-
PDF
- Split View
-
Views
-
Cite
Cite
Mikio Okazaki, Ken Suzawa, Kazuhiko Shien, Hiromasa Yamamoto, Kota Araki, Mototsugu Watanabe, Masanori Okada, Yuho Maki, Tsuyoshi Ueno, Shinji Otani, Ryujiro Sugimoto, Hitoshi Nishikawa, Riki Okita, Makio Hayama, Hiroyuki Tao, Toshiya Fujiwara, Hidetoshi Inokawa, Yuji Hirami, Yoshifumi Sano, Motohiro Yamashita, Osamu Kawamata, Motoki Matsuura, Shinichi Toyooka, Surgical outcome of ipsilateral anatomical resection for lung cancer after pulmonary lobectomy, European Journal of Cardio-Thoracic Surgery, Volume 63, Issue 3, March 2023, ezad048, https://doi.org/10.1093/ejcts/ezad048
Close - Share Icon Share
Abstract
Ipsilateral reoperation after pulmonary lobectomy is often challenging because of adhesions from the previous operation. We retrospectively examined the surgical outcome and prognosis of ipsilateral anatomical resection for lung cancer after pulmonary lobectomy using a multicentre database.
We evaluated the perioperative outcomes and overall survival of 51 patients who underwent pulmonary lobectomy followed by ipsilateral anatomical resection for lung cancer between January 2012 and December 2018. In addition, patients with stage I non-small-cell lung cancer (NSCLC) were compared with 3411 patients with stage I lung cancer who underwent pulmonary resection without a prior ipsilateral lobectomy.
Ipsilateral anatomical resections included 10 completion pneumonectomies, 19 pulmonary lobectomies and 22 pulmonary segmentectomies. Operative time was 312.2 ± 134.5 min, and intraoperative bleeding was 522.2 ± 797.5 ml. Intraoperative and postoperative complications occurred in 9 and 15 patients, respectively. However, the 5-year overall survival rate after anatomical resection followed by ipsilateral lobectomy was 83.5%. Furthermore, in patients with c-stage I NSCLC, anatomical resection followed by ipsilateral lobectomy was not associated with worse survival than anatomical resection without prior ipsilateral lobectomy.
Anatomical resection following ipsilateral lobectomy is associated with a high frequency of intraoperative and postoperative complications. However, the 5-year overall survival in patients with c-stage I NSCLC who underwent ipsilateral anatomical resection after pulmonary lobectomy is comparable to that in patients who underwent anatomical resection without prior pulmonary lobectomy.
INTRODUCTION
With the improvement of postoperative outcomes in lung cancer, the number of pulmonary reoperations for secondary cancers has significantly increased. Although non-surgical options are available, challenging ipsilateral reoperations due to intrathoracic adhesions are still seen [1]. In particular, anatomical resection after pulmonary lobectomy is difficult and involves substantial risk because of severe adhesions surrounding the bronchial stump and pulmonary artery caused by manipulation during the initial operation [2]. Indeed, completion pneumonectomy has been reported to be a technically challenging procedure with significant perioperative mortality [3]. Therefore, surgeons may hesitate to perform ipsilateral anatomical resection after pulmonary lobectomy and choose other non-surgical options, even if the patient has sufficient cardiopulmonary capacity for anatomical resection.
Surgical treatments for metachronous second primary cancers have been well reported [4–8]. However, due to the limited number of cases, analyses of both ipsilateral and contralateral operations and the efficacy of anatomical resection after ipsilateral lobectomy remain unclear. The goal of this study was to review the surgical outcome and efficacy of ipsilateral anatomical pulmonary resection for lung cancer after pulmonary lobectomy.
PATIENTS AND METHODS
Ethics statement
This retrospective multicentre study was approved by the Ethics Committee of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama University Hospital, Okayama, Japan (approval number: 2009–057), followed by each of the participating institutions. Written informed consent was waived by each participating institution.
From January 2012 to December 2018, a total of 6297 patients underwent pulmonary resection for lung cancer at 23 different institutes belonging to the Okayama University Thoracic Surgery Study Group. Of these, 51 who underwent anatomical resection after ipsilateral lobectomy for lung cancer were enrolled in this study. The International Association for the Study of Lung Cancer TNM staging system for lung cancer (eighth edition) was used to determine the disease stage and nodal location [9]. Moreover, 4049 patients with c-stage I non-small-cell lung cancer (NSCLC) who underwent pulmonary resection without prior ipsilateral pulmonary resection were also enrolled in this study for comparison with 34 patients who underwent lobectomy followed by ipsilateral pulmonary resection for c-stage I NSCLC. The postoperative follow-up protocol was basically as follows: a computed tomography (CT) scan of the chest was repeated every 6 months for 5 years. After 5 years, a chest (CT) scan was repeated every year. Metachronous lung cancer was diagnosed according to the Martini-Melamed criteria [10]: (A) different histological types; or (B) the same histological types, if (i) the disease-free interval between the initial and the second tumours was >2 years, or (ii) the origin was carcinoma in situ or (iii) the second cancer was in a different lobe or lung without carcinoma in lymphatics common to both sites and extrapulmonary metastases at the time of diagnosis. The recurrent lung cancers included local recurrences and pulmonary metastases. Pulmonary metastases were also distinguished from metachronous lung cancers using the Martini-Melamed criteria.
The following data were collected from the patients’ medical records: age, sex, presence of comorbidities, performance status, preoperative pulmonary function test results, surgery, histological diagnosis, clinical stage and treatment course.
All statistical analyses were performed using the John Macintosh Project (JMP) Pro 16 software (SAS Institute, Cary, NC, USA). Categorical variables are shown as frequency and percentage. Continuous variables were expressed as mean ± standard deviation if normally distributed and as median values (range) if not normally distributed. For categorical variables, comparisons between groups were made using the χ2 test or Fisher’s exact test. Continuous variables were compared by the Wilcoxon rank sum test. The overall survival (OS) rate was analysed using the Kaplan–Meier method, and the differences between groups were calculated using the log-rank test. Differences were considered statistically significant at P < 0.05.
RESULTS
Characteristics and surgical outcomes of patients who underwent pulmonary lobectomy followed by ipsilateral anatomical resection
This study included 36 men and 15 women, with a mean age of 69.3 ± 8.5 years (range, 49–91 years) at the second operation. Patient characteristics are shown in Table 1. The median interval between the initial and second operations was 55 months (range, 18–335 months). The same histological diagnosis was observed between the initial and second tumours in 36 patients (70.6%), and a different histological diagnosis was observed in 12 patients (23.5%). The histological diagnoses of the initial tumours were unclear in 3 patients. Forty-five patients were diagnosed with multiple lung cancers, and 6 patients were diagnosed with recurrent tumours. The second operations included 10 completion pneumonectomies (19.6%), 19 lobectomies (37.3%) and 22 segmentectomies (43.1%), which included 33 thoracotomies (64.7%), 10 hybrid video-assisted thoracoscopic surgeries (VATS) (19.6%) and 8 complete VATS (15.7%). The median operative time was 274.5 min (range, 122–641 min), and the median volume of intraoperative bleeding was 220 ml (range, 0–3750 ml). Intraoperative complications occurred in 9 patients (17.6%), and postoperative complications occurred in 15 patients (29.4%). However, no patients died. The median follow-up period from the second operation was 59 months (range, 7–121 months), and the 5-year OS rate was 83.5% (95% CI: 0.736–0.947) (Fig. 1).
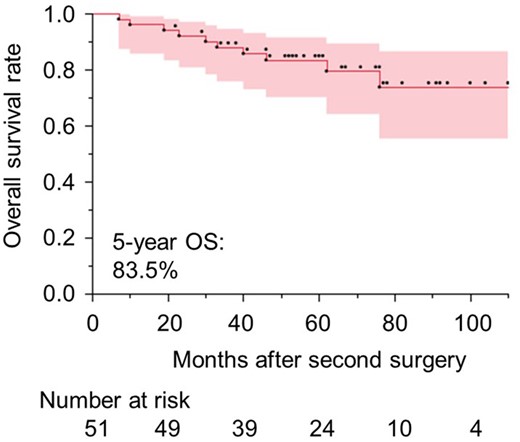
Overall survival rates of patients who underwent anatomical resections after having an ipsilateral lobectomy. OS: overall survival.
