-
PDF
- Split View
-
Views
-
Cite
Cite
Klara Lodin, Cristina Oliveira Da Silva, Anne Wang Gottlieb, Ivana Bulatovic, Andreas Rück, Isaac George, David J Cohen, Frieder Braunschweig, Peter Svenarud, Maria J Eriksson, Kristina H Haugaa, Magnus Dalén, Bahira Shahim, Mitral annular disjunction and mitral valve prolapse: long-term risk of ventricular arrhythmias after surgery, European Heart Journal, 2025;, ehaf195, https://doi.org/10.1093/eurheartj/ehaf195
Close - Share Icon Share
Abstract
Mitral valve prolapse (MVP) is associated with progressive mitral regurgitation (MR) requiring surgical correction. A subset of patients with MVP experience ventricular arrhythmias (VA), and mitral annular disjunction (MAD) has been reported as a risk factor. This study aimed to assess the long-term risk of VA in patients with MAD and MVP undergoing mitral valve surgery for MR.
Patients with MVP with moderate or severe degenerative MR undergoing mitral valve surgery (repair or replacement) in 2010–22 at Karolinska University Hospital were included. Mitral annular disjunction length, referring to true MAD, was measured at end systole on pre- and post-operative transthoracic echocardiography. The primary outcome consisted of VA including hospitalizations, outpatient visits or ablation for confirmed sustained or non-sustained ventricular tachycardia, or high burden of premature ventricular complexes and assessed from medical records.
Of 599 patients undergoing mitral valve surgery, 96 (16%) had pre-operative MAD. The median MAD length was 8.0 [inter-quartile range (IQR) 5.0–10.0] mm. Compared with patients without MAD, patients with MAD were younger (55 ± 15 vs 63 ± 11 years), were more often women (31% vs 17%), and had more Barlow’s disease (70% vs 27%). Mitral annular disjunction was surgically corrected in all patients. During a median follow-up time of 5.4 (IQR 2.8–7.5) years, patients with pre-operative MAD had a higher risk of VA (hazard ratio adjusted for age and sex 3.33, 95% confidence interval 1.37–8.08) regardless of repair/replacement (Pinteraction = .18).
Mitral annular disjunction in patients with MVP and MR was associated with a three-fold increased long-term risk of VA post-mitral valve surgery, despite anatomical correction of MAD.
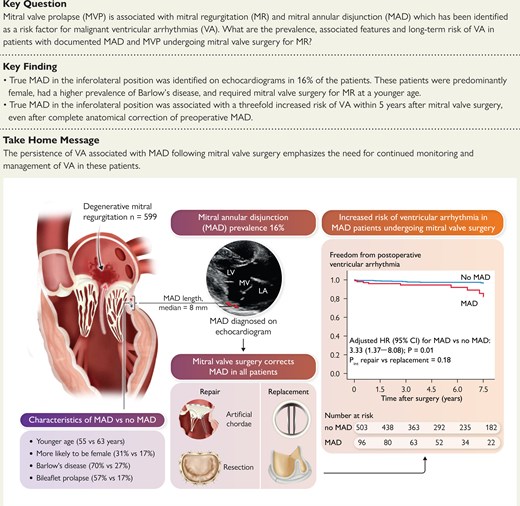
CI, confidence interval; HR, hazard ratio; MAD, mitral annular disjunction.
Introduction
Mitral valve prolapse (MVP) is the most prevalent valve disorder in Western countries, with underlying aetiologies that include Barlow’s disease, fibroelastic deficiency, and mixed pathologies.1 Although MVP has a good overall prognosis, ∼25% of patients develop progressive mitral regurgitation (MR).2 The presence of MR and its effect on left ventricular (LV) function and size are believed to increase the risk of mortality and sudden cardiac death. However, a subset of patients with MVP, with or without MR, experience a more malignant course due to an increased risk of ventricular arrhythmia (VA).3 Several features on the mitral valve apparatus, commonly coexisting, have been associated with VA including mitral annular disjunction (MAD), Barlow’s disease, and bileaflet MVP.4,5 Other risk features include late gadolinium enhancement in the LV inferolateral wall or papillary muscles, frequent premature ventricular complexes (PVC),6 ECG T-wave inversion,7 and non-sustained ventricular tachycardia (VT) at exercise stress test.8 Although surgical correction of MR has been shown to normalize mortality risk overall among patients with MVP,9 whether patients with arrhythmic features still face an increased VA risk post-surgery remains unclear.
Mitral annular disjunction is characterized by detachment of the roots of the mitral annulus from the posterolateral atrioventricular junction. During systole, as the posterolateral myocardium contracts, the annulus ‘slides’ and becomes detached from the ventricular myocardium.10 Recently, MAD has been classified as either ‘true’ or ‘pseudo’ MAD, depending on whether the disjunction is observed during both systole and diastole or only during systole.11 The excess mobility and traction in the presence of MAD are believed to accelerate valvular degeneration, leaflet prolapse, and papillary muscle fibrosis, constituting a substrate for VA.3 In theory, mitral valve surgery should relieve the mechanical stress on the mitral leaflets, chordae, and papillary muscles and mitigate the risk of VA. However, given the complexity of mitral valve pathology in the presence of MAD, it has been speculated that mitral valve surgery may not effectively correct MAD. Additionally, as severe MR generally develops over many years,12 the risk of VA may remain even after mitral valve surgery owing to irreversible structural changes.13 It is also possible that MAD may constitute a marker of an underlying cardiomyopathy with progressive LV remodelling that persists even after surgical correction of MR.14,15
To better characterize these complex relationships, we sought to assess the prevalence, features, and long-term risk of VA in patients with MAD and MVP undergoing either surgical mitral valve repair or replacement for degenerative MR in a well-characterized cohort without coronary artery disease or any other known arrhythmic substrates.
Methods
Study population and outcomes
The study cohort consisted of patients with MVP and moderate or severe degenerative MR undergoing surgical mitral valve repair or replacement between 2010 and 2022 at Karolinska University Hospital, Stockholm, Sweden (see Supplementary data online, Figure S1). Patients were excluded if pre- and post-operative echocardiograms were not stored at Karolinska University Hospital or if they had known ischaemic heart disease including stable or unstable angina, myocardial infarction, history of percutaneous coronary intervention or coronary bypass grafting, previous or concomitant aortic valve interventions, mitral valve stenosis or rheumatic mitral valve disease, endocarditis, a second mitral valve procedure, primary cardiomyopathy, or channelopathy.
Data on patient characteristics and outcomes were collected from electronic medical records, ECG recordings, and echocardiograms blinded to the presence of MAD. Ethical approval was granted by the Swedish Ethical Review Authority.
The primary outcome consisted of VA including hospitalizations, outpatient visits or ablation for confirmed sustained VT or non-sustained VT (≥3 consecutive ventricular beats <30 s with heart rate >100 b.p.m.), or high burden of PVC (≥5% total PVC burden)16 (see Supplementary data online, Table S1). Other study outcomes included post-operative all-cause mortality, sudden cardiac death, new-onset atrial fibrillation or flutter after mitral valve surgery, endocarditis, permanent pacemaker or implantable cardiac defibrillators, hospitalization for new or worsening heart failure, stroke, and mitral valve reoperation for repair failure or prosthesis degeneration. The primary study exposures were MAD and no MAD.
Follow-up for each patient started on the index date, defined as the date of mitral valve surgery, and ended on 31 August 2023, unless an event occurred earlier. Reasons for censoring included death (if not the primary event) or reaching the end of follow-up without experiencing the event. To avoid immortal time bias, no observation time prior to surgery was included in the analysis. Comprehensive outcome tracking was performed with no patients lost to follow-up.
Assessment of mitral annular disjunction and mitral valve characteristics
Transthoracic echocardiography (TTE) and transoesophageal echocardiography (TEE) were performed in each patient pre- and post-operatively before discharge (Vivid E95 scanner, GE Healthcare and Philips). Standard echocardiographic measurements were available from the original examination. Since MAD was not commonly assessed as part of the standard protocol, all pre- and post-operative echocardiograms were analysed offline during 2022–23 (EchoPac version 209, revision 69.0, GE Healthcare). Echocardiographic analyses were performed by an independent sonographer (C.D.S.) who was certified for TTE, TEE, and 2D and 3D echocardiography by the European Association of Cardiovascular Imaging every 5 years since 2014 (see Supplementary data online, Figure S2) and who was blinded to clinical data.
Mitral annular disjunction assessment was performed according to current recommendations11,16,17 and evaluated in the parasternal long-axis or apical three-chamber views. The disjunction was defined as the distance between the insertion point of the posterior mitral leaflet and the left atrial–left ventricular (AV–LV) junction (Figure 1). To ensure accurate measurement of MAD length, the mitral valve’s movement was tracked throughout the cardiac cycle, systole, and diastole, and the measurements were taken by pausing the echocardiographic loop at the point where both mitral valve leaflets and the AV–LV junction were clearly visualized. Also, zoomed images of the mitral valve were utilized to enhance measurement precision. In the present study, the term MAD refers to true MAD in which the displacement was observed both during systole and diastole and its length measured at end systole (Figure 1). Pseudo-MAD was defined as when the posterior leaflet of the mitral valve is inserted at the AV–LV junction in diastole, without any anatomical separation between the posterior mitral valve annulus and the LV myocardium (Figure 2).11 In pseudo-MAD, the disjunction was apparent only in systole (Figure 2).
