-
PDF
- Split View
-
Views
-
Cite
Cite
Won Young Lee, Sook Kyung Yum, Yu-Mi Seo, Sol Kim, Ju Ae Shin, Cheul Lee, Patent ductus arteriosus management in very-low-birth-weight prematurity: a place for an early operation?, European Journal of Cardio-Thoracic Surgery, Volume 65, Issue 5, May 2024, ezae175, https://doi.org/10.1093/ejcts/ezae175
Close - Share Icon Share
Abstract
The goal was to evaluate neonatal outcomes based on treatment strategies and time points for haemodynamically significant patent ductus arteriosus (hsPDA) in very-low-birth-weight preterm infants, with a particular focus on surgical closure.
This retrospective study included very-low-birth-weight infants born between 2014 and 2021 who received active treatment for hsPDA. Neonatal outcomes were compared between (i) primary surgical closure versus primary ibuprofen; (ii) early (<14th post-natal day) versus late primary surgical closure (≥14th post-natal day); and (iii) primary versus secondary surgical closure after ibuprofen failure. Further analysis using 1:1 propensity score matching was performed. Logistic regression was conducted to analyse the risk factors for post-ligation cardiac syndrome (PLCS) and/or acute kidney injury (AKI).
A total of 145 infants with hsPDA underwent active treatment for closure. The in-hospital death rate and the incidence of severe bronchopulmonary dysplasia (BPD) were similar between the primary surgical closure group and the primary ibuprofen group in a 1:1 matched analysis. Severe BPD was significantly higher in the late surgical closure group than in the early primary surgical closure group with 1:1 propensity score matching (72.7% vs 40.9%, P=0.033). The secondary surgical closure group showed the mildest clinical condition; however, the probability of PLCS/AKI was highest (38.6%) compared to the early (15.2%) or the late primary surgical group (28.1%, P<0.001), especially in extremely premature infants (gestational age < 28 weeks).
Surgical patent ductus arteriosus closure is not inferior to pharmacologic treatment. Considering the harmful effect of a prolonged patent ductus arteriosus shunt exposure, a timely decision and timely efforts should be made to minimize the risk of severe BPD and PLCS/AKI after surgical closure.
INTRODUCTION
Patent ductus arteriosus (PDA) is one of the most frequently discussed issues in perinatal–neonatal medicine, and the controversy over its role in preterm infant outcomes and optimal management approaches remains. Prolonged ductal patency in preterm infants leads to increased left-to-right shunt flow, known as “ductal steal”, which compromises systemic perfusion depending on the extent of the steal [1]. Various neonatal morbidities, including intraventricular haemorrhage (IVH), necrotizing enterocolitis, pulmonary haemorrhage and bronchopulmonary dysplasia (BPD), are associated with this pathophysiology [2]. Historic treatment approaches for PDA comprised active treatment strategies including pharmacologic or surgical closure.
During the last 2 decades, active treatment has been regarded as overtreating, considering exposure to potentially harmful drugs or invasive procedures [3]. Many studies reported non-inferior or superior outcomes after adopting conservative management for PDA [4, 5]. After a worldwide adoption of conservative management for PDA, a new insight has emerged—that prolonged exposure to the left-to-right shunt of haemodynamically significant PDA (hsPDA) causing shear stress in the alveoli increases the risk for BPD and BPD-induced pulmonary hypertension [3, 6, 7]. In addition, during postnatal alveolar-capillary development, PDA may impact the respiratory outcomes differently, depending on the treatment time point and the exposure duration of the left-to-right shunt with individual inherent risk and maturation state.
Therefore, our goal was to evaluate neonatal outcomes depending on different treatment strategies for hsPDA with a particular focus on surgical closure in very-low-birth-weight (VLBW) preterm infants, regarding treatment time point.
MATERIALS AND METHODS
Patient selection and data collection
This retrospective study included VLBW infants (<1500 g) with PDA admitted to our neonatal intensive care unit between September 2014 and March 2021. The exclusion criteria were the lack of echocardiographic examinations during the hospital stay, the presence of major congenital anomalies, death within 48 h of life, and critical intrauterine/perinatal illness. Electronic medical records were reviewed to collect data. This study was approved by the institutional review board of Seoul St Mary’s Hospital (number: 2021-2398-0001; institutional review board approval date: 12 October 2021). The need for informed consent was waived.
Baseline characteristics during the hospital stay were collected, and follow-up data were collected from outpatient records and the National Health Insurance database. PDA was evaluated by experienced paediatric cardiologists. The presence of hsPDA was determined using echocardiographic findings based on the criteria suggested by McNamara et al. (Supplementary Material, Table S1) [8]. Active treatment for PDA was further specified as primary surgical closure, pharmacologic closure or secondary closure after failed pharmacologic closure.
Primary end points were in-hospital death (IHD) and severe BPD based on the 2001 National Institute of Child Health and Human Development definition [9]. The secondary end point was a composite outcome of post-ligation cardiac syndrome (PLCS); the need for inotropic agents within 24 h post ligation, plus either oxygenation or ventilation failure [10] or acute kidney injury (AKI); and daily urine output lower than 1.5 ml/kg/h according to the neonatal RIFLE (RIFLE = Risk, Injury, Failure, Loss of function, End-stage kidney disease) criteria within 72 h post ligation [11] after PDA closure. Culture-proven neonatal early (< 7 days) and late (≥7 days) sepsis, necrotizing enterocolitis ≥ stage 2 based on modified Bell’s criteria [12], retinopathy of prematurity (ROP) ≥ stage 3 and IVH ≥grade 3 based on Papile’s criteria [13] were identified.
Treatment strategies for patent ductus arteriosus
The treatment strategy for PDA in our unit was largely dependent on the severity of clinical and echocardiographic findings and on the haemodynamic significance caused by the left-to-right shunt. In addition, the identification of E3 or higher echocardiographic findings based on the criteria of McNamara [8] was considered significant. Based on these findings, the attending neonatologist decided on active treatment in consultation with paediatric cardiologists.
