-
PDF
- Split View
-
Views
-
Cite
Cite
Giovanni Maria Comacchio, Marco Schiavon, Carmelina Cristina Zirafa, Angela De Palma, Roberto Scaramuzzi, Elisa Meacci, Stefano Bongiolatti, Nicola Monaci, Paraskevas Lyberis, Pierluigi Novellis, Jury Brandolini, Sara Parini, Sara Ricciardi, Antonio D’Andrilli, Edoardo Bottoni, Filippo Tommaso Gallina, Maria Carlotta Marino, Giulia Lorenzoni, Andrea Francavilla, Erino Angelo Rendina, Giuseppe Cardillo, Ottavio Rena, Piergiorgio Solli, Marco Alloisio, Luca Luzzi, Francesco Facciolo, Luca Voltolini, Stefano Margaritora, Carlo Curcio, Giuseppe Marulli, Enrico Ruffini, Giulia Veronesi, Franca Melfi, Federico Rea, Robotic thymectomy in thymic tumours: a multicentre, nation-wide study, European Journal of Cardio-Thoracic Surgery, Volume 65, Issue 5, May 2024, ezae178, https://doi.org/10.1093/ejcts/ezae178
Close - Share Icon Share
Abstract
Robotic thymectomy has been suggested and considered technically feasible for thymic tumours. However, because of small-sample series and the lack of data on long-term results, controversies still exist on surgical and oncological results with this approach. We performed a large national multicentre study sought to evaluate the early and long-term outcomes after robot-assisted thoracoscopic thymectomy in thymic epithelial tumours.
All patients with thymic epithelial tumours operated through a robotic thoracoscopic approach between 2002 and 2022 from 15 Italian centres were enrolled. Demographic characteristics, clinical, intraoperative, postoperative, pathological and follow-up data were retrospectively collected and reviewed.
There were 669 patients (307 men and 362 women), 312 (46.6%) of whom had associated myasthenia gravis. Complete thymectomy was performed in 657 (98%) cases and in 57 (8.5%) patients resection of other structures was necessary, with a R0 resection in all but 9 patients (98.6%). Twenty-three patients (3.4%) needed open conversion, but no perioperative mortality occurred. Fifty-one patients (7.7%) had postoperative complications. The median diameter of tumour resected was 4 cm (interquartile range 3–5.5 cm), and Masaoka stage was stage I in 39.8% of patients, stage II in 56.1%, stage III in 3.5% and stage IV in 0.6%. Thymoma was observed in 90.2% of patients while thymic carcinoma occurred in 2.8% of cases. At the end of the follow-up, only 2 patients died for tumour-related causes. Five- and ten-year recurrence rates were 7.4% and 8.3%, respectively.
Through the largest collection of robotic thymectomy for thymic epithelial tumours we demonstrated that robot-enhanced thoracoscopic thymectomy is a technically sound and safe procedure with a low complication rate and optimal oncological outcomes.
INTRODUCTION
Thymomas are rare tumours, representing 0.2–1.5% of all malignancies, but are the most common tumours of the anterior mediastinum, accounting for 50% of all anterior mediastinal malignancies. Surgery is the mainstay in the treatment of thymomas and radical resection has been demonstrated being the most important prognostic factor [1]. Historically, in the field of thymic epithelial tumours (TETs) sternotomy has long been considered the standard approach and still is the preferred choice in complex cases [1]. However, particularly in early-stage disease or with limited involvement of the surrounding structures, minimally invasive approaches have demonstrated to ensure comparable oncological outcomes while showing superior surgical results, particularly in terms of postoperative complications [2–4]. Robotic thymectomy represents one of the most recent surgical approaches to the thymic gland. Since the first descriptions of this technique for the removal of the thymus in patients with myasthenia gravis (MG), new indications gained attention, being used also for the treatment of thymic tumours in early and advanced stages [5]. While displaying all the advantages of minimally invasive approaches, the robotic system seems to overcome the disadvantages of standard VATS (Video-Asssisted Thoracic Surgery) when dealing with mediastinal structures. Indeed, the three-dimensional view, the articulated instruments with 360° of rotation, the 7 degrees of freedom and the tremor filtering system seem to minimize difficulties when dealing with a narrow-to-reach and delicate region like the mediastinum [6].
According to the literature, there are different studies showing the results of robotic thymectomy but displaying all as main limitations the small number of cases, frequently single-centre experiences, and the short oncological follow-up [6–20].
The aim of this study is therefore to analyse the surgical and oncological outcomes after robot-assisted thoracoscopic thymectomy in TETs through a large national multicentre study.
PATIENTS AND METHODS
Ethical statement
The institutional review board of Padua University Hospital approved the study (date 30 June 2022, n. 5400/AO/22), waiver of informed consent.
Methods
We retrospectively reviewed the data of all patients undergoing robotic thymectomy for TETs using the ‘da Vinci’ surgical system (Intuitive Surgical, Inc., Sunnyvalley, CA) collected between 2002 and December 2022 by fifteen Italian Thoracic Surgery Centers (University of Padua; University of Pisa; University of Bari; Monaldi Hospital, Naples; University Hospital of Florence; University Hospital of Siena; University of Turin; San Raffaele Scientific Institute, Milan; Humanitas Hospital, Milan; University Hospital of Bologna; Hospital of Novara; Catholic University of Sacred Heart, Rome; Sant’Andrea Hospital, Rome; San Camillo Forlanini Hospital, Rome; Regina Elena Hospital, Rome). This study included all patients operated in each centre for thymic tumours, from the beginning of their experience with robotic thymectomy until December 2022. Exclusion criteria were the use of surgical approaches other than robotic (i.e. open or other minimally invasive) and tumours other than TETs. Information on patient demographics, presence of associated MG or other paraneoplastic syndromes, tumour characteristics, pathological stage, intra- and postoperative data (e.g. complications, need for open conversion or additional ports or accesses, operative time, length of hospital stay) and oncological data were collected. The Myasthenia Gravis Foundation of America classification was used to stratify the preoperative class of MG [21]. The Masaoka-Koga stage and the eighth edition of TNM staging system were applied to assess the pathological stage, while the World Health Organization classification was used for histological definition [22, 23]. International Thymic Malignancy Interest Group (ITMIG) classification was used to determine the site of recurrence (local, regional or distant) [24]. STROBE statement checklist was followed (Supplementary Material 1).