Characteristics of patients who underwent anatomical resection after an ipsilateral lobectomy
| Variables . | n = 51 (%) . |
|---|---|
| Gender | |
| Male | 36 (70.6) |
| Female | 15 (29.4) |
| Age | 69.3 ± 8.5 |
| Interval between operations (months) | 55 (18–135) |
| Surgical approach to initial lobectomy | |
| Thoracotomy | 24 (47.1) |
| Hybrid VATS | 11 (21.6) |
| Complete VATS | 16 (31.4) |
| Histological diagnosis of initial tumour | |
| Adenocarcinoma | 37 (72.6) |
| Squamous cell carcinoma | 11 (21.6) |
| Unclear | 3 (5.9) |
| Histological type of second tumour | |
| Adenocarcinoma | 44 (86.3) |
| Squamous cell carcinoma | 6 (11.8) |
| Neuroendocrine tumour | 1 (2.0) |
| Histological type of initial and second tumours | |
| Same | 36 (70.6) |
| Different | 12 (23.5) |
| Unclear | 3 (5.9) |
| Diagnosis | |
| Multiple lung cancer | 45 (88.2) |
| Recurrence | 6 (11.8) |
| %VC | 100.1 ± 15.0 |
| %FEV1.0 | 90.9 (53.9-159.8) |
| Tumour size (cm) | 1.9 (0.7-15) |
| Invasive size (cm) | 1.8 (0-15) |
| c-Stage of second tumours | |
| 0 | 4 (8.9) |
| IA | 28 (62.2) |
| IB | 7 (15.6) |
| II | 6 (13.3) |
| Side of second operation | |
| Right | 43 (84.3) |
| Left | 8 (15.7) |
| Extent of second operation | |
| Completion pneumonectomy | 10 (19.6) |
| Lobectomy | 19 (37.3) |
| Segmentectomy | 22 (43.1) |
| Surgical approach of second operation | |
| Thoracotomy | 33 (64.7) |
| Hybrid VATS | 10 (19.6) |
| Complete VATS | 8 (15.7) |
| Operative time (min) | 274.5 (122-641) |
| Intraoperative bleeding (ml) | 220 (0-3750) |
| Intraoperative complications | 9 (17.6) |
| Postoperative complications | 15 (29.4) |
| Variables . | n = 51 (%) . |
|---|---|
| Gender | |
| Male | 36 (70.6) |
| Female | 15 (29.4) |
| Age | 69.3 ± 8.5 |
| Interval between operations (months) | 55 (18–135) |
| Surgical approach to initial lobectomy | |
| Thoracotomy | 24 (47.1) |
| Hybrid VATS | 11 (21.6) |
| Complete VATS | 16 (31.4) |
| Histological diagnosis of initial tumour | |
| Adenocarcinoma | 37 (72.6) |
| Squamous cell carcinoma | 11 (21.6) |
| Unclear | 3 (5.9) |
| Histological type of second tumour | |
| Adenocarcinoma | 44 (86.3) |
| Squamous cell carcinoma | 6 (11.8) |
| Neuroendocrine tumour | 1 (2.0) |
| Histological type of initial and second tumours | |
| Same | 36 (70.6) |
| Different | 12 (23.5) |
| Unclear | 3 (5.9) |
| Diagnosis | |
| Multiple lung cancer | 45 (88.2) |
| Recurrence | 6 (11.8) |
| %VC | 100.1 ± 15.0 |
| %FEV1.0 | 90.9 (53.9-159.8) |
| Tumour size (cm) | 1.9 (0.7-15) |
| Invasive size (cm) | 1.8 (0-15) |
| c-Stage of second tumours | |
| 0 | 4 (8.9) |
| IA | 28 (62.2) |
| IB | 7 (15.6) |
| II | 6 (13.3) |
| Side of second operation | |
| Right | 43 (84.3) |
| Left | 8 (15.7) |
| Extent of second operation | |
| Completion pneumonectomy | 10 (19.6) |
| Lobectomy | 19 (37.3) |
| Segmentectomy | 22 (43.1) |
| Surgical approach of second operation | |
| Thoracotomy | 33 (64.7) |
| Hybrid VATS | 10 (19.6) |
| Complete VATS | 8 (15.7) |
| Operative time (min) | 274.5 (122-641) |
| Intraoperative bleeding (ml) | 220 (0-3750) |
| Intraoperative complications | 9 (17.6) |
| Postoperative complications | 15 (29.4) |
FEV1: forced expiratory volume in 1 s; VATS: video-assisted thoracoscopic surgery; VC: vital capacity.
Characteristics of patients who underwent anatomical resection after an ipsilateral lobectomy
| Variables . | n = 51 (%) . |
|---|---|
| Gender | |
| Male | 36 (70.6) |
| Female | 15 (29.4) |
| Age | 69.3 ± 8.5 |
| Interval between operations (months) | 55 (18–135) |
| Surgical approach to initial lobectomy | |
| Thoracotomy | 24 (47.1) |
| Hybrid VATS | 11 (21.6) |
| Complete VATS | 16 (31.4) |
| Histological diagnosis of initial tumour | |
| Adenocarcinoma | 37 (72.6) |
| Squamous cell carcinoma | 11 (21.6) |
| Unclear | 3 (5.9) |
| Histological type of second tumour | |
| Adenocarcinoma | 44 (86.3) |
| Squamous cell carcinoma | 6 (11.8) |
| Neuroendocrine tumour | 1 (2.0) |
| Histological type of initial and second tumours | |
| Same | 36 (70.6) |
| Different | 12 (23.5) |
| Unclear | 3 (5.9) |
| Diagnosis | |
| Multiple lung cancer | 45 (88.2) |
| Recurrence | 6 (11.8) |
| %VC | 100.1 ± 15.0 |
| %FEV1.0 | 90.9 (53.9-159.8) |
| Tumour size (cm) | 1.9 (0.7-15) |
| Invasive size (cm) | 1.8 (0-15) |
| c-Stage of second tumours | |
| 0 | 4 (8.9) |
| IA | 28 (62.2) |
| IB | 7 (15.6) |
| II | 6 (13.3) |
| Side of second operation | |
| Right | 43 (84.3) |
| Left | 8 (15.7) |
| Extent of second operation | |
| Completion pneumonectomy | 10 (19.6) |
| Lobectomy | 19 (37.3) |
| Segmentectomy | 22 (43.1) |
| Surgical approach of second operation | |
| Thoracotomy | 33 (64.7) |
| Hybrid VATS | 10 (19.6) |
| Complete VATS | 8 (15.7) |
| Operative time (min) | 274.5 (122-641) |
| Intraoperative bleeding (ml) | 220 (0-3750) |
| Intraoperative complications | 9 (17.6) |
| Postoperative complications | 15 (29.4) |
| Variables . | n = 51 (%) . |
|---|---|
| Gender | |
| Male | 36 (70.6) |
| Female | 15 (29.4) |
| Age | 69.3 ± 8.5 |
| Interval between operations (months) | 55 (18–135) |
| Surgical approach to initial lobectomy | |
| Thoracotomy | 24 (47.1) |
| Hybrid VATS | 11 (21.6) |
| Complete VATS | 16 (31.4) |
| Histological diagnosis of initial tumour | |
| Adenocarcinoma | 37 (72.6) |
| Squamous cell carcinoma | 11 (21.6) |
| Unclear | 3 (5.9) |
| Histological type of second tumour | |
| Adenocarcinoma | 44 (86.3) |
| Squamous cell carcinoma | 6 (11.8) |
| Neuroendocrine tumour | 1 (2.0) |
| Histological type of initial and second tumours | |
| Same | 36 (70.6) |
| Different | 12 (23.5) |
| Unclear | 3 (5.9) |
| Diagnosis | |
| Multiple lung cancer | 45 (88.2) |
| Recurrence | 6 (11.8) |
| %VC | 100.1 ± 15.0 |
| %FEV1.0 | 90.9 (53.9-159.8) |
| Tumour size (cm) | 1.9 (0.7-15) |
| Invasive size (cm) | 1.8 (0-15) |
| c-Stage of second tumours | |
| 0 | 4 (8.9) |
| IA | 28 (62.2) |
| IB | 7 (15.6) |
| II | 6 (13.3) |
| Side of second operation | |
| Right | 43 (84.3) |
| Left | 8 (15.7) |
| Extent of second operation | |
| Completion pneumonectomy | 10 (19.6) |
| Lobectomy | 19 (37.3) |
| Segmentectomy | 22 (43.1) |
| Surgical approach of second operation | |
| Thoracotomy | 33 (64.7) |
| Hybrid VATS | 10 (19.6) |
| Complete VATS | 8 (15.7) |
| Operative time (min) | 274.5 (122-641) |
| Intraoperative bleeding (ml) | 220 (0-3750) |
| Intraoperative complications | 9 (17.6) |
| Postoperative complications | 15 (29.4) |
FEV1: forced expiratory volume in 1 s; VATS: video-assisted thoracoscopic surgery; VC: vital capacity.
