-
PDF
- Split View
-
Views
-
Cite
Cite
Peter Henschke, Laura Chiara Guglielmetti, Sven Hillinger, Gian-Marco Monsch, Didier Schneiter, Isabelle Opitz, Olivia Lauk, Risk factors influencing postoperative pleural empyema in patients with pleural mesothelioma: a retrospective single-centre analysis, European Journal of Cardio-Thoracic Surgery, Volume 65, Issue 4, April 2024, ezae137, https://doi.org/10.1093/ejcts/ezae137
Close - Share Icon Share
Abstract
Postoperative empyema is a severe, potentially lethal complication also present, but poorly studied in patients undergoing surgery for pleural mesothelioma. We aimed to analyse which perioperative characteristics might be associated with an increased risk for postoperative empyema.
From September 1999 to February 2023 a retrospective analysis of consecutive patients undergoing surgery for pleural mesothelioma at the University Hospital of Zurich was performed. Uni- and multivariable logistic regression was used to identify associated risk factors of postoperative empyema after surgery.
A total of 400 PM patients were included in the analysis, of which n = 50 patients developed empyema after surgery (12.5%). Baseline demographics were comparable between patients with (Eyes) and without empyema (Eno). 39% (n = 156) patients underwent extrapleural pneumonectomy (EPP), of whom 22% (n = 35) developed postoperative pleural empyema; 6% (n = 15) of the remaining 244 patients undergoing pleurectomy and decortication (n = 46), extended pleurectomy and decortication (n = 114), partial pleurectomy (n = 54) or explorative thoracotomy (n = 30) resulted in postoperative empyema. In multivariable logistic regression analysis, EPP (odds ratio 2.8, 95% confidence interval 1.5–5.4, P = 0.002) emerged as the only risk factor associated with postoperative empyema when controlled for smoking status. Median overall survival was significantly worse for Eyes (16 months, interquartile range 5–27 months) than for Eno (18 months, interquartile range 8–35 months).
Patients undergoing EPP had a significantly higher risk of developing postoperative pleural empyema compared to patients undergoing other surgery types. Survival of patients with empyema was significantly shorter.
INTRODUCTION
Patients diagnosed with pleural mesothelioma (PM), that are in a curatively treatable tumour stage, are recommended to undergo a multimodal therapy concept. This includes induction chemotherapy followed by macroscopic complete resection according to the latest European Respiratory Society, European Society of Thoracic Surgeons, European Association of Cardio-Thoracic Surgery, European Society for Radiotherapy and Oncology guidelines for the management of PM [1]. Patients undergoing major thoracic surgery, including those with PM, the occurrence of pleural empyema can be a potential problem following surgery, as this complication significantly contributes to postoperative morbidity and mortality rates [2, 3]. Recent advancements in surgical techniques and preoperative optimized management approaches have positively impacted peri- and postoperative morbidity and mortality as well as overall survival (OS) and quality of life for these patients [3–6]. Nevertheless, overall morbidity and mortality in PM surgery remain considerable [7]. Additionally, the risk of postoperative empyema remains a noteworthy challenge in PM treatment [8].
Certain peri- and postoperative risk factors linked to the occurrence of postoperative empyema have been identified so far. Some studies indicate that postoperative empyema is more prevalent among male patients and those of advanced age [9, 10]. Nevertheless, our understanding of modifiable clinical and preoperative risk factors that could help to identify PM with an increased risk of developing postoperative empyema early in the course remains limited.
The objective of this study was to identify perioperative factors linked to a more frequent occurrence of postoperative pleural empyema in patients undergoing surgery for PM. In addition, demographic and clinical parameters were examined.
PATIENTS AND METHODS
Patients
Patients included in this analysis underwent surgical treatment following induction chemotherapy for PM at the Universitätsspital Zürich between September 1999 and February 2023.
Patients who did not fit these criteria of surgical resection have been excluded from analysis (Fig. 1).