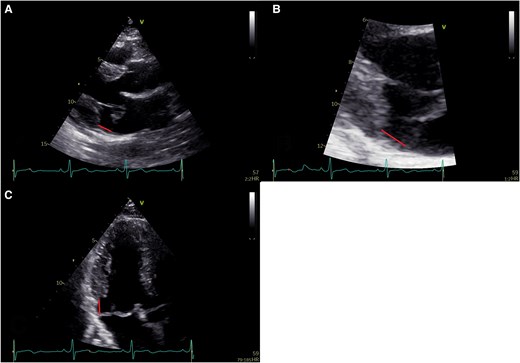
Assessment of mitral annular disjunction by transthoracic echocardiography. (A) Transthoracic echocardiographic parasternal long-axis image demonstrating mitral annular disjunction with mitral valve prolapse near ventricular end systole; (B) transthoracic echocardiographic long-axis zoomed image demonstrating mitral annular disjunction; and (C) transthoracic echocardiographic apical three-chamber view demonstrating mitral annular disjunction with mitral valve prolapse near ventricular end systole
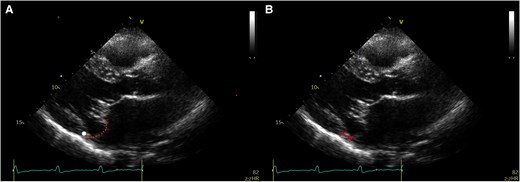
Assessment of pseudo-mitral annular disjunction by transthoracic echocardiography. (A) and (B) Pseudo-mitral annular disjunction. The white point marks the hinge line. The dotted line marks the posterior leaflet. If the annular plane is erroneously set at the leaflet bending point in the atrium, then pseudo-mitral annular disjunction occurs (double dotted arrow)
Given the lack of a standardized threshold for significant end-systolic MAD length, with prior studies using various thresholds, from any visible disjunction to >1, ≥2, and ≥5 mm,18–23 we selected a threshold of ≥3 mm based on previous research indicating measurement variability below this level21 and likely less clinical relevance.5,24,25 Additionally, a sensitivity analysis was performed using a stricter threshold of 5 mm, as this was referred to as ‘relevant’ MAD in two of the previous studies.20,26
Mitral valve prolapse was diagnosed, according to current guidelines in the parasternal long-axis view by observing a systolic displacement of the mitral leaflet into the left atrium (LA) of at least 2 mm from the mitral annular plane.27,28 Patients with MVP were further classified as having Barlow’s disease, characterized by diffuse thickening and redundancy typically involving multiple segments of both leaflets and chordae, along with annular dilation.27,28 Other aetiologies, including fibroelastic deficiency (FED), FED+, and forme fruste, were grouped under the category of ‘other’. The severity of mitral and tricuspid regurgitation was categorized as none—trivial, mild, moderate, or severe.27,28
Statistical analysis
Continuous variables were presented as mean and standard deviation or median [inter-quartile range (IQR)]. Categorical variables were presented as number and percentage. Mean values were compared using t-test for normally distributed data and Mann–Whitney U test for non-parametric data. Categorical variables were tested using χ2 test or Fisher’s exact test. Ordinal variables were tested using Mann–Whitney U test. Kaplan–Meier curves were performed for time-to-event analyses. The association between MAD, as both a categorical and a continuous variable, and clinical outcomes in patients undergoing mitral valve surgery were assessed using Cox proportional hazards regression adjusted for age and sex. In a sensitivity analysis, these models were further adjusted for the different cardiac surgeons involved. In addition, interaction terms were included to determine whether the association between MAD and clinical outcomes differs by surgical mode (repair vs replacement). Furthermore, inverse probability weighting (IPW) for the Cox regression model was applied to address confounder imbalance between patients with and without MAD.29 The propensity score was estimated using a logistic regression model that included the following covariates: age, sex, body mass index (BMI), history of prior stroke, diabetes, hypertension, history of atrial fibrillation or flutter, smoking status, pulmonary hypertension, chronic lung disease, prior pacemaker, hypercholesterolaemia, haemoglobin level (g/L), estimated glomerular filtration rate (mL/min/1.73 m2), New York Heart Association class, critical pre-operative status, EuroSCORE II (%), LV ejection fraction (%), and baseline MR degree. The results of the IPW-adjusted Cox regression analysis were presented as hazard ratios (HRs) with 95% confidence intervals (CI) and were performed for both the entire population and the subset undergoing mitral valve repair. The significance level was defined as a two-sided P < .05. Statistical analyses were performed using RStudio® (R Foundation for Statistical Computing, Vienna, Austria) and IBM SPSS Statistics® (IBM SPSS Statistics 28, IBM, New York, NY, USA).
Results
Study population
The final study population consisted of 599 patients, of whom 485 (81%) underwent mitral valve repair and 114 (19%) underwent mitral valve replacement. Of them, 96 (16%) had MAD with a median MAD length at end systole of 8.0 mm (IQR 5.0–10.0 mm). There were a total of 39 cases of pseudo-MAD.
Baseline characteristics according to the presence or absence of MAD are given in Tables 1–3. Patients with MAD, when compared with those without MAD, were younger (54.9 ± 14.5 vs 62.7 ± 11.4 years), more often female (31% vs 17%), and had lower BMI (23.6 ± 2.9 vs 25.3 ± 4.0 kg/m2). They were also less likely to have atrial fibrillation or flutter, had lower EuroSCORE II scores, and had lower New York Heart Association classification. Additionally, patients with MAD had lower pre-operative serum creatinine levels when compared with patients without MAD.
Baseline characteristics stratified by the presence of mitral annular disjunction among patients undergoing mitral valve repair or replacement
| Baseline characteristics . | No MAD n = 503 . | MAD n = 96 . |
|---|---|---|
| Age, years | 62.7 ± 11.4 | 54.9 ± 14.5 |
| Female sex | 17% (87) | 31% (30) |
| BMI classification, kg/m2 | ||
| <18.5 | 2% (11) | 4% (4) |
| 18.5–24.9 | 51% (257) | 70% (67) |
| ≥25 | 47% (235) | 26% (25) |
| Prior stroke | 4% (21) | 2% (2) |
| Diabetes | 2% (12) | 2% (2) |
| Hypertension | 31% (157) | 22% (21) |
| History of atrial fibrillation or flutter | 37% (187) | 24% (23) |
| CHA2DS2-VASc score | ||
| 0 | 9% (46) | 12% (11) |
| 1 | 30% (150) | 37% (35) |
| 2 | 30% (151) | 30% (29) |
| 3 | 17% (87) | 10% (10) |
| 4 | 11% (56) | 7% (7) |
| 5 | 2% (11) | 3% (3) |
| 6 | <1% (2) | 0% (0) |
| 7 | 0% (0) | 1% (1) |
| Pulmonary hypertension | 14% (68) | 12% (11) |
| Chronic lung disease | 5% (25) | 3% (3) |
| Prior pacemaker | 3% (14) | 1% (1) |
| Extracardiac vascular disease | 1% (5) | 0% (0) |
| Hypercholesterolaemiaa | 9% (44) | 4% (4) |
| Smoking status | ||
| Never | 73% (367) | 78% (75) |
| Prior | 24% (118) | 18% (17) |
| Current | 4% (18) | 4% (4) |
| Haemoglobin, g/L | 141.6 ± 13.2 | 140.1 ± 13.3 |
| Serum creatinine, µmol/L | 88.9 ± 22.4 | 80.3 ± 18.7 |
| eGFRb, mL/min/1.73 m2 | ||
| <60 | 14% (68) | 8% (8) |
| ≥60 | 86% (435) | 92% (88) |
| New York Heart Association class | ||
| I or II | 47% (238) | 65% (62) |
| III or IV | 53% (265) | 35% (34) |
| Critical pre-operative status | 2% (8) | 2% (2) |
| EuroSCORE II, % | ||
| <4 | 86% (434) | 91% (87) |
| ≥4 | 14% (69) | 9% (9) |
| Baseline characteristics . | No MAD n = 503 . | MAD n = 96 . |
|---|---|---|
| Age, years | 62.7 ± 11.4 | 54.9 ± 14.5 |
| Female sex | 17% (87) | 31% (30) |
| BMI classification, kg/m2 | ||
| <18.5 | 2% (11) | 4% (4) |
| 18.5–24.9 | 51% (257) | 70% (67) |
| ≥25 | 47% (235) | 26% (25) |
| Prior stroke | 4% (21) | 2% (2) |
| Diabetes | 2% (12) | 2% (2) |
| Hypertension | 31% (157) | 22% (21) |
| History of atrial fibrillation or flutter | 37% (187) | 24% (23) |
| CHA2DS2-VASc score | ||
| 0 | 9% (46) | 12% (11) |
| 1 | 30% (150) | 37% (35) |
| 2 | 30% (151) | 30% (29) |
| 3 | 17% (87) | 10% (10) |
| 4 | 11% (56) | 7% (7) |
| 5 | 2% (11) | 3% (3) |
| 6 | <1% (2) | 0% (0) |
| 7 | 0% (0) | 1% (1) |
| Pulmonary hypertension | 14% (68) | 12% (11) |
| Chronic lung disease | 5% (25) | 3% (3) |
| Prior pacemaker | 3% (14) | 1% (1) |
| Extracardiac vascular disease | 1% (5) | 0% (0) |
| Hypercholesterolaemiaa | 9% (44) | 4% (4) |
| Smoking status | ||
| Never | 73% (367) | 78% (75) |
| Prior | 24% (118) | 18% (17) |
| Current | 4% (18) | 4% (4) |
| Haemoglobin, g/L | 141.6 ± 13.2 | 140.1 ± 13.3 |
| Serum creatinine, µmol/L | 88.9 ± 22.4 | 80.3 ± 18.7 |
| eGFRb, mL/min/1.73 m2 | ||
| <60 | 14% (68) | 8% (8) |
| ≥60 | 86% (435) | 92% (88) |
| New York Heart Association class | ||
| I or II | 47% (238) | 65% (62) |
| III or IV | 53% (265) | 35% (34) |
| Critical pre-operative status | 2% (8) | 2% (2) |
| EuroSCORE II, % | ||
| <4 | 86% (434) | 91% (87) |
| ≥4 | 14% (69) | 9% (9) |
Values are presented as mean ± standard deviation, or % (n).