Statistical analysis
Continuous variables are expressed as median and interquartile range (IQR), while categorical variables are expressed as absolute numbers and percentages. Survival at follow-up was evaluated using the Kaplan–Meier method, while cumulative incidence functions were employed to evaluate recurrence at follow-up to account for competing risks. Univariable Fine-Gray subdistribution hazard competing risk regression models were employed to assess the role of baseline characteristics on recurrence at follow-up. Results were reported as hazard ration (HR), 95% confidence interval (CI) and P-values. Complete case analysis was performed. Analysis was performed using the R software.
RESULTS
In the study period, 1104 patients underwent thymectomy for TETs in 15 Italian Thoracic Surgery Centers. Among these, 669 (60.5%) underwent robotic thymectomy and represent the population of this study. Supplementary Material, Fig. S1 shows the year of the first robotic thymectomy reported in the database and the number of patients enrolled by each centre. Three hundred and seven (45.8%) patients were males and 362 (54.2%) females, with a median age of 60 years (IQR 50–70 years). Three hundred and twelve (46.6%) patients were affected by MG and 5 (0.7%) had other paraneoplastic syndromes reported (2 red cell aplasia, 1 cerebellar ataxia, 1 systemic lupus erythematosus-like syndrome, 1 fever). Table 1 shows the demographic characteristics of the population.
| Variable . | Study population (n = 669) . |
|---|---|
| Gender, n (%) | |
| M | 307 (45.8%) |
| F | 362 (54.2%) |
| Age, years, median (IQR) | 60 (50–70) |
| Myasthenia gravis, n (%) | 312 (46.6%) |
| Myasthenia gravis duration, months, median (IQR) | 7 (4,12) |
| Preoperative MGFA class (n = 244), n (%) | |
| I | 73 (30%) |
| II | 104 (42.6%) |
| III | 48 (19.6%) |
| IV | 15 (6.1%) |
| V | 4 (1.7%) |
| Other paraneoplastic syndromes, n (%) | 5 (0.7%) |
| Induction therapy, n (%) | 0 (0%) |
| Variable . | Study population (n = 669) . |
|---|---|
| Gender, n (%) | |
| M | 307 (45.8%) |
| F | 362 (54.2%) |
| Age, years, median (IQR) | 60 (50–70) |
| Myasthenia gravis, n (%) | 312 (46.6%) |
| Myasthenia gravis duration, months, median (IQR) | 7 (4,12) |
| Preoperative MGFA class (n = 244), n (%) | |
| I | 73 (30%) |
| II | 104 (42.6%) |
| III | 48 (19.6%) |
| IV | 15 (6.1%) |
| V | 4 (1.7%) |
| Other paraneoplastic syndromes, n (%) | 5 (0.7%) |
| Induction therapy, n (%) | 0 (0%) |
F: female; IQR: interquartile range; M: male; MGFA: Myasthenia Gravis Foundation of America
| Variable . | Study population (n = 669) . |
|---|---|
| Gender, n (%) | |
| M | 307 (45.8%) |
| F | 362 (54.2%) |
| Age, years, median (IQR) | 60 (50–70) |
| Myasthenia gravis, n (%) | 312 (46.6%) |
| Myasthenia gravis duration, months, median (IQR) | 7 (4,12) |
| Preoperative MGFA class (n = 244), n (%) | |
| I | 73 (30%) |
| II | 104 (42.6%) |
| III | 48 (19.6%) |
| IV | 15 (6.1%) |
| V | 4 (1.7%) |
| Other paraneoplastic syndromes, n (%) | 5 (0.7%) |
| Induction therapy, n (%) | 0 (0%) |
| Variable . | Study population (n = 669) . |
|---|---|
| Gender, n (%) | |
| M | 307 (45.8%) |
| F | 362 (54.2%) |
| Age, years, median (IQR) | 60 (50–70) |
| Myasthenia gravis, n (%) | 312 (46.6%) |
| Myasthenia gravis duration, months, median (IQR) | 7 (4,12) |
| Preoperative MGFA class (n = 244), n (%) | |
| I | 73 (30%) |
| II | 104 (42.6%) |
| III | 48 (19.6%) |
| IV | 15 (6.1%) |
| V | 4 (1.7%) |
| Other paraneoplastic syndromes, n (%) | 5 (0.7%) |
| Induction therapy, n (%) | 0 (0%) |
F: female; IQR: interquartile range; M: male; MGFA: Myasthenia Gravis Foundation of America
Intraoperative data are described in Table 2. The majority of patients (525, 78.4%) were operated on through a left-side approach and underwent a complete thymectomy (656 cases, 98%), rather than a thymomectomy (10 cases, 1.4%) or a hemi-thymectomy (3 cases, 0.6%). Resection of adjacent structures was necessary in 57 (8.5%) patients, with the most frequent one involving the lung parenchyma, while lymphadenectomy was performed only in 59 (8.8%).