Comparison of characteristics and surgical outcomes based on the extent of the pulmonary resection of the second operation
Table 2 shows the comparison of patient characteristics and surgical outcomes between the surgical procedures. Tumour size did not differ among patients who underwent completion pneumonectomy, lobectomy or segmentectomy. However, the invasive size of the tumour was significantly smaller in patients who underwent segmentectomy than in those who underwent completion pneumonectomy or pulmonary lobectomy (P = 0.0051). All cases of completion pneumonectomy and lobectomy required a thoracotomy, whereas 8 segmentectomies were performed using the complete VATS approach. Although operative time was not significantly different between the groups (P = 0.0810), the median operative time of completion pneumonectomy and lobectomy was approximately 100 min longer than that of segmentectomy (330, 297 and 238, respectively). Intraoperative bleeding was significantly higher in patients with completion pneumonectomy than in others (P = 0.0386). The incidence of intraoperative complications was not significantly different among the surgical procedures (P = 1.0000). Two patients who underwent completion pneumonectomy experienced major bleeding from the pulmonary artery. Of those patients who had a pulmonary lobectomy, 1 experienced major bleeding from the pulmonary artery; 1 had major bleeding from the azygos vein; and 1 sustained an injury to the bronchus. Of those who had a segmentectomy, 3 patients had major bleeding from the pulmonary artery and 1 patient, from the chest wall and lung. The incidence of postoperative complications did not differ between the surgical procedures.
Comparison of characteristics based on the extent of pulmonary resection of the second operation
| . | Completion pneumonectomy . | Lobectomy . | Segmentectomy . | P-value . |
|---|---|---|---|---|
| . | n = 10 . | n = 19 . | n = 22 . | . |
| Gender | 0.6086 | |||
| Male | 8 | 12 | 16 | |
| Female | 2 | 7 | 6 | |
| Age | 70 (58-73) | 68 (53-91) | 70 (49-87) | 0.5393 |
| Interval between operations (months) | 59 (24-146) | 55 (18-335) | 118 (20-206) | 0.9918 |
| Surgical approach to initial lobectomy | 0.0062 | |||
| Thoracotomy | 7 | 13 | 4 | |
| Hybrid VATS | 2 | 2 | 7 | |
| Complete VATS | 1 | 4 | 11 | |
| %VC | 98.5 ± 14.7 | 98.3 ± 16.9 | 102.3 ± 13.8 | 0.6613 |
| %FEV1.0 | 88.6 (67.6-105.0) | 101 (53.9-143.2) | 84.4 (53.9-159.8) | 0.1902 |
| Tumour size | 1.9 (1.2-6.0) | 2.5 (1.0-15.0) | 1.7 (0.7-4.5) | 0.0527 |
| Invasive size | 1.9 (1.2-6.0) | 2.1 (0-15) | 1.4 (0-2.8) | 0.0051 |
| Histological type | 0.0381 | |||
| Adenocarcinoma | 7 | 16 | 21 | |
| Squamous cell carcinoma | 3 | 3 | 0 | |
| Neuroendocrine tumour | 0 | 0 | 1 | |
| Diagnosis | 0.3282 | |||
| Multiple lung cancer | 8 | 15 | 21 | |
| Recurrence | 2 | 4 | 1 | |
| c-Stage of second tumours | 0.2131 | |||
| 0 | 0 | 1 | 3 | |
| IA | 4 | 9 | 15 | |
| IB | 1 | 3 | 3 | |
| II | 3 | 3 | 0 | |
| Side of second operation | 0.0068 | |||
| Right | 6 | 19 | 18 | |
| Left | 4 | 0 | 4 | |
| Surgical approach of second operation | 0.0070 | |||
| Thoracotomy | 9 | 14 | 10 | |
| Hybrid VATS | 1 | 5 | 4 | |
| Complete VATS | 0 | 0 | 8 | |
| Operative time (min) | 330 (171-641) | 297 (157-623) | 238 (122-436) | 0.0810 |
| Intraoperative bleeding (ml) | 550 (50-3575) | 220 (0-3750) | 150 (0-1700) | 0.0386 |
| Intraoperative complications | 2 (20.0%) | 3 (15.8%) | 4 (18.2%) | 1.0000 |
| Major bleeding from PA | 2 | 1 | 3 | |
| Major bleeding from azygos vein | 1 | |||
| Major bleeding from chest wall and lung | 1 | |||
| Injury of bronchus | 1 | |||
| Postoperative complications | 5 (50.0%) | 3 (15.8%) | 7 (31.8%) | 0.1424 |
| Prolonged air leak | 2 | 5 | ||
| Arrythmia | 1 | 1 | 1 | |
| Empyema | 2 | |||
| Recurrent nerve paralysis | 2 | |||
| Bronchopleural fistula | 1 |
| . | Completion pneumonectomy . | Lobectomy . | Segmentectomy . | P-value . |
|---|---|---|---|---|
| . | n = 10 . | n = 19 . | n = 22 . | . |
| Gender | 0.6086 | |||
| Male | 8 | 12 | 16 | |
| Female | 2 | 7 | 6 | |
| Age | 70 (58-73) | 68 (53-91) | 70 (49-87) | 0.5393 |
| Interval between operations (months) | 59 (24-146) | 55 (18-335) | 118 (20-206) | 0.9918 |
| Surgical approach to initial lobectomy | 0.0062 | |||
| Thoracotomy | 7 | 13 | 4 | |
| Hybrid VATS | 2 | 2 | 7 | |
| Complete VATS | 1 | 4 | 11 | |
| %VC | 98.5 ± 14.7 | 98.3 ± 16.9 | 102.3 ± 13.8 | 0.6613 |
| %FEV1.0 | 88.6 (67.6-105.0) | 101 (53.9-143.2) | 84.4 (53.9-159.8) | 0.1902 |
| Tumour size | 1.9 (1.2-6.0) | 2.5 (1.0-15.0) | 1.7 (0.7-4.5) | 0.0527 |
| Invasive size | 1.9 (1.2-6.0) | 2.1 (0-15) | 1.4 (0-2.8) | 0.0051 |
| Histological type | 0.0381 | |||
| Adenocarcinoma | 7 | 16 | 21 | |
| Squamous cell carcinoma | 3 | 3 | 0 | |
| Neuroendocrine tumour | 0 | 0 | 1 | |
| Diagnosis | 0.3282 | |||
| Multiple lung cancer | 8 | 15 | 21 | |
| Recurrence | 2 | 4 | 1 | |
| c-Stage of second tumours | 0.2131 | |||
| 0 | 0 | 1 | 3 | |
| IA | 4 | 9 | 15 | |
| IB | 1 | 3 | 3 | |
| II | 3 | 3 | 0 | |
| Side of second operation | 0.0068 | |||
| Right | 6 | 19 | 18 | |
| Left | 4 | 0 | 4 | |
| Surgical approach of second operation | 0.0070 | |||
| Thoracotomy | 9 | 14 | 10 | |
| Hybrid VATS | 1 | 5 | 4 | |
| Complete VATS | 0 | 0 | 8 | |
| Operative time (min) | 330 (171-641) | 297 (157-623) | 238 (122-436) | 0.0810 |
| Intraoperative bleeding (ml) | 550 (50-3575) | 220 (0-3750) | 150 (0-1700) | 0.0386 |
| Intraoperative complications | 2 (20.0%) | 3 (15.8%) | 4 (18.2%) | 1.0000 |
| Major bleeding from PA | 2 | 1 | 3 | |
| Major bleeding from azygos vein | 1 | |||
| Major bleeding from chest wall and lung | 1 | |||
| Injury of bronchus | 1 | |||
| Postoperative complications | 5 (50.0%) | 3 (15.8%) | 7 (31.8%) | 0.1424 |
| Prolonged air leak | 2 | 5 | ||
| Arrythmia | 1 | 1 | 1 | |
| Empyema | 2 | |||
| Recurrent nerve paralysis | 2 | |||
| Bronchopleural fistula | 1 |
FEV1: forced expiratory volume in 1 s; PA: pulmonary artery; VATS: video-assisted thoracoscopic surgery; VC: vital capacity.
