-
PDF
- Split View
-
Views
-
Cite
Cite
Christelle M Vandervelde, Stephanie Everaerts, Walter Weder, Siebe Orolé, Pieter-Jan Hermans, Paul De Leyn, Philippe Nafteux, Herbert Decaluwé, Hans Van Veer, Lieven Depypere, Steve Coppens, Arne P Neyrinck, Sofian Bouneb, Johan De Coster, Johan Coolen, Christophe Dooms, Dirk E Van Raemdonck, Wim Janssens, Laurens J Ceulemans, Implementation of an enhanced recovery protocol for lung volume reduction surgery: an observational cohort study, European Journal of Cardio-Thoracic Surgery, Volume 65, Issue 4, April 2024, ezae109, https://doi.org/10.1093/ejcts/ezae109
Close - Share Icon Share
Abstract
Lung volume reduction surgery (LVRS) is an established therapeutic option for advanced emphysema. To improve patients’ safety and reduce complications, an enhanced recovery protocol (ERP) was implemented. This study aims to describe and evaluate the short-term outcome of this ERP.
This retrospective single-centre study included all consecutive LVRS patients (1 January 2017 until 15 September 2020). An ERP for LVRS was implemented and stepwise optimised from 1 August 2019, it consisted of changes in pre-, peri- and postoperative care pathways. Patients were compared before and after implementation of ERP. Primary outcome was incidence of postoperative complications (Clavien-Dindo), and secondary outcomes included chest tube duration, incidence of prolonged air leak (PAL), length of stay (LOS) and 90-day mortality. Lung function and exercise capacity were evaluated at 3 and 6 months post-LVRS.
Seventy-six LVRS patients were included (pre-ERP: n=41, ERP: n=35). The ERP cohort presented with lower incidence of postoperative complications (42% vs 83%, P=0.0002), shorter chest tube duration (4 vs 12 days, P<0.0001) with a lower incidence of PAL (21% vs 61%, P=0.0005) and shorter LOS (6 vs 14 days, P<0.0001). No in-hospital mortality occurred in the ERP cohort versus 4 pre-ERP. Postoperative forced expiratory volume in 1 s was higher in the ERP cohort compared to pre-ERP at 3 months (1.35 vs 1.02 l) and at 6 months (1.31 vs 1.01 l).
Implementation of ERP as part of a comprehensive reconceptualisation towards LVRS, demonstrated fewer postoperative complications, including PAL, resulting in reduced LOS. Improved short-term functional outcomes were observed at 3 and 6 months.
INTRODUCTION
Chronic obstructive pulmonary disease is a highly prevalent disease and is the third leading cause of death worldwide [1]. Lung volume reduction surgery (LVRS) reduces hyperinflation of advanced emphysema by targeting the excess residual volume (RV) [2]. After careful patient selection, several studies demonstrated improvement of dyspnoea, lung function, exercise tolerance, quality-of-life and survival compared to medical therapy [3–9].
Enhanced recovery protocols (ERP) are multidisciplinary pre-, peri- and postoperative care pathways designed to achieve early recovery after surgery by preserving preoperative organ function and lowering the stress response [10]. This concept, introduced in 1997 by Henrik Kehlet [11] was originally implemented in colorectal surgery where it resulted in fewer postoperative complications and reduced length of stay (LOS) [12–14]. Over the years, numerous surgical specialties demonstrated the benefits of ERP [15]. In thoracic surgery, reduced pulmonary and cardiac complications, reduced LOS and decreased hospital costs have been attributed to ERP [16, 17], resulting in specific ERP guidelines [18, 19]. However, to our knowledge, no studies reported outcomes after ERP for LVRS. We introduced an ERP in our centre for patients undergoing LVRS with the objective to standardise the surgical approach and patient care and thereby improve postoperative recovery. The aim of this study is to describe the implementation of an ERP for LVRS and the impact on short-term outcome as compared to the historical non-ERP.
PATIENTS AND METHODS
Ethical statement
The study was approved by the research ethics committee UZ/KU Leuven on 17 March 2020 (MP012568). Because of the retrospective nature of the study, the need for individual patient consent was waived.