When PDA closure was not immediately necessary (stage <E3), the patient was treated conservatively, including fluid restriction and diuretic administration. If relevant symptoms persisted or were aggravated despite such supportive measures, follow-up echocardiography was performed at the discretion of the neonatologist on duty—which was usually within 24 to 72 h—to identify any changes compared to the prior examination, and the need for active treatment was reassessed. For medical closure, ibuprofen, the only cyclooxygenase inhibitor available in our unit, was administered either intravenously or orally. When treatment was initiated within the first week of life, a loading dose of 10 mg/kg/day and a subsequent maintenance dose of 5 mg/kg/day were administered for the next 2 days. When the treatment started after the second week of life, a loading dose of 18 mg/kg/day followed by maintenance doses of 9 mg/kg/day for 2 days was administered.
Surgical closure of the PDA was considered when pharmacologic closure failed or was contraindicated. The contraindications for ibuprofen use were pulmonary haemorrhage and IVH grade ≥3, coagulopathy, necrotizing enterocolitis and kidney injury (oliguria or increased serum creatinine >1.8 mg/dl). Early primary closure was defined as surgical closure of PDA within 14 days of post-natal age, and late primary closure was defined as surgical closure performed ≥14 days after birth. The operation was performed through a limited left posterior thoracotomy via the third or fourth intercostal space. After identifying the left vagus and recurrent laryngeal nerves, minimal dissection was performed immediately above and below the ductus, and a medium- or large-sized horizontal titanium clip (Teleflex Medical, Research Triangle Park, NC, USA) was applied around the PDA. No chest tubes were placed at the end of the procedure.
Statistical analysis
Categorical data are presented as frequencies and percentages, and continuous variables are presented as mean or median with standard deviation or interquartile range. Differences between groups were evaluated using t-tests for continuous variables and the χ2 or Fisher’s exact test for categorical variables. A 1:1 propensity score matching was performed for applicable groups to compare neonatal outcomes. The following covariates were entered for logistic regression analysis: gestational age (GA), birthweight, small for GA, oligohydramnios, histologic chorioamnionitis, Apgar score at 1 min, high-stage resuscitation at birth, mean airway pressure and FiO2 at surgery and pulmonary hypertension at the initial echocardiogram.
Adjusted logistic regression was conducted to analyse risk factors for the composite outcome of PLCS/AKI. Variables were evaluated in the models, and those with P-values < 0.20 in the univariate analyses were candidates for multivariate analysis, in which P-values < 0.05 were considered significant. Predicted probabilities (with 95% confidence intervals) of the PLCS/AKI according to GA were plotted with marginal standardization by adjusting other risk factors identified on multivariable logistic regression to control confounding factors. The 95% confidence intervals were not adjusted for multiple comparisons, and the inferences drawn from them may not be reproducible. Probabilities were compared between the treatment strategies using a one-way analysis of variance, followed by Bonferroni multiple comparisons when the distribution of the variables was normally examined using the Kolmogorov-Smirnov test. All statistical analyses were conducted using the R version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/).
RESULTS
A total of 517 VLBW infants with PDA were admitted to the neonatal intensive care unit during the study period. Excluding 95 patients, 145 hsPDA infants (145/422, 34.4%) underwent surgical or medical treatment for closure and were included in the final analysis (Fig. 1).
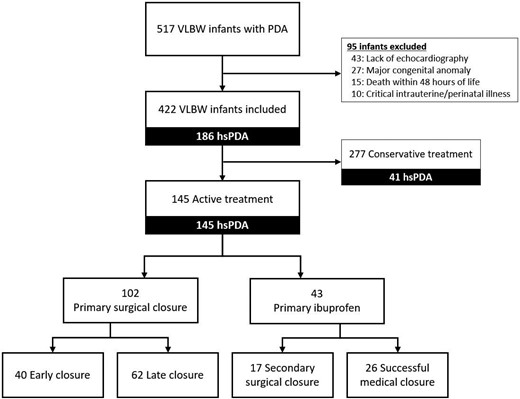
Flow diagram of very-low-birth-weight infants included in the study. hsPDA: haemodynamically significant patent ductus arteriosus; PDA: patent ductus arteriosus; VLBW: very-low-birth-weight.
Primary surgical closure versus primary ibuprofen
Baseline characteristics and outcomes of all the patients and a comparison between 102 primary surgical closures versus 43 primary ibuprofen are listed in Table 1. The preterm neonates in the primary surgical closure group and the primary ibuprofen group showed significant differences in the antenatal corticosteroid administration rate (77.5% vs 97.7%, P=0.003) and the proportion of outborn infants (7.8% vs 30.2%, P<0.001). Otherwise the 2 groups had similar baseline characteristics, including GA and birthweight.
