-
PDF
- Split View
-
Views
-
Cite
Cite
Yeke Huang, Xipeng Wang, Yajie Zhang, Yuqin Cao, Yunjiu Gou, Shumin Wang, Hecheng Li, Comparison between robot- and video-assisted thoracoscopic surgeries for anterior mediastinal lesions, European Journal of Cardio-Thoracic Surgery, Volume 67, Issue 5, May 2025, ezaf113, https://doi.org/10.1093/ejcts/ezaf113
Close - Share Icon Share
Abstract
Video-assisted thoracic surgery (VATS) and robot-assisted thoracic surgery (RATS) are widely used in the treatment of anterior mediastinal lesions. However, recent reports comparing the efficacy of VATS and RATS remain unclear, owing to limitations, including territorial constraints, small sample sizes or lack of subgroup analysis. Thus, we conducted a multi-centre retrospective study to compare perioperative outcomes of VATS and RATS via lateral thoracic or subxiphoid approach for anterior mediastinal lesions.
Patients with anterior mediastinal lesions from 3 high-volume Chinese centres were included. VATS and RATS via lateral thoracic or subxiphoid approaches were performed. A propensity score-matching analysis was conducted with covariates including sex, smoking, alcohol, hypertension, diabetes, myasthenia gravis symptoms, lesion diameter, pathology and blood test results.
A total of 1076 patients (954 VATS, 122 RATS) were included. For the lateral thoracic approach, 122 VATS and 62 RATS patients were matched. RATS resulted in shorter catheter retention (P < 0.001), shorter postoperative stays (P = 0.002) and lower complication rates (P < 0.001), with no conversions or re-surgeries. For the subxiphoid approach, 98 VATS and 52 RATS patients were matched. RATS demonstrated higher drainage volume (P < 0.001), longer catheter retention (P = 0.03) and greater albumin reduction (P < 0.001), with no conversions or re-surgeries.
Using the lateral thoracic approach, RATS offered shorter catheter retention, shorter postoperative stays and fewer complications. However, with the subxiphoid approach, RATS led to higher drainage volume and longer catheter retention. Our study indicates that surgical approach impacts outcomes, with RATS being more beneficial for lateral thoracic cases and VATS for subxiphoid cases.
INTRODUCTION
Mediastinal lesions, including anterior, median and posterior lesions, have various origins, including thymoma, thymic carcinoma, bronchogenic cyst and neurinoma, and usually require surgical intervention. Anterior lesions are more common in clinical practice [1].
Video-assisted thoracic surgery (VATS) has long been used as a minimally invasive method for mediastinal surgery, offering advantages over conventional thoracotomy, in terms of improved quality of life, safety and reduced postoperative complications [2, 3]. In recent years, robot-assisted thoracic surgery (RATS) has become widely used for mediastinal procedures. Numerous studies have explored differences in clinical efficacy between RATS and VATS for the resection of thymomas, thymic carcinoma and other mediastinal lesions, mostly focusing on operation time, postoperative hospital stay, blood loss and drainage volume; however, opinions on these comparisons vary [4–11].
Early resection of anterior mediastinal lesions using VATS or RATS is often performed via a lateral thoracic approach. This approach can easily damage the intercostal nerves and muscles of the patient, leading to both acute and chronic postoperative pain, which can hinder postoperative recovery [9, 12]. Conversely, the subxiphoid approach maintains the integrity of the thorax and avoids the intercostal space, thereby preventing postoperative pain caused by intercostal nerve injury. Additionally, this approach facilitates easier identification of the bilateral phrenic nerves and the superior pole of the thymus, results in less pulmonary function impairment and provides a more aesthetically pleasing surgical incision. Consequently, it has been widely adopted in clinical practice in recent years [13].
Some studies have investigated the differences in efficacy and safety between VATS and RATS in anterior mediastinal lesions resections; however, the results differ, probably because of limitations such as territorial constraints, small sample sizes or lack of subgroup analysis. Therefore, we conducted a multi-centre retrospective study focusing on the perioperative efficacy and safety in the resection of anterior mediastinal lesions between VATS and RATS using either the lateral or subxiphoid approach.
PATIENTS AND METHODS
Clinical information
This multi-centre retrospective cohort study included 1076 consecutive patients with anterior mediastinal tumours treated using VATS and RATS, between March 2013 and March 2024, at the Department of Thoracic Surgery at Ruijin Hospital, Gansu Provincial People’s Hospital, and Northern Theater General Hospital.
Clinical data were collected retrospectively through the medical history systems of each centre. Medical records were retrieved from previous clinical visits and met the 4 principles of waiving informed consent. All information was kept strictly confidential and handled in accordance with the information security protocol of Ruijin Hospital. Access to relevant medical records was restricted to authorized personnel to verify data accuracy and ensure that the study was conducted properly. Study findings may be presented at medical meetings and published in scientific journals, but no identifiable personal information will be disclosed. Additionally, all specimens obtained in this study will be used solely for research purposes and not for commercial use.
Inclusion criteria were as follows:
Preoperative examination using enhanced computed tomography that identified anterior mediastinal tumours.
No previous history of tuberculosis, pleurisy or surgery, and no preoperative findings suggesting pleural thickening or adhesion.
Surgical and perioperative missions and care were provided by the 3 participating medical centres.
Exclusion criteria were as follows:
Presence of middle and posterior mediastinal tumours.
Poor cardiopulmonary function or severe cardiac arrhythmias that render the patient unable to tolerate surgery.
Surgical method
Lateral approach
The anaesthesia and artificial pneumothorax techniques were the same as those used in the subxiphoid approach. The patient was positioned semi-supine, with the upper body elevated 30–45°, and the affected-side upper limb abducted and secured. Port placement followed the ‘5–3–5’ configuration: an observation port was placed in the 5th intercostal space along the anterior axillary line, with the first operational arm port in the 3rd intercostal space, and the second operational arm port in the 5th intercostal space along the midclavicular line (Fig. 1A).
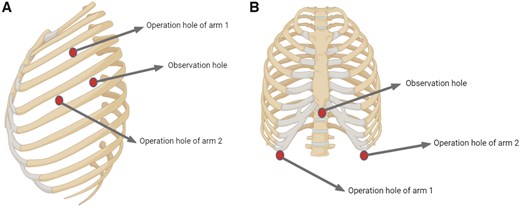
Schematic diagram of the surgical approach. (A) Lateral approach. (B) Subxiphoid approach.