BMI, body mass index; eGFR, estimated glomerular filtration rate; MAD, mitral annular disjunction.
aHypercholesterolaemia was defined as being on lipid-lowering therapy.
beGFR was calculated using the 2009 CKD-EPI equation.
Baseline characteristics stratified by the presence of mitral annular disjunction among patients undergoing mitral valve repair or replacement
| Baseline characteristics . | No MAD n = 503 . | MAD n = 96 . |
|---|---|---|
| Age, years | 62.7 ± 11.4 | 54.9 ± 14.5 |
| Female sex | 17% (87) | 31% (30) |
| BMI classification, kg/m2 | ||
| <18.5 | 2% (11) | 4% (4) |
| 18.5–24.9 | 51% (257) | 70% (67) |
| ≥25 | 47% (235) | 26% (25) |
| Prior stroke | 4% (21) | 2% (2) |
| Diabetes | 2% (12) | 2% (2) |
| Hypertension | 31% (157) | 22% (21) |
| History of atrial fibrillation or flutter | 37% (187) | 24% (23) |
| CHA2DS2-VASc score | ||
| 0 | 9% (46) | 12% (11) |
| 1 | 30% (150) | 37% (35) |
| 2 | 30% (151) | 30% (29) |
| 3 | 17% (87) | 10% (10) |
| 4 | 11% (56) | 7% (7) |
| 5 | 2% (11) | 3% (3) |
| 6 | <1% (2) | 0% (0) |
| 7 | 0% (0) | 1% (1) |
| Pulmonary hypertension | 14% (68) | 12% (11) |
| Chronic lung disease | 5% (25) | 3% (3) |
| Prior pacemaker | 3% (14) | 1% (1) |
| Extracardiac vascular disease | 1% (5) | 0% (0) |
| Hypercholesterolaemiaa | 9% (44) | 4% (4) |
| Smoking status | ||
| Never | 73% (367) | 78% (75) |
| Prior | 24% (118) | 18% (17) |
| Current | 4% (18) | 4% (4) |
| Haemoglobin, g/L | 141.6 ± 13.2 | 140.1 ± 13.3 |
| Serum creatinine, µmol/L | 88.9 ± 22.4 | 80.3 ± 18.7 |
| eGFRb, mL/min/1.73 m2 | ||
| <60 | 14% (68) | 8% (8) |
| ≥60 | 86% (435) | 92% (88) |
| New York Heart Association class | ||
| I or II | 47% (238) | 65% (62) |
| III or IV | 53% (265) | 35% (34) |
| Critical pre-operative status | 2% (8) | 2% (2) |
| EuroSCORE II, % | ||
| <4 | 86% (434) | 91% (87) |
| ≥4 | 14% (69) | 9% (9) |
| Baseline characteristics . | No MAD n = 503 . | MAD n = 96 . |
|---|---|---|
| Age, years | 62.7 ± 11.4 | 54.9 ± 14.5 |
| Female sex | 17% (87) | 31% (30) |
| BMI classification, kg/m2 | ||
| <18.5 | 2% (11) | 4% (4) |
| 18.5–24.9 | 51% (257) | 70% (67) |
| ≥25 | 47% (235) | 26% (25) |
| Prior stroke | 4% (21) | 2% (2) |
| Diabetes | 2% (12) | 2% (2) |
| Hypertension | 31% (157) | 22% (21) |
| History of atrial fibrillation or flutter | 37% (187) | 24% (23) |
| CHA2DS2-VASc score | ||
| 0 | 9% (46) | 12% (11) |
| 1 | 30% (150) | 37% (35) |
| 2 | 30% (151) | 30% (29) |
| 3 | 17% (87) | 10% (10) |
| 4 | 11% (56) | 7% (7) |
| 5 | 2% (11) | 3% (3) |
| 6 | <1% (2) | 0% (0) |
| 7 | 0% (0) | 1% (1) |
| Pulmonary hypertension | 14% (68) | 12% (11) |
| Chronic lung disease | 5% (25) | 3% (3) |
| Prior pacemaker | 3% (14) | 1% (1) |
| Extracardiac vascular disease | 1% (5) | 0% (0) |
| Hypercholesterolaemiaa | 9% (44) | 4% (4) |
| Smoking status | ||
| Never | 73% (367) | 78% (75) |
| Prior | 24% (118) | 18% (17) |
| Current | 4% (18) | 4% (4) |
| Haemoglobin, g/L | 141.6 ± 13.2 | 140.1 ± 13.3 |
| Serum creatinine, µmol/L | 88.9 ± 22.4 | 80.3 ± 18.7 |
| eGFRb, mL/min/1.73 m2 | ||
| <60 | 14% (68) | 8% (8) |
| ≥60 | 86% (435) | 92% (88) |
| New York Heart Association class | ||
| I or II | 47% (238) | 65% (62) |
| III or IV | 53% (265) | 35% (34) |
| Critical pre-operative status | 2% (8) | 2% (2) |
| EuroSCORE II, % | ||
| <4 | 86% (434) | 91% (87) |
| ≥4 | 14% (69) | 9% (9) |
Values are presented as mean ± standard deviation, or % (n).
BMI, body mass index; eGFR, estimated glomerular filtration rate; MAD, mitral annular disjunction.
aHypercholesterolaemia was defined as being on lipid-lowering therapy.
beGFR was calculated using the 2009 CKD-EPI equation.