Baseline characteristics and outcomes of all the patients and comparison between the primary surgical closure group and the primary ibuprofen group
| Characteristics . | Total . | Primary surgical closure . | Primary ibuprofen . | P-value . |
|---|---|---|---|---|
| (n = 145) . | (n = 102) . | (n = 43) . | . | |
| GA, weeks, median (IQR) | 26.7 [25.6−28.5] | 26.6 [25.4−28.6] | 27.1 [26.0−28.4] | 0.49 |
| Birth weight (g), median (IQR) | 910 [757−1079] | 896 [731−1061] | 951 [844−1090] | 0.41 |
| Small for GA, <10 percentile (%) | 18 (12.4%) | 14(13.7%) | 4(9.3%) | 0.59 |
| Male (%) | 67 (46.2%) | 46 (45.1%) | 21 (48.8%) | 0.68 |
| Preterm premature rupture of membrane (%) | 48 (33.1%) | 37 (36.3%) | 11 (25.6%) | 0.21 |
| Oligohydramnios (%) | 12 (8.3%) | 10 (9.8%) | 2 (4.7%) | 0.51 |
| Histologic chorioamnionitis (%) | 60 (41.4%) | 44 (43.1%) | 16 (37.2%) | 0.51 |
| Antenatal corticosteroids (%) | 121 (83.4%) | 79 (77.5%) | 42 (97.7%) | 0.003 |
| Cesarean section (%) | 124 (85.5%) | 90 (88.2%) | 34 (79.1%) | 0.15 |
| Multiple gestation (%) | 52 (35.9%) | 33 (32.4%) | 19 (44.2%) | 0.18 |
| Apgar score at 1 min, mean (SD) | 2.7 (1.7) | 2.6 (1.7) | 3.0 (1.8) | 0.20 |
| Apgar score at 5 min, mean (SD) | 5.0 (1.9) | 5.0 (1.9) | 5.2 (1.9) | 0.56 |
| High stage resuscitation at birth (%) | 12 (8.3%) | 11(10.8%) | 1(2.3%) | 0.11 |
| Pulmonary hypertension at initial echocardiography (%) | 23 (15.9%) | 19 (18.6%) | 4 (9.3%) | 0.22 |
| Outborn (%) | 21 (14.5%) | 8 (7.8%) | 13 (30.2%) | <0.001 |
| Outcomes | ||||
| Age at PDA closure (days), median (IQR) | 16.0 [9.0−24.0] | 16.0 [9.0−23.0] | 18 [8.0−37.0] | 0.35 |
| In-hospital deaths (%) | 12 (8.3%) | 10 (9.8%) | 2 (4.7%) | 0.51 |
| Severe BPD (%) | 83 (57.2%) | 62 (60.8%) | 21 (48.8%) | 0.18 |
| Culture proven sepsis (%) | 42 (29.0%) | 31 (30.4%) | 11 (25.6%) | 0.56 |
| Early sepsis (<7 days) (%) | 8 (5.6%) | 6 (5.9%) | 2 (5.0%) | >0.99 |
| Late sepsis (≥7 days) (%) | 34 (23.9%) | 25 (24.5%) | 9 (22.5%) | >0.99 |
| Necrotizing enterocolitis, ≥ stage 2 (%) | 9 (6.2%) | 8 (7.8%) | 1 (2.3%) | 0.28 |
| ROP, ≥ stage 3 (%) | 38 (27.3%) | 25 (25.5%) | 13 (31.7%) | 0.46 |
| IVH, ≥ grade 3 (%) | 41 (28.5%) | 35 (34.7%) | 6 (14.0%) | 0.015 |
| Characteristics . | Total . | Primary surgical closure . | Primary ibuprofen . | P-value . |
|---|---|---|---|---|
| (n = 145) . | (n = 102) . | (n = 43) . | . | |
| GA, weeks, median (IQR) | 26.7 [25.6−28.5] | 26.6 [25.4−28.6] | 27.1 [26.0−28.4] | 0.49 |
| Birth weight (g), median (IQR) | 910 [757−1079] | 896 [731−1061] | 951 [844−1090] | 0.41 |
| Small for GA, <10 percentile (%) | 18 (12.4%) | 14(13.7%) | 4(9.3%) | 0.59 |
| Male (%) | 67 (46.2%) | 46 (45.1%) | 21 (48.8%) | 0.68 |
| Preterm premature rupture of membrane (%) | 48 (33.1%) | 37 (36.3%) | 11 (25.6%) | 0.21 |
| Oligohydramnios (%) | 12 (8.3%) | 10 (9.8%) | 2 (4.7%) | 0.51 |
| Histologic chorioamnionitis (%) | 60 (41.4%) | 44 (43.1%) | 16 (37.2%) | 0.51 |
| Antenatal corticosteroids (%) | 121 (83.4%) | 79 (77.5%) | 42 (97.7%) | 0.003 |
| Cesarean section (%) | 124 (85.5%) | 90 (88.2%) | 34 (79.1%) | 0.15 |
| Multiple gestation (%) | 52 (35.9%) | 33 (32.4%) | 19 (44.2%) | 0.18 |
| Apgar score at 1 min, mean (SD) | 2.7 (1.7) | 2.6 (1.7) | 3.0 (1.8) | 0.20 |
| Apgar score at 5 min, mean (SD) | 5.0 (1.9) | 5.0 (1.9) | 5.2 (1.9) | 0.56 |
| High stage resuscitation at birth (%) | 12 (8.3%) | 11(10.8%) | 1(2.3%) | 0.11 |
| Pulmonary hypertension at initial echocardiography (%) | 23 (15.9%) | 19 (18.6%) | 4 (9.3%) | 0.22 |
| Outborn (%) | 21 (14.5%) | 8 (7.8%) | 13 (30.2%) | <0.001 |
| Outcomes | ||||
| Age at PDA closure (days), median (IQR) | 16.0 [9.0−24.0] | 16.0 [9.0−23.0] | 18 [8.0−37.0] | 0.35 |
| In-hospital deaths (%) | 12 (8.3%) | 10 (9.8%) | 2 (4.7%) | 0.51 |
| Severe BPD (%) | 83 (57.2%) | 62 (60.8%) | 21 (48.8%) | 0.18 |
| Culture proven sepsis (%) | 42 (29.0%) | 31 (30.4%) | 11 (25.6%) | 0.56 |
| Early sepsis (<7 days) (%) | 8 (5.6%) | 6 (5.9%) | 2 (5.0%) | >0.99 |
| Late sepsis (≥7 days) (%) | 34 (23.9%) | 25 (24.5%) | 9 (22.5%) | >0.99 |
| Necrotizing enterocolitis, ≥ stage 2 (%) | 9 (6.2%) | 8 (7.8%) | 1 (2.3%) | 0.28 |
| ROP, ≥ stage 3 (%) | 38 (27.3%) | 25 (25.5%) | 13 (31.7%) | 0.46 |
| IVH, ≥ grade 3 (%) | 41 (28.5%) | 35 (34.7%) | 6 (14.0%) | 0.015 |
BPD: bronchopulmonary dysplasia; GA: gestational age; IQR: interquartile range; IVH: Intraventricular haemorrhage; PDA: patent ductus arteriosus; ROP: retinopathy of prematurity.