Study population and design
This single-centre retrospective study of an intention-to-treat cohort was performed at University Hospitals Leuven (Belgium) and included all patients who underwent LVRS between 1 January 2017 and 15 September 2020 (n = 93). Patients operated during the transition period (1 July to 31 July 2019) (n = 5) were excluded, to ensure that basic elements of the ERP (e.g. change of surgeon, avoiding intensive care, extubation on the operating table, surgical material) were implemented. From 1 August 2019, all patients were managed within ERP, and procedures were performed by the same surgeon (Laurens J. Ceulemans). During a preceding 6-month fellowship at the University Hospital Zürich, Switzerland, a world-leading expert centre regarding LVRS, he assisted 25 cases (under mentorship of W. Weder). Procedures during the pre-ERP were performed by Dirk Van Raemdonck. Bullectomy (n = 10) and urgent LVRS procedures (e.g. secondary pneumothorax), not followed by contralateral elective LVRS were excluded (n = 2). Eventually, 76 consecutive LVRS patients were included for further analysis [pre-ERP: n = 41 (Dirk E. Van Raemdonck), ERP: n = 35 (Laurens J. Ceulemans)] (Fig. 1).
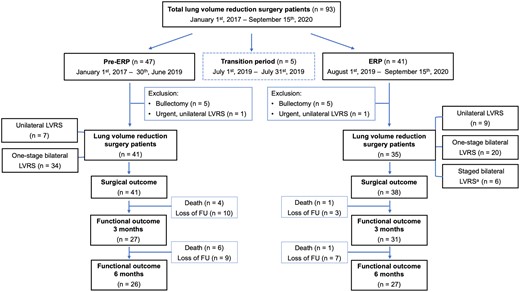
Flowchart diagram of the study cohort. Patients who underwent LVRS from 1 January 2017 to 15 September 2020. Five patients were excluded during the transition period. Analysis was performed on patients in the pre- (n = 41) and ERP era (n = 35) after exclusion of bullectomies and urgent unilateral LVRS. ERP: enhanced recovery protocol; LVRS: lung volume reduction surgery.
Baseline, postoperative and follow-up data were collected from electronic patient files. The morphological classification system, based on chest computed tomography was used to define the type of emphysema [20]. Preoperative baseline data of each patient included age, sex, oral steroids, oxygen, pulmonary function, exercise capacity, body mass index and emphysema distribution. Postoperative data were collected from routine follow-up records.
Enhanced recovery protocol
The LVRS-ERP is built upon our own experience with ERP for oesophagectomy and lobectomy, complemented by insights gained from W. Weder, is consistent with previously reported ERP guidelines in thoracic surgery [18, 19] and was optimised over time by in-clinic observations. This comprehensive reconceptualisation of LVRS on pre- and postoperative care pathways and adjustments of the surgical procedure are included in a multimodality approach (Table 1).
Summary of care elements before and after implementation of an enhanced recovery protocol for lung volume reduction surgery patients
| . | Pre-ERP . | ERP . |
|---|---|---|
| Preoperative | ||
| Investigations | Non-complete testing at peripheral centres | Broad and centralized testing at University Hospitals Leuven |
| Radiological imaging | Sometimes only visual assessment of CT data | Always including emphysema quantification (StratX-Pulmon©) |
| Referral | Ad hoc multidisciplinary discussion | Systematic multidisciplinary assessment |
| Education | Non-standardised education provided by surgeon’s office | Optimal education by surgeon, social worker, dietitian, physiotherapist and program coordinator |
| Intra-operative | ||
| Anaesthesia |
|
|
| Positioning | Dorsal decubitus | Lateral decubitus with mid-term conversion to contralateral decubitus |
| Approach | Anterior two-port VATS | One-, two-, three-, or four-port VATS |
| Incision |
|
|
| Material | Echelon Flex™ Endopath® Staplers (non-buttressed), beige and black cartridge | Signia™ Stapling System (buttressed), purple cartridge |
| Parenchyma stapling | More centrally, emphysema directed approach | Volume-based lung reshaping, more peripheral approach with respect to the lungs original shape |
| Drainage | Bilateral double chest tube | Bilateral, single or double chest tube |
| Postoperative | ||
| Recovery | Transfer to ICU for at least 1 night |
|
| Analgesia | Standardised analgesic protocol with the standard use of PCEA until POD3 |
|
| Prophylactic antibiotics | 5 days amoxycillin/clavulanic acid | |
| Urinary catheter | Non-standardised management | Removal POD1 |
| Fluids and intake |
|
|
| Physiotherapy and ambulation | Respiratory physiotherapy starting on POD1 |
|