| Variable . | Study population (n = 669) . |
|---|---|
| Surgical approach side, n (%) | |
| Left | 525 (78.4%) |
| Right | 130 (19.4%) |
| Bilateral | 9 (1.3%) |
| Subxiphoid | 5 (0.9%) |
| Operative time (min), median (IQR) | 140 (114–175) |
| Extent of thymectomy, n (%) | |
| Complete thymectomy | 656 (98%) |
| Hemithymectomy | 3 (0.6%) |
| Thymomectomy | 10 (1.4%) |
| Resection of other structures, n (%) | 57 (8.5%) |
| Pericardium | 15 (2.2%) |
| Lung | 40 (6%) |
| Pleural lesions | 5 (0.7%) |
| Phrenic nerve | 8 (1.2%) |
| Other | 2 (0.3%) |
| Lymphadenectomy, n (%) | 59 (8.8%) |
| Intraoperative complications, n (%) | 9 (1.3%) |
| Vascular injury | 3 |
| Phrenic nerve injury | 2 |
| Tumour capsule breach | 1 |
| Lung lesion | 1 |
| Cardiac tamponade | 1 |
| Diaphragmatic breach | 1 |
| Open conversion, n (%) | 23 (3.4%) |
| Intraoperative death, n (%) | 0 (0%) |
| Postoperative chest tube length of stay (days), median (IQR) | 2 (1–4) |
| Postoperative in-hospital length of stay (days), median (IQR) | 4 (3–5) |
| Postoperative complications, n (%) | 51 (7.7%) |
| Myasthenic crisis | 10 (15%) |
| Hemothorax | 6 (9%) |
| Chylothorax | 3 (0.4%) |
| Pneumothorax | 1 (0.1%) |
| Pleural effusion | 3 (0.4%) |
| Prolonged air leaks | 4 (0.5%) |
| Other medical complications | 27 (4%) |
| Clavien-Dindo grade ≥3 complications, n (%) | 11 (1.6%) |
| In-hospital mortality, n (%) | 1 (0.1%) |
| Variable . | Study population (n = 669) . |
|---|---|
| Surgical approach side, n (%) | |
| Left | 525 (78.4%) |
| Right | 130 (19.4%) |
| Bilateral | 9 (1.3%) |
| Subxiphoid | 5 (0.9%) |
| Operative time (min), median (IQR) | 140 (114–175) |
| Extent of thymectomy, n (%) | |
| Complete thymectomy | 656 (98%) |
| Hemithymectomy | 3 (0.6%) |
| Thymomectomy | 10 (1.4%) |
| Resection of other structures, n (%) | 57 (8.5%) |
| Pericardium | 15 (2.2%) |
| Lung | 40 (6%) |
| Pleural lesions | 5 (0.7%) |
| Phrenic nerve | 8 (1.2%) |
| Other | 2 (0.3%) |
| Lymphadenectomy, n (%) | 59 (8.8%) |
| Intraoperative complications, n (%) | 9 (1.3%) |
| Vascular injury | 3 |
| Phrenic nerve injury | 2 |
| Tumour capsule breach | 1 |
| Lung lesion | 1 |
| Cardiac tamponade | 1 |
| Diaphragmatic breach | 1 |
| Open conversion, n (%) | 23 (3.4%) |
| Intraoperative death, n (%) | 0 (0%) |
| Postoperative chest tube length of stay (days), median (IQR) | 2 (1–4) |
| Postoperative in-hospital length of stay (days), median (IQR) | 4 (3–5) |
| Postoperative complications, n (%) | 51 (7.7%) |
| Myasthenic crisis | 10 (15%) |
| Hemothorax | 6 (9%) |
| Chylothorax | 3 (0.4%) |
| Pneumothorax | 1 (0.1%) |
| Pleural effusion | 3 (0.4%) |
| Prolonged air leaks | 4 (0.5%) |
| Other medical complications | 27 (4%) |
| Clavien-Dindo grade ≥3 complications, n (%) | 11 (1.6%) |
| In-hospital mortality, n (%) | 1 (0.1%) |
IQR: interquartile range.
| Variable . | Study population (n = 669) . |
|---|---|
| Surgical approach side, n (%) | |
| Left | 525 (78.4%) |
| Right | 130 (19.4%) |
| Bilateral | 9 (1.3%) |
| Subxiphoid | 5 (0.9%) |
| Operative time (min), median (IQR) | 140 (114–175) |
| Extent of thymectomy, n (%) | |
| Complete thymectomy | 656 (98%) |
| Hemithymectomy | 3 (0.6%) |
| Thymomectomy | 10 (1.4%) |
| Resection of other structures, n (%) | 57 (8.5%) |
| Pericardium | 15 (2.2%) |
| Lung | 40 (6%) |
| Pleural lesions | 5 (0.7%) |
| Phrenic nerve | 8 (1.2%) |
| Other | 2 (0.3%) |
| Lymphadenectomy, n (%) | 59 (8.8%) |
| Intraoperative complications, n (%) | 9 (1.3%) |
| Vascular injury | 3 |
| Phrenic nerve injury | 2 |
| Tumour capsule breach | 1 |
| Lung lesion | 1 |
| Cardiac tamponade | 1 |
| Diaphragmatic breach | 1 |
| Open conversion, n (%) | 23 (3.4%) |
| Intraoperative death, n (%) | 0 (0%) |
| Postoperative chest tube length of stay (days), median (IQR) | 2 (1–4) |
| Postoperative in-hospital length of stay (days), median (IQR) | 4 (3–5) |
| Postoperative complications, n (%) | 51 (7.7%) |
| Myasthenic crisis | 10 (15%) |
| Hemothorax | 6 (9%) |
| Chylothorax | 3 (0.4%) |
| Pneumothorax | 1 (0.1%) |
| Pleural effusion | 3 (0.4%) |
| Prolonged air leaks | 4 (0.5%) |
| Other medical complications | 27 (4%) |
| Clavien-Dindo grade ≥3 complications, n (%) | 11 (1.6%) |
| In-hospital mortality, n (%) | 1 (0.1%) |
| Variable . | Study population (n = 669) . |
|---|---|
| Surgical approach side, n (%) | |
| Left | 525 (78.4%) |
| Right | 130 (19.4%) |
| Bilateral | 9 (1.3%) |
| Subxiphoid | 5 (0.9%) |
| Operative time (min), median (IQR) | 140 (114–175) |
| Extent of thymectomy, n (%) | |
| Complete thymectomy | 656 (98%) |
| Hemithymectomy | 3 (0.6%) |
| Thymomectomy | 10 (1.4%) |
| Resection of other structures, n (%) | 57 (8.5%) |
| Pericardium | 15 (2.2%) |
| Lung | 40 (6%) |
| Pleural lesions | 5 (0.7%) |
| Phrenic nerve | 8 (1.2%) |
| Other | 2 (0.3%) |
| Lymphadenectomy, n (%) | 59 (8.8%) |
| Intraoperative complications, n (%) | 9 (1.3%) |
| Vascular injury | 3 |
| Phrenic nerve injury | 2 |
| Tumour capsule breach | 1 |
| Lung lesion | 1 |
| Cardiac tamponade | 1 |
| Diaphragmatic breach | 1 |
| Open conversion, n (%) | 23 (3.4%) |
| Intraoperative death, n (%) | 0 (0%) |
| Postoperative chest tube length of stay (days), median (IQR) | 2 (1–4) |
| Postoperative in-hospital length of stay (days), median (IQR) | 4 (3–5) |
| Postoperative complications, n (%) | 51 (7.7%) |
| Myasthenic crisis | 10 (15%) |
| Hemothorax | 6 (9%) |
| Chylothorax | 3 (0.4%) |
| Pneumothorax | 1 (0.1%) |
| Pleural effusion | 3 (0.4%) |
| Prolonged air leaks | 4 (0.5%) |
| Other medical complications | 27 (4%) |
| Clavien-Dindo grade ≥3 complications, n (%) | 11 (1.6%) |
| In-hospital mortality, n (%) | 1 (0.1%) |
IQR: interquartile range.