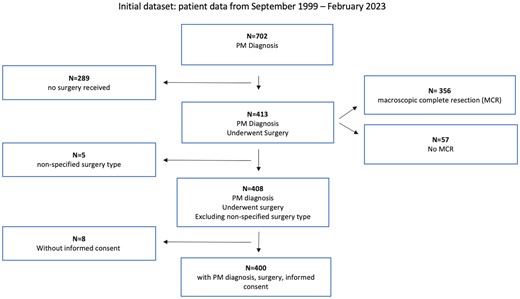
Flow chart detailing the subsequent exclusions leading to the enriched dataset used in the results section. The initial dataset ranging from September 1999 to February 2023 was taken from the institutional database. Excluding for the parameters of PM diagnosis, undergoing surgery, non-specific type of surgery and without informed consent, the dataset used for the subsequent analysis had N = 400 patients. MCR: macroscopic complete resection; PM: pleural mesothelioma.
Ethics statement
Local ethics committee approval was granted on 9 February 2021 under the BASEC-number 2020-02566, entailing the retrospective as well as prospective data collection.
Methods
In this retrospective data analysis, patients’ medical records were reviewed, and data extracted including baseline demographics, clinical characteristics and values as well as the therapy schemes and operation types. Detailed depictions of the baseline demographics can be found in the Supplementary Material.
The following clinical characteristics and values available before surgery have been investigated and analysed: weight loss; preoperative blood levels of C-reactive Protein (being a marker of general inflammation); the histological subtype of the tumour; and the clinical International Mesothelioma Interest Group classification.
Furthermore, the multimodality prognostic score was analysed. Multimodality prognostic score is a performance score routinely used at our institution since 2015 to stratify PM patients suitable for a multimodal therapy approach. The score ranges from 0 to 4, based on the following factors: tumour volume before chemotherapy, histological subtype, C-reactive protein before chemotherapy and tumour progression after chemotherapy. Each parameter is given 1 point if applicable. The cut-off that favours or unfavours surgery is set by 2 as described by our group in previous papers [11, 12]. Unfortunately, the score could only be included for, as it bases itself on clinical data that was, in part, not routinely collected before its implementation.
If information is missing, we included an ‘unknown’ category for subgroups, and report results accordingly. No outcome or covariate data was missing in our regression model.
The most common therapy schemes included induction chemotherapy; induction chemo- and immunotherapy by the addition of a vascular endothelial growth factor called bevacizumab according to the landmark trial by Zalcman et al. [5]; as well as no induction therapy at all.
Some patients additionally received various schemes of adjuvant chemotherapy, with pemetrexed, carboplatin and cisplatin being the most common substances that have been applied. Two patients received adjuvant combined chemo- and immunotherapy including bevacizumab.
Furthermore, some patients received adjuvant radiotherapy. This was done partly as part of a planned therapy scheme, partly for palliation. Reasons for a palliative adjuvant radiotherapy included either R1 resection, meaning microscopic tumour tissue left in situ after the operation could not be ruled out safely; as well as postoperative chronic pain at the resection site. Planned radiotherapy was conducted as part of a planned trimodal therapy scheme as part of the SAKK 17/04 study [13]. This was a study to investigate the routine use of haemithoracic radiotherapy for PM treatment after induction chemotherapy and extrapleural pneumonectomy (EPP). It was conducted from 2005 to 2017 as a multicentre phase 2 study with pathologically conformed PM patients eligible for surgery.
The most common surgery types included EPP (meaning the removal of both pleural layers (namely visceralis and parietalis) as well as the whole lung and in some cases parts of the pericardium and the diaphragm), pleurectomy and decortication (P/D, meaning the removal of both pleural layers as well as all of the tumour tissue, sparing the uninfiltrated lung tissue, without the resection of the pericardium and/or the diaphragm) and extended pleurectomy and decortication (EP/D, sparing the uninfiltrated lung tissue while removing the pleura and tumour tissue as well as parts of the pericardium and/or the diaphragm). A few cases have been treated with either partial pleurectomy, where only parts of the parietal pleura have been removed, or explorative thoracotomy of only explorative nature and closing without resection of any parts. However, the patients receiving partial pleurectomy or explorative thoracotomy have been excluded from the regression analysis, as mentioned below in the ‘Statistical analysis’ section (see Table 1).