Comparison of characteristics based on the extent of pulmonary resection of the second operation
| . | Completion pneumonectomy . | Lobectomy . | Segmentectomy . | P-value . |
|---|---|---|---|---|
| . | n = 10 . | n = 19 . | n = 22 . | . |
| Gender | 0.6086 | |||
| Male | 8 | 12 | 16 | |
| Female | 2 | 7 | 6 | |
| Age | 70 (58-73) | 68 (53-91) | 70 (49-87) | 0.5393 |
| Interval between operations (months) | 59 (24-146) | 55 (18-335) | 118 (20-206) | 0.9918 |
| Surgical approach to initial lobectomy | 0.0062 | |||
| Thoracotomy | 7 | 13 | 4 | |
| Hybrid VATS | 2 | 2 | 7 | |
| Complete VATS | 1 | 4 | 11 | |
| %VC | 98.5 ± 14.7 | 98.3 ± 16.9 | 102.3 ± 13.8 | 0.6613 |
| %FEV1.0 | 88.6 (67.6-105.0) | 101 (53.9-143.2) | 84.4 (53.9-159.8) | 0.1902 |
| Tumour size | 1.9 (1.2-6.0) | 2.5 (1.0-15.0) | 1.7 (0.7-4.5) | 0.0527 |
| Invasive size | 1.9 (1.2-6.0) | 2.1 (0-15) | 1.4 (0-2.8) | 0.0051 |
| Histological type | 0.0381 | |||
| Adenocarcinoma | 7 | 16 | 21 | |
| Squamous cell carcinoma | 3 | 3 | 0 | |
| Neuroendocrine tumour | 0 | 0 | 1 | |
| Diagnosis | 0.3282 | |||
| Multiple lung cancer | 8 | 15 | 21 | |
| Recurrence | 2 | 4 | 1 | |
| c-Stage of second tumours | 0.2131 | |||
| 0 | 0 | 1 | 3 | |
| IA | 4 | 9 | 15 | |
| IB | 1 | 3 | 3 | |
| II | 3 | 3 | 0 | |
| Side of second operation | 0.0068 | |||
| Right | 6 | 19 | 18 | |
| Left | 4 | 0 | 4 | |
| Surgical approach of second operation | 0.0070 | |||
| Thoracotomy | 9 | 14 | 10 | |
| Hybrid VATS | 1 | 5 | 4 | |
| Complete VATS | 0 | 0 | 8 | |
| Operative time (min) | 330 (171-641) | 297 (157-623) | 238 (122-436) | 0.0810 |
| Intraoperative bleeding (ml) | 550 (50-3575) | 220 (0-3750) | 150 (0-1700) | 0.0386 |
| Intraoperative complications | 2 (20.0%) | 3 (15.8%) | 4 (18.2%) | 1.0000 |
| Major bleeding from PA | 2 | 1 | 3 | |
| Major bleeding from azygos vein | 1 | |||
| Major bleeding from chest wall and lung | 1 | |||
| Injury of bronchus | 1 | |||
| Postoperative complications | 5 (50.0%) | 3 (15.8%) | 7 (31.8%) | 0.1424 |
| Prolonged air leak | 2 | 5 | ||
| Arrythmia | 1 | 1 | 1 | |
| Empyema | 2 | |||
| Recurrent nerve paralysis | 2 | |||
| Bronchopleural fistula | 1 |
| . | Completion pneumonectomy . | Lobectomy . | Segmentectomy . | P-value . |
|---|---|---|---|---|
| . | n = 10 . | n = 19 . | n = 22 . | . |
| Gender | 0.6086 | |||
| Male | 8 | 12 | 16 | |
| Female | 2 | 7 | 6 | |
| Age | 70 (58-73) | 68 (53-91) | 70 (49-87) | 0.5393 |
| Interval between operations (months) | 59 (24-146) | 55 (18-335) | 118 (20-206) | 0.9918 |
| Surgical approach to initial lobectomy | 0.0062 | |||
| Thoracotomy | 7 | 13 | 4 | |
| Hybrid VATS | 2 | 2 | 7 | |
| Complete VATS | 1 | 4 | 11 | |
| %VC | 98.5 ± 14.7 | 98.3 ± 16.9 | 102.3 ± 13.8 | 0.6613 |
| %FEV1.0 | 88.6 (67.6-105.0) | 101 (53.9-143.2) | 84.4 (53.9-159.8) | 0.1902 |
| Tumour size | 1.9 (1.2-6.0) | 2.5 (1.0-15.0) | 1.7 (0.7-4.5) | 0.0527 |
| Invasive size | 1.9 (1.2-6.0) | 2.1 (0-15) | 1.4 (0-2.8) | 0.0051 |
| Histological type | 0.0381 | |||
| Adenocarcinoma | 7 | 16 | 21 | |
| Squamous cell carcinoma | 3 | 3 | 0 | |
| Neuroendocrine tumour | 0 | 0 | 1 | |
| Diagnosis | 0.3282 | |||
| Multiple lung cancer | 8 | 15 | 21 | |
| Recurrence | 2 | 4 | 1 | |
| c-Stage of second tumours | 0.2131 | |||
| 0 | 0 | 1 | 3 | |
| IA | 4 | 9 | 15 | |
| IB | 1 | 3 | 3 | |
| II | 3 | 3 | 0 | |
| Side of second operation | 0.0068 | |||
| Right | 6 | 19 | 18 | |
| Left | 4 | 0 | 4 | |
| Surgical approach of second operation | 0.0070 | |||
| Thoracotomy | 9 | 14 | 10 | |
| Hybrid VATS | 1 | 5 | 4 | |
| Complete VATS | 0 | 0 | 8 | |
| Operative time (min) | 330 (171-641) | 297 (157-623) | 238 (122-436) | 0.0810 |
| Intraoperative bleeding (ml) | 550 (50-3575) | 220 (0-3750) | 150 (0-1700) | 0.0386 |
| Intraoperative complications | 2 (20.0%) | 3 (15.8%) | 4 (18.2%) | 1.0000 |
| Major bleeding from PA | 2 | 1 | 3 | |
| Major bleeding from azygos vein | 1 | |||
| Major bleeding from chest wall and lung | 1 | |||
| Injury of bronchus | 1 | |||
| Postoperative complications | 5 (50.0%) | 3 (15.8%) | 7 (31.8%) | 0.1424 |
| Prolonged air leak | 2 | 5 | ||
| Arrythmia | 1 | 1 | 1 | |
| Empyema | 2 | |||
| Recurrent nerve paralysis | 2 | |||
| Bronchopleural fistula | 1 |
FEV1: forced expiratory volume in 1 s; PA: pulmonary artery; VATS: video-assisted thoracoscopic surgery; VC: vital capacity.
