | 1.29 (0.48–3.46), 0.61 |
| Males: 0.71–0.80 | |||||
| Females: 0.79–0.92 | 442 | 12 | 1.64 (0.63–4.25), 0.31 | 1.59 (0.60–4.21), 0.35 | 1.45 (0.54–3.87), 0.46 |
| Males: 0.80–0.91 | |||||
| Females: 0.93–2.25 | 416 | 37 | 5.26 (2.25–12.30), <0.001 | 4.86 (1.98–11.89), <0.001 | 3.77 (1.50–9.46), 0.005 |
| Males: 0.91–2.48 | |||||
| Fourth quartile vs. all others pooled | 3.87 (2.33–6.42), <0.001 | 3.59 (2.09–6.14), <0.001 | 2.91 (1.67–5.05), <0.001 | ||
| Associated with quartiles of creatinine | |||||
| Creatinine (standardized) | 1816 | 66 | 1.12 (0.85–1.48), 0.43 | 1.12 (0.85–1.48), 0.43 | 1.00 (0.76–1.33), 0.99 |
| Females: 0.38–0.68 | 430 | 12 | Referent | Referent | Referent |
| Males: 0.28–0.84 | |||||
| Females: 0.69–0.77 | 442 | 15 | 1.11 (0.52–2.38), 0.79 | 1.04 (0.48–2.23), 0.92 | 0.99 (0.46–2.14), 0.99 |
| Males: 0.85–0.94 | |||||
| Females: 0.78–0.86 | 463 | 14 | 0.93 (0.43–2.01), 0.85 | 0.90 (0.41–1.95), 0.78 | 0.77 (0.35–1.68), 0.51 |
| Males: 0.95–1.05 | |||||
| Females: 0.87–1.02 | 415 | 25 | 1.67 (0.83–3.34), 0.15 | 1.62 (0.80–3.28), 0.18 | 1.27 (0.62–2.61), 0.51 |
| Males: 1.06–1.32 | |||||
Model 1: age- and sex-adjusted.
Model 2: additionally adjusted for risk factors (body mass index, smoking status, diabetes, hypertension, LDL/HDL ratio, and C-reactive protein).
Model 3: additionally adjusted for Nt-proBNP.
No significant association was observed between creatinine and cardiovascular death (Table 3).
Discussion
In this prospective study in 1827 consecutive patients with CAD, baseline cystatin C concentrations were related to future cardiovascular mortality in individuals with normal or mildly reduced renal function (eGFR > 60 mL/min). This association remained robust after adjustment for potential cardiovascular confounders. We can therefore extend and refine the knowledge about cystatin C for cardiovascular risk prediction in CAD patients in the range of no or mild renal impairment with cystatin C concentrations in the lowest percentiles reported in recent investigations.13,14 A potential threshold effect for individuals in the upper quartile, similarly seen in recent publications of patients with CAD in relation to overall mortality, needs further exploration.13,15 The lower bound of quartile 4 in males is 0.91 mg/L and thus close to the upper range of normal values provided by the manufacturer which were derived from 413 individuals, 194 males, aged between 1 and 78 years. Interestingly, although serum creatinine was one of the strongest predictors of cystatin C concentrations, it was not related to the outcome in this subgroup with maintained renal function. Overall, clinical variables only explained 29% of the variability in cystatin C.
Cystatin C as described in heart failure might just occur as a bystander of GFR.22 However, mirroring GFR more closely than creatinine-dependent estimates might be a valuable and more accurate tool compared with previous methods. In particular, in the setting of mild renal impairment, which is not adequately reflected by creatinine, it can support the identification of patients with adverse outcome. This ability of cystatin C to be very sensitive for mild decrease in GFR has been reported to be predictive of cardiovascular events and mortality in the community.23
In acute heart failure patients with creatinine concentrations in the normal range, cystatin C was independently associated with mortality at 12 months.17 This characteristic holds true in our CAD patient cohort with normal or only slightly elevated creatinine and renders it an interesting risk indicator in addition to classical cardiovascular risk factors and Nt-proBNP. Furthermore, cystatin C is less affected by gender, age, and body composition24,25 in comparison with creatinine and has little intra-individual variation26 which is essential for a potential biomarker for routine diagnostics.