Univariable and multivariable logistic regression analyses of risk factors associated with postoperative empyema
| . | Univariable . | Multivariable . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | OR . | Lower CI . | Upper CI . | P-value . | OR . | Lower CI . | Upper CI . | P-value . |
| EPP | 2.796 | 1.458 | 5.363 | 0.002 | 2.260 | 1.130 | 4.521 | 0.021 |
| Current smoker | 2.111 | 0.880 | 5.061 | 0.094 | 2.299 | 0.931 | 5.678 | 0.071 |
| Diaphragm replacement | 2.597 | 0.893 | 7.553 | 0.080 | 2.171 | 0.597 | 7.900 | 0.240 |
| cT | 1.356 | 0.719 | 2.558 | 0.347 | ||||
| cN | 1.198 | 0.631 | 2.274 | 0.581 | ||||
| Female Gender | 0.636 | 0.215 | 1.885 | 0.414 | ||||
| Blood loss (dl) | 1.007 | 0.984 | 1.031 | 0.559 | ||||
| Sarcomatoid | 1.929 | 0.666 | 5.588 | 0.226 | ||||
| Induction therapy | 1.784 | 0.670 | 4.748 | 0.247 | ||||
| . | Univariable . | Multivariable . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | OR . | Lower CI . | Upper CI . | P-value . | OR . | Lower CI . | Upper CI . | P-value . |
| EPP | 2.796 | 1.458 | 5.363 | 0.002 | 2.260 | 1.130 | 4.521 | 0.021 |
| Current smoker | 2.111 | 0.880 | 5.061 | 0.094 | 2.299 | 0.931 | 5.678 | 0.071 |
| Diaphragm replacement | 2.597 | 0.893 | 7.553 | 0.080 | 2.171 | 0.597 | 7.900 | 0.240 |
| cT | 1.356 | 0.719 | 2.558 | 0.347 | ||||
| cN | 1.198 | 0.631 | 2.274 | 0.581 | ||||
| Female Gender | 0.636 | 0.215 | 1.885 | 0.414 | ||||
| Blood loss (dl) | 1.007 | 0.984 | 1.031 | 0.559 | ||||
| Sarcomatoid | 1.929 | 0.666 | 5.588 | 0.226 | ||||
| Induction therapy | 1.784 | 0.670 | 4.748 | 0.247 | ||||
Hosmer–Lemeshow X2 1.801, P = 0.615, area under the curve 0.64, 95% CI: 0.55–0.72. Sarcomatoid = sarcomatoid histology type compared to any other type.
CI: 95% confidence interval; cN: nodal staging of the TNM classification, dichotomized in cN positive versus negative; cT: tumour staging of the TNM classification of malignant tumours, dichotomized into cT0-2 versus cT3-4; EPP: extrapleural pneumonectomy; OR: odds ratio.
Univariable and multivariable logistic regression analyses of risk factors associated with postoperative empyema
| . | Univariable . | Multivariable . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | OR . | Lower CI . | Upper CI . | P-value . | OR . | Lower CI . | Upper CI . | P-value . |
| EPP | 2.796 | 1.458 | 5.363 | 0.002 | 2.260 | 1.130 | 4.521 | 0.021 |
| Current smoker | 2.111 | 0.880 | 5.061 | 0.094 | 2.299 | 0.931 | 5.678 | 0.071 |
| Diaphragm replacement | 2.597 | 0.893 | 7.553 | 0.080 | 2.171 | 0.597 | 7.900 | 0.240 |
| cT | 1.356 | 0.719 | 2.558 | 0.347 | ||||
| cN | 1.198 | 0.631 | 2.274 | 0.581 | ||||
| Female Gender | 0.636 | 0.215 | 1.885 | 0.414 | ||||
| Blood loss (dl) | 1.007 | 0.984 | 1.031 | 0.559 | ||||
| Sarcomatoid | 1.929 | 0.666 | 5.588 | 0.226 | ||||
| Induction therapy | 1.784 | 0.670 | 4.748 | 0.247 | ||||
| . | Univariable . | Multivariable . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | OR . | Lower CI . | Upper CI . | P-value . | OR . | Lower CI . | Upper CI . | P-value . |
| EPP | 2.796 | 1.458 | 5.363 | 0.002 | 2.260 | 1.130 | 4.521 | 0.021 |
| Current smoker | 2.111 | 0.880 | 5.061 | 0.094 | 2.299 | 0.931 | 5.678 | 0.071 |
| Diaphragm replacement | 2.597 | 0.893 | 7.553 | 0.080 | 2.171 | 0.597 | 7.900 | 0.240 |
| cT | 1.356 | 0.719 | 2.558 | 0.347 | ||||
| cN | 1.198 | 0.631 | 2.274 | 0.581 | ||||
| Female Gender | 0.636 | 0.215 | 1.885 | 0.414 | ||||
| Blood loss (dl) | 1.007 | 0.984 | 1.031 | 0.559 | ||||
| Sarcomatoid | 1.929 | 0.666 | 5.588 | 0.226 | ||||
| Induction therapy | 1.784 | 0.670 | 4.748 | 0.247 | ||||
Hosmer–Lemeshow X2 1.801, P = 0.615, area under the curve 0.64, 95% CI: 0.55–0.72. Sarcomatoid = sarcomatoid histology type compared to any other type.