Baseline characteristics and outcomes of all the patients and comparison between the primary surgical closure group and the primary ibuprofen group
| Characteristics . | Total . | Primary surgical closure . | Primary ibuprofen . | P-value . |
|---|---|---|---|---|
| (n = 145) . | (n = 102) . | (n = 43) . | . | |
| GA, weeks, median (IQR) | 26.7 [25.6−28.5] | 26.6 [25.4−28.6] | 27.1 [26.0−28.4] | 0.49 |
| Birth weight (g), median (IQR) | 910 [757−1079] | 896 [731−1061] | 951 [844−1090] | 0.41 |
| Small for GA, <10 percentile (%) | 18 (12.4%) | 14(13.7%) | 4(9.3%) | 0.59 |
| Male (%) | 67 (46.2%) | 46 (45.1%) | 21 (48.8%) | 0.68 |
| Preterm premature rupture of membrane (%) | 48 (33.1%) | 37 (36.3%) | 11 (25.6%) | 0.21 |
| Oligohydramnios (%) | 12 (8.3%) | 10 (9.8%) | 2 (4.7%) | 0.51 |
| Histologic chorioamnionitis (%) | 60 (41.4%) | 44 (43.1%) | 16 (37.2%) | 0.51 |
| Antenatal corticosteroids (%) | 121 (83.4%) | 79 (77.5%) | 42 (97.7%) | 0.003 |
| Cesarean section (%) | 124 (85.5%) | 90 (88.2%) | 34 (79.1%) | 0.15 |
| Multiple gestation (%) | 52 (35.9%) | 33 (32.4%) | 19 (44.2%) | 0.18 |
| Apgar score at 1 min, mean (SD) | 2.7 (1.7) | 2.6 (1.7) | 3.0 (1.8) | 0.20 |
| Apgar score at 5 min, mean (SD) | 5.0 (1.9) | 5.0 (1.9) | 5.2 (1.9) | 0.56 |
| High stage resuscitation at birth (%) | 12 (8.3%) | 11(10.8%) | 1(2.3%) | 0.11 |
| Pulmonary hypertension at initial echocardiography (%) | 23 (15.9%) | 19 (18.6%) | 4 (9.3%) | 0.22 |
| Outborn (%) | 21 (14.5%) | 8 (7.8%) | 13 (30.2%) | <0.001 |
| Outcomes | ||||
| Age at PDA closure (days), median (IQR) | 16.0 [9.0−24.0] | 16.0 [9.0−23.0] | 18 [8.0−37.0] | 0.35 |
| In-hospital deaths (%) | 12 (8.3%) | 10 (9.8%) | 2 (4.7%) | 0.51 |
| Severe BPD (%) | 83 (57.2%) | 62 (60.8%) | 21 (48.8%) | 0.18 |
| Culture proven sepsis (%) | 42 (29.0%) | 31 (30.4%) | 11 (25.6%) | 0.56 |
| Early sepsis (<7 days) (%) | 8 (5.6%) | 6 (5.9%) | 2 (5.0%) | >0.99 |
| Late sepsis (≥7 days) (%) | 34 (23.9%) | 25 (24.5%) | 9 (22.5%) | >0.99 |
| Necrotizing enterocolitis, ≥ stage 2 (%) | 9 (6.2%) | 8 (7.8%) | 1 (2.3%) | 0.28 |
| ROP, ≥ stage 3 (%) | 38 (27.3%) | 25 (25.5%) | 13 (31.7%) | 0.46 |
| IVH, ≥ grade 3 (%) | 41 (28.5%) | 35 (34.7%) | 6 (14.0%) | 0.015 |
| Characteristics . | Total . | Primary surgical closure . | Primary ibuprofen . | P-value . |
|---|---|---|---|---|
| (n = 145) . | (n = 102) . | (n = 43) . | . | |
| GA, weeks, median (IQR) | 26.7 [25.6−28.5] | 26.6 [25.4−28.6] | 27.1 [26.0−28.4] | 0.49 |
| Birth weight (g), median (IQR) | 910 [757−1079] | 896 [731−1061] | 951 [844−1090] | 0.41 |
| Small for GA, <10 percentile (%) | 18 (12.4%) | 14(13.7%) | 4(9.3%) | 0.59 |
| Male (%) | 67 (46.2%) | 46 (45.1%) | 21 (48.8%) | 0.68 |
| Preterm premature rupture of membrane (%) | 48 (33.1%) | 37 (36.3%) | 11 (25.6%) | 0.21 |
| Oligohydramnios (%) | 12 (8.3%) | 10 (9.8%) | 2 (4.7%) | 0.51 |
| Histologic chorioamnionitis (%) | 60 (41.4%) | 44 (43.1%) | 16 (37.2%) | 0.51 |
| Antenatal corticosteroids (%) | 121 (83.4%) | 79 (77.5%) | 42 (97.7%) | 0.003 |
| Cesarean section (%) | 124 (85.5%) | 90 (88.2%) | 34 (79.1%) | 0.15 |
| Multiple gestation (%) | 52 (35.9%) | 33 (32.4%) | 19 (44.2%) | 0.18 |
| Apgar score at 1 min, mean (SD) | 2.7 (1.7) | 2.6 (1.7) | 3.0 (1.8) | 0.20 |
| Apgar score at 5 min, mean (SD) | 5.0 (1.9) | 5.0 (1.9) | 5.2 (1.9) | 0.56 |
| High stage resuscitation at birth (%) | 12 (8.3%) | 11(10.8%) | 1(2.3%) | 0.11 |
| Pulmonary hypertension at initial echocardiography (%) | 23 (15.9%) | 19 (18.6%) | 4 (9.3%) | 0.22 |
| Outborn (%) | 21 (14.5%) | 8 (7.8%) | 13 (30.2%) | <0.001 |
| Outcomes | ||||
| Age at PDA closure (days), median (IQR) | 16.0 [9.0−24.0] | 16.0 [9.0−23.0] | 18 [8.0−37.0] | 0.35 |
| In-hospital deaths (%) | 12 (8.3%) | 10 (9.8%) | 2 (4.7%) | 0.51 |
| Severe BPD (%) | 83 (57.2%) | 62 (60.8%) | 21 (48.8%) | 0.18 |
| Culture proven sepsis (%) | 42 (29.0%) | 31 (30.4%) | 11 (25.6%) | 0.56 |
| Early sepsis (<7 days) (%) | 8 (5.6%) | 6 (5.9%) | 2 (5.0%) | >0.99 |
| Late sepsis (≥7 days) (%) | 34 (23.9%) | 25 (24.5%) | 9 (22.5%) | >0.99 |
| Necrotizing enterocolitis, ≥ stage 2 (%) | 9 (6.2%) | 8 (7.8%) | 1 (2.3%) | 0.28 |
| ROP, ≥ stage 3 (%) | 38 (27.3%) | 25 (25.5%) | 13 (31.7%) | 0.46 |
| IVH, ≥ grade 3 (%) | 41 (28.5%) | 35 (34.7%) | 6 (14.0%) | 0.015 |
BPD: bronchopulmonary dysplasia; GA: gestational age; IQR: interquartile range; IVH: Intraventricular haemorrhage; PDA: patent ductus arteriosus; ROP: retinopathy of prematurity.