Consistent with prior studies, we report a statistically significant positive correlation of cystatin C with C-reactive protein as a marker of inflammation.15,27 The coefficients in our cohort were small, and cystatin C concentrations were predictive of cardiovascular mortality independent of this inflammatory marker.
Renal function has an important influence on circulating concentrations of natriuretic peptides, in particular for Nt-proBNP28–30 that belongs to the strongest currently available cardiovascular risk indicators.31 Our data confirm a moderate correlation (r = 0.32) of cystatin C with Nt-proBNP.15 The association of cystatin C with the outcome remained similar after adjustment for Nt-proBNP.
It is well known that patients with renal dysfunction are at significantly higher risk for in-hospital mortality, thrombotic and bleeding events and adverse long-term outcome after acute coronary syndrome compared to patients without kidney disease.32–34 A higher risk has not only been observed in severe renal insufficiency but even in patients with a mild stage of chronic kidney disease.35 It is important to identify these patients with increased risk immediately in the chest pain unit in order to be able to plan interventions and target specific therapy.36,37 Our data across the whole spectrum of CAD suggest that cystatin C is a marker that supports the diagnosis of high-risk patients in the acute care setting with a multiple marker strategy in addition to Nt-proBNP and necrosis markers. The prompt availability of cystatin C concentrations might not only help therapeutic decision-making but can also support clinical trials to optimize interventional concepts and provide more aggressive medical care to this patient group.37
Overall, our findings indicate that cystatin C is a potential marker for identification of CAD patients at high risk for whom effective measures of prevention and protection are available. This will become of socioeconomic relevance since with longer survival in patients with CAD we are facing an increasing burden of coexisting subclinical chronic kidney disease and cardiovascular disease.38
Limitations
During a median follow-up of 3.65 years, only a relatively small number of deaths were registered which may cause unstable results, especially in the multivariate survival analysis. The sample size is determined through the number of patients eligible and willing to participate. The observed effects are consistent with our pathophysiological hypothesis and the results are, in part, confirmatory to recent investigations; yet, our data have to be assessed as exploratory and need confirmation in independent cohorts. As known from studies enrolling patients with manifest CAD, the majority of participants was of male sex, and the transportability of results to females has to be shown.
To date, no standardized assay for cystatin C determination is available, although the nephelometric measurement seems to be the one most widely used method in recent investigations and no recommendations for uniform cut-off values exist which renders clinical application and comparability between studies difficult.
We cannot provide direct measurements of GFR but estimated it by the generally used modified MDRD formula which has some minor flaws that may lead to inaccuracies in the assessment; in particular, the potential measurement error in eGFR in persons with mildly reduced renal function (eGFR > 60 mL/min/1.73 m2) may be substantial.39,40 If direct measurement of the GFR is not possible, the eGFR has provided a good approximation, but may be inferior to cystatin C concentrations.16
In conclusion, cystatin C as a marker of renal function can enhance cardiovascular risk prediction in addition to classical risk factors, inflammation, and Nt-proBNP in patients across the whole spectrum of patients with CAD and mild chronic kidney disease. Future studies have to demonstrate whether cardiovascular survival can be improved when cystatin C concentrations are used for prognostic assessment and therapeutic decision-making.
Supplementary material
Supplementary Material is available at European Heart Journal online.
Funding
The AtheroGene study is supported by a grant of the ‘Stiftung Rheinland-Pfalz für Innovation’, Ministry for Science and Education (AZ 15202–386261/545), Mainz.
Conflict of interest: none declared.
Acknowledgements
The authors are indebted to Margot Neuser for her graphical work and to Beatrix C. Mattes for critical review of the manuscript.