Intraoperative complications occurred in 9 (1.3%) cases and in 3 of them, an emergency open conversion was necessary. In other 19 (2.8%) patients, the open conversion was required because of oncological or technical issues that made the robotic resection unsafe.
In the postoperative period, onset of any kind of complications was reported in 51 (7.7%) patients, while the median duration of chest tube stay and the in-hospital length of stay were, respectively, 2 (IQR 1–4) and 4 (IQR 3–5) postoperative days (Table 2). There was a single death recorded in the postoperative period, due to a severe myasthenic crisis. No deaths were recorded in the 90 days after surgery.
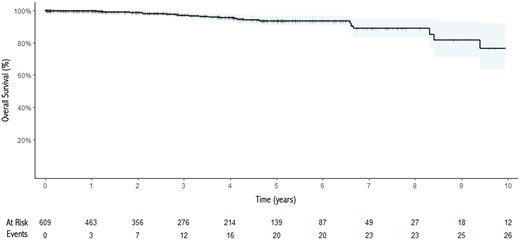
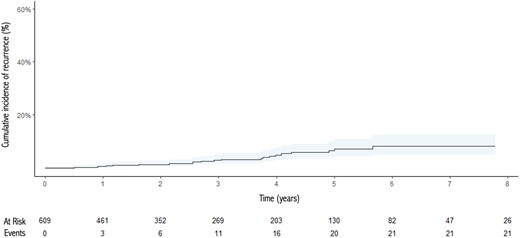
Pathological and oncological outcomes are described in Table 3. An R0 resection was obtained in 660 (98.6%) patients. One hundred sixty (24%) patients needed for an adjuvant treatment, mainly radiotherapy (149 cases, 93%). At the end of the follow-up period (median 29 months, IQR 10–56), there were 21 (3.4%) disease recurrences, mainly regional according to the ITMIG classification. At the same time point, there were 25 (3.7%) deaths among the entire population, only 2 related to the thymic tumour. The 5- and 10-year recurrence and survival rates were 7.4% and 8.3% and 94% and 77%, respectively. Kaplan–Meier and cumulative incidence function curves for survival and recurrence, respectively, are shown in Figs 1 and 2. Univariable Fine-Gray models identified the need for extended resections [HR (95% CI): 5.68 (2.34–13.8), P < 0.001], lymphadenectomy [HR (95% CI): 7.91 (3.0–20.9), P < 0.001], lack of radicality [HR (95% CI): 8.46 (2.39–29.9), P < 0.001], need for conversion [HR (95% CI): 6.62 (2.54–17.3), P < 0.001], thymic carcinoma histology [HR (95 CI): 48.4 (4.71–496), P = 0.001] and higher Masaoka stage [I vs IIb HR (95% CI): 5.73 (1.14–28.8), P = 0.034; I vs III HR (95% CI): 34.1 (6.78–171), P < 0.001; I vs IV HR (95% CI): 36.6 (6.1–219), P < 0.001] all to be associated with the risk for tumour recurrence (Table 4).
| Variable . | Study population (n = 669) . |
|---|---|
| Histology, n (%) | |
| A thymoma | 94 (14.1%) |
| AB thymoma | 156 (23.3%) |
| B1 thymoma | 121 (18.1%) |
| B2 thymoma | 175 (26.2%) |
| B3 thymoma | 59 (8.8%) |
| Thymic carcinoma | 19 (2.8%) |
| Microscopic thymoma | 18 (2.7%) |
| Micronodular thymoma | 16 (2.4%) |
| Thymic neuroendocrine tumour | 7 (0.9%) |
| Sclerosing thymoma | 4 (0.6%) |
| Myasthenia gravis/histology, n (%) | |
| A thymoma | 29 (30.8%) |
| AB thymoma | 57 (36.5%) |
| B1 thymoma | 57 (47.1%) |
| B2 thymoma | 113 (64.5%) |
| B3 thymoma | 38 (62.7%) |
| Thymic carcinoma | 1 (5.2%) |
| Microscopic thymoma | 13 (72.2%) |
| Micronodular thymoma | 1 (6.3%) |
| Sclerosing thymoma | 3 (75%) |
| Masaoka-Koga staging (n = 632), n (%) | |
| I | 251 (39.8%) |
| IIa | 241 (37.9%) |
| IIb | 115 (18.2%) |
| III | 22 (3.5%) |
| IVa | 3 (0.6%) |
| TNM staging (n = 653), n (%) | |
| T | |
| 1 | 631 (96.6%) |
| 2 | 6 (0.9%) |
| 3 | 16 (2.4%) |
| N | |
| 0 | 653 (100%) |
| M | |
| 1a | 3 (0.6%) |
| Stage | |
| I | 629 (96.3%) |
| II | 6 (0.9%) |
| III | 15 (2.3%) |
| IV | 3 (0.6%) |
| Tumour dimension, cm, median (IQR) | 4 (3–5.5) |
| R0 resection, n (%) | 660 (98.6%) |
| Adjuvant therapy (n = 663) | 160 (24%) |
| Radiotherapy | 149 (22.3%) |
| Chemotherapy | 11 (1.6%) |
| Follow-up, months, median (IQR) | 29 (10–56) |
| Lost at follow-up, n (%) | 60 (8.9%) |
| Disease status (n = 609), n (%) | |
| NED | 577 (95.8%) |
| AWD | 7 (1.1%) |
| DOD | 2 (0.4%) |
| DOC | 24 (3.9%) |
| Recurrence (n = 609), n (%) | 21 (3.4%) |
| Local | 5 (0.8%) |
| Regional | 14 (2.2%) |
| Distant | 1 (0.2%) |
| Unknown | 1 (0.2%) |