Comparison of characteristics and surgical outcomes of anatomical resection for patients with stage I non-small-cell lung cancer with and without a history of ipsilateral lobectomy
To test the validity of anatomical lung resection for stage I NSCLC after ipsilateral lobectomy, we compared the results with those of patients without a history of ipsilateral lobectomy (Table 3). A total of 3411 patients underwent anatomical resections for stage I NSCLC without a history of ipsilateral lobectomy; they had a 5-year OS rate of 83.1% (95% CI: 0.810–0.851). The 5-year OS rate of patients with a history of ipsilateral lobectomy who underwent anatomical resection was 96.7% (95% CI: 0.898–1.000), which was not worse than that of patients without a history of ipsilateral lobectomy (P = 0.0556) (Fig. 2).
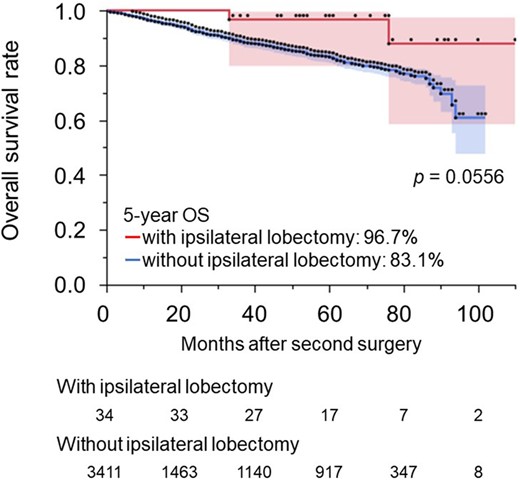
Overall survival rates for patients with stage I non-small-cell lung cancer who underwent anatomical resection with and without a history of an ipsilateral lobectomy. NSCLC: non-small-cell lung cancer.
Comparison of characteristics of anatomical resection for stage I non-small-cell lung cancer with and without a history of ipsilateral lobectomy
| Variables . | With a history of ipsilateral lobectomy . | Without a history of ipsilateral lobectomy . | . |
|---|---|---|---|
| . | n = 34 . | n = 3411 . | P-value . |
| Gender | 0.4120 | ||
| Male | 25 | 1929 | |
| Female | 9 | 1482 | |
| Age | 70 (53-90) | 71 (28-96) | 0.8388 |
| Histological type | 0.4951 | ||
| Adenocarcinoma | 31 | 1700 | |
| Squamous cell carcinoma | 3 | 321 | |
| Other | 0 | 90 | |
| Performance status | 0.8231 | ||
| 0 | 27 | 2865 | |
| 1 | 6 | 427 | |
| 2 | 1 | 75 | |
| >3 | 0 | 37 | |
| %VC | 101.3 (75.6-134.1) | 101.0 (36.9-167.2) | 0.5512 |
| %FEV1.0 | 90.5 (53.9-159.8) | 91.8 (24.6-205.8) | 0.6815 |
| Tumour size (cm) | 1.9 (0.9-4.5) | 2.1 (0.4-22.1) | 0.2454 |
| Invasive size (cm) | 1.8 (0.8-3.7) | 1.6 (0-4.0) | 0.5073 |
| c-Stage | 0.5335 | ||
| IA1 | 7 | 888 | |
| IA2 | 16 | 1200 | |
| IA3 | 5 | 686 | |
| IB | 6 | 637 | |
| Surgical approach | <0.0001 | ||
| Thoracotomy | 20 | 238 | |
| Hybrid VATS | 8 | 1073 | |
| Complete VATS | 6 | 2097 | |
| Pulmonary resection | <0.0001 | ||
| Pneumonectomy | 5 | 3 | |
| Lobectomy | 12 | 2572 | |
| Segmentectomy | 17 | 836 | |
| Operative time (min) | 271 (122-641) | 215 (78-1395) | 0.0011 |
| Intraoperative bleeding (ml) | 185 (0-1980) | 50 (0-8500) | <0.0001 |
| Intraoperative complications | 4 (11.8%) | 130 (3.8%) | 0.0524 |
| Postoperative complications | 9 (26.5%) | 686 (20.4%) | 0.4013 |
| Variables . | With a history of ipsilateral lobectomy . | Without a history of ipsilateral lobectomy . | . |
|---|---|---|---|
| . | n = 34 . | n = 3411 . | P-value . |
| Gender | 0.4120 | ||
| Male | 25 | 1929 | |
| Female | 9 | 1482 | |
| Age | 70 (53-90) | 71 (28-96) | 0.8388 |
| Histological type | 0.4951 | ||
| Adenocarcinoma | 31 | 1700 | |
| Squamous cell carcinoma | 3 | 321 | |
| Other | 0 | 90 | |
| Performance status | 0.8231 | ||
| 0 | 27 | 2865 | |
| 1 | 6 | 427 | |
| 2 | 1 | 75 | |
| >3 | 0 | 37 | |
| %VC | 101.3 (75.6-134.1) | 101.0 (36.9-167.2) | 0.5512 |
| %FEV1.0 | 90.5 (53.9-159.8) | 91.8 (24.6-205.8) | 0.6815 |
| Tumour size (cm) | 1.9 (0.9-4.5) | 2.1 (0.4-22.1) | 0.2454 |
| Invasive size (cm) | 1.8 (0.8-3.7) | 1.6 (0-4.0) | 0.5073 |
| c-Stage | 0.5335 | ||
| IA1 | 7 | 888 | |
| IA2 | 16 | 1200 | |
| IA3 | 5 | 686 | |
| IB | 6 | 637 | |
| Surgical approach | <0.0001 | ||
| Thoracotomy | 20 | 238 | |
| Hybrid VATS | 8 | 1073 | |
| Complete VATS | 6 | 2097 | |
| Pulmonary resection | <0.0001 | ||
| Pneumonectomy | 5 | 3 | |
| Lobectomy | 12 | 2572 | |
| Segmentectomy | 17 | 836 | |
| Operative time (min) | 271 (122-641) | 215 (78-1395) | 0.0011 |
| Intraoperative bleeding (ml) | 185 (0-1980) | 50 (0-8500) | <0.0001 |
| Intraoperative complications | 4 (11.8%) | 130 (3.8%) | 0.0524 |
| Postoperative complications | 9 (26.5%) | 686 (20.4%) | 0.4013 |
FEV1, forced expiratory volume in 1 s; VC, vital capacity; VATS, video-assisted thoracoscopic surgery.