Baseline echocardiographic characteristics stratified by the presence of mitral annular disjunction among patients undergoing mitral valve repair or replacement
| Baseline echocardiographic characteristics . | No MAD n = 503 . | MAD n = 96 . | ||
|---|---|---|---|---|
| n . | Mean ± SD or % (n) or median (IQR) . | n . | Mean ± SD or % (n) or median (IQR) . | |
| LV end-diastolic dimension, mm | 491 | 58.2 ± 6.3 | 93 | 57.6 ± 6.5 |
| LV end-diastolic volume, mL | 417 | 162.9 ± 44.0 | 81 | 170.4 ± 43.4 |
| LV end-systolic dimension, mm | 452 | 36.5 ± 7.5 | 90 | 35.7 ± 6.2 |
| LV end-systolic volume, mL | 396 | 67.2 ± 23.3 | 77 | 70.9 ± 21.4 |
| Left atrial volume index, mL/m2 | 455 | 65.1 ± 23.2 | 90 | 63.5 ± 20.5 |
| Pulmonary artery systolic pressure, mmHg | 391 | 43.3 ± 15.2 | 75 | 38.6 ± 15.5 |
| LV ejection fraction, % | 503 | 59.3 ± 7.5 | 96 | 58.2 ± 7.1 |
| LV ejection fraction ≤ 35% | 6 | 30.8 ± 5.8 | 2 | 32.5 ± 3.5 |
| Mitral regurgitation, degree | 503 | 96 | ||
| None, trivial | 0% (0) | 0% (0) | ||
| Mild | 0% (0) | 0% (0) | ||
| Moderate | 20% (102) | 22% (21) | ||
| Severe | 80% (401) | 78% (75) | ||
| Tricuspid regurgitation, degree | 503 | 96 | ||
| None, trivial | 48% (240) | 42% (40) | ||
| Mild | 41% (205) | 46% (44) | ||
| Moderate | 8% (40) | 9% (9) | ||
| Severe | 4% (18) | 3% (3) | ||
| Tricuspid regurgitation peak velocity, m/s | 371 | 2.9 ± 0.55 | 72 | 2.7 ± 0.54 |
| Mitral valve characteristics | ||||
| Posterior leaflet prolapse | 503 | 70% (353) | 96 | 43% (41) |
| Anterior leaflet prolapse | 503 | 13% (65) | 96 | 0% (0) |
| Bileaflet prolapse | 503 | 17% (84) | 96 | 57% (55) |
| Barlow’s disease | 503 | 27% (135) | 96 | 70% (67) |
| Other aetiologies | 503 | 73% (368) | 96 | 30% (29) |
| Length of MAD, mm | 23 | 2.0 (1.0–2.0) | 96 | 8.0 (5.0–10.0) |
| Pseudo-MAD | 503 | 8% (39) | 96 | 0% (0) |
| Baseline echocardiographic characteristics . | No MAD n = 503 . | MAD n = 96 . | ||
|---|---|---|---|---|
| n . | Mean ± SD or % (n) or median (IQR) . | n . | Mean ± SD or % (n) or median (IQR) . | |
| LV end-diastolic dimension, mm | 491 | 58.2 ± 6.3 | 93 | 57.6 ± 6.5 |
| LV end-diastolic volume, mL | 417 | 162.9 ± 44.0 | 81 | 170.4 ± 43.4 |
| LV end-systolic dimension, mm | 452 | 36.5 ± 7.5 | 90 | 35.7 ± 6.2 |
| LV end-systolic volume, mL | 396 | 67.2 ± 23.3 | 77 | 70.9 ± 21.4 |
| Left atrial volume index, mL/m2 | 455 | 65.1 ± 23.2 | 90 | 63.5 ± 20.5 |
| Pulmonary artery systolic pressure, mmHg | 391 | 43.3 ± 15.2 | 75 | 38.6 ± 15.5 |
| LV ejection fraction, % | 503 | 59.3 ± 7.5 | 96 | 58.2 ± 7.1 |
| LV ejection fraction ≤ 35% | 6 | 30.8 ± 5.8 | 2 | 32.5 ± 3.5 |
| Mitral regurgitation, degree | 503 | 96 | ||
| None, trivial | 0% (0) | 0% (0) | ||
| Mild | 0% (0) | 0% (0) | ||
| Moderate | 20% (102) | 22% (21) | ||
| Severe | 80% (401) | 78% (75) | ||
| Tricuspid regurgitation, degree | 503 | 96 | ||
| None, trivial | 48% (240) | 42% (40) | ||
| Mild | 41% (205) | 46% (44) | ||
| Moderate | 8% (40) | 9% (9) | ||
| Severe | 4% (18) | 3% (3) | ||
| Tricuspid regurgitation peak velocity, m/s | 371 | 2.9 ± 0.55 | 72 | 2.7 ± 0.54 |
| Mitral valve characteristics | ||||
| Posterior leaflet prolapse | 503 | 70% (353) | 96 | 43% (41) |
| Anterior leaflet prolapse | 503 | 13% (65) | 96 | 0% (0) |
| Bileaflet prolapse | 503 | 17% (84) | 96 | 57% (55) |
| Barlow’s disease | 503 | 27% (135) | 96 | 70% (67) |
| Other aetiologies | 503 | 73% (368) | 96 | 30% (29) |
| Length of MAD, mm | 23 | 2.0 (1.0–2.0) | 96 | 8.0 (5.0–10.0) |
| Pseudo-MAD | 503 | 8% (39) | 96 | 0% (0) |
LV, left ventricle; MAD, mitral annular disjunction; SD, standard deviation.
Baseline echocardiographic characteristics stratified by the presence of mitral annular disjunction among patients undergoing mitral valve repair or replacement
| Baseline echocardiographic characteristics . | No MAD n = 503 . | MAD n = 96 . | ||
|---|---|---|---|---|
| n . | Mean ± SD or % (n) or median (IQR) . | n . | Mean ± SD or % (n) or median (IQR) . | |
| LV end-diastolic dimension, mm | 491 | 58.2 ± 6.3 | 93 | 57.6 ± 6.5 |
| LV end-diastolic volume, mL | 417 | 162.9 ± 44.0 | 81 | 170.4 ± 43.4 |
| LV end-systolic dimension, mm | 452 | 36.5 ± 7.5 | 90 | 35.7 ± 6.2 |
| LV end-systolic volume, mL | 396 | 67.2 ± 23.3 | 77 | 70.9 ± 21.4 |
| Left atrial volume index, mL/m2 | 455 | 65.1 ± 23.2 | 90 | 63.5 ± 20.5 |
| Pulmonary artery systolic pressure, mmHg | 391 | 43.3 ± 15.2 | 75 | 38.6 ± 15.5 |
| LV ejection fraction, % | 503 | 59.3 ± 7.5 | 96 | 58.2 ± 7.1 |
| LV ejection fraction ≤ 35% | 6 | 30.8 ± 5.8 | 2 | 32.5 ± 3.5 |
| Mitral regurgitation, degree | 503 | 96 | ||
| None, trivial | 0% (0) | 0% (0) | ||
| Mild | 0% (0) | 0% (0) | ||
| Moderate | 20% (102) | 22% (21) | ||
| Severe | 80% (401) | 78% (75) | ||
| Tricuspid regurgitation, degree | 503 | 96 | ||
| None, trivial | 48% (240) | 42% (40) | ||
| Mild | 41% (205) | 46% (44) | ||
| Moderate | 8% (40) | 9% (9) | ||
| Severe | 4% (18) | 3% (3) | ||
| Tricuspid regurgitation peak velocity, m/s | 371 | 2.9 ± 0.55 | 72 | 2.7 ± 0.54 |
| Mitral valve characteristics | ||||
| Posterior leaflet prolapse | 503 | 70% (353) | 96 | 43% (41) |
| Anterior leaflet prolapse | 503 | 13% (65) | 96 | 0% (0) |
| Bileaflet prolapse | 503 | 17% (84) | 96 | 57% (55) |
| Barlow’s disease | 503 | 27% (135) | 96 | 70% (67) |
| Other aetiologies | 503 | 73% (368) | 96 | 30% (29) |
| Length of MAD, mm | 23 | 2.0 (1.0–2.0) | 96 | 8.0 (5.0–10.0) |
| Pseudo-MAD | 503 | 8% (39) | 96 | 0% (0) |
| Baseline echocardiographic characteristics . | No MAD n = 503 . | MAD n = 96 . | ||
|---|---|---|---|---|
| n . | Mean ± SD or % (n) or median (IQR) . | n . | Mean ± SD or % (n) or median (IQR) . | |
| LV end-diastolic dimension, mm | 491 | 58.2 ± 6.3 | 93 | 57.6 ± 6.5 |
| LV end-diastolic volume, mL | 417 | 162.9 ± 44.0 | 81 | 170.4 ± 43.4 |
| LV end-systolic dimension, mm | 452 | 36.5 ± 7.5 | 90 | 35.7 ± 6.2 |
| LV end-systolic volume, mL | 396 | 67.2 ± 23.3 | 77 | 70.9 ± 21.4 |
| Left atrial volume index, mL/m2 | 455 | 65.1 ± 23.2 | 90 | 63.5 ± 20.5 |
| Pulmonary artery systolic pressure, mmHg | 391 | 43.3 ± 15.2 | 75 | 38.6 ± 15.5 |
| LV ejection fraction, % | 503 | 59.3 ± 7.5 | 96 | 58.2 ± 7.1 |
| LV ejection fraction ≤ 35% | 6 | 30.8 ± 5.8 | 2 | 32.5 ± 3.5 |
| Mitral regurgitation, degree | 503 | 96 | ||
| None, trivial | 0% (0) | 0% (0) | ||
| Mild | 0% (0) | 0% (0) | ||
| Moderate | 20% (102) | 22% (21) | ||
| Severe | 80% (401) | 78% (75) | ||
| Tricuspid regurgitation, degree | 503 | 96 | ||
| None, trivial | 48% (240) | 42% (40) | ||
| Mild | 41% (205) | 46% (44) | ||
| Moderate | 8% (40) | 9% (9) | ||
| Severe | 4% (18) | 3% (3) | ||
| Tricuspid regurgitation peak velocity, m/s | 371 | 2.9 ± 0.55 | 72 | 2.7 ± 0.54 |
| Mitral valve characteristics | ||||
| Posterior leaflet prolapse | 503 | 70% (353) | 96 | 43% (41) |
| Anterior leaflet prolapse | 503 | 13% (65) | 96 | 0% (0) |
| Bileaflet prolapse | 503 | 17% (84) | 96 | 57% (55) |
| Barlow’s disease | 503 | 27% (135) | 96 | 70% (67) |
| Other aetiologies | 503 | 73% (368) | 96 | 30% (29) |
| Length of MAD, mm | 23 | 2.0 (1.0–2.0) | 96 | 8.0 (5.0–10.0) |
| Pseudo-MAD | 503 | 8% (39) | 96 | 0% (0) |
LV, left ventricle; MAD, mitral annular disjunction; SD, standard deviation.