Subxiphoid approach
A 3 cm subxiphoid incision served as the observation port, with operational arm ports placed along the midclavicular line and at the lower rib borders on both sides (Fig. 1B). If necessary, an auxiliary port is positioned in the 6th intercostal space along the midaxillary line. CO2 was insufflated at a pressure of 6–8 mmHg to create an artificial pneumothorax, expanding the posterior sternal space and exposing the mediastinal structures. After blunt dissection, an operational arm was inserted, and the mediastinal pleura was bilaterally opened to enhance visibility. In cases of severe myasthenia gravis, anterior adipose tissue near the phrenic nerve is removed to alleviate symptoms. Following tumour resection, the specimen was placed in a retrieval bag and extracted through the subxiphoid incision, which can be extended if necessary. A mediastinal drainage tube was inserted, the lungs re-expanded and the chest closed, with the drainage tube connected to negative pressure.
Observation indicators
Baseline information included: sex, age, smoking history, alcohol intake, comorbidities (such as hypertension and diabetes), myasthenia gravis, tumour size, pathology and peripheral blood test. Surgical and postoperative data include conversion to open surgery, drainage volume, catheter retention time, postoperative hospital stay, re-operation, readmission within 30 days and postoperative complications (such as myasthenia gravis, hoarseness, pulmonary infection, pulmonary atelectasis, pleural effusion, arrhythmia, DVT, incision infection, chylothorax and postoperative haemorrhage). Additionally, we observed postoperative changes in white blood cell (WBC) count (×109/l), haemoglobin levels (g/l) and albumin levels (g/l).
Statistical analysis
To minimize the influence of potential confounders and reduce selection bias, we performed a propensity score-matching (PSM) analysis. Factors associated with the combined treatment were incorporated into the propensity model, including sex, smoking history, alcohol consumption, hypertension history, diabetes, myasthenia gravis, lesion diameter, pathology, haemoglobin levels, WBC count and albumin levels. Greedy nearest-neighbour matching with a calliper width of 0.25 standard deviations of the propensity score was applied in a 1:2 ratio (RATS:VATS) without replacement.
Statistical analysis was conducted using SAS software. For normally distributed continuous data (determined using the Shapiro–Wilk test, P ≥ 0.05 indicates normality), results are presented as mean±standard deviation (x ± s), with comparisons between groups performed using the t-test. For non-normally distributed continuous data, results were presented as median [M(P25, P75)], and group comparisons were conducted using the Mann–Whitney U-test. The signed rank test was used to analyse paired samples post-PSM. Categorical variables were expressed as frequencies and percentages, with group comparisons carried out using the chi-square test. McNemar’s test was used to analyse paired samples post-PSM, and Fisher’s exact test was applied for small samples. Statistical significance was set at P < 0.05.
RESULTS
Baseline patient information
A total of 1076 patients who underwent anterior mediastinal surgery using either VATS (954 patients) or RATS (122 patients) via a lateral or subxiphoid approach from March 2013 to March 2024 were enrolled at 3 hospitals in mainland China: Ruijin Hospital (660 patients), Gansu Provincial People’s Hospital (88 patients) and General Hospital of Northern Theater Command (328 patients). The number of each type of surgery performed per centre is listed in Supplementary Material, Table S10. Baseline patient information before PSM is presented in Supplementary Material, Table S1 (left), and detailed pathology profiles are listed in Supplementary Material, Table S9. In the VATS group, 523 patients (54.82%) were female, with a median age of 55 years. Eleven (1.15%) of 954 VATS patients had a history of myasthenia gravis, and the median lesion size was 3.0 cm. In the RATS group, 55 patients (45.08%) were women, with a median age of 52 years. Of the 122 VATS patients, 6 (4.92%) had a history of myasthenia gravis, and the median lesion size was 3.1 cm.
Comparison of outcomes between VATS and RATS patients
After PSM, 234 VATS patients and 120 RATS patients were included in each group (Supplementary Material, Table S1, right). No significant differences were observed between the 2 groups after matching. No significant differences were found in drainage volume (ml) [VATS: 240 (100–420), RATS: 250 (80–500), P = 0.69, Fig. 2A], postoperative stay (day) [VATS: 3.0 (3.0–5.0), RATS: 3.0 (2.0–5.0), P = 0.15, Fig. 2C], the ratio of conversion to open surgery (%) [VATS: 2 (0.85), RATS: 0 (0.00), P = 0.55], and no patients needed re-operation (%) (Supplementary Material, Table S2). RATS group showed shorter catheter retention time (day) [VATS: 2.0 (2.0–3.0), RATS: 2.0 (2.0–3.0), P = 0.04, Fig. 2B]. No significant difference was discovered in haemoglobin decrease (g/l) [VATS: 9.0 (2.0–16.0), RATS: 11.0 (4.0–18.0), P = 0.24, Fig. 2D] and WBC increase (×109/l) between 2 groups [VATS: 4.40 (2.92–5.78), RATS: 4.20 (2.70–5.80), P = 0.40, Fig. 2E] (Supplementary Material, Table S3). The decrease in albumin was more significant in RATS [VATS: 4.6 (2.0–8.0), RATS: 7.0 (3.2–9.0), P = 0.008, Fig. 2F]. Finally, no significant difference was observed in the overall incidence of recorded complications or in specific complications [VATS: 34 (14.53), RATS: 11 (9.17), P = 0.08], except for postoperative haemorrhage which was significantly higher in the VATS group [VATS: 12 (5.13), RATS: 1 (0.83), P = 0.008] (Supplementary Material, Table S4).
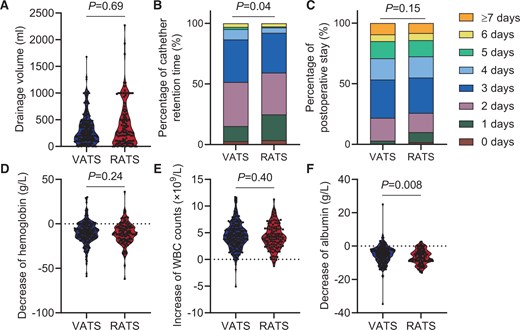
Outcomes of surgery between the VATS and RATS groups. (A) The medium drainage volume was 240 ml in the VATS group and 250 ml in the RATS group (P = 0.69). (B) The medium catheter retention time was 3.0 days in both the VATS and RATS groups; however, the P90 of the VATS group (4.0) was higher than that of the RATS group (3.0) (P = 0.04). (C) The medium postoperative stay was 3 days in both the VATS and RATS groups (P = 0.15). (D–F) Blood test results showing the change in blood constituent 1 day before and after the surgery. The median increase in WBC counts was 4.40 × 109/l in the VATS group and 4.20 × 109/l in the RATS group (P = 0.40). The median decrease in haemoglobin was 9.0 g/l in the VATS group and 11.0 g/l in the RATS group (P = 0.24). The median decrease in albumin was 4.6 g/l in the VATS group and 7.0 g/l in the RATS group (P = 0.008).