The age at PDA closure was also similar (16 vs 18 days) between the 2 groups. Regarding in-hospital neonatal outcomes, IVH ≥ grade 3 was significantly greater in the primary surgical group (34.7%) than in the primary ibuprofen group (14.0%, P=0.015). With the exception of ROP ≥ stage 3, other neonatal morbidities including IHD rate and severe BPD were higher in the primary surgical closure group without statistical significance. After 1:1 propensity score matching (Supplementary Material, Table S2), none of the outcomes was significantly different between the 2 groups including IHD and severe BPD (Table 2).
| Outcomes . | Primary surgical closure . | Primary ibuprofen . | P-value . |
|---|---|---|---|
| (n = 42) . | (n = 42) . | ||
| In-hospital deaths (%) | 5 (11.9%) | 2 (4.8%) | 0.43 |
| Severe BPD (%) | 22 (52.4%) | 21 (50.0%) | 0.83 |
| Culture-proven sepsis (%) | 10 (23.8%) | 11 (26.2%) | 0.80 |
| Early sepsis (< 7 days) (%) | 2 (4.8%) | 2 (4.8%) | >0.99 |
| Late sepsis (≥ 7 days) (%) | 8 (19.0%) | 9 (21.4%) | 0.79 |
| Necrotizing enterocolitis, ≥ stage 2 (%) | 3 (7.1%) | 1 (2.4%) | 0.62 |
| ROP, ≥ stage 3 (%) | 10 (23.8%) | 13 (31.0%) | 0.46 |
| IVH, ≥ grade 3 (%) | 14 (33.3%) | 6 (14.3%) | 0.071 |
| Outcomes . | Primary surgical closure . | Primary ibuprofen . | P-value . |
|---|---|---|---|
| (n = 42) . | (n = 42) . | ||
| In-hospital deaths (%) | 5 (11.9%) | 2 (4.8%) | 0.43 |
| Severe BPD (%) | 22 (52.4%) | 21 (50.0%) | 0.83 |
| Culture-proven sepsis (%) | 10 (23.8%) | 11 (26.2%) | 0.80 |
| Early sepsis (< 7 days) (%) | 2 (4.8%) | 2 (4.8%) | >0.99 |
| Late sepsis (≥ 7 days) (%) | 8 (19.0%) | 9 (21.4%) | 0.79 |
| Necrotizing enterocolitis, ≥ stage 2 (%) | 3 (7.1%) | 1 (2.4%) | 0.62 |
| ROP, ≥ stage 3 (%) | 10 (23.8%) | 13 (31.0%) | 0.46 |
| IVH, ≥ grade 3 (%) | 14 (33.3%) | 6 (14.3%) | 0.071 |
BPD: bronchopulmonary dysplasia; IVH: Intraventricular haemorrhage; ROP: retinopathy of prematurity.
| Outcomes . | Primary surgical closure . | Primary ibuprofen . | P-value . |
|---|---|---|---|
| (n = 42) . | (n = 42) . | ||
| In-hospital deaths (%) | 5 (11.9%) | 2 (4.8%) | 0.43 |
| Severe BPD (%) | 22 (52.4%) | 21 (50.0%) | 0.83 |
| Culture-proven sepsis (%) | 10 (23.8%) | 11 (26.2%) | 0.80 |
| Early sepsis (< 7 days) (%) | 2 (4.8%) | 2 (4.8%) | >0.99 |
| Late sepsis (≥ 7 days) (%) | 8 (19.0%) | 9 (21.4%) | 0.79 |
| Necrotizing enterocolitis, ≥ stage 2 (%) | 3 (7.1%) | 1 (2.4%) | 0.62 |
| ROP, ≥ stage 3 (%) | 10 (23.8%) | 13 (31.0%) | 0.46 |
| IVH, ≥ grade 3 (%) | 14 (33.3%) | 6 (14.3%) | 0.071 |
| Outcomes . | Primary surgical closure . | Primary ibuprofen . | P-value . |
|---|---|---|---|
| (n = 42) . | (n = 42) . | ||
| In-hospital deaths (%) | 5 (11.9%) | 2 (4.8%) | 0.43 |
| Severe BPD (%) | 22 (52.4%) | 21 (50.0%) | 0.83 |
| Culture-proven sepsis (%) | 10 (23.8%) | 11 (26.2%) | 0.80 |
| Early sepsis (< 7 days) (%) | 2 (4.8%) | 2 (4.8%) | >0.99 |
| Late sepsis (≥ 7 days) (%) | 8 (19.0%) | 9 (21.4%) | 0.79 |
| Necrotizing enterocolitis, ≥ stage 2 (%) | 3 (7.1%) | 1 (2.4%) | 0.62 |
| ROP, ≥ stage 3 (%) | 10 (23.8%) | 13 (31.0%) | 0.46 |
| IVH, ≥ grade 3 (%) | 14 (33.3%) | 6 (14.3%) | 0.071 |
BPD: bronchopulmonary dysplasia; IVH: Intraventricular haemorrhage; ROP: retinopathy of prematurity.
Primary versus secondary surgical closure
Primary and secondary surgical groups (surgery after ibuprofen failure) are compared in Supplementary Material, Table S3. The postnatal age at surgery was significantly greater in the secondary surgical closure group. Except for a lower 1-minute Apgar score in the primary surgical closure group, primary versus secondary surgical closure groups did not show significant differences in baseline neonatal characteristics.
Regarding preoperative findings, the secondary surgical closure group showed the mildest condition postoperatively, including a significantly lower rate of high-frequency oscillatory ventilation support, mean airway pressure at surgery and systemic corticosteroid administration within 72 h prior to surgery compared to those in the primary surgical closure groups. Neonatal outcomes were similar; however, the PLCS rate was more than twofold higher in the secondary surgical closure group than in the primary surgical closure group (35.3% vs 13.7%, P=0.028).