Procedural characteristics and in-hospital outcomes stratified by the presence of mitral annular disjunction among patients undergoing mitral valve repair or replacement
| Procedural characteristics and in-hospital outcomes . | No MAD n = 503 . | MAD n = 96 . | ||
|---|---|---|---|---|
| n . | Mean ± SD or % (n) . | n . | Mean ± SD or % (n) . | |
| Mitral valve surgery performed | 503 | 96 | ||
| Repair | 80% (404) | 84% (81) | ||
| Replacement | 20% (99) | 16% (15) | ||
| Repair technique | 404 | 81 | ||
| Artificial chordae | 87% (352) | 89% (72) | ||
| Resection | 13% (52) | 11% (9) | ||
| Extracorporeal circulation time, min | 468 | 142.3 ± 58.3 | 92 | 144.7 ± 57.2 |
| Aortic cross-clamp time, min | 468 | 99.0 ± 41.1 | 92 | 101.7 ± 41.1 |
| Residual MAD | N/A | N/A | 96 | 0% (0) |
| Residual mitral regurgitation, degree | 503 | 96 | ||
| none, trivial | 83% (420) | 87% (84) | ||
| mild | 16% (77) | 12% (11) | ||
| moderate | 1% (6) | 1% (1) | ||
| severe | 0% (0) | 0% (0) | ||
| Tricuspid regurgitation, degree | 503 | 96 | ||
| None, trivial | 46% (230) | 57% (55) | ||
| Mild | 48% (240) | 37% (35) | ||
| Moderate | 6% (28) | 5% (5) | ||
| Severe | 1% (5) | 1% (1) | ||
| Tricuspid regurgitation peak velocity, m/s | 288 | 2.7 ± 0.44 | 61 | 2.4 ± 0.42 |
| Mitral valve gradient, mmHg | 496 | 3.9 ± 1.7 | 95 | 4.0 ± 1.9 |
| In-hospital death | 503 | 1% (5) | 96 | 0% (0) |
| Reoperation for bleeding | 503 | 6% (29) | 96 | 3% (3) |
| Procedural characteristics and in-hospital outcomes . | No MAD n = 503 . | MAD n = 96 . | ||
|---|---|---|---|---|
| n . | Mean ± SD or % (n) . | n . | Mean ± SD or % (n) . | |
| Mitral valve surgery performed | 503 | 96 | ||
| Repair | 80% (404) | 84% (81) | ||
| Replacement | 20% (99) | 16% (15) | ||
| Repair technique | 404 | 81 | ||
| Artificial chordae | 87% (352) | 89% (72) | ||
| Resection | 13% (52) | 11% (9) | ||
| Extracorporeal circulation time, min | 468 | 142.3 ± 58.3 | 92 | 144.7 ± 57.2 |
| Aortic cross-clamp time, min | 468 | 99.0 ± 41.1 | 92 | 101.7 ± 41.1 |
| Residual MAD | N/A | N/A | 96 | 0% (0) |
| Residual mitral regurgitation, degree | 503 | 96 | ||
| none, trivial | 83% (420) | 87% (84) | ||
| mild | 16% (77) | 12% (11) | ||
| moderate | 1% (6) | 1% (1) | ||
| severe | 0% (0) | 0% (0) | ||
| Tricuspid regurgitation, degree | 503 | 96 | ||
| None, trivial | 46% (230) | 57% (55) | ||
| Mild | 48% (240) | 37% (35) | ||
| Moderate | 6% (28) | 5% (5) | ||
| Severe | 1% (5) | 1% (1) | ||
| Tricuspid regurgitation peak velocity, m/s | 288 | 2.7 ± 0.44 | 61 | 2.4 ± 0.42 |
| Mitral valve gradient, mmHg | 496 | 3.9 ± 1.7 | 95 | 4.0 ± 1.9 |
| In-hospital death | 503 | 1% (5) | 96 | 0% (0) |
| Reoperation for bleeding | 503 | 6% (29) | 96 | 3% (3) |
MAD, mitral annular disjunction.
Procedural characteristics and in-hospital outcomes stratified by the presence of mitral annular disjunction among patients undergoing mitral valve repair or replacement
| Procedural characteristics and in-hospital outcomes . | No MAD n = 503 . | MAD n = 96 . | ||
|---|---|---|---|---|
| n . | Mean ± SD or % (n) . | n . | Mean ± SD or % (n) . | |
| Mitral valve surgery performed | 503 | 96 | ||
| Repair | 80% (404) | 84% (81) | ||
| Replacement | 20% (99) | 16% (15) | ||
| Repair technique | 404 | 81 | ||
| Artificial chordae | 87% (352) | 89% (72) | ||
| Resection | 13% (52) | 11% (9) | ||
| Extracorporeal circulation time, min | 468 | 142.3 ± 58.3 | 92 | 144.7 ± 57.2 |
| Aortic cross-clamp time, min | 468 | 99.0 ± 41.1 | 92 | 101.7 ± 41.1 |
| Residual MAD | N/A | N/A | 96 | 0% (0) |
| Residual mitral regurgitation, degree | 503 | 96 | ||
| none, trivial | 83% (420) | 87% (84) | ||
| mild | 16% (77) | 12% (11) | ||
| moderate | 1% (6) | 1% (1) | ||
| severe | 0% (0) | 0% (0) | ||
| Tricuspid regurgitation, degree | 503 | 96 | ||
| None, trivial | 46% (230) | 57% (55) | ||
| Mild | 48% (240) | 37% (35) | ||
| Moderate | 6% (28) | 5% (5) | ||
| Severe | 1% (5) | 1% (1) | ||
| Tricuspid regurgitation peak velocity, m/s | 288 | 2.7 ± 0.44 | 61 | 2.4 ± 0.42 |
| Mitral valve gradient, mmHg | 496 | 3.9 ± 1.7 | 95 | 4.0 ± 1.9 |
| In-hospital death | 503 | 1% (5) | 96 | 0% (0) |
| Reoperation for bleeding | 503 | 6% (29) | 96 | 3% (3) |
| Procedural characteristics and in-hospital outcomes . | No MAD n = 503 . | MAD n = 96 . | ||
|---|---|---|---|---|
| n . | Mean ± SD or % (n) . | n . | Mean ± SD or % (n) . | |
| Mitral valve surgery performed | 503 | 96 | ||
| Repair | 80% (404) | 84% (81) | ||
| Replacement | 20% (99) | 16% (15) | ||
| Repair technique | 404 | 81 | ||
| Artificial chordae | 87% (352) | 89% (72) | ||
| Resection | 13% (52) | 11% (9) | ||
| Extracorporeal circulation time, min | 468 | 142.3 ± 58.3 | 92 | 144.7 ± 57.2 |
| Aortic cross-clamp time, min | 468 | 99.0 ± 41.1 | 92 | 101.7 ± 41.1 |
| Residual MAD | N/A | N/A | 96 | 0% (0) |
| Residual mitral regurgitation, degree | 503 | 96 | ||
| none, trivial | 83% (420) | 87% (84) | ||
| mild | 16% (77) | 12% (11) | ||
| moderate | 1% (6) | 1% (1) | ||
| severe | 0% (0) | 0% (0) | ||
| Tricuspid regurgitation, degree | 503 | 96 | ||
| None, trivial | 46% (230) | 57% (55) | ||
| Mild | 48% (240) | 37% (35) | ||
| Moderate | 6% (28) | 5% (5) | ||
| Severe | 1% (5) | 1% (1) | ||
| Tricuspid regurgitation peak velocity, m/s | 288 | 2.7 ± 0.44 | 61 | 2.4 ± 0.42 |
| Mitral valve gradient, mmHg | 496 | 3.9 ± 1.7 | 95 | 4.0 ± 1.9 |
| In-hospital death | 503 | 1% (5) | 96 | 0% (0) |
| Reoperation for bleeding | 503 | 6% (29) | 96 | 3% (3) |
MAD, mitral annular disjunction.
Patients with MAD had similar LV ejection fraction, left atrial volume index, and LV dimensions but lower pulmonary artery systolic pressure and lower maximum velocity for tricuspid regurgitation when compared with patients without MAD. Compared with patients without MAD, patients with MAD had more Barlow’s disease (70% vs 27%) and bileaflet prolapse (57% vs 17%).
Baseline characteristics of patients with and without MAD stratified by mode of surgery (mitral valve repair vs mitral valve replacement) are given in Supplementary data online, Tables S2 and S3.The differences in baseline characteristics of patients with and without MAD remained similar to those in the overall cohort, except that patients with MAD in the mitral valve replacement group tended to have a lower LV ejection fraction.