Comparison of outcomes between VATS and RATS patients via the lateral approach
The baseline information of 725 VATS patients and 68 RATS patients are detailed in Supplementary Material, Table S5 (left). Using PSM, we matched 122 VATS patients with 62 RATS patients and observed no significant differences in baseline information (Supplementary Material, Table S5, right). Our finding revealed no significant difference in the conversion rate to open surgery (%) [VATS: 1 (0.82), RATS: 0 (0.00), P > 0.99], and drainage volume (ml) [VATS: 260 (130–400)], RATS: 208 (65–390), P = 0.24, Fig. 3A]. Additionally, no patients in either group needed re-operation (Table 1, left). The RATS group had significantly shorter catheter retention time (day) [VATS: 3.0 (2.0–3.0), RATS: 2.0 (1.0–3.0), P < 0.001, Fig. 3B] and shorter postoperative stay (day) [VATS: 3.0 (3.0–5.0), RATS: 3.0 (2.0–4.0), P = 0.002, Fig. 3C] than the VATS group (Table 1, left). No significant difference was observed in haemoglobin decrease (g/l) [VATS: 9.0 (1.0–16.0), RATS: 11.0 (4.5–21.0), P = 0.07, Fig. 3D]. However, the RATS group exhibited a significantly lower increase in WBC counts (×109/l) [VATS: 4.40 (2.60–6.10), RATS: 3.80 (2.33–5.64), P = 0.02, Fig. 3E] and a greater albumin decline (g/l) [VATS: 4.3 (2.0–8.9), RATS: 7.0 (4.0–9.0), P = 0.008, Fig. 3F] than the VATS group (Table 2, left). Among patients who underwent surgery via lateral approach, 17.21% of the VATS group developed complications within 30 days which was significantly higher than 3.23% in the RATS group (P < 0.001). Additionally, complication (Clavien–Dindo≥III) rates were significantly higher (P < 0.001) in the VATS group (12.30%) than in the RATS group (1.61%). We analysed the proportion of different complications in the total incidence of complications and found no significant difference in the proportion of each complication between the VATS group and RATS group (P > 0.99, Supplementary Material, Table S11, left). However, the incidence of pleural effusion (%) [VATS: 9 (7.38), RATS: 1 (1.16), P = 0.03] and postoperative haemorrhage (%) [VATS: 7 (5.74), RATS: 0 (0.00), P = 0.02] was significantly higher in the VATS group. No other statistically significant difference was observed in the incidence of specific complications. No patients required readmission within 30 days (Table 1, left). To eliminate potential bias due to different pathological types (benign or malignant), we analysed patients with malignant lesions. The results suggested that the differences between VATS and RATS patients were consistent with those previously described (Supplementary Material, Tables S7 and S8, left).
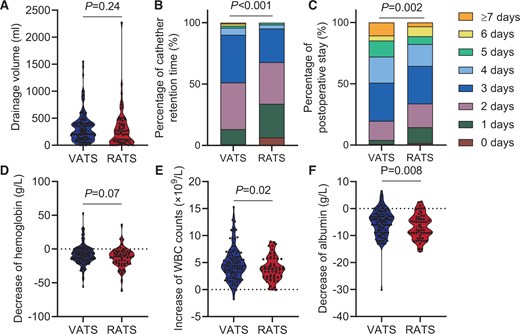
Surgical outcomes using the lateral approach in the VATS and RATS groups. (A) The median drainage volume was 260 ml in the VATS group and 208 ml in the RATS group (P = 0.24). (B) The median catheter retention time was 3.0 days in the VATS group and 2.0 days in the RATS group (P < 0.001). (C) The median postoperative stay was 3 days in both the VATS and RATS groups; however, the (Q1, Q3) of the VATS group (3.0–5.0) is higher than that of the RATS group (2.0–4.0) (P = 0.002). (D–F) Blood test results showing changes in blood constituents 1 day before and after the surgery. The median increase in WBC counts was 4.40 × 109/l in the VATS group and 3.80 × 109/l in the RATS group (P = 0.02). The medium decrease in haemoglobin was 9.0 g/l in the VATS group and 11.0 g/l in the RATS group (P = 0.07). The median decrease in albumin was 4.3 g/l in the VATS group and 7.0 g/l in the RATS group (P = 0.008).
| Characteristics . | Lateral approach . | Subxiphoid approach . | ||||
|---|---|---|---|---|---|---|
| VATS (n = 122) . | RATS (n = 62) . | P-value . | VATS (n = 98) . | RATS (n = 52) . | P-value . | |
| Conversion to open surgery (%) | 1 (0.82) | 0 (0.00) | >0.99 | 0.00 | 0.00 | / |
| Drainage volume (ml) | 260 (130–400) | 208 (65–390) | 0.24 | 180 (60–310) | 283 (110–600) | <0.001 |
| Catheter retention time (day) | 3.0 (2.0–3.0) | 2.0 (1.0–3.0) | <0.001 | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 0.03 |
| Postoperative stay (day) | 3.0 (3.0–5.0) | 3.0 (2.0–4.0) | 0.002 | 3.0 (3.0–5.0) | 4.0 (3.0–5.0) | 0.17 |
| Second operation (%) | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Complications occurred within 30 days after surgery (%) | 21 (17.21) | 2 (3.23) | <0.001 | 13 (13.27) | 8 (15.38) | 0.68 |
| Clavien Dindo≥III | 15 (12.30) | 1 (1.61) | <0.001 | 10 (10.20) | 5 (9.62) | >0.99 |
| Myasthenia gravis | 0.00 | 0.00 | / | 1 (1.02) | 0 (0.00) | >0.99 |
| Hoarseness | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Pulmonary infection | 6 (4.92) | 1 (1.61) | 0.16 | 2 (2.04) | 4 (7.69) | 0.06 |
| Pulmonary atelectasis | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Pleural effusion | 9 (7.38) | 1 (1.61) | 0.03 | 5 (5.10) | 4 (7.69) | 0.56 |
| Arrhythmia | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| DVT | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Incision infection | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Chylothorax | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Postoperative haemorrhage | 7 (5.74) | 0 (0.00) | 0.02 | 5 (5.10) | 1 (1.92) | 0.26 |