| Rehabilitation | 30-Session pulmonary rehabilitation | 60-Session pulmonary rehabilitation: 3 weeks at home, 2–3 months ambulant pulmonary rehabilitation centre |
| . | Pre-ERP . | ERP . |
|---|---|---|
| Preoperative | ||
| Investigations | Non-complete testing at peripheral centres | Broad and centralized testing at University Hospitals Leuven |
| Radiological imaging | Sometimes only visual assessment of CT data | Always including emphysema quantification (StratX-Pulmon©) |
| Referral | Ad hoc multidisciplinary discussion | Systematic multidisciplinary assessment |
| Education | Non-standardised education provided by surgeon’s office | Optimal education by surgeon, social worker, dietitian, physiotherapist and program coordinator |
| Intra-operative | ||
| Anaesthesia |
|
|
| Positioning | Dorsal decubitus | Lateral decubitus with mid-term conversion to contralateral decubitus |
| Approach | Anterior two-port VATS | One-, two-, three-, or four-port VATS |
| Incision |
|
|
| Material | Echelon Flex™ Endopath® Staplers (non-buttressed), beige and black cartridge | Signia™ Stapling System (buttressed), purple cartridge |
| Parenchyma stapling | More centrally, emphysema directed approach | Volume-based lung reshaping, more peripheral approach with respect to the lungs original shape |
| Drainage | Bilateral double chest tube | Bilateral, single or double chest tube |
| Postoperative | ||
| Recovery | Transfer to ICU for at least 1 night |
|
| Analgesia | Standardised analgesic protocol with the standard use of PCEA until POD3 |
|
| Prophylactic antibiotics | 5 days amoxycillin/clavulanic acid | |
| Urinary catheter | Non-standardised management | Removal POD1 |
| Fluids and intake |
|
|
| Physiotherapy and ambulation | Respiratory physiotherapy starting on POD1 |
|
| Rehabilitation | 30-Session pulmonary rehabilitation | 60-Session pulmonary rehabilitation: 3 weeks at home, 2–3 months ambulant pulmonary rehabilitation centre |
CT: computed tomography; CVC: central venous catheter; ERP: enhanced recovery protocol; ICU: intensive care unit; PACU: postanaesthesia care unit; PCEA: patient-controlled epidural anaesthesia; POD: postoperative day; TIVA: total intravenous anaesthesia; VATS: video-assisted thoracoscopic surgery.
Summary of care elements before and after implementation of an enhanced recovery protocol for lung volume reduction surgery patients
| . | Pre-ERP . | ERP . |
|---|---|---|
| Preoperative | ||
| Investigations | Non-complete testing at peripheral centres | Broad and centralized testing at University Hospitals Leuven |
| Radiological imaging | Sometimes only visual assessment of CT data | Always including emphysema quantification (StratX-Pulmon©) |
| Referral | Ad hoc multidisciplinary discussion | Systematic multidisciplinary assessment |
| Education | Non-standardised education provided by surgeon’s office | Optimal education by surgeon, social worker, dietitian, physiotherapist and program coordinator |
| Intra-operative | ||
| Anaesthesia |
|
|
| Positioning | Dorsal decubitus | Lateral decubitus with mid-term conversion to contralateral decubitus |
| Approach | Anterior two-port VATS | One-, two-, three-, or four-port VATS |
| Incision |
|
|
| Material | Echelon Flex™ Endopath® Staplers (non-buttressed), beige and black cartridge | Signia™ Stapling System (buttressed), purple cartridge |
| Parenchyma stapling | More centrally, emphysema directed approach | Volume-based lung reshaping, more peripheral approach with respect to the lungs original shape |
| Drainage | Bilateral double chest tube | Bilateral, single or double chest tube |
| Postoperative | ||
| Recovery | Transfer to ICU for at least 1 night |
|
| Analgesia | Standardised analgesic protocol with the standard use of PCEA until POD3 |
|
| Prophylactic antibiotics | 5 days amoxycillin/clavulanic acid | |
| Urinary catheter | Non-standardised management | Removal POD1 |
| Fluids and intake |
|
|
| Physiotherapy and ambulation | Respiratory physiotherapy starting on POD1 |
|
| Rehabilitation | 30-Session pulmonary rehabilitation | 60-Session pulmonary rehabilitation: 3 weeks at home, 2–3 months ambulant pulmonary rehabilitation centre |
| . | Pre-ERP . | ERP . |
|---|---|---|
| Preoperative | ||
| Investigations | Non-complete testing at peripheral centres | Broad and centralized testing at University Hospitals Leuven |