Comparison of characteristics of anatomical resection for stage I non-small-cell lung cancer with and without a history of ipsilateral lobectomy
| Variables . | With a history of ipsilateral lobectomy . | Without a history of ipsilateral lobectomy . | . |
|---|---|---|---|
| . | n = 34 . | n = 3411 . | P-value . |
| Gender | 0.4120 | ||
| Male | 25 | 1929 | |
| Female | 9 | 1482 | |
| Age | 70 (53-90) | 71 (28-96) | 0.8388 |
| Histological type | 0.4951 | ||
| Adenocarcinoma | 31 | 1700 | |
| Squamous cell carcinoma | 3 | 321 | |
| Other | 0 | 90 | |
| Performance status | 0.8231 | ||
| 0 | 27 | 2865 | |
| 1 | 6 | 427 | |
| 2 | 1 | 75 | |
| >3 | 0 | 37 | |
| %VC | 101.3 (75.6-134.1) | 101.0 (36.9-167.2) | 0.5512 |
| %FEV1.0 | 90.5 (53.9-159.8) | 91.8 (24.6-205.8) | 0.6815 |
| Tumour size (cm) | 1.9 (0.9-4.5) | 2.1 (0.4-22.1) | 0.2454 |
| Invasive size (cm) | 1.8 (0.8-3.7) | 1.6 (0-4.0) | 0.5073 |
| c-Stage | 0.5335 | ||
| IA1 | 7 | 888 | |
| IA2 | 16 | 1200 | |
| IA3 | 5 | 686 | |
| IB | 6 | 637 | |
| Surgical approach | <0.0001 | ||
| Thoracotomy | 20 | 238 | |
| Hybrid VATS | 8 | 1073 | |
| Complete VATS | 6 | 2097 | |
| Pulmonary resection | <0.0001 | ||
| Pneumonectomy | 5 | 3 | |
| Lobectomy | 12 | 2572 | |
| Segmentectomy | 17 | 836 | |
| Operative time (min) | 271 (122-641) | 215 (78-1395) | 0.0011 |
| Intraoperative bleeding (ml) | 185 (0-1980) | 50 (0-8500) | <0.0001 |
| Intraoperative complications | 4 (11.8%) | 130 (3.8%) | 0.0524 |
| Postoperative complications | 9 (26.5%) | 686 (20.4%) | 0.4013 |
| Variables . | With a history of ipsilateral lobectomy . | Without a history of ipsilateral lobectomy . | . |
|---|---|---|---|
| . | n = 34 . | n = 3411 . | P-value . |
| Gender | 0.4120 | ||
| Male | 25 | 1929 | |
| Female | 9 | 1482 | |
| Age | 70 (53-90) | 71 (28-96) | 0.8388 |
| Histological type | 0.4951 | ||
| Adenocarcinoma | 31 | 1700 | |
| Squamous cell carcinoma | 3 | 321 | |
| Other | 0 | 90 | |
| Performance status | 0.8231 | ||
| 0 | 27 | 2865 | |
| 1 | 6 | 427 | |
| 2 | 1 | 75 | |
| >3 | 0 | 37 | |
| %VC | 101.3 (75.6-134.1) | 101.0 (36.9-167.2) | 0.5512 |
| %FEV1.0 | 90.5 (53.9-159.8) | 91.8 (24.6-205.8) | 0.6815 |
| Tumour size (cm) | 1.9 (0.9-4.5) | 2.1 (0.4-22.1) | 0.2454 |
| Invasive size (cm) | 1.8 (0.8-3.7) | 1.6 (0-4.0) | 0.5073 |
| c-Stage | 0.5335 | ||
| IA1 | 7 | 888 | |
| IA2 | 16 | 1200 | |
| IA3 | 5 | 686 | |
| IB | 6 | 637 | |
| Surgical approach | <0.0001 | ||
| Thoracotomy | 20 | 238 | |
| Hybrid VATS | 8 | 1073 | |
| Complete VATS | 6 | 2097 | |
| Pulmonary resection | <0.0001 | ||
| Pneumonectomy | 5 | 3 | |
| Lobectomy | 12 | 2572 | |
| Segmentectomy | 17 | 836 | |
| Operative time (min) | 271 (122-641) | 215 (78-1395) | 0.0011 |
| Intraoperative bleeding (ml) | 185 (0-1980) | 50 (0-8500) | <0.0001 |
| Intraoperative complications | 4 (11.8%) | 130 (3.8%) | 0.0524 |
| Postoperative complications | 9 (26.5%) | 686 (20.4%) | 0.4013 |
FEV1, forced expiratory volume in 1 s; VC, vital capacity; VATS, video-assisted thoracoscopic surgery.
Comparison of characteristics and surgical outcomes between residual lobes after right-sided ipsilateral relobectomy
Left-sided ipsilateral relobectomy implies pneumonectomy, whereas right-sided ipsilateral relobectomy can preserve 1 lobe. Sixteen patients with NSCLC underwent a right-sided ipsilateral relobectomy. The upper lobe was preserved in 5 cases, and the second operations were an inferior lobectomy and 4 middle lobectomies. The middle lobe was preserved in 7 cases, and all second operations were upper lobectomies. The lower lobe was preserved in 4 cases, and the second operations were 2 upper lobectomies and 2 middle lobectomies. Patient characteristics and surgical outcomes were compared between residual, upper/lower and middle lobes, as shown in Table 4. There were no significant differences in patient characteristics and perioperative outcomes between the groups. The 5-year OS rate of patients with remaining upper or lower lobes was 88.9% (95% CI: 0.706–1.000). On the other hand, the 5-year OS rate of patients with the remaining middle lobe was 42.9% (95% CI: 0.182–1.000), which was worse than those with the upper or lower lobe; however, there was no statistical difference (P = 0.0646) (Fig. 3). The postoperative complications after an operation to preserve the middle lobe included prolonged air leakage in 2 patients (28.6%). The causes of death after operations to preserve the middle lobe were cancer-related in 2 patients and pneumonia in 2 patients.
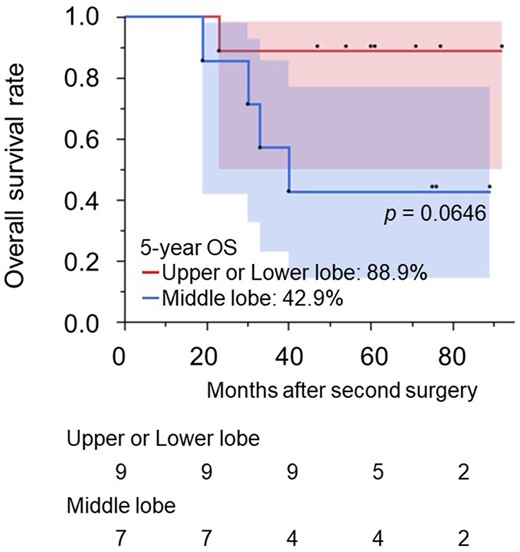
Overall survival rates for patients with non-small-cell lung cancer who underwent a second ipsilateral lobectomy based on the residual lobe. NSCLC: non-small-cell lung cancer.
Comparison of characteristics and surgical outcomes between residual lobes after ipsilateral relobectomy
| . | Upper or Lower Lobe . | Middle Lobe . | P-value . |
|---|---|---|---|
| . | n = 9 . | n = 7 . | . |
| Gender | 0.3077 | ||
| Male | 5 | 6 | |
| Female | 4 | 1 | |
| Age | 71.8 ± 8.9 | 66.4 ± 13.6 | 0.3584 |
| Interval between operations (months) | 55 (19-335) | 50 (18-113) | 0.3357 |
| Surgical approach to initial lobectomy | 1.0000 | ||
| Thoracotomy | 6 | 5 | |
| Hybrid VATS | 1 | 1 | |
| Complete VATS | 2 | 1 | |
| %VC | 101.1 ± 21.0 | 94.4 ± 8.2 | 0.4392 |
| %FEV1.0 | 100.7 (75.1-106.9) | 105.9 (72.3-143.2) | 0.3163 |
| Tumour size | 2.6 ± 1.6 | 2.3 ± 1.1 | 0.7137 |
| Invasive size | 2.6 ± 1.6 | 2.0 ± 1.4 | 0.4453 |
| c-Stage | 0.5500 | ||
| I | 8 | 5 | |
| II | 1 | 2 | |
| Histological type | 1.0000 | ||
| Adenocarcinoma | 7 | 6 | |
| Squamous cell carcinoma | 2 | 1 | |
| Surgical approach of second operation | 0.3077 | ||
| Thoracotomy | 5 | 6 | |
| Hybrid VATS | 4 | 1 | |
| Operative time (min) | 342.1 ± 163.6 | 346.3 ± 158.2 | 0.9598 |
| Intraoperative bleeding (ml) | 179.4 ± 139.1 | 308.6 ± 303.9 | 0.2740 |
| Intraoperative complications | 1 (11.1%) | 1 (14.3%) | 1.0000 |
| Major bleeding from PA | 1 | ||
| Major bleeding from azygos vein | 1 | ||
| Postoperative complications | 1 (11.1%) | 2 (28.6%) | 0.5500 |
| Prolonged air leak | 2 | ||
| Arrythmia | 1 |
| . | Upper or Lower Lobe . | Middle Lobe . | P-value . |
|---|---|---|---|
| . | n = 9 . | n = 7 . | . |
| Gender | 0.3077 | ||
| Male | 5 | 6 | |
| Female | 4 | 1 | |
| Age | 71.8 ± 8.9 | 66.4 ± 13.6 | 0.3584 |
| Interval between operations (months) | 55 (19-335) | 50 (18-113) | 0.3357 |
| Surgical approach to initial lobectomy | 1.0000 | ||
| Thoracotomy | 6 | 5 | |
| Hybrid VATS | 1 | 1 | |
| Complete VATS | 2 | 1 | |
| %VC | 101.1 ± 21.0 | 94.4 ± 8.2 | 0.4392 |
| %FEV1.0 | 100.7 (75.1-106.9) | 105.9 (72.3-143.2) | 0.3163 |
| Tumour size | 2.6 ± 1.6 | 2.3 ± 1.1 | 0.7137 |
| Invasive size | 2.6 ± 1.6 | 2.0 ± 1.4 | 0.4453 |
| c-Stage | 0.5500 | ||
| I | 8 | 5 | |
| II | 1 | 2 | |
| Histological type | 1.0000 | ||
| Adenocarcinoma | 7 | 6 | |
| Squamous cell carcinoma | 2 | 1 | |
| Surgical approach of second operation | 0.3077 | ||
| Thoracotomy | 5 | 6 | |
| Hybrid VATS | 4 | 1 | |
| Operative time (min) | 342.1 ± 163.6 | 346.3 ± 158.2 | 0.9598 |
| Intraoperative bleeding (ml) | 179.4 ± 139.1 | 308.6 ± 303.9 | 0.2740 |
| Intraoperative complications | 1 (11.1%) | 1 (14.3%) | 1.0000 |
| Major bleeding from PA | 1 | ||
| Major bleeding from azygos vein | 1 | ||
| Postoperative complications | 1 (11.1%) | 2 (28.6%) | 0.5500 |
| Prolonged air leak | 2 | ||
| Arrythmia | 1 |
FEV1: forced expiratory volume in 1 s; VC: vital capacity; PA: pulmonary artery; VATS: video-assisted thoracoscopic surgery.