Procedural characteristics and in-hospital outcomes
The number of cardiac surgeons performing mitral valve surgeries is given in Supplementary data online, Table S4. About 87% of the mitral valve surgeries were performed by four experienced cardiac surgeons, and the average annual volume of mitral valve surgeries at the study centre was 167 per year. Procedural and in-hospital outcomes are given in Table 3. In general, the surgical approach was dictated by valvular anatomy without consideration of the presence or absence of MAD. Mitral valve repair was the most frequent type of surgery regardless of the presence of MAD (84% in patients with MAD and 80% in patients without MAD). The most common repair technique (89% in patients with MAD and 87% in patients without MAD) included the use of artificial chordae with annuloplasty ring (Carpentier-Edwards Physio II, Edward Lifesciences, Irvine, CA, USA).
There was no residual MAD on post-operative echocardiograms. The presence and severity of residual MR and post-operative tricuspid regurgitation were similar in patients with and without pre-operative MAD. Five patients experienced in-hospital death, and none of them had pre-operative MAD. The rates of reoperation for bleeding were similar in patients with and without pre-operative MAD.
Procedural and in-hospital outcomes stratified by mode of surgery (repair or replacement) are given in Supplementary data online, Table S5. Among patients undergoing mitral valve repair, there were no significant differences in the distribution of annuloplasty with artificial chordae or resection with regard to the presence of pre-operative MAD and all other procedural outcomes were also similar between patients with and without pre-operative MAD.
Long-term clinical outcomes in the overall cohort
Patient follow-up visits through the study period are outlined in Supplementary data online, Figure S3. No patients were lost to follow-up, and all patients were followed until the occurrence of a clinical outcome (e.g. post-operative VA or death) or until the end of the study period 31 August 2023. The median time to first and second visits after mitral valve surgery was 44 days (IQR 37–55) and 109 days (IQR 80–185), respectively, followed by annual visits. Clinical outcomes are shown in Table 4, Structured Graphical Abstract, Figure 3, and Supplementary data online, Table S6. During a median follow-up of 5.4 (IQR 2.8–7.5) years, patients with pre-operative MAD had a significantly higher risk of post-operative VA (adjusted HR 3.33, 95% CI 1.37–8.08; P = .01) compared with patients without pre-operative MAD. The results remained similar when further adjusting for the different cardiac surgeons involved (see Supplementary data online, Table S7). Additionally, each 1-mm increase in MAD length was associated with a higher risk of post-operative VA (adjusted HR 1.25, 95% CI 1.01–1.54; P = .04). Using a cut-off of 5 mm for MAD length, the observed risk of post-operative VA in patients with MAD was significantly higher compared with those without MAD (adjusted HR 4.23, 95% CI 1.71–10.5; P = .002). There was no significant interaction between MAD status and surgical mode (repair vs replacement) on the risk of post-operative VA (Pinteraction = .18) (see Supplementary data online, Table S6). Among patients with LV ejection fraction ≤35%, one patient experienced post-surgical VA and this patient had also MAD.

Cox regression survival age-adjusted analysis for all-cause death (A) and Kaplan–Meier survival analysis for heart failure hospitalization (B), new-onset atrial fibrillation or flutter (C), and new-onset stroke (D) stratified by the presence of MAD in patients undergoing mitral valve repair or replacement
Clinical outcomes during 7.5 years of follow-up stratified by the presence of mitral annular disjunction among patients undergoing mitral valve repair or replacement
| Outcomes . | no MAD (n = 503) No. of events (%), IR/100 PY (95% CI) . | MAD (n = 96) No. of events (%), IR/100 PY (95% CI) . | Crude HR (95% CI), P-value . | Adjusted HRa (95% CI), P-value . | IPW-adjusted HR (95% CI), P-value . |
|---|---|---|---|---|---|
| Post-operative VA VAb | 14 (3%)c, 0.56 (0.30–0.93) | 9 (9%)c, 2.08 (0.95–3.95) | 3.79 (1.64–8.79), .002 | 3.33 (1.37–8.08), .01 | 2.89 (1.08–7.73), .03 |
| All-cause death | 33 (7%), 1.29 (0.89–1.81) | 0 (0%) | |||
| Sudden cardiac death | 6 (1%), 0.23 (0.09–0.51) | 0 (0%) | |||
| Stroke | 44 (9%), 1.85 (1.34–2.48) | 9 (9%), 2.09 (0.95–3.96) | 1.06 (0.52–2.18), .87 | 1.32 (0.64–2.75), .45 | 1.14 (0.54–2.43), .73 |
| Post-operative new-onset atrial fibrillation or flutter | 153 (30%), 8.87 (7.52–10.4) | 22 (23%), 6.30 (3.95–9.54) | 0.71 (0.45–1.11), .13 | 0.81 (0.51–1.28), .36 | 0.92 (0.53–1.61), .77 |
| Pacemaker implantation | 46 (9%), 1.93 (1.41–2.58) | 6 (6%), 1.41 (0.52–3.07) | 0.68 (0.29–1.60), .38 | 0.99 (0.42–2.33), .98 | 0.83 (0.30–2.32), .72 |
| Cardiac implantable defibrillator | 5 (1%), 0.20 (0.06–0.46) | 2 (2%), 0.45 (0.05–1.62) | 2.31 (0.45–11.9), .32 | 1.97 (0.35–11.2), .44 | 1.41 (0.24–8.36), .70 |
| Infective endocarditis | 10 (2%), 0.40 (0.19–0.73) | 0 (0%) | |||
| Myocardial infarction | 7 (1%), 0.28 (0.11–0.57) | 1 (1%), 0.22 (0.01–1.25) | 0.76 (0.09–6.19), .80 | 1.23 (0.15–10.2), .85 | 0.77 (0.09–6.46), .81 |
| Heart failure hospitalization | 51 (10%), 2.17 (1.62–2.86) | 7 (7%), 1.70 (0.68–3.50) | 0.72 (0.33–1.58), .41 | 0.89 (0.40–2.00), .79 | 0.74 (0.27–2.00), .55 |
| Mitral valve reoperation for repair failure or prosthesis degeneration | 14 (3%), 0.56 (0.30–0.93) | 3 (3%), 0.67 (0.14–1.96) | 1.18 (0.34–4.12), .79 | 1.21 (0.33–4.38), .78 | 1.11 (0.31–4.00), .88 |
| Outcomes . | no MAD (n = 503) No. of events (%), IR/100 PY (95% CI) . | MAD (n = 96) No. of events (%), IR/100 PY (95% CI) . | Crude HR (95% CI), P-value . | Adjusted HRa (95% CI), P-value . | IPW-adjusted HR (95% CI), P-value . |
|---|---|---|---|---|---|
| Post-operative VA VAb | 14 (3%)c, 0.56 (0.30–0.93) | 9 (9%)c, 2.08 (0.95–3.95) | 3.79 (1.64–8.79), .002 | 3.33 (1.37–8.08), .01 | 2.89 (1.08–7.73), .03 |
| All-cause death | 33 (7%), 1.29 (0.89–1.81) | 0 (0%) | |||
| Sudden cardiac death | 6 (1%), 0.23 (0.09–0.51) | 0 (0%) | |||
| Stroke | 44 (9%), 1.85 (1.34–2.48) | 9 (9%), 2.09 (0.95–3.96) | 1.06 (0.52–2.18), .87 | 1.32 (0.64–2.75), .45 | 1.14 (0.54–2.43), .73 |
| Post-operative new-onset atrial fibrillation or flutter | 153 (30%), 8.87 (7.52–10.4) | 22 (23%), 6.30 (3.95–9.54) | 0.71 (0.45–1.11), .13 | 0.81 (0.51–1.28), .36 | 0.92 (0.53–1.61), .77 |
| Pacemaker implantation | 46 (9%), 1.93 (1.41–2.58) | 6 (6%), 1.41 (0.52–3.07) | 0.68 (0.29–1.60), .38 | 0.99 (0.42–2.33), .98 | 0.83 (0.30–2.32), .72 |
| Cardiac implantable defibrillator | 5 (1%), 0.20 (0.06–0.46) | 2 (2%), 0.45 (0.05–1.62) | 2.31 (0.45–11.9), .32 | 1.97 (0.35–11.2), .44 | 1.41 (0.24–8.36), .70 |
| Infective endocarditis | 10 (2%), 0.40 (0.19–0.73) | 0 (0%) | |||
| Myocardial infarction | 7 (1%), 0.28 (0.11–0.57) | 1 (1%), 0.22 (0.01–1.25) | 0.76 (0.09–6.19), .80 | 1.23 (0.15–10.2), .85 | 0.77 (0.09–6.46), .81 |
| Heart failure hospitalization | 51 (10%), 2.17 (1.62–2.86) | 7 (7%), 1.70 (0.68–3.50) | 0.72 (0.33–1.58), .41 | 0.89 (0.40–2.00), .79 | 0.74 (0.27–2.00), .55 |
| Mitral valve reoperation for repair failure or prosthesis degeneration | 14 (3%), 0.56 (0.30–0.93) | 3 (3%), 0.67 (0.14–1.96) | 1.18 (0.34–4.12), .79 | 1.21 (0.33–4.38), .78 | 1.11 (0.31–4.00), .88 |
IPW analysis using a Cox proportional hazards regression model to adjust for potential confounding factors. The model was weighted based on the following baseline characteristics and clinical variables: age, sex, BMI, history of prior stroke, diabetes, hypertension, history of atrial fibrillation or flutter, smoking status, pulmonary hypertension, chronic lung disease, prior pacemaker, hypercholesterolaemia, haemoglobin level (g/L), eGFR (mL/min/1.73 m2), New York Heart Association class, critical pre-operative status, EuroSCORE II (%), LV ejection fraction (%), and baseline mitral regurgitation degree.