| Readmission within 30 days | 0.00 | 0.00 | / | 1 (1.02) | 0.00 | >0.99 |
| Characteristics . | Lateral approach . | Subxiphoid approach . | ||||
|---|---|---|---|---|---|---|
| VATS (n = 122) . | RATS (n = 62) . | P-value . | VATS (n = 98) . | RATS (n = 52) . | P-value . | |
| Conversion to open surgery (%) | 1 (0.82) | 0 (0.00) | >0.99 | 0.00 | 0.00 | / |
| Drainage volume (ml) | 260 (130–400) | 208 (65–390) | 0.24 | 180 (60–310) | 283 (110–600) | <0.001 |
| Catheter retention time (day) | 3.0 (2.0–3.0) | 2.0 (1.0–3.0) | <0.001 | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 0.03 |
| Postoperative stay (day) | 3.0 (3.0–5.0) | 3.0 (2.0–4.0) | 0.002 | 3.0 (3.0–5.0) | 4.0 (3.0–5.0) | 0.17 |
| Second operation (%) | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Complications occurred within 30 days after surgery (%) | 21 (17.21) | 2 (3.23) | <0.001 | 13 (13.27) | 8 (15.38) | 0.68 |
| Clavien Dindo≥III | 15 (12.30) | 1 (1.61) | <0.001 | 10 (10.20) | 5 (9.62) | >0.99 |
| Myasthenia gravis | 0.00 | 0.00 | / | 1 (1.02) | 0 (0.00) | >0.99 |
| Hoarseness | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Pulmonary infection | 6 (4.92) | 1 (1.61) | 0.16 | 2 (2.04) | 4 (7.69) | 0.06 |
| Pulmonary atelectasis | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Pleural effusion | 9 (7.38) | 1 (1.61) | 0.03 | 5 (5.10) | 4 (7.69) | 0.56 |
| Arrhythmia | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| DVT | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Incision infection | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Chylothorax | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Postoperative haemorrhage | 7 (5.74) | 0 (0.00) | 0.02 | 5 (5.10) | 1 (1.92) | 0.26 |
| Readmission within 30 days | 0.00 | 0.00 | / | 1 (1.02) | 0.00 | >0.99 |
| Characteristics . | Lateral approach . | Subxiphoid approach . | ||||
|---|---|---|---|---|---|---|
| VATS (n = 122) . | RATS (n = 62) . | P-value . | VATS (n = 98) . | RATS (n = 52) . | P-value . | |
| Conversion to open surgery (%) | 1 (0.82) | 0 (0.00) | >0.99 | 0.00 | 0.00 | / |
| Drainage volume (ml) | 260 (130–400) | 208 (65–390) | 0.24 | 180 (60–310) | 283 (110–600) | <0.001 |
| Catheter retention time (day) | 3.0 (2.0–3.0) | 2.0 (1.0–3.0) | <0.001 | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 0.03 |
| Postoperative stay (day) | 3.0 (3.0–5.0) | 3.0 (2.0–4.0) | 0.002 | 3.0 (3.0–5.0) | 4.0 (3.0–5.0) | 0.17 |
| Second operation (%) | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Complications occurred within 30 days after surgery (%) | 21 (17.21) | 2 (3.23) | <0.001 | 13 (13.27) | 8 (15.38) | 0.68 |
| Clavien Dindo≥III | 15 (12.30) | 1 (1.61) | <0.001 | 10 (10.20) | 5 (9.62) | >0.99 |
| Myasthenia gravis | 0.00 | 0.00 | / | 1 (1.02) | 0 (0.00) | >0.99 |
| Hoarseness | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Pulmonary infection | 6 (4.92) | 1 (1.61) | 0.16 | 2 (2.04) | 4 (7.69) | 0.06 |
| Pulmonary atelectasis | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Pleural effusion | 9 (7.38) | 1 (1.61) | 0.03 | 5 (5.10) | 4 (7.69) | 0.56 |
| Arrhythmia | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| DVT | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Incision infection | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Chylothorax | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Postoperative haemorrhage | 7 (5.74) | 0 (0.00) | 0.02 | 5 (5.10) | 1 (1.92) | 0.26 |
| Readmission within 30 days | 0.00 | 0.00 | / | 1 (1.02) | 0.00 | >0.99 |
| Characteristics . | Lateral approach . | Subxiphoid approach . | ||||
|---|---|---|---|---|---|---|
| VATS (n = 122) . | RATS (n = 62) . | P-value . | VATS (n = 98) . | RATS (n = 52) . | P-value . | |
| Conversion to open surgery (%) | 1 (0.82) | 0 (0.00) | >0.99 | 0.00 | 0.00 | / |
| Drainage volume (ml) | 260 (130–400) | 208 (65–390) | 0.24 | 180 (60–310) | 283 (110–600) | <0.001 |
| Catheter retention time (day) | 3.0 (2.0–3.0) | 2.0 (1.0–3.0) | <0.001 | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 0.03 |
| Postoperative stay (day) | 3.0 (3.0–5.0) | 3.0 (2.0–4.0) | 0.002 | 3.0 (3.0–5.0) | 4.0 (3.0–5.0) | 0.17 |
| Second operation (%) | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Complications occurred within 30 days after surgery (%) | 21 (17.21) | 2 (3.23) | <0.001 | 13 (13.27) | 8 (15.38) | 0.68 |
| Clavien Dindo≥III | 15 (12.30) | 1 (1.61) | <0.001 | 10 (10.20) | 5 (9.62) | >0.99 |
| Myasthenia gravis | 0.00 | 0.00 | / | 1 (1.02) | 0 (0.00) | >0.99 |
| Hoarseness | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Pulmonary infection | 6 (4.92) | 1 (1.61) | 0.16 | 2 (2.04) | 4 (7.69) | 0.06 |
| Pulmonary atelectasis | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Pleural effusion | 9 (7.38) | 1 (1.61) | 0.03 | 5 (5.10) | 4 (7.69) | 0.56 |
| Arrhythmia | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| DVT | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Incision infection | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Chylothorax | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Postoperative haemorrhage | 7 (5.74) | 0 (0.00) | 0.02 | 5 (5.10) | 1 (1.92) | 0.26 |
| Readmission within 30 days | 0.00 | 0.00 | / | 1 (1.02) | 0.00 | >0.99 |