Comparison of characteristics and surgical outcomes between residual lobes after ipsilateral relobectomy
| . | Upper or Lower Lobe . | Middle Lobe . | P-value . |
|---|---|---|---|
| . | n = 9 . | n = 7 . | . |
| Gender | 0.3077 | ||
| Male | 5 | 6 | |
| Female | 4 | 1 | |
| Age | 71.8 ± 8.9 | 66.4 ± 13.6 | 0.3584 |
| Interval between operations (months) | 55 (19-335) | 50 (18-113) | 0.3357 |
| Surgical approach to initial lobectomy | 1.0000 | ||
| Thoracotomy | 6 | 5 | |
| Hybrid VATS | 1 | 1 | |
| Complete VATS | 2 | 1 | |
| %VC | 101.1 ± 21.0 | 94.4 ± 8.2 | 0.4392 |
| %FEV1.0 | 100.7 (75.1-106.9) | 105.9 (72.3-143.2) | 0.3163 |
| Tumour size | 2.6 ± 1.6 | 2.3 ± 1.1 | 0.7137 |
| Invasive size | 2.6 ± 1.6 | 2.0 ± 1.4 | 0.4453 |
| c-Stage | 0.5500 | ||
| I | 8 | 5 | |
| II | 1 | 2 | |
| Histological type | 1.0000 | ||
| Adenocarcinoma | 7 | 6 | |
| Squamous cell carcinoma | 2 | 1 | |
| Surgical approach of second operation | 0.3077 | ||
| Thoracotomy | 5 | 6 | |
| Hybrid VATS | 4 | 1 | |
| Operative time (min) | 342.1 ± 163.6 | 346.3 ± 158.2 | 0.9598 |
| Intraoperative bleeding (ml) | 179.4 ± 139.1 | 308.6 ± 303.9 | 0.2740 |
| Intraoperative complications | 1 (11.1%) | 1 (14.3%) | 1.0000 |
| Major bleeding from PA | 1 | ||
| Major bleeding from azygos vein | 1 | ||
| Postoperative complications | 1 (11.1%) | 2 (28.6%) | 0.5500 |
| Prolonged air leak | 2 | ||
| Arrythmia | 1 |
| . | Upper or Lower Lobe . | Middle Lobe . | P-value . |
|---|---|---|---|
| . | n = 9 . | n = 7 . | . |
| Gender | 0.3077 | ||
| Male | 5 | 6 | |
| Female | 4 | 1 | |
| Age | 71.8 ± 8.9 | 66.4 ± 13.6 | 0.3584 |
| Interval between operations (months) | 55 (19-335) | 50 (18-113) | 0.3357 |
| Surgical approach to initial lobectomy | 1.0000 | ||
| Thoracotomy | 6 | 5 | |
| Hybrid VATS | 1 | 1 | |
| Complete VATS | 2 | 1 | |
| %VC | 101.1 ± 21.0 | 94.4 ± 8.2 | 0.4392 |
| %FEV1.0 | 100.7 (75.1-106.9) | 105.9 (72.3-143.2) | 0.3163 |
| Tumour size | 2.6 ± 1.6 | 2.3 ± 1.1 | 0.7137 |
| Invasive size | 2.6 ± 1.6 | 2.0 ± 1.4 | 0.4453 |
| c-Stage | 0.5500 | ||
| I | 8 | 5 | |
| II | 1 | 2 | |
| Histological type | 1.0000 | ||
| Adenocarcinoma | 7 | 6 | |
| Squamous cell carcinoma | 2 | 1 | |
| Surgical approach of second operation | 0.3077 | ||
| Thoracotomy | 5 | 6 | |
| Hybrid VATS | 4 | 1 | |
| Operative time (min) | 342.1 ± 163.6 | 346.3 ± 158.2 | 0.9598 |
| Intraoperative bleeding (ml) | 179.4 ± 139.1 | 308.6 ± 303.9 | 0.2740 |
| Intraoperative complications | 1 (11.1%) | 1 (14.3%) | 1.0000 |
| Major bleeding from PA | 1 | ||
| Major bleeding from azygos vein | 1 | ||
| Postoperative complications | 1 (11.1%) | 2 (28.6%) | 0.5500 |
| Prolonged air leak | 2 | ||
| Arrythmia | 1 |
FEV1: forced expiratory volume in 1 s; VC: vital capacity; PA: pulmonary artery; VATS: video-assisted thoracoscopic surgery.
DISCUSSION
An ipsilateral reoperation after a pulmonary lobectomy is often challenging due to adhesions from the previous operation. In this study, we retrospectively examined the surgical outcome and prognosis of patients who had ipsilateral anatomical resection for lung cancer after a pulmonary lobectomy using a multicentre database. Although these resections required long operative times, large amounts of intraoperative bleeding and a high incidence of intraoperative complications, the prognoses of these patients were much better than expected.
Previous groups have reported that repeat lung resection has a high mortality rate of 5% or more [11–13]. Linden et al. reported that the mortality rate of ipsilateral and contralateral lung resections after a prior lobectomy was 11% [14]. On the contrary, it has recently been reported that the surgical treatment of a metachronous second primary cancer was performed with lower mortality and morbidity rates than before [5, 15]. However, these reports included both ipsilateral/contralateral resections and anatomical/wedge resections, and there have been no reports focusing on an ipsilateral resection after a pulmonary lobectomy. The present study did not include wedge resection cases in either the initial or the second operation. If the initial operation is a wedge resection, the ipsilateral reoperation is not difficult because adhesions occur only between the lung and the chest wall and not around the bronchial stump and pulmonary vessels. In addition, ipsilateral wedge resection after pulmonary lobectomy is technically not complicated, even if the initial operation was a lobectomy. This situation occurs because it only requires an adhesiotomy from the chest wall and does not require exposure of the pulmonary vessels, which carries the risk of major bleeding. Sato et al. reported that the OS of patients with stage I ipsilateral metachronous second primary lung cancer was comparable between those who had wedge resection and anatomical resection in the second operation [16]. However, this is not what we intended to elucidate because they included wedge resection cases in the initial operation. Although Hattori et al. reported repeated anatomical pulmonary resection for a metachronous ipsilateral second NSCLC, the study included segmentectomies in the initial operation and completion lobectomies in the second operation [17].