CI: 95% confidence interval; cN: nodal staging of the TNM classification, dichotomized in cN positive versus negative; cT: tumour staging of the TNM classification of malignant tumours, dichotomized into cT0-2 versus cT3-4; EPP: extrapleural pneumonectomy; OR: odds ratio.
For all the aforementioned surgery types a lateral thoracotomy in the sixth intercostal space was performed. The parietal pleura was mobilized as a whole towards the lung’s hilum and apex, as well as dorsolaterally towards the diaphragm. The visceral pleura was removed from all lobes including the interlobar fissures. For extended P/D, the pericardium and/or the diaphragm were resected depending on extent of tumour infiltration, assessed by intraoperative frozen sections, and based on the surgeon’s choice (2 experienced surgeons). Reconstruction was performed using a porcine pericardial patch (Supple PeriGuard VR, Pericardium with Apex Processing VR; Synovis Surgical Innovations, St Paul, Minnesota, USA) and Gore-Tex patch (Dualmesh VR Biomaterial; W.L. Gore & Associates, Inc., Flagstaff, Arizona, USA). Chest wall resection was performed if tumour infiltration was confirmed by frozen section (EPP: n = 3; P/D: n = 1) and reconstruction was done using a Gore-Tex Patch if necessary.
In case of postoperative empyema, patients underwent the installation of intrathoracic vacuum-assisted closure dressings, repeatedly changed until the chest cavity presented clean without any signs of an ongoing infection, macroscopically and proven by intraoperative microbiological negative samples.
Statistical analysis
Descriptive statistics were used to summarize patients’ characteristics. Continuous variables were reported as mean and standard deviation or median and interquartile range (IQR). Categorical variables were summarized as frequencies (%) and compared using Pearson’s chi-squared test or Fisher’s Exact test if applicable.
Risk factors associated with postoperative empyema were determined using logistic regression analyses. Patients undergoing either partial pleurectomy or explorative thoracotomy were excluded from this analysis. Results are reported as odds ratio with a corresponding 95% confidence interval. Goodness of fit was tested using the Hosmer–Lemeshow test. The area under the curve is reported to bestow more informative value on the model. All variables retained in the multivariable model had a weak correlation (Spearman correlation coefficient ρ < 0.39, as suggested by Evans [14]).
Additionally, Kaplan–Meier curves were used to compare median survival times (Fig. 2). Follow-up was calculated from the date of operation until the last follow-up or death, using the reverse Kaplan–Meier method. For survival analyses, we performed an additional subgroup analysis excluding patients undergoing partial pleurectomy or explorative thoracotomy (Fig. 3).
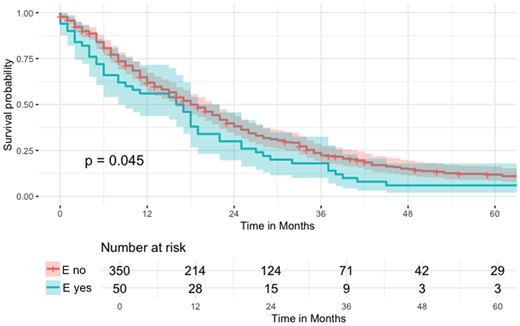
Kaplan–Meier curve regarding overall survival (OS) of the n = 400 patients undergoing PM (pleural mesothelioma) surgery (logrank P = 0.045).