| Characteristics . | Lateral approach . | Subxiphoid approach . | ||||
|---|---|---|---|---|---|---|
| VATS (n = 122) . | RATS (n = 62) . | P-value . | VATS (n = 98) . | RATS (n = 52) . | P-value . | |
| WBC increase (×109/l) | 4.40 (2.60–6.10) | 3.80 (2.33–5.64) | 0.02 | 4.13 (2.90–5.45) | 4.64 (3.16–6.35) | 0.21 |
| Haemoglobin decrease (g/l) | 9.0 (1.0–16.0) | 11.0 (4.5–21.0) | 0.07 | 6.0 (1.0–12.0) | 10.0 (2.5–14.0) | 0.08 |
| Albumin decrease (g/l) | 4.3 (2.0–8.9) | 7.0 (4.0–9.0) | 0.008 | 2.9 (1.0–5.2) | 6.5 (3.0–8.50) | <0.001 |
| Characteristics . | Lateral approach . | Subxiphoid approach . | ||||
|---|---|---|---|---|---|---|
| VATS (n = 122) . | RATS (n = 62) . | P-value . | VATS (n = 98) . | RATS (n = 52) . | P-value . | |
| WBC increase (×109/l) | 4.40 (2.60–6.10) | 3.80 (2.33–5.64) | 0.02 | 4.13 (2.90–5.45) | 4.64 (3.16–6.35) | 0.21 |
| Haemoglobin decrease (g/l) | 9.0 (1.0–16.0) | 11.0 (4.5–21.0) | 0.07 | 6.0 (1.0–12.0) | 10.0 (2.5–14.0) | 0.08 |
| Albumin decrease (g/l) | 4.3 (2.0–8.9) | 7.0 (4.0–9.0) | 0.008 | 2.9 (1.0–5.2) | 6.5 (3.0–8.50) | <0.001 |
| Characteristics . | Lateral approach . | Subxiphoid approach . | ||||
|---|---|---|---|---|---|---|
| VATS (n = 122) . | RATS (n = 62) . | P-value . | VATS (n = 98) . | RATS (n = 52) . | P-value . | |
| WBC increase (×109/l) | 4.40 (2.60–6.10) | 3.80 (2.33–5.64) | 0.02 | 4.13 (2.90–5.45) | 4.64 (3.16–6.35) | 0.21 |
| Haemoglobin decrease (g/l) | 9.0 (1.0–16.0) | 11.0 (4.5–21.0) | 0.07 | 6.0 (1.0–12.0) | 10.0 (2.5–14.0) | 0.08 |
| Albumin decrease (g/l) | 4.3 (2.0–8.9) | 7.0 (4.0–9.0) | 0.008 | 2.9 (1.0–5.2) | 6.5 (3.0–8.50) | <0.001 |
| Characteristics . | Lateral approach . | Subxiphoid approach . | ||||
|---|---|---|---|---|---|---|
| VATS (n = 122) . | RATS (n = 62) . | P-value . | VATS (n = 98) . | RATS (n = 52) . | P-value . | |
| WBC increase (×109/l) | 4.40 (2.60–6.10) | 3.80 (2.33–5.64) | 0.02 | 4.13 (2.90–5.45) | 4.64 (3.16–6.35) | 0.21 |
| Haemoglobin decrease (g/l) | 9.0 (1.0–16.0) | 11.0 (4.5–21.0) | 0.07 | 6.0 (1.0–12.0) | 10.0 (2.5–14.0) | 0.08 |
| Albumin decrease (g/l) | 4.3 (2.0–8.9) | 7.0 (4.0–9.0) | 0.008 | 2.9 (1.0–5.2) | 6.5 (3.0–8.50) | <0.001 |
Comparison of outcomes between VATS and RATS patients via the subxiphoid approach
For the subxiphoid approach, 229 VATS patients and 54 RATS patients were included (Supplementary Material, Table S6, left), with 98 VATS patients and 52 RATS patients matched (Supplementary Material, Table S6, right). No patients in VATS and RATS groups needed conversion to open surgery or re-operation. No significant difference in postoperative stay was observed (day) [VATS: 3.0 (3.0–5.0), RATS: 4.0 (3.0–5.0), P = 0.17, Fig. 4C]. Notably, higher drainage volume (ml) [VATS: 180 (60–310), RATS: 283 (110–600), P < 0.001, Fig. 4A] and catheter retention time (day) [VATS: 2.0 (2.0–3.0), RATS: 3.0 (2.0–3.0), P = 0.03, Fig. 4B] were observed in RATS group than in VATS group (Table 1, right). A significantly higher albumin decrease (g/l) was observed in the RATS group [VATS: 2.9 (1.0–5.2), RATS: 6.5 (3.0–8.5), P < 0.001, Fig. 4F]. However, no significant difference was found in WBC increase (×109/l) [VATS: 4.13 (2.90–5.45), RATS: 4.64 (3.16–6.35), P = 0.21, Fig. 4E] and haemoglobin (g/l) [VATS: 6.0 (1.0–12.0), RATS: 10.0 (2.5–14.0), P = 0.08, Fig. 4D] (Table 2, right). Moreover, no significant difference was observed in the total incidence of complications within 30 days (%) (VATS: 13.27, RATS: 15.38, P = 0.68) or in any specific complication (Table 1, right). The complication (Clavien–Dindo≥III) rates were not significantly different (P > 0.99) between the VATS group (10.20%) and the RATS group (3.85%). Additionally, no significant difference was observed in the proportion of each complication between the VATS group and RATS group (P > 0.34, Supplementary Material, Table S11, right). Similarly, we analysed patients with malignant lesions in the subxiphoid group. The results suggest that in malignant lesions, the differences between VATS and RATS patients are consistent with those previously described (Supplementary Material, Tables S7 and S8, right).
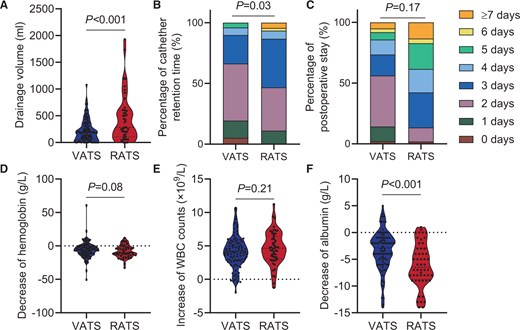
Surgical outcomes using the subxiphoid approach in the VATS and RATS groups. (A) The median drainage volume was 180 ml in the VATS group and 283 ml in the RATS group (P < 0.001). (B) The median catheter retention time was 2.0 days in the VATS group and 3.0 days in the RATS group (P = 0.03). (C) The medium postoperative stay was 3 days in the VATS group and 4 days in the RATS group (P = 0.17). (D–F) Blood test results showing changes in blood constituents 1 day before and after the surgery. The median increase in WBC counts was 4.13 × 109/l in the VATS group and 4.64 × 109/l in the RATS group (P = 0.21). The median decrease in haemoglobin was 6.0 g/l in the VATS group and 10.0 g/l in the RATS group (P = 0.08). The median decrease in albumin was 2.9 g/l in the VATS group and 6.5 g/l in the RATS group (P < 0.001).
DISCUSSION
Mediastinal lesion resection via VATS or RATS using the lateral or subxiphoid approach has been widely adopted in clinical practice. However, the efficacy discrepancy between VATS and RATS in the treatment of anterior mediastinal lesions remains controversial.