| Variable . | Study population (n = 669) . |
|---|---|
| Histology, n (%) | |
| A thymoma | 94 (14.1%) |
| AB thymoma | 156 (23.3%) |
| B1 thymoma | 121 (18.1%) |
| B2 thymoma | 175 (26.2%) |
| B3 thymoma | 59 (8.8%) |
| Thymic carcinoma | 19 (2.8%) |
| Microscopic thymoma | 18 (2.7%) |
| Micronodular thymoma | 16 (2.4%) |
| Thymic neuroendocrine tumour | 7 (0.9%) |
| Sclerosing thymoma | 4 (0.6%) |
| Myasthenia gravis/histology, n (%) | |
| A thymoma | 29 (30.8%) |
| AB thymoma | 57 (36.5%) |
| B1 thymoma | 57 (47.1%) |
| B2 thymoma | 113 (64.5%) |
| B3 thymoma | 38 (62.7%) |
| Thymic carcinoma | 1 (5.2%) |
| Microscopic thymoma | 13 (72.2%) |
| Micronodular thymoma | 1 (6.3%) |
| Sclerosing thymoma | 3 (75%) |
| Masaoka-Koga staging (n = 632), n (%) | |
| I | 251 (39.8%) |
| IIa | 241 (37.9%) |
| IIb | 115 (18.2%) |
| III | 22 (3.5%) |
| IVa | 3 (0.6%) |
| TNM staging (n = 653), n (%) | |
| T | |
| 1 | 631 (96.6%) |
| 2 | 6 (0.9%) |
| 3 | 16 (2.4%) |
| N | |
| 0 | 653 (100%) |
| M | |
| 1a | 3 (0.6%) |
| Stage | |
| I | 629 (96.3%) |
| II | 6 (0.9%) |
| III | 15 (2.3%) |
| IV | 3 (0.6%) |
| Tumour dimension, cm, median (IQR) | 4 (3–5.5) |
| R0 resection, n (%) | 660 (98.6%) |
| Adjuvant therapy (n = 663) | 160 (24%) |
| Radiotherapy | 149 (22.3%) |
| Chemotherapy | 11 (1.6%) |
| Follow-up, months, median (IQR) | 29 (10–56) |
| Lost at follow-up, n (%) | 60 (8.9%) |
| Disease status (n = 609), n (%) | |
| NED | 577 (95.8%) |
| AWD | 7 (1.1%) |
| DOD | 2 (0.4%) |
| DOC | 24 (3.9%) |
| Recurrence (n = 609), n (%) | 21 (3.4%) |
| Local | 5 (0.8%) |
| Regional | 14 (2.2%) |
| Distant | 1 (0.2%) |
| Unknown | 1 (0.2%) |
AWD: alive with disease; DOC: died of other causes; DOD: died of disease; IQR: interquartile range; NED: no evidence of disease
| Variable . | Study population (n = 669) . |
|---|---|
| Histology, n (%) | |
| A thymoma | 94 (14.1%) |
| AB thymoma | 156 (23.3%) |
| B1 thymoma | 121 (18.1%) |
| B2 thymoma | 175 (26.2%) |
| B3 thymoma | 59 (8.8%) |
| Thymic carcinoma | 19 (2.8%) |
| Microscopic thymoma | 18 (2.7%) |
| Micronodular thymoma | 16 (2.4%) |
| Thymic neuroendocrine tumour | 7 (0.9%) |
| Sclerosing thymoma | 4 (0.6%) |
| Myasthenia gravis/histology, n (%) | |
| A thymoma | 29 (30.8%) |
| AB thymoma | 57 (36.5%) |
| B1 thymoma | 57 (47.1%) |
| B2 thymoma | 113 (64.5%) |
| B3 thymoma | 38 (62.7%) |
| Thymic carcinoma | 1 (5.2%) |
| Microscopic thymoma | 13 (72.2%) |
| Micronodular thymoma | 1 (6.3%) |
| Sclerosing thymoma | 3 (75%) |
| Masaoka-Koga staging (n = 632), n (%) | |
| I | 251 (39.8%) |
| IIa | 241 (37.9%) |
| IIb | 115 (18.2%) |
| III | 22 (3.5%) |
| IVa | 3 (0.6%) |
| TNM staging (n = 653), n (%) | |
| T | |
| 1 | 631 (96.6%) |
| 2 | 6 (0.9%) |
| 3 | 16 (2.4%) |
| N | |
| 0 | 653 (100%) |
| M | |
| 1a | 3 (0.6%) |
| Stage | |
| I | 629 (96.3%) |
| II | 6 (0.9%) |
| III | 15 (2.3%) |
| IV | 3 (0.6%) |
| Tumour dimension, cm, median (IQR) | 4 (3–5.5) |
| R0 resection, n (%) | 660 (98.6%) |
| Adjuvant therapy (n = 663) | 160 (24%) |
| Radiotherapy | 149 (22.3%) |
| Chemotherapy | 11 (1.6%) |
| Follow-up, months, median (IQR) | 29 (10–56) |
| Lost at follow-up, n (%) | 60 (8.9%) |
| Disease status (n = 609), n (%) | |
| NED | 577 (95.8%) |
| AWD | 7 (1.1%) |
| DOD | 2 (0.4%) |
| DOC | 24 (3.9%) |
| Recurrence (n = 609), n (%) | 21 (3.4%) |
| Local | 5 (0.8%) |
| Regional | 14 (2.2%) |
| Distant | 1 (0.2%) |
| Unknown | 1 (0.2%) |
| Variable . | Study population (n = 669) . |
|---|---|
| Histology, n (%) | |
| A thymoma | 94 (14.1%) |
| AB thymoma | 156 (23.3%) |
| B1 thymoma | 121 (18.1%) |
| B2 thymoma | 175 (26.2%) |
| B3 thymoma | 59 (8.8%) |
| Thymic carcinoma | 19 (2.8%) |
| Microscopic thymoma | 18 (2.7%) |
| Micronodular thymoma | 16 (2.4%) |