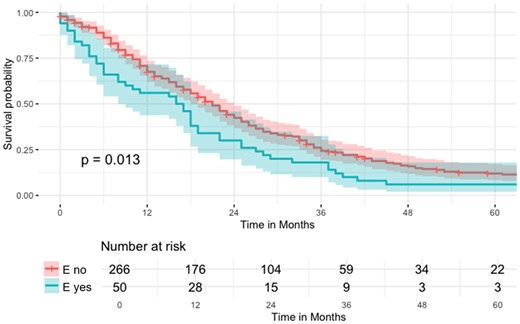
Kaplan–Meier curve regarding OS of the n = 316 patients not receiving partial pleurectomy or explorative thoracotomy (logrank P = 0.013).
SPSS version 28 (IBM Corp., Armonk, New York State, USA) and R Studio version 1.2.1335 (RStudio, Inc., Boston, Massachusetts, USA) were used for data analysis. P-values <0.05 are considered statistically significant.
RESULTS
A total of 400 patients undergoing surgical treatment for PM were analysed. 39% of patients (n = 156) underwent EPP, 40% of patients (n = 160) underwent P/D or EP/D, 13.5% of patients (n = 54) underwent only partial pleurectomy. Finally, the remaining 7.5% of patients (n = 30) underwent explorative thoracotomy. In 12.5% of all patients (n = 50), pleural empyema was reported with 30% (n = 15) as an ‘early empyema’ within the first 30 days and 70% (n = 35) as a ‘late empyema’ >30 days after surgery.
Of the n = 156 patients undergoing EPP, 9% (n = 14) developed a bronchopleural fistula postoperatively. Of all patients with postoperative empyema (n = 50), 26% (n = 13) developed a pleural empyema following a bronchopleural fistula.
Baseline demographics
Baseline demographics were comparable between patients with (Eyes) and without postoperative empyema (Eno). The overall median age at the time of surgery was slightly lower for Eyes patients [61 years (IQR = 9) vs 63 years (IQR of 9) for Eno patients].
Therapy
Eno patients received EPP in 35% (n = 121), compared to 70% (n = 35) of Eyes patients. P/D or EP/D was received by 41% (n = 145) of Eno patients, compared to only 30% (n = 15) of Eyes patients. While no Eyes patients received partial pleurectomy, 15% (n = 54) of Eno patients underwent this type of surgery. Finally, 9% (n = 30) of Eno patients received explorative thoracotomy, with again no Eyes patients undergoing this treatment (see Table 1).
None of the Eyes patients received any adjuvant chemotherapy before developing the empyema. However, 11% (n = 4) of late empyema patients developed a late empyema (>30 days postoperatively) immediately after receiving adjuvant radiotherapy (see Table 2). Of these n = 4 patients, n = 3 received planned adjuvant radiotherapy as part of the aforementioned SAKK 17/04 study [13]. The fourth patient received radiation due to R1 resection.
Adjuvant treatments for (n = 50) empyema patients, received between resection and developing empyema
| . | Eyes overall, 50 (100%) . | Early empyema, 15 (100%) . | Late empyema, 35 (100%) . |
|---|---|---|---|
| Adjuvant chemotherapy before empyema | 0 | 0 | 0 |
| Adjuvant radiotherapy before empyema | 4 (8%) | 0 | 4 (11%) |
| No adjuvant therapy before empyema | 46 (92%) | 15 (100%) | 31 (89%) |
| . | Eyes overall, 50 (100%) . | Early empyema, 15 (100%) . | Late empyema, 35 (100%) . |
|---|---|---|---|
| Adjuvant chemotherapy before empyema | 0 | 0 | 0 |
| Adjuvant radiotherapy before empyema | 4 (8%) | 0 | 4 (11%) |
| No adjuvant therapy before empyema | 46 (92%) | 15 (100%) | 31 (89%) |
None of the patients with early empyema received any adjuvant treatments before developing empyema. Of the patients with late empyema, n = 4 received adjuvant radiotherapy before developing empyema, with none receiving adjuvant chemotherapy before developing empyema.