Most published studies have focused on the lateral approach, with many highlighting the advantages of RATS in mediastinal lesion resection. Ochi et al. [4] reported that RATS via lateral approach led to shorter postoperative stay, and no significant differences were found between blood loss, incidence of conversion to open chest surgery, and duration of chest drainage. Similarly, in the surgical treatment of Masaoka stage I thymoma, Ye et al. [11] found that RATS led to shorter postoperative and reduced drainage volume after surgery in the RATS group, although the blood loss was not significantly different between the 2 approaches. However, some studies have reported no significant differences in postoperative stay, with RATS even causing slightly higher blood loss during surgery [8, 9]. Suda et al. [13] demonstrated that, using the subxiphoid approach, no significant differences were observed between trans-subxiphoid robotic thymectomy and single-port thymectomy in terms of blood loss and postoperative stay.
Our study results revealed no significant difference between the 2 groups in terms of drainage volume and postoperative stay; however, catheter retention time was significantly shorter in the RATS group. In the subgroup analysis, the lateral approach group showed significantly shorter catheter retention time and postoperative stay, which was partially consistent with previous studies [4, 10, 11]. The drainage volume was slightly lower in the RATS group but not significantly. Unexpectedly, for the subxiphoid approach, our findings indicated that the VATS group had less drainage volume and shorter catheter retention time than the RATS group, and the postoperative stay was slightly higher in the RATS group though not significantly. Thus, regarding perioperative surgical outcomes, these results indicate that RATS appeared to be superior in reducing the catheter retention time and postoperative stay for the lateral approach, whereas it showed limited advantages for the subxiphoid approach.
Next, we analysed the changes in peripheral blood test results before and after surgery, including WBC counts, haemoglobin decrease and albumin decrease, which reflect surgical stress and fluid loss during surgery [14, 15]. Regardless of the approaches, VATS patients exhibited a higher increase in WBC counts and a greater decrease in haemoglobin than RATS patients; however, these differences were not statistically significant. Conversely, RATS showed a significantly greater decrease in albumin. Using the lateral approach, VATS showed a significantly higher increase in WBC than that in RATS patients, aligning with previous reports [14]; however, for the subxiphoid approach, the increase in WBC appeared to be higher in RATS but not significantly, while the albumin decrease was also significantly higher in RATS than in VATS. In summary, RATS led to significantly more loss of albumin regardless of the approach, and caused a lower increase in WBC in the lateral approach but not in the subxiphoid approach.
Our findings highlight that while RATS may offer advantages in the lateral approach, its benefits in the subxiphoid approach are less clear, with increased drainage volume and catheter retention time observed. This discrepancy suggests that the robotic system may not yet offer optimal efficiency in subxiphoid procedures. Potential reasons include the relatively limited manoeuvrability of robotic instruments in the subxiphoid space compared to the lateral thoracic cavity, as well as differences in tissue handling and haemostatic control. Furthermore, the increased albumin loss in RATS suggests a possibly higher inflammatory response or more extensive surgical stress, which warrants further investigation. These results emphasize the need for careful surgical approach selection based on individual patient characteristics and lesion location. Future studies should explore whether refinements in robotic instrumentation, enhanced surgeon experience, or modifications in perioperative management can improve RATS outcomes in the subxiphoid approach. Additionally, prospective trials comparing long-term oncological and functional outcomes between VATS and RATS in different approaches would be valuable in guiding surgical decision-making.
Further, we analysed the incidence of different types of complications occurring within 30 days after surgery. Ochi et al. [4] and Chendaer et al. [14] have previously demonstrated that the lateral approach showed no significant difference in the occurrence of complications. In our study, after PSM, no significant difference was observed between the VATS and RATS groups regardless of surgical approach. However, in the lateral approach, RATS patients showed a significantly lower incidence of total complications, whereas in the subxiphoid approach, no significant difference was observed. Consequently, our study showed that RATS resulted in fewer complications than VATS 30 days after surgery using lateral approach but not the subxiphoid approach.
Additionally, in our study, the inclusion period was substantially long with considerable changes in the standard of care which may impact the outcome of this comparison. To clarify the potential impact of calendar time on the outcomes of our comparison, we conducted a subgroup analysis comparing the first half and second half of the included patients (Table 3). The results of this analysis were consistent, indicating that changes in the standard of care during the inclusion period did not significantly affect the overall outcomes. In the lateral approach, the incidence of total complications in the VATS group (15.63%) was significantly higher than that in the RATS group (0.00%) in the first half of the inclusion period (P = 0.03). In the second half, the incidence of total complications in the VATS group (18.97%) was higher than that in the RATS group (5.88%), although this difference was not statistically significant (P = 0.12). In the subxiphoid approach, no significant difference in the overall complication rate was observed between the VATS and RATS groups in either the first (P>0.99) or the second half of the inclusion period (P = 0.49). These findings suggest that the calendar effect had little influence on the study results, supporting the reliability of our conclusion.