| Thymic neuroendocrine tumour | 7 (0.9%) |
| Sclerosing thymoma | 4 (0.6%) |
| Myasthenia gravis/histology, n (%) | |
| A thymoma | 29 (30.8%) |
| AB thymoma | 57 (36.5%) |
| B1 thymoma | 57 (47.1%) |
| B2 thymoma | 113 (64.5%) |
| B3 thymoma | 38 (62.7%) |
| Thymic carcinoma | 1 (5.2%) |
| Microscopic thymoma | 13 (72.2%) |
| Micronodular thymoma | 1 (6.3%) |
| Sclerosing thymoma | 3 (75%) |
| Masaoka-Koga staging (n = 632), n (%) | |
| I | 251 (39.8%) |
| IIa | 241 (37.9%) |
| IIb | 115 (18.2%) |
| III | 22 (3.5%) |
| IVa | 3 (0.6%) |
| TNM staging (n = 653), n (%) | |
| T | |
| 1 | 631 (96.6%) |
| 2 | 6 (0.9%) |
| 3 | 16 (2.4%) |
| N | |
| 0 | 653 (100%) |
| M | |
| 1a | 3 (0.6%) |
| Stage | |
| I | 629 (96.3%) |
| II | 6 (0.9%) |
| III | 15 (2.3%) |
| IV | 3 (0.6%) |
| Tumour dimension, cm, median (IQR) | 4 (3–5.5) |
| R0 resection, n (%) | 660 (98.6%) |
| Adjuvant therapy (n = 663) | 160 (24%) |
| Radiotherapy | 149 (22.3%) |
| Chemotherapy | 11 (1.6%) |
| Follow-up, months, median (IQR) | 29 (10–56) |
| Lost at follow-up, n (%) | 60 (8.9%) |
| Disease status (n = 609), n (%) | |
| NED | 577 (95.8%) |
| AWD | 7 (1.1%) |
| DOD | 2 (0.4%) |
| DOC | 24 (3.9%) |
| Recurrence (n = 609), n (%) | 21 (3.4%) |
| Local | 5 (0.8%) |
| Regional | 14 (2.2%) |
| Distant | 1 (0.2%) |
| Unknown | 1 (0.2%) |
AWD: alive with disease; DOC: died of other causes; DOD: died of disease; IQR: interquartile range; NED: no evidence of disease
| Characteristic . | N . | HR . | 95% CI . | P-Value . |
|---|---|---|---|---|
| Gender | 608 | |||
| M | – | – | ||
| F | 0.91 | 0.38, 2.13 | 0.8 | |
| Myasthenia gravis | 608 | |||
| Yes | – | – | ||
| No | 1.43 | 0.61, 3.36 | 0.4 | |
| Associated resections | 609 | |||
| No | – | – | ||
| Yes | 5.68 | 2.34, 13.8 | <0.001 | |
| Lymphadenectomy | 599 | 7.91 | 3.00, 20.9 | <0.001 |
| Radicality | 605 | |||
| R0 | – | – | ||
| R1 | 8.46 | 2.39, 29.9 | <0.001 | |
| Conversion | 609 | 6.62 | 2.54, 17.3 | <0.001 |
| Histology | 606 | |||
| Thymoma A | – | – | ||
| Thymoma AB | 0.74 | 0.05, 11.9 | 0.8 | |
| Thymoma B1 | 2.06 | 0.21, 20.1 | 0.5 | |
| Thymoma B2 | 3.61 | 0.44, 29.5 | 0.2 | |
| Thymoma B3 | 3.27 | 0.3, 36.2 | 0.3 | |
| Thymic carcinoma | 48.4 | 4.71, 496 | 0.001 | |
| Other | 9.19 | 0.91, 92.8 | 0.06 | |
| Masaoka stage | 569 | |||
| I | – | – | ||
| IIa | 2.33 | 0.43, 12.7 | 0.3 | |
| IIb | 5.73 | 1.14, 28.8 | 0.034 | |
| III | 34.1 | 6.78, 171 | <0.001 | |
| IV | 36.6 | 6.10, 219 | <0.001 | |
| Tumour dimension | 593 | 1.01 | 1.00, 1.02 | 0.13 |
| Adjuvant therapy | 606 | 2.27 | 0.96, 5.35 | 0.061 |
| Characteristic . | N . | HR . | 95% CI . | P-Value . |
|---|---|---|---|---|
| Gender | 608 | |||
| M | – | – | ||
| F | 0.91 | 0.38, 2.13 | 0.8 | |
| Myasthenia gravis | 608 | |||
| Yes | – | – | ||
| No | 1.43 | 0.61, 3.36 | 0.4 | |
| Associated resections | 609 | |||
| No | – | – | ||
| Yes | 5.68 | 2.34, 13.8 | <0.001 | |
| Lymphadenectomy | 599 | 7.91 | 3.00, 20.9 | <0.001 |
| Radicality | 605 | |||
| R0 | – | – | ||
| R1 | 8.46 | 2.39, 29.9 | <0.001 | |
| Conversion | 609 | 6.62 | 2.54, 17.3 | <0.001 |
| Histology | 606 | |||
| Thymoma A | – | – | ||
| Thymoma AB | 0.74 | 0.05, 11.9 | 0.8 | |
| Thymoma B1 | 2.06 | 0.21, 20.1 | 0.5 | |
| Thymoma B2 | 3.61 | 0.44, 29.5 | 0.2 | |
| Thymoma B3 | 3.27 | 0.3, 36.2 | 0.3 | |
| Thymic carcinoma | 48.4 | 4.71, 496 | 0.001 | |
| Other | 9.19 | 0.91, 92.8 | 0.06 | |
| Masaoka stage | 569 | |||
| I | – | – | ||
| IIa | 2.33 | 0.43, 12.7 | 0.3 | |
| IIb | 5.73 | 1.14, 28.8 | 0.034 | |
| III | 34.1 | 6.78, 171 | <0.001 | |
| IV | 36.6 | 6.10, 219 | <0.001 | |
| Tumour dimension | 593 | 1.01 | 1.00, 1.02 | 0.13 |
| Adjuvant therapy | 606 | 2.27 | 0.96, 5.35 | 0.061 |
Data are hazard ration (HR), 95% confidence interval (CI) and P-value.