CI, confidence interval; HR, hazard ratio; MAD, mitral annular disjunction; IR, incidence rate; PY, person-years.
aAdjusted for age and sex.
bThe composite endpoint of post-operative VA including hospitalizations, outpatient visits or ablation for confirmed non-sustained or sustained VT, or high burden of PVC.
cCensoring reasons using post-operative VA as example: total number of events: 23. Reasons for censoring included death (n = 33) and reaching the end of the study period without experiencing the event (n = 543).
Clinical outcomes during 7.5 years of follow-up stratified by the presence of mitral annular disjunction among patients undergoing mitral valve repair or replacement
| Outcomes . | no MAD (n = 503) No. of events (%), IR/100 PY (95% CI) . | MAD (n = 96) No. of events (%), IR/100 PY (95% CI) . | Crude HR (95% CI), P-value . | Adjusted HRa (95% CI), P-value . | IPW-adjusted HR (95% CI), P-value . |
|---|---|---|---|---|---|
| Post-operative VA VAb | 14 (3%)c, 0.56 (0.30–0.93) | 9 (9%)c, 2.08 (0.95–3.95) | 3.79 (1.64–8.79), .002 | 3.33 (1.37–8.08), .01 | 2.89 (1.08–7.73), .03 |
| All-cause death | 33 (7%), 1.29 (0.89–1.81) | 0 (0%) | |||
| Sudden cardiac death | 6 (1%), 0.23 (0.09–0.51) | 0 (0%) | |||
| Stroke | 44 (9%), 1.85 (1.34–2.48) | 9 (9%), 2.09 (0.95–3.96) | 1.06 (0.52–2.18), .87 | 1.32 (0.64–2.75), .45 | 1.14 (0.54–2.43), .73 |
| Post-operative new-onset atrial fibrillation or flutter | 153 (30%), 8.87 (7.52–10.4) | 22 (23%), 6.30 (3.95–9.54) | 0.71 (0.45–1.11), .13 | 0.81 (0.51–1.28), .36 | 0.92 (0.53–1.61), .77 |
| Pacemaker implantation | 46 (9%), 1.93 (1.41–2.58) | 6 (6%), 1.41 (0.52–3.07) | 0.68 (0.29–1.60), .38 | 0.99 (0.42–2.33), .98 | 0.83 (0.30–2.32), .72 |
| Cardiac implantable defibrillator | 5 (1%), 0.20 (0.06–0.46) | 2 (2%), 0.45 (0.05–1.62) | 2.31 (0.45–11.9), .32 | 1.97 (0.35–11.2), .44 | 1.41 (0.24–8.36), .70 |
| Infective endocarditis | 10 (2%), 0.40 (0.19–0.73) | 0 (0%) | |||
| Myocardial infarction | 7 (1%), 0.28 (0.11–0.57) | 1 (1%), 0.22 (0.01–1.25) | 0.76 (0.09–6.19), .80 | 1.23 (0.15–10.2), .85 | 0.77 (0.09–6.46), .81 |
| Heart failure hospitalization | 51 (10%), 2.17 (1.62–2.86) | 7 (7%), 1.70 (0.68–3.50) | 0.72 (0.33–1.58), .41 | 0.89 (0.40–2.00), .79 | 0.74 (0.27–2.00), .55 |
| Mitral valve reoperation for repair failure or prosthesis degeneration | 14 (3%), 0.56 (0.30–0.93) | 3 (3%), 0.67 (0.14–1.96) | 1.18 (0.34–4.12), .79 | 1.21 (0.33–4.38), .78 | 1.11 (0.31–4.00), .88 |
| Outcomes . | no MAD (n = 503) No. of events (%), IR/100 PY (95% CI) . | MAD (n = 96) No. of events (%), IR/100 PY (95% CI) . | Crude HR (95% CI), P-value . | Adjusted HRa (95% CI), P-value . | IPW-adjusted HR (95% CI), P-value . |
|---|---|---|---|---|---|
| Post-operative VA VAb | 14 (3%)c, 0.56 (0.30–0.93) | 9 (9%)c, 2.08 (0.95–3.95) | 3.79 (1.64–8.79), .002 | 3.33 (1.37–8.08), .01 | 2.89 (1.08–7.73), .03 |
| All-cause death | 33 (7%), 1.29 (0.89–1.81) | 0 (0%) | |||
| Sudden cardiac death | 6 (1%), 0.23 (0.09–0.51) | 0 (0%) | |||
| Stroke | 44 (9%), 1.85 (1.34–2.48) | 9 (9%), 2.09 (0.95–3.96) | 1.06 (0.52–2.18), .87 | 1.32 (0.64–2.75), .45 | 1.14 (0.54–2.43), .73 |
| Post-operative new-onset atrial fibrillation or flutter | 153 (30%), 8.87 (7.52–10.4) | 22 (23%), 6.30 (3.95–9.54) | 0.71 (0.45–1.11), .13 | 0.81 (0.51–1.28), .36 | 0.92 (0.53–1.61), .77 |
| Pacemaker implantation | 46 (9%), 1.93 (1.41–2.58) | 6 (6%), 1.41 (0.52–3.07) | 0.68 (0.29–1.60), .38 | 0.99 (0.42–2.33), .98 | 0.83 (0.30–2.32), .72 |
| Cardiac implantable defibrillator | 5 (1%), 0.20 (0.06–0.46) | 2 (2%), 0.45 (0.05–1.62) | 2.31 (0.45–11.9), .32 | 1.97 (0.35–11.2), .44 | 1.41 (0.24–8.36), .70 |
| Infective endocarditis | 10 (2%), 0.40 (0.19–0.73) | 0 (0%) | |||
| Myocardial infarction | 7 (1%), 0.28 (0.11–0.57) | 1 (1%), 0.22 (0.01–1.25) | 0.76 (0.09–6.19), .80 | 1.23 (0.15–10.2), .85 | 0.77 (0.09–6.46), .81 |
| Heart failure hospitalization | 51 (10%), 2.17 (1.62–2.86) | 7 (7%), 1.70 (0.68–3.50) | 0.72 (0.33–1.58), .41 | 0.89 (0.40–2.00), .79 | 0.74 (0.27–2.00), .55 |
| Mitral valve reoperation for repair failure or prosthesis degeneration | 14 (3%), 0.56 (0.30–0.93) | 3 (3%), 0.67 (0.14–1.96) | 1.18 (0.34–4.12), .79 | 1.21 (0.33–4.38), .78 | 1.11 (0.31–4.00), .88 |
IPW analysis using a Cox proportional hazards regression model to adjust for potential confounding factors. The model was weighted based on the following baseline characteristics and clinical variables: age, sex, BMI, history of prior stroke, diabetes, hypertension, history of atrial fibrillation or flutter, smoking status, pulmonary hypertension, chronic lung disease, prior pacemaker, hypercholesterolaemia, haemoglobin level (g/L), eGFR (mL/min/1.73 m2), New York Heart Association class, critical pre-operative status, EuroSCORE II (%), LV ejection fraction (%), and baseline mitral regurgitation degree.
CI, confidence interval; HR, hazard ratio; MAD, mitral annular disjunction; IR, incidence rate; PY, person-years.
aAdjusted for age and sex.
bThe composite endpoint of post-operative VA including hospitalizations, outpatient visits or ablation for confirmed non-sustained or sustained VT, or high burden of PVC.
cCensoring reasons using post-operative VA as example: total number of events: 23. Reasons for censoring included death (n = 33) and reaching the end of the study period without experiencing the event (n = 543).
During the follow-up period, 17 patients (3%) required redo mitral valve surgery for recurrent MR which was not a predictor of post-operative VA (adjusted HR 1.54, 95% CI 0.21–11.5; P = .67), regardless of MAD status (Pinteraction = .98).
Interventions for post-operative VA are given in Supplementary data online, Table S8, and the type of intervention for post-operative VA did not differ between patients with and without MAD. Causes of death are outlined in Supplementary data online, Table S9, and the risk of sudden cardiac death did not differ between patients with and without MAD. In addition, there were no significant differences in the adjusted risk of other outcomes between patients with vs without pre-operative MAD.