| Characteristics . | Lateral approach . | Subxiphoid approach . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First half . | Second half . | First half . | Second half . | |||||||||
| VATS (n = 64) . | RATS (n = 28) . | P-value . | VATS (n = 58) . | RATS (n = 34) . | P-value . | VATS (n = 48) . | RATS (n = 27) . | P-value . | VATS (n = 50) . | RATS (n = 25) . | P-value . | |
| Conversion to open surgery (%) | 0.00 | 0.00 | / | 1 (1.72) | 0 (0.00) | 0.99 | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Drainage volume (ml) | 210 (130–380) | 262 (90–395) | 0.83 | 285 (115–420) | 160 (50–370) | 0.07 | 130 (48–223) | 260 (95–550) | 0.01 | 200 (75–375) | 380 (140–610) | 0.08 |
| Catheter retention time (day) | 2.0 (2.0–3.0) | 2.0 (1.0–3.0) | 0.10 | 2.5 (2.0–3.0) | 2.0 (1.0–3.0) | 0.004 | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 0.03 | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 0.07 |
| Postoperative stay (day) | 4.0 (3.0–5.0) | 3.0 (2.0–4.0) | 0.02 | 3.0 (3.0–4.0) | 3.0 (2.0–4.0) | 0.26 | 3.0 (3.0–4.0) | 4.0 (3.0–5.0) | 0.33 | 3.5 (3.0–5.0) | 4.0 (3.0–5.0) | 0.31 |
| Second operation (%) | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Complications occurred within 30 days after surgery (%) | 10 (15.63) | 0 (0.00) | 0.03 | 11 (18.97) | 2 (5.88) | 0.12 | 7 (14.58) | 3 (11.11) | >0.99 | 6 (12.00) | 5 (20.00) | 0.49 |
| Myasthenia gravis | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 1 (2.00) | 0 (0.00) | >0.99 |
| Hoarseness | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Pulmonary infection | 4 (6.25) | 0 (0.00) | 0.31 | 2 (3.45) | 1 (2.94) | >0.99 | 0 (0.00) | 2 (8.00) | 0.13 | 2 (4.00) | 2 (8.00) | 0.60 |
| Pulmonary atelectasis | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Pleural effusion | 3 (4.69) | 0 (0.00) | 0.55 | 6 (10.34) | 1 (2.94) | 0.25 | 4 (8.33) | 1 (3.70) | 0.65 | 1 (2.00) | 3 (12.00) | 0.11 |
| Arrhythmia | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| DVT | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Incision infection | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Chylothorax | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Postoperative haemorrhage | 4 (6.25) | 0 (0.00) | 0.31 | 3 (5.17) | 0 (0.00) | 0.29 | 3 (6.25) | 0 (0.00) | 0.55 | 2 (4.00) | 1 (4.00) | >0.99 |
| Readmission within 30 days | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 1 (2.00) | 0 (0.00) | >0.99 |
| Characteristics . | Lateral approach . | Subxiphoid approach . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First half . | Second half . | First half . | Second half . | |||||||||
| VATS (n = 64) . | RATS (n = 28) . | P-value . | VATS (n = 58) . | RATS (n = 34) . | P-value . | VATS (n = 48) . | RATS (n = 27) . | P-value . | VATS (n = 50) . | RATS (n = 25) . | P-value . | |
| Conversion to open surgery (%) | 0.00 | 0.00 | / | 1 (1.72) | 0 (0.00) | 0.99 | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Drainage volume (ml) | 210 (130–380) | 262 (90–395) | 0.83 | 285 (115–420) | 160 (50–370) | 0.07 | 130 (48–223) | 260 (95–550) | 0.01 | 200 (75–375) | 380 (140–610) | 0.08 |
| Catheter retention time (day) | 2.0 (2.0–3.0) | 2.0 (1.0–3.0) | 0.10 | 2.5 (2.0–3.0) | 2.0 (1.0–3.0) | 0.004 | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 0.03 | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 0.07 |
| Postoperative stay (day) | 4.0 (3.0–5.0) | 3.0 (2.0–4.0) | 0.02 | 3.0 (3.0–4.0) | 3.0 (2.0–4.0) | 0.26 | 3.0 (3.0–4.0) | 4.0 (3.0–5.0) | 0.33 | 3.5 (3.0–5.0) | 4.0 (3.0–5.0) | 0.31 |
| Second operation (%) | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Complications occurred within 30 days after surgery (%) | 10 (15.63) | 0 (0.00) | 0.03 | 11 (18.97) | 2 (5.88) | 0.12 | 7 (14.58) | 3 (11.11) | >0.99 | 6 (12.00) | 5 (20.00) | 0.49 |
| Myasthenia gravis | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 1 (2.00) | 0 (0.00) | >0.99 |
| Hoarseness | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Pulmonary infection | 4 (6.25) | 0 (0.00) | 0.31 | 2 (3.45) | 1 (2.94) | >0.99 | 0 (0.00) | 2 (8.00) | 0.13 | 2 (4.00) | 2 (8.00) | 0.60 |
| Pulmonary atelectasis | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Pleural effusion | 3 (4.69) | 0 (0.00) | 0.55 | 6 (10.34) | 1 (2.94) | 0.25 | 4 (8.33) | 1 (3.70) | 0.65 | 1 (2.00) | 3 (12.00) | 0.11 |
| Arrhythmia | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| DVT | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Incision infection | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Chylothorax | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Postoperative haemorrhage | 4 (6.25) | 0 (0.00) | 0.31 | 3 (5.17) | 0 (0.00) | 0.29 | 3 (6.25) | 0 (0.00) | 0.55 | 2 (4.00) | 1 (4.00) | >0.99 |
| Readmission within 30 days | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 1 (2.00) | 0 (0.00) | >0.99 |
| Characteristics . | Lateral approach . | Subxiphoid approach . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First half . | Second half . | First half . | Second half . | |||||||||
| VATS (n = 64) . | RATS (n = 28) . | P-value . | VATS (n = 58) . | RATS (n = 34) . | P-value . | VATS (n = 48) . | RATS (n = 27) . | P-value . | VATS (n = 50) . | RATS (n = 25) . | P-value . | |
| Conversion to open surgery (%) | 0.00 | 0.00 | / | 1 (1.72) | 0 (0.00) | 0.99 | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Drainage volume (ml) | 210 (130–380) | 262 (90–395) | 0.83 | 285 (115–420) | 160 (50–370) | 0.07 | 130 (48–223) | 260 (95–550) | 0.01 | 200 (75–375) | 380 (140–610) | 0.08 |
| Catheter retention time (day) | 2.0 (2.0–3.0) | 2.0 (1.0–3.0) | 0.10 | 2.5 (2.0–3.0) | 2.0 (1.0–3.0) | 0.004 | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 0.03 | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 0.07 |
| Postoperative stay (day) | 4.0 (3.0–5.0) | 3.0 (2.0–4.0) | 0.02 | 3.0 (3.0–4.0) | 3.0 (2.0–4.0) | 0.26 | 3.0 (3.0–4.0) | 4.0 (3.0–5.0) | 0.33 | 3.5 (3.0–5.0) | 4.0 (3.0–5.0) | 0.31 |
| Second operation (%) | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Complications occurred within 30 days after surgery (%) | 10 (15.63) | 0 (0.00) | 0.03 | 11 (18.97) | 2 (5.88) | 0.12 | 7 (14.58) | 3 (11.11) | >0.99 | 6 (12.00) | 5 (20.00) | 0.49 |
| Myasthenia gravis | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 1 (2.00) | 0 (0.00) | >0.99 |
| Hoarseness | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Pulmonary infection | 4 (6.25) | 0 (0.00) | 0.31 | 2 (3.45) | 1 (2.94) | >0.99 | 0 (0.00) | 2 (8.00) | 0.13 | 2 (4.00) | 2 (8.00) | 0.60 |
| Pulmonary atelectasis | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Pleural effusion | 3 (4.69) | 0 (0.00) | 0.55 | 6 (10.34) | 1 (2.94) | 0.25 | 4 (8.33) | 1 (3.70) | 0.65 | 1 (2.00) | 3 (12.00) | 0.11 |