| Characteristic . | N . | HR . | 95% CI . | P-Value . |
|---|---|---|---|---|
| Gender | 608 | |||
| M | – | – | ||
| F | 0.91 | 0.38, 2.13 | 0.8 | |
| Myasthenia gravis | 608 | |||
| Yes | – | – | ||
| No | 1.43 | 0.61, 3.36 | 0.4 | |
| Associated resections | 609 | |||
| No | – | – | ||
| Yes | 5.68 | 2.34, 13.8 | <0.001 | |
| Lymphadenectomy | 599 | 7.91 | 3.00, 20.9 | <0.001 |
| Radicality | 605 | |||
| R0 | – | – | ||
| R1 | 8.46 | 2.39, 29.9 | <0.001 | |
| Conversion | 609 | 6.62 | 2.54, 17.3 | <0.001 |
| Histology | 606 | |||
| Thymoma A | – | – | ||
| Thymoma AB | 0.74 | 0.05, 11.9 | 0.8 | |
| Thymoma B1 | 2.06 | 0.21, 20.1 | 0.5 | |
| Thymoma B2 | 3.61 | 0.44, 29.5 | 0.2 | |
| Thymoma B3 | 3.27 | 0.3, 36.2 | 0.3 | |
| Thymic carcinoma | 48.4 | 4.71, 496 | 0.001 | |
| Other | 9.19 | 0.91, 92.8 | 0.06 | |
| Masaoka stage | 569 | |||
| I | – | – | ||
| IIa | 2.33 | 0.43, 12.7 | 0.3 | |
| IIb | 5.73 | 1.14, 28.8 | 0.034 | |
| III | 34.1 | 6.78, 171 | <0.001 | |
| IV | 36.6 | 6.10, 219 | <0.001 | |
| Tumour dimension | 593 | 1.01 | 1.00, 1.02 | 0.13 |
| Adjuvant therapy | 606 | 2.27 | 0.96, 5.35 | 0.061 |
| Characteristic . | N . | HR . | 95% CI . | P-Value . |
|---|---|---|---|---|
| Gender | 608 | |||
| M | – | – | ||
| F | 0.91 | 0.38, 2.13 | 0.8 | |
| Myasthenia gravis | 608 | |||
| Yes | – | – | ||
| No | 1.43 | 0.61, 3.36 | 0.4 | |
| Associated resections | 609 | |||
| No | – | – | ||
| Yes | 5.68 | 2.34, 13.8 | <0.001 | |
| Lymphadenectomy | 599 | 7.91 | 3.00, 20.9 | <0.001 |
| Radicality | 605 | |||
| R0 | – | – | ||
| R1 | 8.46 | 2.39, 29.9 | <0.001 | |
| Conversion | 609 | 6.62 | 2.54, 17.3 | <0.001 |
| Histology | 606 | |||
| Thymoma A | – | – | ||
| Thymoma AB | 0.74 | 0.05, 11.9 | 0.8 | |
| Thymoma B1 | 2.06 | 0.21, 20.1 | 0.5 | |
| Thymoma B2 | 3.61 | 0.44, 29.5 | 0.2 | |
| Thymoma B3 | 3.27 | 0.3, 36.2 | 0.3 | |
| Thymic carcinoma | 48.4 | 4.71, 496 | 0.001 | |
| Other | 9.19 | 0.91, 92.8 | 0.06 | |
| Masaoka stage | 569 | |||
| I | – | – | ||
| IIa | 2.33 | 0.43, 12.7 | 0.3 | |
| IIb | 5.73 | 1.14, 28.8 | 0.034 | |
| III | 34.1 | 6.78, 171 | <0.001 | |
| IV | 36.6 | 6.10, 219 | <0.001 | |
| Tumour dimension | 593 | 1.01 | 1.00, 1.02 | 0.13 |
| Adjuvant therapy | 606 | 2.27 | 0.96, 5.35 | 0.061 |
Data are hazard ration (HR), 95% confidence interval (CI) and P-value.
DISCUSSION
Robotic thymectomy may be considered a relatively new surgical approach to the thymic gland. The first description of this technique dates back at the beginning of the century, when Yoshino described the use of a robotic system in the treatment of a small thymoma [25]. In the following years, attention was mainly directed to the field of MG treatment rather than the oncological field. Indeed, as for all minimally invasive techniques, there were many doubts over the application of robotic surgery in the treatment of TETs, mainly represented by the possible rupture of the capsule with implantation of the tumour during endoscopic manipulations and the supposed increased risk of local recurrence (due to reduced safety margins after minimally invasive resection) [2, 13]. Still in 2008, Davenport considered minimally invasive approaches to thymic tumours as experimental [26].
Despite these considerations, different experiences in the treatment of TETs started to be published [6–20], with encouraging results compared to open approaches [4, 7, 14]. However, these studies are generally characterized by being single-centre series with small number of patients and relatively short follow-up. To overcome these limitations, through our work, we intended to analyse the nation-wide experience of robotic thymectomy for TETs in Italy. Indeed, this is the largest cohort of patients up to date.
Interestingly, the study cohort represents 60% of all thymectomies performed in the participating centres during the study period, demonstrating how robotic thymectomy is widely performed for thymic tumours when the robotic system is available. Unfortunately, because of the retrospective design of the study and the difference in selection criteria and in expertise among the different centres, we were not able to depict a clear picture of current indications for this technique. Certainly, early-stage tumours or tumours incidentally found during thymectomy for other indications (i.e., for myasthenia gravis) represent the majority of our population.
Based on previous reports, there are some general radiological criteria that may guide in the selection of patients with suspicious thymic tumour to propose for minimally invasive thymectomy: the tumour location in the anterior mediastinum and its encapsulation, the presence between the tumour and vital organs of a fat plane, the absence of compression on the surrounding structures and the unilateral tumour predominance. Even dimension seems to play an important role in patients’ selection, with most studies in the literature dealing with tumours that are 5 cm or less [2]. Comparably, in our work, we found that the median diameter was 4 cm, but more experienced centres performed resection of tumours up to 16 cm. Indeed, as previously reported, tumour dimension is not an absolute contraindication for the robotic approach but may increase the need for open conversion.
While there is some debate over the best side to perform thymectomy in TETs, analysis of our data shows that the majority of surgeons prefer a left-side approach, using a contralateral approach only in case of right-side predominant lesions. Moreover, complete thymectomy accounts for 98% of resections, while limited resections are rarely performed. A recent work from the ESTS confirmed that complete thymectomy has an advantage in terms of recurrence compared to simple thymomectomy in stage I disease [27]. Additionally, MG was present in nearly a half of the patients, and this is an additional factor when considering the need for a complete removal of the thymic tissue. Notably, the rate of intentional lymphadenectomy performed is low. This could be partially in contrast with the ITMIG recommendations that suggest the removal of anterior mediastinal nodes in all cases and removal or at least sampling of the deeper levels in case of advanced disease of thymic carcinoma [28]. However, extended thymectomy includes the lymph nodes of the anterior region, but we were not able to define the extent and mapping of lymphadenectomy. From the surgical point of view, our study confirms that robotic thymectomy is a safe procedure, with no intraoperative deaths. This is particularly valuable considering that in 57 cases there was a need for resection of other structures, therefore may be considered complex cases. Moreover, the conversion rate is low (23 cases, 3.4%) and only in 3 (0.5%) cases this was related to an intraoperative emergency. This is comparable to previous works that report a conversion rate between 1% and 10% [6–20]. Even operative time, which accounts also for docking and undocking times, is in line with the previously reported ones [6–20]. These aspects have an influence also on the postoperative data, allowing an early removal of the chest drain and a reduced length of stay. This is particularly important when comparing robotic thymectomy and sternotomy, both in terms of patients’ outcomes and overall costs, as previously reported [4, 17, 18].