Adjuvant treatments for (n = 50) empyema patients, received between resection and developing empyema
| . | Eyes overall, 50 (100%) . | Early empyema, 15 (100%) . | Late empyema, 35 (100%) . |
|---|---|---|---|
| Adjuvant chemotherapy before empyema | 0 | 0 | 0 |
| Adjuvant radiotherapy before empyema | 4 (8%) | 0 | 4 (11%) |
| No adjuvant therapy before empyema | 46 (92%) | 15 (100%) | 31 (89%) |
| . | Eyes overall, 50 (100%) . | Early empyema, 15 (100%) . | Late empyema, 35 (100%) . |
|---|---|---|---|
| Adjuvant chemotherapy before empyema | 0 | 0 | 0 |
| Adjuvant radiotherapy before empyema | 4 (8%) | 0 | 4 (11%) |
| No adjuvant therapy before empyema | 46 (92%) | 15 (100%) | 31 (89%) |
None of the patients with early empyema received any adjuvant treatments before developing empyema. Of the patients with late empyema, n = 4 received adjuvant radiotherapy before developing empyema, with none receiving adjuvant chemotherapy before developing empyema.
A first patient was treated according to SAKK 17/04 protocol [13], receiving radiotherapy of the high-risk area in the right dorsal costodiaphragmatic recess, with the therapy being suspended after a dose of 32 Gy in 16 fractions due to a pleural empyema in the right dorsolateral thoracic cavity. A second patient being treated according to the SAKK 17/04 protocol [13] received radiotherapy of the high-risk area in the right ventral and lateral costodiaphragmatic recess, with the therapy being suspended after a dose of 42 Gy in 21 fractions due to the pleural empyema in the thoracic cavity of the same side. The third and final patient being treated as part of the SAKK 17/04 study [13] received RapidArc radiotherapy in the right pleural cavity with a dose of 55.9 Gy in 26 fractions. Four days after the completed radiotherapy protocol, this patient was diagnosed with a pleural empyema on the same side. Finally, 1 patient received radiotherapy of the left thoracic side, due to R1 resection. The therapy had to be suspended after a dose of 18 Gy in 6 fractions due to the pleural empyema on the same side.
The question of a possible connection between adjuvant radiotherapy and a subsequent development of pleural empyema was not analysed statistically due to the low sample size and therefore remains a mere observation.
Risk factors associated with postoperative empyema: logistic regression analysis
Risk factors associated with postoperative empyema were determined using logistic regression analyses. As mentioned in the ‘Patients and methods’ section, patients undergoing partial pleurectomy or explorative thoracotomy were excluded from regression analyses.
A total of 9 variables were tested in univariable models. The variables included for univariable analysis were a priori determined after literature review and based on clinical parameters.
All variables with a P-value of ≤ 0.15 were retained in the multivariable regression model.
When controlled for smoking status, EPP (odds ratio 2.8, 95% confidence interval 1.5–5.4) emerged as only statistically significant risk factor associated with postoperative empyema.
Survival
OS was 18 months (IQR 8–34 months), and it was significantly worse for patients with postoperative empyema with a median survival of 16 months (IQR 5–27 months) compared to 18 months (IQR 8–35 months) for patients without postoperative empyema (also see Fig. 2: survival function of patients with and without postoperative empyema compared with the logrank test). The median follow-up time was 112 months (IQR 59–159 months).
Subgroup analysis
Similarly to the cohort used for the investigation of potential risk factors associated with postoperative empyema in the regression model, a subgroup analysis was performed excluding patients undergoing partial pleurectomy or explorative thoracotomy (see Fig. 3). The findings were comparable to the analysis of the complete cohort: patients with postoperative empyema had a statistically significantly worse survival compared to those without postoperative empyema (logrank P = 0.013).
DISCUSSION
With this retrospective single-centre analysis, to our knowledge one of the largest surgical series addressing the question, we aimed to identify potential risk factors associated with the occurrence of postoperative empyema. The surgical approach showed a statistically significant difference with a higher percentage of patients undergoing EPP compared to EP/D or P/D. EPP is known for a high postoperative morbidity and mortality rate in comparison to other types of surgery, which is the main reason for it to become more and more abundant as a standard of care over the last decade, as reflected in Fig. 4 [4, 15]. This is reflected in the fact that the type of operation was associated significantly with the occurrence of pleural empyema. It should be noted, that in our institution, covering of the bronchial stump was usually performed only after the occurrence of a bronchopleural fistula. This might have some influence on this procedure’s aforementioned morbidity rate.