| Arrhythmia | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| DVT | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Incision infection | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Chylothorax | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Postoperative haemorrhage | 4 (6.25) | 0 (0.00) | 0.31 | 3 (5.17) | 0 (0.00) | 0.29 | 3 (6.25) | 0 (0.00) | 0.55 | 2 (4.00) | 1 (4.00) | >0.99 |
| Readmission within 30 days | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 1 (2.00) | 0 (0.00) | >0.99 |
| Characteristics . | Lateral approach . | Subxiphoid approach . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First half . | Second half . | First half . | Second half . | |||||||||
| VATS (n = 64) . | RATS (n = 28) . | P-value . | VATS (n = 58) . | RATS (n = 34) . | P-value . | VATS (n = 48) . | RATS (n = 27) . | P-value . | VATS (n = 50) . | RATS (n = 25) . | P-value . | |
| Conversion to open surgery (%) | 0.00 | 0.00 | / | 1 (1.72) | 0 (0.00) | 0.99 | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Drainage volume (ml) | 210 (130–380) | 262 (90–395) | 0.83 | 285 (115–420) | 160 (50–370) | 0.07 | 130 (48–223) | 260 (95–550) | 0.01 | 200 (75–375) | 380 (140–610) | 0.08 |
| Catheter retention time (day) | 2.0 (2.0–3.0) | 2.0 (1.0–3.0) | 0.10 | 2.5 (2.0–3.0) | 2.0 (1.0–3.0) | 0.004 | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 0.03 | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) | 0.07 |
| Postoperative stay (day) | 4.0 (3.0–5.0) | 3.0 (2.0–4.0) | 0.02 | 3.0 (3.0–4.0) | 3.0 (2.0–4.0) | 0.26 | 3.0 (3.0–4.0) | 4.0 (3.0–5.0) | 0.33 | 3.5 (3.0–5.0) | 4.0 (3.0–5.0) | 0.31 |
| Second operation (%) | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Complications occurred within 30 days after surgery (%) | 10 (15.63) | 0 (0.00) | 0.03 | 11 (18.97) | 2 (5.88) | 0.12 | 7 (14.58) | 3 (11.11) | >0.99 | 6 (12.00) | 5 (20.00) | 0.49 |
| Myasthenia gravis | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 1 (2.00) | 0 (0.00) | >0.99 |
| Hoarseness | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Pulmonary infection | 4 (6.25) | 0 (0.00) | 0.31 | 2 (3.45) | 1 (2.94) | >0.99 | 0 (0.00) | 2 (8.00) | 0.13 | 2 (4.00) | 2 (8.00) | 0.60 |
| Pulmonary atelectasis | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Pleural effusion | 3 (4.69) | 0 (0.00) | 0.55 | 6 (10.34) | 1 (2.94) | 0.25 | 4 (8.33) | 1 (3.70) | 0.65 | 1 (2.00) | 3 (12.00) | 0.11 |
| Arrhythmia | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| DVT | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Incision infection | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Chylothorax | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / |
| Postoperative haemorrhage | 4 (6.25) | 0 (0.00) | 0.31 | 3 (5.17) | 0 (0.00) | 0.29 | 3 (6.25) | 0 (0.00) | 0.55 | 2 (4.00) | 1 (4.00) | >0.99 |
| Readmission within 30 days | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 0.00 | 0.00 | / | 1 (2.00) | 0 (0.00) | >0.99 |
Finally, the results may indicate that RATS via the lateral approach may provide patients with benefits, including lower complication rate, less hospital stay and drainage time in anterior mediastinal lesions resection. However, in the subxiphoid approach, RATS did not show significant advantages and even resulted in increased drainage volume and drainage time. However, considering the relatively higher hospitalization costs of the RATS group [16], weighing the merits and demerits when choosing the appropriate surgical method in clinical practice remains crucial.
Limitations
First, this retrospective study included patients from 3 centres, and the sample size was larger than that of other similar studies. However, the number of patients undergoing robotic surgery, especially thoracoscopic surgery via the subxiphoid approach, was relatively small, and the distribution of these patients in the 3 centres may have introduced bias into the study results. Second, patients with both benign and malignant pathology were included. Although the results in patients with malignancies were consistent with those of the overall cohort (Supplementary Material, Tables S7 and S8), the influence of different pathological types on the selection of surgical techniques warrants further verification. Third, robotic surgery is more limited than thoracoscopic surgery, and the bias caused by the clinical experience of independent surgeons may affect the accuracy of some results. Additionally, patient selection biases, surgeon experience and centre-specific differences may influence the outcomes. Moreover, in the analysis of the baseline data of the patients, some baseline indicators were unbalanced. Although the difference was partially corrected after PSM, it could reflect the possible bias of patient selection. Finally, this study focused on short-term perioperative metrics, and the absence of malignancy-specific parameters (e.g. R0 resection rates) limits conclusions regarding the oncological superiority of either approach. Future randomized controlled trials may be needed to further clarify the relevant results.
CONCLUSION
Overall, our study indicates that RATS outperforms VATS in anterior mediastinal lesion resection via the lateral approach; however, its advantages in the subxiphoid approach are limited. Given the higher cost of RATS than that of VATS, it remains essential to weigh the advantages and disadvantages when selecting an appropriate surgical method in clinical practice to maximize benefits to patients.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
FUNDING
This study was supported by National Natural Science Foundation of China (82372855 and 82072557), Interdisciplinary Program of Shanghai Jiao Tong University (YG2023ZD04), Novel Interdisciplinary Research Project from Shanghai Municipal Health Commission (2022JC023), Shanghai Municipal Education Commission—Gaofeng Clinical Medicine grant (20172005, the 2nd round of disbursement), National Key Research and Development Program of China (2021YFC2500900).
Conflict of interest: none declared.
DATA AVAILABILITY
The datasets are not publicly available due to ethical restrictions but are available from the corresponding author on reasonable request.
Author contributions
Yeke Huang: Data curation; Formal analysis; Writing—original draft. Xipeng Wang: Data curation; Writing—original draft. Yuqin Cao: Methodology; Writing—review & editing. Yunjiu Gou: Data curation; Resources. Shumin Wang: Data curation; Resources. Yajie Zhang: Conceptualization; Writing—review & editing. Hecheng Li: Conceptualization; Funding acquisition; Resources; Writing—review & editing
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Daniel A. Valdivia, Alex Fourdrain and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
Buentzel J, Heinz J, Hinterthaner M, et al. Robotic versus thoracoscopic thymectomy: the current evidence.
ABBREVIATIONS
- PSM
Propensity score matching
- RATS
Robot-assisted thoracic surgery
- VATS
Video-assisted thoracic surgery
- WBC
White blood cell
Author notes
Yeke Huanga, Xipeng Wanga, Yajie Zhanga and Yuqin Cao authors contributed equally to this work.