As for previous reports, the main indications for robotic thymectomy are early-stage TETs, but 4.1% of our cases were classified as advanced stage (Masaoka III–IV). Instead, none of the patients in our cohort was operated after a neoadjuvant treatment. This is probably related to the indications for an induction treatment, that generally accounts for advanced disease with extensive and diffuse pleural involvement, large lesions with extensive invasion of the surrounding structures, all conditions that would definitively contraindicate a minimally invasive approach of every sort.
Obviously, being a national database, our study accounts for both centres that have recently started their robotic program and more experienced ones that have the confidence and expertise to perform resection that otherwise would be performed through an open approach. In complex cases, dexterity of robotic instruments enhances dissection and allows for a safe resection of surrounding structures, with a higher degree of confidence for the surgeon. In our experience, we don’t report any case of vascular resections. This type of resections, although performed and described by means of a robotic approach [18], are the most challenging, particularly because of the narrow space between the sternum and the vascular plane not mentioning the possible catastrophic consequences of a lesion of a major vascular structure. Moreover, previous reports deal only with resection of the innominate veins that, probably, with an open approach would have been only partially resected or reconstructed.
Because of the relatively recent adoption of this technique in many centres, the oncological follow-up is not adequate for an indolent tumour such as thymoma, which accounts for most cases (90.2%). This is common with previous reports, that rarely report a follow-up longer than 2 years. Nevertheless, with a median follow-up time of 29 months and the longest duration of the follow-up of nearly 20 years, we describe only 21 recurrences (3.4%), mainly located in the pleural space, with the 5- and 10-year recurrence rates respectively of 7.4% and 8.3%. These data are consistent with previous reports and indeed represent a better evaluation of the effectiveness of the surgical procedure than overall survival [29].
Although outside the main end points of the study, we analysed the risk factors for tumour recurrence. As for previous studies, we confirmed that advanced stage disease, thymic carcinoma histology and incomplete resections to be strongly associated with an increased risk for disease recurrence [29].
Limitations
This study has several limitations, first of all because of the retrospective nature of the analysis. Second, the nationwide approach, while giving a complete view in terms of practice and approaches, gathers centres with large differences in terms of experience and indications. Also, the absence of a control group obviously makes it impossible to know how good or bad the results of the technique are in comparison with other approaches. However, there are several papers comparing robotic thymectomy with other approaches, and our data are in line with previous experiences. Finally, the length of follow-up, as said before, may be insufficient in allowing for a definitive conclusion on the oncological outcome.
However, we were able to gather the largest cohort of patients undergoing robotic thymectomy for TETs and describe a nation-wide picture of indications and results for this technique.
CONCLUSIONS
We conducted the largest retrospective, nation-wide analysis of robotic thymectomy for thymic tumours. Data confirm that robotic thymectomy is a safe procedure, with optimal perioperative surgical outcomes. From the oncological point of view, long-term oncological follow-up, while still non-sufficient for an indolent tumour as thymoma, nevertheless, shows a low rate of recurrence.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
FUNDING
No funding is declared.
Conflict of interest: Franca Melfi and Carmelina Cristina Zirafa are official proctors for Intuitive Surgical, Inc.; Edoardo Bottoni has received honoraria from AB Medica SpA. The other authors have no conflicts of interest to declare.
DATA AVAILABILITY
The data underlying this article cannot be shared publicly for the privacy of individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.
Author contributions
Giovanni Maria Comacchio: Conceptualization; Formal analysis; Methodology; Project administration; Validation; Visualization; Writing—original draft; Writing—review & editing. Marco Schiavon: Conceptualization; Formal analysis; Methodology; Project administration; Validation; Visualization; Writing—original draft; Writing—review & editing. Carmelina Cristina Zirafa: Data curation; Investigation; Writing—review & editing. Angela De Palma: Data curation; Investigation; Writing—review & editing. Roberto Scaramuzzi: Data curation; Investigation; Writing—review & editing. Elisa Meacci: Data curation; Investigation; Writing—review & editing. Stefano Bongiolatti: Data curation; Investigation; Writing—review & editing. Nicola Monaci: Data curation; Investigation; Writing—review & editing. Paraskevas Lyberis: Data curation; Investigation; Writing—review & editing. Pierluigi Novellis: Data curation; Investigation; Writing—review & editing. Jury Brandolini: Data curation; Investigation; Writing—review & editing. Sara Parini: Data curation; Investigation; Writing—review & editing. Sara Ricciardi: Data curation; Investigation; Writing—review & editing. Antonio D’Andrilli: Data curation; Investigation; Writing—review & editing. Edoardo Bottoni: Data curation; Investigation; Writing—review & editing. Filippo Tommaso Gallina: Data curation; Investigation; Writing—review & editing. Maria Carlotta Marino: Data curation; Investigation; Writing—review & editing. Giulia Lorenzoni: Data curation; Formal analysis; Software; Visualization; Writing—review & editing. Andrea Francavilla: Data curation; Formal analysis; Software; Visualization; Writing—review & editing. Erino Angelo Rendina: Resources; Supervision; Writing—review & editing. Giuseppe Cardillo: Resources; Validation; Writing—review & editing. Ottavio Rena: Resources; Supervision; Writing—review & editing. Piergiorgio Solli: Resources; Supervision; Writing—review & editing. Marco Alloisio: Resources; Supervision; Writing—review & editing. Luca Luzzi: Resources; Supervision; Writing—review & editing. Francesco Facciolo: Resources; Supervision; Writing—review & editing. Luca Voltolini: Supervision; Writing—review & editing. Stefano Margaritora: Resources; Supervision; Writing—review & editing. Carlo Curcio: Resources; Supervision; Writing—review & editing. Giuseppe Marulli: Resources; Supervision; Writing—review & editing. Enrico Ruffini: Resources; Supervision; Writing—review & editing. Giulia Veronesi: Resources; Supervision; Writing—review & editing. Franca Melfi: Resources; Supervision; Writing—review & editing. Federico Rea: Conceptualization; Supervision; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Toru Bando, Clemens Aigner and the other anonymous reviewer for their contribution to the peer review process of this article.
Presented at the 31st Annual Meeting of the European Society for Thoracic Surgeons, Milan, Italy, 4–6 June 2023.
REFERENCES
ABBREVIATIONS
- CI
Confidence interval
- HR
Hazard ration
- IQR
Interquartile range
- ITMIG
International Thymic Malignancy Interest Group
- MG
Myasthenia gravis
- TETs
Thymic epithelial tumours
Author notes
Giovanni Maria Comacchio and Marco Schiavon authors contributed equally to this work.