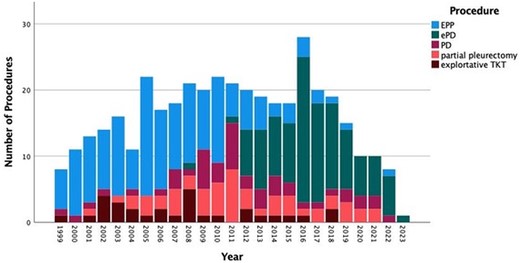
Histogram depicting the number of combined procedures for pleural mesotheliomas in each year of the study period.
Another notable concern emerged regarding some of the late empyema patients receiving adjuvant radiotherapy prior to developing pleural empyema. Only a very small number of patients (n = 4) developed pleural empyema shortly after receiving adjuvant radiotherapy, compared to a larger number of patients (n = 88) receiving adjuvant radiotherapy without developing postoperative pleural empyema. Nevertheless, we postulate a possible connection between tissue damage through adjuvant radiotherapy and the late onset of pleural empyema, that might be worth further investigation.
Our group, Kostron et al. [15], published a paper in 2017, that assessed perioperative outcomes and OS of n = 78 patients receiving the major types of operations, comparing EPP with P/D and EP/D. In this cohort, 30% of patients receiving EPP (n = 16) developed pleural empyema postoperatively, compared to only 4% of patients receiving P/D or EP/D. Overall major morbidity factors such as prolonged air leak, reoperation, chylothorax, as well as postoperative pleural empyema, were found to have a statistically higher occurrence of pleural empyema in patients receiving EPP than in patients receiving P/D or EP/D.
A recently published paper by Bueno et al. [9] investigated the incidence, risk factors and prognosis of postoperative empyema for n = 354 patients undergoing P/D for PM, with 6.8% (n = 24) of these patients suffering postoperative empyema. They could show that the occurrence of postoperative empyema in patients receiving this type of surgery is associated with male sex, prolonged air leak and the use of prosthetic mesh (ie Gore-Tex). These factors might present as further risk factors, additionally to the type of operation. Based on the fact that the use of prosthetic mesh showed significant differences, they proposed the possibility of a lower risk of infection and consequently postoperative empyema by the use of meshes from absorbable materials (Vicryl, Ethicon, Dexon) as opposed to Gore-Tex.
Additionally, in a large publication of 4772 patients undergoing surgery for lung cancer, Matsutani et al. [2] studied the occurrence of postoperative empyema following aforementioned surgery in 2018. From this large cohort, only 0.9% of patients (n = 43) developed postoperative empyema. 32.6% (n = 14) of the empyema cases in their cohort occurred following a case of bronchopleural fistula after EPP. These results are comparable to our data, where 26% (n = 13) of pleural empyema cases occurred following a bronchopleural fistula after EPP. Additionally, Matsutani et al. described the occurrence of pleural empyema to be associated with male gender, in addition to lung cancer-specific risk factors (such as squamous cell lung carcinoma compared to other types of lung cancer). Furthermore, they discovered the occurrence of postoperative empyema to be significantly more frequent in patients receiving wider resections in particular pneumonectomy, as well as bilobectomy. This might suggest the hypothesis, that the occurrence of postoperative empyema gets less likely, the less lung tissue is removed, and vice versa. Our data also suggest this possibility.
In 2006, Brunelli et al. [16] compared 85 patients with prolonged air leak (of 7 days or more) after pulmonary lobectomy with 85 patients with no air leak at all after pulmonary lobectomy. They discovered patients with prolonged air leak to be showing significantly higher rates of postoperative empyema (8.2% or n = 7) compared to patients with a no air leak at all (n = 0). However, while EPP usually shows greater overall postoperative morbidity than P/D or EP/D, specifically prolonged air leak is absent as a cause of postoperative morbidity after EPP [15, 17].