Early versus late primary surgical closure
The same variables as in the primary surgical versus secondary surgical groups were compared between the early versus late primary surgical closure groups (Supplementary Material, Table S3). The postnatal age at surgery was a median of 9 and 22 days in the early and late primary surgical groups, respectively (P<0.001). The late primary surgical group infants had shorter GAs (26.1 vs 27.8 weeks, P<0.001) and lighter birthweights (837 vs 1064 g, P<0.001). In regard to in-hospital outcomes, the late primary surgical closure group showed a significantly higher rate of severe BPD and necrotizing enterocolitis ≥ stage 2. Because the late primary surgical closure group presented more critical baseline features, the 2 groups were analysed for neonatal outcomes after 1:1 propensity score matching (Supplementary Material, Table S4 and Table 3). Severe BPD rates were significantly higher in the late surgical closure group (72.7% vs 40.9%, P=0.033).
Comparison of the outcomes between early and late primary surgical closure after propensity score matching
| Outcomes . | Early closure . | Late closure . | P-value . |
|---|---|---|---|
| (n = 22) . | (n = 22) . | ||
| Age at surgery (days), median (IQR) | 8.5 [7.0−10.3] | 22.0 [17.0−34.5] | <0.001 |
| PMA at surgery (weeks), median (IQR) | 27.5 [26.5−28.8] | 30.4 [28.9−31.8] | <0.001 |
| In-hospital death (%) | 4 (18.2%) | 2 (9.1%) | 0.66 |
| Severe BPD (%) | 9 (40.9%) | 16 (72.7%) | 0.033 |
| Culture proven sepsis (%) | 4 (18.2%) | 9 (40.9%) | 0.19 |
| ROP, ≥ stage 3 (%) | 4 (18.2%) | 4 (18.2%) | >0.99 |
| IVH, ≥ grade 3 (%) | 9 (40.9%) | 5 (22.7%) | 0.20 |
| Necrotizing enterocolitis, ≥ stage 2 (%) | 0 | 0 | NA |
| Outcomes . | Early closure . | Late closure . | P-value . |
|---|---|---|---|
| (n = 22) . | (n = 22) . | ||
| Age at surgery (days), median (IQR) | 8.5 [7.0−10.3] | 22.0 [17.0−34.5] | <0.001 |
| PMA at surgery (weeks), median (IQR) | 27.5 [26.5−28.8] | 30.4 [28.9−31.8] | <0.001 |
| In-hospital death (%) | 4 (18.2%) | 2 (9.1%) | 0.66 |
| Severe BPD (%) | 9 (40.9%) | 16 (72.7%) | 0.033 |
| Culture proven sepsis (%) | 4 (18.2%) | 9 (40.9%) | 0.19 |
| ROP, ≥ stage 3 (%) | 4 (18.2%) | 4 (18.2%) | >0.99 |
| IVH, ≥ grade 3 (%) | 9 (40.9%) | 5 (22.7%) | 0.20 |
| Necrotizing enterocolitis, ≥ stage 2 (%) | 0 | 0 | NA |
BPD: bronchopulmonary dysplasia; IQR: interquartile range; IVH: Intraventricular haemorrhage; PMA: postmenstrual age; ROP: retinopathy of prematurity.
Comparison of the outcomes between early and late primary surgical closure after propensity score matching
| Outcomes . | Early closure . | Late closure . | P-value . |
|---|---|---|---|
| (n = 22) . | (n = 22) . | ||
| Age at surgery (days), median (IQR) | 8.5 [7.0−10.3] | 22.0 [17.0−34.5] | <0.001 |
| PMA at surgery (weeks), median (IQR) | 27.5 [26.5−28.8] | 30.4 [28.9−31.8] | <0.001 |
| In-hospital death (%) | 4 (18.2%) | 2 (9.1%) | 0.66 |
| Severe BPD (%) | 9 (40.9%) | 16 (72.7%) | 0.033 |
| Culture proven sepsis (%) | 4 (18.2%) | 9 (40.9%) | 0.19 |
| ROP, ≥ stage 3 (%) | 4 (18.2%) | 4 (18.2%) | >0.99 |
| IVH, ≥ grade 3 (%) | 9 (40.9%) | 5 (22.7%) | 0.20 |
| Necrotizing enterocolitis, ≥ stage 2 (%) | 0 | 0 | NA |
| Outcomes . | Early closure . | Late closure . | P-value . |
|---|---|---|---|
| (n = 22) . | (n = 22) . | ||
| Age at surgery (days), median (IQR) | 8.5 [7.0−10.3] | 22.0 [17.0−34.5] | <0.001 |
| PMA at surgery (weeks), median (IQR) | 27.5 [26.5−28.8] | 30.4 [28.9−31.8] | <0.001 |
| In-hospital death (%) | 4 (18.2%) | 2 (9.1%) | 0.66 |
| Severe BPD (%) | 9 (40.9%) | 16 (72.7%) | 0.033 |
| Culture proven sepsis (%) | 4 (18.2%) | 9 (40.9%) | 0.19 |
| ROP, ≥ stage 3 (%) | 4 (18.2%) | 4 (18.2%) | >0.99 |
| IVH, ≥ grade 3 (%) | 9 (40.9%) | 5 (22.7%) | 0.20 |
| Necrotizing enterocolitis, ≥ stage 2 (%) | 0 | 0 | NA |
BPD: bronchopulmonary dysplasia; IQR: interquartile range; IVH: Intraventricular haemorrhage; PMA: postmenstrual age; ROP: retinopathy of prematurity.
Risk factors for post-ligation cardiac syndrome/acute kidney injury
Table 4 shows the results of logistic regression analyses to evaluate the risk factors associated with the development of PLCS/AKI after surgical PDA closure. Oligohydramnios (odds ratio: 3.93, 95% confidence interval: 1.03 − 14.59, P=0.041) and secondary surgical closure (odds ratio: 3.40, 95% confidence interval: 1.12 − 10.33, P=0.031) were 2 significant risk factors for PLCS/AKI.