Yang et al. compared the survival outcomes of lobectomy and sublobectomy in patients with metachronous secondary primary lung cancer in a propensity score matching study [18]. Although their study included both ipsilateral and contralateral resections, they indicated that lobectomy remains a valid choice for metachronous secondary primary lung cancer. However, several reports prefer wedge resection for metachronous secondary primary lung cancer [7, 16]. Although wedge resection may be a good option for an ipsilateral reoperation, anatomical resection may be necessary instead of wedge resection, depending on the size and location of the tumour. In such cases, some surgeons might avoid an operation and choose chemotherapy or radiation therapy. Indeed, we have shown that anatomical resection after ipsilateral lobectomy carries a high risk for intraoperative and postoperative complications. However, no patients died, and it was suggested that overcoming the perioperative period would provide a survival benefit to these patients.
The 5-year OS rate of patients with a history of ipsilateral lobectomy who underwent anatomical resection was not worse than that of patients without a history of ipsilateral lobectomy. There was no statistically significant difference; however, the prognosis was better with a reoperation. The reason for this is unclear because there were no significant differences in patient backgrounds. However, one possible reason could be the difference in time when the cancer was detected. The time to cancer detection may be shorter in reoperation cases than in initial surgery cases. Cancer in reoperation cases is more likely to be detected during the intensive postoperative follow-up period, which includes a chest CT scan. On the other hand, patients diagnosed with lung cancer for the first time have a longer period of time between the microscopic occurrence of cancer and the time of clinical diagnosis than those diagnosed for the second time. This finding indicates that the possibility of micrometastasis is greater for patients diagnosed for the first time than for those diagnosed for the second time despite the same clinical stage. Furthermore, the pulmonary function of patients with a history of an ipsilateral lobectomy was not worse than that of patients without a history of ipsilateral lobectomy, suggesting that patients with a history of ipsilateral lobectomy had extremely good pulmonary function before the initial operation. Furthermore, ipsilateral anatomical resections were performed in patients with sufficient residual pulmonary function, and the indications for the operation were considered appropriate.
When the remaining lobe after a right-sided ipsilateral relobectomy is the middle lobe, it is controversial whether to preserve the middle lobe or perform a completion pneumonectomy. Operations to preserve the middle lobe have generally been avoided because a residual middle lobe could result in torsion and emphysematous changes. Nonetheless, this operation has some advantages: preservation of pulmonary function and prevention of excessive mediastinal shift [2, 19]. We previously reported 6 cases of middle lobe-preserving operations for patients with secondary lung cancer [2]. Although torsion of the middle lobe did not occur in any of these cases and a middle lobe-preserving operation was acceptable, the incidence of perioperative complications was high, and the long-term outcome was unclear. Other case reports of operations to preserve the middle lobe documented fewer cases than our previous report and lacked long-term outcomes [19, 20]. In the present study, operations to preserve the middle lobe after ipsilateral relobectomy were performed in 7 patients with NSCLC . The results were compared with those of patients who had upper or lower lobe-preserving operations. The number of cases was small, with no significant differences. However, it was suggested that the prognosis of middle lobe-preserving operations may be poorer than that of the upper or lower lobe. It was also suggested that the amount of remaining lung volume of the middle lobe was less than that of the upper and lower lobes. However, there was no difference in the incidence of perioperative complications between operations to preserve the middle lobe and the upper/lower lobe. Although we previously reported that an operation to preserve the middle lobe carries a high risk of perioperative complications [2], this increased risk could be due to the risk of the relobectomy itself.
When ipsilateral secondary lung cancer is diagnosed after a lobectomy, treatment options include wedge resection, anatomical resections and other non-operative treatments such as stereotactic body radiotherapy (SBRT) and radiofrequency ablation (RFA). SBRT has demonstrated good local control and morbidity comparable to that of lung resection [21–24] and has proved to be standard treatment for inoperable patients. RFA has also been reported as an alternative option for inoperative patients [25]. Pulmonary function is a major factor influencing the treatment strategy. First, if the patient's pulmonary function is too poor to tolerate even a wedge resection, SBRT or RFA should be chosen. Second, if the patient has pulmonary function that precludes anatomical resection but allows wedge resection and the tumour is a peripheral lesion, we choose wedge resection. However, SBRT or RFA is chosen if the tumour is located where wedge resection is difficult. Although it has not been clear whether sublobar resection or SBRT had a better prognosis, the authors of a meta-analysis comparing partial resection with SBRT have reported that the OS of patients with stage I NSCLC who underwent wedge resections was superior to that of patients with SBRT [26]. Third, if the patient has adequate pulmonary function, even if the tumour is peripheral and wedge resection is possible, we prefer anatomical resection rather than wedge resection if it is oncologically necessary. In this study, the prognosis of patients with wedge resection after ipsilateral lobectomy was poorer than that of those with anatomical resection (Supplementary Table 1; Supplementary Fig. 1). Furthermore, when considering comparisons between anatomical resection and SBRT, most studies comparing SBRT with pulmonary lobectomy report a better prognosis for those who have a lobectomy [27–29]. Moreover, one of the major differences between anatomical resection and SBRT is the evaluation of lymph nodes. Anatomical resection allows more accurate staging than SBRT and can provide appropriate additional treatment in cases of upstaging. Although the results of prospective randomized controlled trials are required, at this time it seems reasonable to consider anatomical resection first in patients who require ipsilateral anatomical resection after pulmonary lobectomy and have sufficient residual cardiopulmonary function.
This study had several limitations. First, the sample size was small, even though a multicentre database was used for analysis. Second, it was a retrospective study. In the future, we plan to conduct a prospective study to elucidate the optimal strategy for ipsilateral resection after lobectomy. Finally, patients with metachronous lung cancer were diagnosed using Martini-Melamed criteria; however, patients with lung metastases may have been inadvertently included because they were not genetically proven.
CONCLUSION
Although ipsilateral anatomical resection after pulmonary lobectomy was associated with high rates of perioperative complications, the 5-year OS in patients with c-stage I NSCLC who underwent ipsilateral anatomical resection after a pulmonary lobectomy was comparable to that in patients with c-stage I NSCLC who underwent anatomical resection without a prior pulmonary lobectomy. However, the prognosis of patients who have operations to preserve the middle lobe after ipsilateral pulmonary lobectomy may be poor.
ACKNOWLEDGEMENT
We would like to thank Editage (http://www.editage.com) for editing and reviewing this manuscript for English language usage.
Funding
This study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest: None declared.
Author contributions
Mikio Okazaki: Conceptualization, Data curation, Investigation, Writing—original draft. Ken Suzawa: Supervision. Kazuhiko Shien: Supervision. Hiromasa Yamamoto: Supervision. Kota Araki: Data curation. Mototsugu Watanabe: Data curation. Masanori Okada: Data curation. Yuho Maki: Data curation. Tsuyoshi Ueno: Data curation. Shinji Otani: Data curation. Ryujiro Sugimoto: Data curation. Hitoshi Nishikawa: Data curation. Riki Okita: Supervision. Makio Hayama: Data curation, Supervision. Hiroyuki Tao: Data curation, Supervision. Toshiya Fujiwara: Supervision. Hidetoshi Inokawa: Supervision. Yuji Hirami: Data curation, Supervision. Yoshifumi Sano: Supervision. Motohiro Yamashita: Supervision. Osamu Kawamata: Data curation, Supervision. Motoki Matsuura: Supervision. Shinichi Toyooka: Project administration, Writing—review & editing.
DATA AVAILABILITY
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
ABBREVIATIONS:
- NSCLC
non-small-cell lung cancer
- OS
overall survival
- RFA
radiofrequency ablation
- SBRT
stereotactic body radiotherapy
- VATS
video-assisted thoracoscopic surgery