Furthermore, we divided the empyema in early (≤30 days) and late (>30 days) with the majority of late empyema cases (70%, compared to 30% of early empyema cases). 98% of these patients had to undergo any kind of reoperation as a therapy approach for postoperative empyema. Besides this high rate of reoperation our morbidity and mortality rates stayed very low with 4% and 7.5%, respectively. This might be due to our institutional accelerated treatment approach, which has been previously described by our group, as well as others [18, 19].
The OS for Eyes patients was statistically significantly impacted, which goes along with findings by other current literature on the topic [9, 20]. Whether the postoperative empyema was the reason (or 1 contributing factor) for the earlier deaths or the result of preceding factors who are in fact responsible for the decline in survival, could not be determined with our study setting. In most cases, the empyema led to repeated reoperations, therefore presumably having an impact on a prolonged recovery time.
Limitations and strengths
This retrospective analysis ranged over a time span of >2 decades. Over this vast time span, many aspects of the clinical management changed significantly, including diagnosis and treatment. Nevertheless, this diversity is represented in both Eyes and Eno patients. However, the inclusion of diverse patients was deemed necessary to ensure a sufficient sample size. Despite the aforementioned inhomogeneity, baseline demographics were comparable between Eyes and Eno patients. Therapy schemes showed a similar distribution among both Eyes and Eno patients.
Caution is to be given when it comes to interpretation and comparison of these data due to the reduced sample size of 50 empyema cases. Nevertheless, the numbers are representative especially looking at the literature for this rare and devastating disease of PM [6, 9, 15].
Furthermore, due to the retrospective nature of this study, the data allowed no time-dependent analysis. Further prospective studies are needed, including time-dependent analyses of postoperative empyema in PM patients.
CONCLUSION
In conclusion, our findings further support the current standard of care, which overall favours surgical therapy by P/D or EP/D over EPP [4, 21], which over the past years has become a rare procedure for PM surgery in most specialized centres. Furthermore, our data suggest a potential context between adjuvant radiotherapy and postoperative pleural empyema.
Postoperative pleural empyema remains a significant risk for PM patients undergoing surgery, including those not receiving EPP, with an impact on their quality of life, although not separately evaluated by quality of life-specific questionnaires. Hence, we propose that further research is necessary to allow a better risk stratification for patients that have a higher risk of developing postoperative pleural empyema.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
ACKNOWLEDGEMENTS
We would like to acknowledge the support of Alessandra Matter of the Department of Thoracic Surgery of the University Hospital Zurich, who contributed to generating and organizing the raw data.
FUNDING
The project received no funding from any institution.
Conflict of interest: No conflicts of interest present for any of the authors.
DATA AVAILABILITY
The data underlying this article were accessed from the University Hospital of Zurich. The derived data generated in this research will be shared on reasonable request to the corresponding Author.
Author contributions
Peter Henschke: Data curation; Formal analysis; Methodology; Visualization; Writing—original draft; Writing—review & editing. Laura Chiara Guglielmetti: Data curation; Formal analysis; Statistical Analysis; Methodology; Visualization; Writing—original draft; Writing—review & editing. Sven Hillinger: Writing—review & editing. Gian-Marco Monsch: Writing—review & editing. Didier Schneiter: Writing—review & editing. Isabelle Opitz: Methodology; Supervision; Writing—review & editing. Olivia Lauk: Conceptualization; Data curation; Methodology; Project administration; Supervision; Visualization; Writing—original draft; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Francoise Le Pimpec-Barthes, Mohamed Rahouma and the other anonymous reviewers for their contribution to the peer review process of this article.
Presented at the ESTS Annual Meeting 2023, Milan, Italy, 6th of June, 2023.
Presented at the IMIG 2023, Lille, France, 27th of June, 2023.
Presented at the Swiss College of Surgeon Annual Meeting 2023, Basel, Switzerland, 8th of June, 2023.
REFERENCES
ABBREVIATIONS
- Eyes
Patients with postoperative Empyema
- Eno
Patients without postoperative Empyema
- EP/D
Extended pleurectomy and decortication
- EPP
Extrapleural pneumonectomy
- IQR
Interquartile range
- OS
Overall survival
- P/D
Pleurectomy and decortication
- PM
Pleural mesothelioma
Author notes
Peter Henschke and Laura Chiara Guglielmetti authors contributed equally to this work.