Risk factor analysis of post-ligation cardiac syndrome/acute kidney injury in the surgical group
| . | Univariable analysis . | Multivariable analysis . | ||
|---|---|---|---|---|
| OR (95% CI) . | P-value . | OR (95% CI) . | P-value . | |
| Gestational age (weeks) | 0.83 (0.66−1.05) | 0.13 | ||
| Age at surgery (days) | 1.01 (0.98−1.04) | 0.54 | ||
| Small for gestational age | 0.83 (0.22−3.20) | 0.79 | ||
| Oligohydramnios | 3.45 (0.96−12.40) | 0.058 | 3.93 (1.06−14.59) | 0.041 |
| Histologic chorioamnionitis | 0.97 (0.40−2.34) | 0.95 | ||
| Preoperative inotropes ≥2 | 1.68 (0.67−4.18) | 0.27 | ||
| Pulmonary hypertension at initial echocardiography | 1.15 (0.38−3.49) | 0.81 | ||
| Duration of surgery | 1.01 (0.96−1.05) | 0.80 | ||
| Secondary closure | 3.06 (1.03−9.07) | 0.044 | 3.40 (1.12−10.33) | 0.031 |
| . | Univariable analysis . | Multivariable analysis . | ||
|---|---|---|---|---|
| OR (95% CI) . | P-value . | OR (95% CI) . | P-value . | |
| Gestational age (weeks) | 0.83 (0.66−1.05) | 0.13 | ||
| Age at surgery (days) | 1.01 (0.98−1.04) | 0.54 | ||
| Small for gestational age | 0.83 (0.22−3.20) | 0.79 | ||
| Oligohydramnios | 3.45 (0.96−12.40) | 0.058 | 3.93 (1.06−14.59) | 0.041 |
| Histologic chorioamnionitis | 0.97 (0.40−2.34) | 0.95 | ||
| Preoperative inotropes ≥2 | 1.68 (0.67−4.18) | 0.27 | ||
| Pulmonary hypertension at initial echocardiography | 1.15 (0.38−3.49) | 0.81 | ||
| Duration of surgery | 1.01 (0.96−1.05) | 0.80 | ||
| Secondary closure | 3.06 (1.03−9.07) | 0.044 | 3.40 (1.12−10.33) | 0.031 |
AKI: acute kidney injury; BPD: bronchopulmonary dysplasia; CI: confidence interval; OR: odds ratio; PLCS: post-ligation cardiac syndrome; PMA: postmenstrual age.
Risk factor analysis of post-ligation cardiac syndrome/acute kidney injury in the surgical group
| . | Univariable analysis . | Multivariable analysis . | ||
|---|---|---|---|---|
| OR (95% CI) . | P-value . | OR (95% CI) . | P-value . | |
| Gestational age (weeks) | 0.83 (0.66−1.05) | 0.13 | ||
| Age at surgery (days) | 1.01 (0.98−1.04) | 0.54 | ||
| Small for gestational age | 0.83 (0.22−3.20) | 0.79 | ||
| Oligohydramnios | 3.45 (0.96−12.40) | 0.058 | 3.93 (1.06−14.59) | 0.041 |
| Histologic chorioamnionitis | 0.97 (0.40−2.34) | 0.95 | ||
| Preoperative inotropes ≥2 | 1.68 (0.67−4.18) | 0.27 | ||
| Pulmonary hypertension at initial echocardiography | 1.15 (0.38−3.49) | 0.81 | ||
| Duration of surgery | 1.01 (0.96−1.05) | 0.80 | ||
| Secondary closure | 3.06 (1.03−9.07) | 0.044 | 3.40 (1.12−10.33) | 0.031 |
| . | Univariable analysis . | Multivariable analysis . | ||
|---|---|---|---|---|
| OR (95% CI) . | P-value . | OR (95% CI) . | P-value . | |
| Gestational age (weeks) | 0.83 (0.66−1.05) | 0.13 | ||
| Age at surgery (days) | 1.01 (0.98−1.04) | 0.54 | ||
| Small for gestational age | 0.83 (0.22−3.20) | 0.79 | ||
| Oligohydramnios | 3.45 (0.96−12.40) | 0.058 | 3.93 (1.06−14.59) | 0.041 |
| Histologic chorioamnionitis | 0.97 (0.40−2.34) | 0.95 | ||
| Preoperative inotropes ≥2 | 1.68 (0.67−4.18) | 0.27 | ||
| Pulmonary hypertension at initial echocardiography | 1.15 (0.38−3.49) | 0.81 | ||
| Duration of surgery | 1.01 (0.96−1.05) | 0.80 | ||
| Secondary closure | 3.06 (1.03−9.07) | 0.044 | 3.40 (1.12−10.33) | 0.031 |
AKI: acute kidney injury; BPD: bronchopulmonary dysplasia; CI: confidence interval; OR: odds ratio; PLCS: post-ligation cardiac syndrome; PMA: postmenstrual age.
The predicted probabilities of PLCS/AKI stratified by GA were compared between the 3 surgical closure groups (early, late and secondary) in Fig. 2. After adjusting for non-linear effects, the early primary closure group showed significantly lower probability than other 2 groups in extremely premature infants (GA <28 weeks). The probability of PLCS/AKI was highest in the secondary closure group compared to the other 2 groups in extremely premature infants (early 15.2% vs late 28.1%, P <0.001; late 28.1% vs secondary 38.6%, P <0.001, Fig. 2). After 28 weeks of GA, the probabilities of PLCS/AKI were low without statistical difference between the groups (early 9.8% vs late 6.7% vs secondary 10.3%).
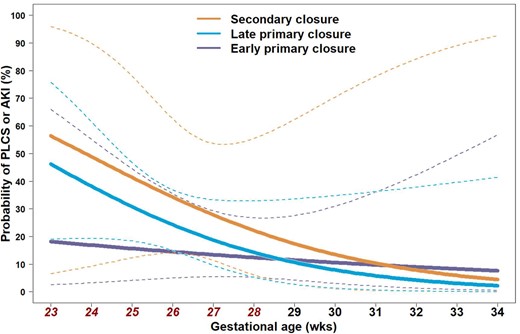
Probability of post-ligation cardiac syndrome/acute kidney injury was compared by surgical strategies (early vs late vs secondary) to determine which secondary closure group showed the highest rate when infants were born before 28 weeks of gestational age (early 15.2% vs late, 28.1%, P<0.001; late 28.1% vs secondary 38.6%, P<0.001). Dashed lines enclose the 95% confidence interval. AKI: acute kidney injury; PLCS: post-ligation cardiac syndrome.