Long-term clinical outcomes after inverse probability weighting
After applying weighting, all covariates achieved balance, except for minor residual imbalances in BMI and hypertension, which were further adjusted for in the IPW Cox regression models (see Supplementary data online, Table S10 and Figure S4). The results of the IPW-adjusted Cox regression analyses for clinical outcomes are given in Table 4 and Supplementary data online, Table S11. In these analysis, patients with pre-operative MAD had a significantly higher risk of post-operative VA (adjusted HR 2.89, 95% CI 1.08–7.73; P = .03). Additionally, each 1-mm increase in MAD length was associated with a higher risk of post-operative VA (adjusted HR 1.33, 95% CI 1.15–1.54; P < .001). Using a 5-mm threshold for MAD length further indicated a markedly elevated risk of post-operative VA (HR 4.53, 95% CI 1.70–12.1; P = .003).
Discussion
To our knowledge, this study constitutes the hitherto largest cohort of mitral valve surgery patients featuring detailed echocardiographic characterization of MAD and complete long-term outcome tracking. We report three main novel findings: (i) patients with MAD were, on average, 8 years younger when presenting for MR requiring mitral valve surgery and had a nearly three-fold increased risk of post-operative VAs during long-term follow-up, irrespective of surgical technique; (ii) each 1-mm increase in the length of MAD was associated with a 35% increase in the risk of post-surgical VAs; and (iii) MAD was successfully corrected with mitral valve surgery without an increased risk of repair failure, re-do procedures, or residual MR when compared with patients without MAD.
Prevalence of mitral annular disjunction
The prevalence of MAD (true MAD) in our study (16%) was at the lower end of previously reported estimates of 16–50%.22,30–34 However, it is likely that the prevalence of MAD has been overestimated in earlier studies for several reasons. First, there have been recent clarifications in the anatomical definition of MAD.11 In patients with MVP, especially in the presence of Barlow’s disease, the posterior mitral valve leaflet bulges into the LA in systole which may mimic MAD if the annular plane is set at the leaflet bending point in the LA (pseudo-MAD). However, true MAD is characterized by the posterior leaflet being inserted into the LA in both systole and diastole with a perceptible distance from the crest of the left ventricle. Second, a recent study from the UK Biobank of 2607 cases from the general population reported a MAD prevalence of 76% assessed by cardiac magnetic resonance (CMR) imaging,23 suggesting that MAD should be considered a normal finding in most cases. Disjunction was found inferiorly in 58% of the cases, anteriorly in 54%, anterolaterally in 13%, and inferolaterally in 5%. Interestingly, inferolateral MAD was strongly associated with systolic curling and VA. In line with these observations, another study of patients with MAD undergoing CMR found that only inferolateral MAD length was a marker of VA.19,35 Based on these recent clarifications, our study focused only on inferolateral true MAD observed during both systole and diastole. Additionally, most previous studies have defined MAD as any disjunction length, either >1 or ≥2 mm.18–20,22,23 Our threshold of a MAD length of ≥3 mm was conservatively chosen as measurement variability below this level has been previously reported21 and less clinical relevance.5,24,25
Correction of mitral annular disjunction
It has been speculated that the presence of MAD may increase the risk of repair failure in patients undergoing surgical correction of MR owing to paradoxical systolic expansion and flattening of the mitral annulus, leaflet and chordal tissue deficiency, as well as the risk of attaching the annuloplasty ring to the displaced annulus.33,36 A modified technique for mitral valve repair in the presence of MAD was proposed for the first time in 2005 in which the displaced annulus was repaired with sutures, the prolapsing segment was resected, and all chordae tendineae attached to the ventricular surface of the remaining posterior leaflet were excised.18 In recent years, the trend in mitral valve repair has shifted from ‘resect’ towards a ‘respect’ approach with implantation of artificial chordae, without any distinction being made between patients with or without MAD.37 Accordingly, at our centre, most patients underwent mitral valve repair with placement of artificial chordae and annular ring. Our study showed that patients with pre-operative MAD had no residual MAD after surgery and no increased risk of repair failure.
Long-term outcomes
Despite younger age and complete surgical correction of MAD, patients with pre-operative MAD had a substantial increase in the long-term risk of VA after mitral valve surgery. The mechanisms underlying these observations are largely unknown. Although MAD was corrected by mitral valve surgery, it is possible that the longstanding pre-operative stress on the native chordae and papillary muscles had induced cardiac fibrosis serving as the substrate for VA.38 The arrhythmic risk may also remain because of surgical correction with placement of an insufficient number of artificial chordae, inappropriate ring form or size, excessive over-tensioning of the artificial chordae which may continue the stress on these structures, or progression of Barlow’s disease in the treated or untreated leaflets and adverse LV remodelling. The coexistence of MVP, MAD, Barlow’s disease, and female sex, as observed in the present study, constitutes the hallmarks of the recently proposed arrhythmogenic mitral valve prolapse (AMVP),16 which should alert the clinicians to offer arrhythmic monitoring and timely interventions. Our results also show that the risk of VA in patients with MVP and MAD does not necessarily decrease after successful mitral valve surgery and emphasize the need for continued monitoring and management of VA even after surgery.
Only three previous studies have reported on arrhythmic outcomes of patients with MVP, with and without MAD, who underwent mitral valve surgery for degenerative MR. While two of these previous studies reported an increased risk of post-operative VA in patients with pre-operative MAD,21,30 one did not find any such associations.32 However, these studies were limited by significantly smaller sample sizes (n = 111, 183, and 185, respectively) and included heterogeneous patient populations with a mix of patients with secondary MR, those undergoing coronary bypass grafting, and no differentiation between mitral valve repair or replacement procedures. In contrast, our study not only represents the largest cohort but also includes the most well-characterized group of patients undergoing mitral valve surgery at a high-volume centre, with an average of 167 mitral valve surgeries per year.39,40 Our centre’s extensive experience with complex mitral valve procedures, coupled with low procedural complication rates, strengthens the reliability of our findings compared with previous studies. Additionally, we have been able to achieve comprehensive, nationwide follow-up without any patients being lost to follow-up since their initial mitral valve surgery, allowing for complete outcome tracking, and outcome data were collected blinded to the presence of MAD.
Limitations
Our study has limitations. First, it is possible that the prevalence of MAD as evaluated by echocardiography was underestimated. However, a previous study reported an almost complete concordance between echocardiography and CMR in assessing the presence of inferolateral MAD.19 Additionally, echocardiographic images were reviewed by an independent and experienced sonographer blinded to clinical outcomes to reduce interpretation variability. Second, data were collected retrospectively and there was no central event adjudication committee. Third, patients were not screened for VA unless they sought medical care due to VA-related symptoms, which may have led to an underestimation of the incidence of silent VA. However, the incidence rates of VA were likely underestimated similarly in both groups rendering the relative difference between groups unchanged. Fourth, data on pre-operative VA were not available and changes in arrhythmic burden over time could not be evaluated. Additionally, the number of patients in the replacement group was small, and our study was not powered to assess any differences in the risk of VA with regard to surgical repair techniques such as use of artificial chordae vs leaflet resection.
Conclusions
Mitral annular disjunction was more common in women and was associated with Barlow’s disease, the need for mitral valve surgery for MR at a younger age, and a substantial increased long-term risk of VA after mitral valve surgery, despite complete anatomical correction of pre-operative MAD. These findings highlight the importance of close monitoring for MR progression among the subset of patients with MVP with MAD to offer a timely intervention. Our results also show that the risk of VA in MVP/MAD does not necessarily decrease after successful mitral valve surgery and emphasize the need for continued monitoring, and management of VA after surgery.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
A.R. has received grants from the Boston Scientific Corporation, and Edward Lifesciences. I.G. has served as a consultant for Edwards Lifesciences, Medtronic, Boston Scientific Corporation, and Abbott Laboratories. D.J.C. has received research grant support and consulting income by Abbott Vascular, Medtronic, Edwards Lifesciences, Boston Scientific Corporation. F.B. has held leadership roles with Medtronic, Biotronik. M.J.E. has received grants from Novartis Foundation for Medical-Biological Research. All other authors report no conflict of interest.
Data Availability
The data that support the findings of this study are available from the corresponding author, B.S., upon reasonable request.
Funding
A.W.G. was supported by the Swedish Heart-Lung Foundation (grant 20210578). A.R. has received grants from the Swedish Heart-Lung Foundation. F.B. has received grants from the Swedish Heart-Lung Foundation. K.H.H. is funded by the Norwegian Research Council by the grant Precision Health Center for Optimized Cardiac Care (ProCardio). M.D. has received donations from Mr Fredrik Lundberg, the Schörling Foundation. B.S. was supported by the Stockholm County Council (FoUI-988371), the Swedish Heart-Lung Foundation (grant 20220524), the Swedish Research Council (grant 2022-01472), the Swedish Society for Medical Research (SSMF; SG-23-0142-B), the Swedish Society of Medicine (SLS; grant 987010), Karolinska Institutet (2-116/2023).
Ethical Approval
Ethical approval was granted by the Swedish Ethical Review Authority (dnrs 2017/934-31, 2018/909-32, and 2024-02861-01).
Pre-registered Clinical Trial Number
Not applicable.