| Radiological imaging | Sometimes only visual assessment of CT data | Always including emphysema quantification (StratX-Pulmon©) |
| Referral | Ad hoc multidisciplinary discussion | Systematic multidisciplinary assessment |
| Education | Non-standardised education provided by surgeon’s office | Optimal education by surgeon, social worker, dietitian, physiotherapist and program coordinator |
| Intra-operative | ||
| Anaesthesia |
|
|
| Positioning | Dorsal decubitus | Lateral decubitus with mid-term conversion to contralateral decubitus |
| Approach | Anterior two-port VATS | One-, two-, three-, or four-port VATS |
| Incision |
|
|
| Material | Echelon Flex™ Endopath® Staplers (non-buttressed), beige and black cartridge | Signia™ Stapling System (buttressed), purple cartridge |
| Parenchyma stapling | More centrally, emphysema directed approach | Volume-based lung reshaping, more peripheral approach with respect to the lungs original shape |
| Drainage | Bilateral double chest tube | Bilateral, single or double chest tube |
| Postoperative | ||
| Recovery | Transfer to ICU for at least 1 night |
|
| Analgesia | Standardised analgesic protocol with the standard use of PCEA until POD3 |
|
| Prophylactic antibiotics | 5 days amoxycillin/clavulanic acid | |
| Urinary catheter | Non-standardised management | Removal POD1 |
| Fluids and intake |
|
|
| Physiotherapy and ambulation | Respiratory physiotherapy starting on POD1 |
|
| Rehabilitation | 30-Session pulmonary rehabilitation | 60-Session pulmonary rehabilitation: 3 weeks at home, 2–3 months ambulant pulmonary rehabilitation centre |
CT: computed tomography; CVC: central venous catheter; ERP: enhanced recovery protocol; ICU: intensive care unit; PACU: postanaesthesia care unit; PCEA: patient-controlled epidural anaesthesia; POD: postoperative day; TIVA: total intravenous anaesthesia; VATS: video-assisted thoracoscopic surgery.
Preoperative phase
Eligible lung volume reduction candidates, with advanced emphysema who are not sufficiently responsive to medical treatment and rehabilitation, are assessed multidisciplinary based on high-resolution chest computed tomography, ventilation/perfusion-scintigraphy, spirometry, body plethysmography, 6-min walking distance and cardiac assessment to evaluate coronary disease, pulmonary artery pressure and right ventricular function (with a low threshold to perform left/right catheterisation) (Supplementary Material, Table S1).
Patients with a low functional reserve capacity [forced expiratory volume in 1 s (FEV1) <15% of predicted and/or diffusion capacity for carbon monoxide (DLCO) <20%pred] are only considered if a heterogeneous bullous-like morphology is present. Bronchiectasis, more than 2 exacerbations in the past year, a BODE-index of >7 and any comorbid disease rendering them unfit for surgery are considered ineligible. Pulmonary arterial hypertension (systolic pressure >45 mmHg at right heart catheterisation) with signs of right ventricular failure is contraindicated. Age (>75 years) and body mass index (>30 kg/m2) are taken into consideration as risk factors for developing postoperative complications.
Patients are preoperatively counselled by a social worker, dietitian and physiotherapist. They are encouraged to follow preoperative pulmonary rehabilitation.
Perioperative phase
At induction, prophylactic antibiotics (Amoxicillin/Clavulanic acid 4 × 1000 mg/200 mg intravenously or 875/125 mg orally, 5 days) are administered, arterial, peripheral venous and urinary catheters are placed. Patient-controlled epidural analgesia is used for pain control. Standardised anaesthesia regimen is used for all patients. Propofol (target-controlled infusion), sufentanil 0.2 mg kg−1, followed by remifentanil infusion 0.1–0.3 mg kg−1 and rocuronium 0.6–0.8 mg kg−1 are administered for induction. Patients are installed in lateral decubitus which allows safer adhesiolysis with an improved angle for stapling compared to supine positioning, although this can be considered in patients with markedly heterogeneous upper-lobe morphology, when no adhesions are expected. Low-tidal volume ventilation, controlled reinflation of the lung, systematic reversal of neuromuscular block and early on-table extubation are critical to reduce incidence of air leak caused by stress-tearing of the lung tissue.