DISCUSSION
Our study findings show that primary surgical closure of hsPDA is not inferior to primary ibuprofen treatment regarding in-hospital neonatal outcomes, based on propensity score matching analysis. Among the infants who underwent primary surgical closure, late closure may be associated with an increased risk of severe BPD compared with early closure. In addition, extremely premature infants in the secondary surgical closure group seem to be at the greatest risk of developing PLCS/AKI.
Surgical closure of the PDA has been accused of being responsible for the invasiveness and the potentially harmful effects on neonatal outcomes [14, 15]. However, Weisz et al. [16] pointed out the selection and the confounding biases in the previous publications and reported the lack of association of surgical ligation with the composite outcome of death or neuro-developmental impairment in the preterm infants < 28 weeks compared to medical treatment. Also, PDA ligation was not associated with BPD or ROP. Therefore, surgical ligation for hsPDA remains a valid treatment modality in a certain proportion of preterm infants. In our study, the late primary surgical closure group had a higher incidence of severe BPD and necrotizing enterocolitis than the early closure group, although in the propensity score matched group comparison, an analysis of the incidence of necrotizing enterocolitis was not performed due to the lack of cases. Lee et al. [17] previously reported that early PDA ligation (within 10 postnatal days) was associated with a lower rate of adverse neonatal outcomes, highlighting the importance of avoiding delayed PDA closure in the infants who did not respond to pharmacologic treatment. Other related studies have also underscored that longer duration of hsPDA is associated with increased suboptimal outcomes, including BPD [18–20]. These results reflect the adverse effect of pulmonary overcirculation, leading to changes in lung compliance and decreased effective surface area for alveolar-capillary gas exchange [21].
The alveolar-capillary membranes normally undergo considerable development between 22 and 32 weeks of gestation, whereas the preterm infants might expect a “catch-up” of the lung function by secondary septation of the alveoli in the saccular stage (28–36 GA weeks) [22, 23]. Postnatal maturational process patterns are altered during this period by postnatal hyperoxia/hypoxia, mechanical ventilator support, inflammatory diseases and prolonged ductal shunt flow [24]. The early primary closure group might have experienced shorter and fewer exposures in the “catch up” process, resulting in a beneficial outcome in patients with BPD.
Our study results also indicate that the secondary surgical closure of hsPDA after medical treatment failure seems to be associated with an increased probability of PLCS/AKI. This result also is potentially due to the prolonged duration of hsPDA exposure with the latest closure age, which complicates the development of the preterm heart and kidney to different extents according to organ maturation status at birth and throughout the postnatal period. Normal fetuses undergo nephrogenesis between 20 and 28 weeks, which is completed after 32 weeks of GA. Preterm infants with incomplete nephrons continue to develop; however, exposure to ibuprofen and compromised kidney perfusion caused by “ductal steal” might affect the postnatal maturation of the renal system, leading to altered kidney function. Meanwhile, PLCS, which develops in the setting of impaired left ventricular systolic performance against increased afterload, is affected by age-dependent maturation of the myocardium in preterm infants [25, 26]. Based on our study results, suboptimal outcomes of PLCS/AKI according to surgical strategies were significantly different before 28 weeks of GA; however, these suboptimal outcomes seem to be alleviated after 28 weeks of GA, which reflects the role of organ maturation status.
The limitations of our study are rooted largely in its retrospective nature, which included difficulties in identifying potential clinical confounders not captured in medical records. The transferred infants experienced different strategies with incomplete assessment. In addition, although the mainstay of the PDA treatment approach of our unit was based on previous criteria, the final decision was dependent on the choices made by the attending physician. Meanwhile, it is reassuring that the overall rate of postoperative complications other than PLCS was lower than that previously reported [26, 27]. We believe that the safety and effectiveness of bedside PDA closure can be achieved by a dedicated PDA closure team.
CONCLUSIONS
Surgical PDA closure remains a valid treatment modality for hsPDA. Establishing a timely decision and efforts to minimize the risk of severe BPD and PLCS/AKI are as important as considering the harmful effect of prolonged PDA shunt exposure.
SUPPLEMENTARY MATERIAL
Supplementary material is available at the European Journal of Cardio-Thoracic Surgery online.
FUNDING
No funding was secured for this study.
Conflict of interest: The authors have indicated they have no conflicts of interest to disclose.
DATA AVAILABILITY
De-identified individual participant data will not be made available.
Author contributions
Won Young Lee: Conceptualization; Data curation; Formal analysis; Visualization; Writing—original draft; Writing—review and editing. Sook Kyung Yum: Conceptualization; Data curation; Formal analysis; Visualization; Writing—original draft; Writing—review and editing. Yu-Mi Seo: Conceptualization; Data curation; Investigation; Writing—review and editing. Sol Kim: Conceptualization; Data curation; Investigation; Writing—review and editing. Ju Ae Shin: Conceptualization; Data curation; Investigation; Writing—review and editing. Cheul Lee: Conceptualization; Data curation; Methodology; Project administration; Supervision; Validation; Writing—review and editing.
Reviewer information
The European Journal of Cardio-Thoracic Surgery thanks Katrien Francois and the other anonymous reviewers for their contribution to the peer review process of this article.
Presented at the 103rd Annual Meeting of the American Association for Thoracic Surgery, Los Angeles, CA, USA, 6–9 May 2023.
REFERENCES
ABBREVIATIONS
- AKI
Acute kidney injury
- BPD
Bronchopulmonary dysplasia
- GA
Gestational age
- hsPDA
Haemodynamically significant patent ductus arteriosus
- IHD
In-hospital death
- IVH
Intraventricular haemorrhage
- PDA
Patent ductus arteriosus
- PLCS
Post-ligation cardiac syndrome
- ROP
Retinopathy of prematurity
- VLBW
Very-low-birth-weight
Author notes
Won Young Lee and Sook Kyung Yum authors contributed equally to this study.