The number of ports depends on the location of the target zones, ranging from uniportal up to 4 ports combining upper and lower lobe targets. Ipsilateral lung ventilation is stopped at incision, so that the most severely destroyed areas remain inflated and resection of the lung volume by peripheral volume reshaping (lung contouring) can be achieved without sacrificing functional tissue [to remodel the lungs to the predicted total lung capacity (TLC)]. The lungs are manipulated by minimally invasive forceps or cherry dissectors (Ethicon Endo-Surgery, Cincinnati, OH) to avoid tissue damage (no-touch concept) and a complete adhesiolysis allowing maximal elastic recoil is performed. Paraffine lubricated Signia Tri-Staple™ 2.0 (45 or 60 mm) purple Reinforced Reload system (Medtronic, Minneapolis, MN, USA) is used after pre-staple compression. Polyglycolic acid sheet (Neoveil® sheet; Gunze Kyoto, Japan) is used over the staple lines to reinforce the surrounding tissue, in combination with a polymeric biodegradable hydrogel sealant (Progel™ Pleural Air Leak Sealant; Becton Dickinson Company, Franklin Lakes, NJ) in case of very fragile tissue.
One or 2 chest tubes are left behind, and suction is reserved for patients with postoperative air leak (−5 to −10 cm H2O). In patients for whom a bilateral plan was intended, the option of a two-staged procedure is considered in case of extensive pleural adhesions (increasing risk of air leaks), prolonged surgical time, presence of air leak, cardiopulmonary instability or hypercapnia.
Postoperative phase
The patient is extubated in the operating room. Special attention is taken to avoid coughing (Lidocaine 1 mg/kg) and standard reconversion of muscular block is performed. A laryngeal mask can prevent bronchospasm, coughing or valsalva. Use of non-invasive ventilatory support is considered if progressive hypercapnia occurs. Adequate use of opioid analgesics, and early mobilisation (at postanaesthesia care unit) is implemented to prevent respiratory failure or infection. In case of sufficient recovery, the patient is transferred to the ward the same day.
Patient-controlled epidural analgesia is removed at postoperative day (POD) 2/3 in case of unilateral (u)-/b-LVRS, respectively. Daily administered drugs are proton-pump inhibitor, beta-2 adrenergic agonist aerosol, prophylactic dose of low-molecular-weight heparin and osmotic laxative. On POD1 the urinary catheter is removed, and intravenous fluid administration is limited (500 cc/24 h). Respiratory physiotherapy and general ambulation are continued during hospitalisation. Patients receive protein-enriched meals from POD1. Chest drains are removed in the absence of air leaks and drainages <200 cc. Postoperative investigations include blood analysis and daily chest X-rays. Cardiac situation is followed by telemetry (POD2) (Supplementary Material, Table S2). In case of prolonged air leak (PAL) or worsening of air leak, patient is evaluated for reintervention. All patients are included in a 3-month (60-session) respiratory rehabilitation program. In patients with symmetrical disease, the duration between both procedures is determined based on recovery and functional improvement after first surgery.
Outcome
Primary outcome was the incidence of any postoperative complication within the first 30 days after LVRS or until discharge from the hospital. Complications were classified according Clavien-Dindo as minor (grade I–II), major (grade III–IV) and any complication leading to death (grade V) [21]. Secondary outcomes included chest tube duration (days), incidence of PAL (persistent air leak > 7 days), LOS (days) and in-hospital and 90-day mortality. Functional outcome comprised pulmonary function (FEV1, RV, TLC, DLCO) and 6-min walking distance at 3 and 6 months post-LVRS. In two-stage b-LVRS patients, functional outcomes were assessed after the second procedure.
Regarding surgical outcomes, procedures were compared (pre-ERP: n = 41, ERP: n = 38) to accurately assess the postoperative risks of each procedure. Regarding functional outcomes, the assessment was made based on individual patients’ follow-up (pre-ERP: n = 41, ERP: n = 35).
Statistical analysis
Continuous variables were reported as means and standard deviations, in case of non-normal distribution are presented as median (interquartile range). Categorical variables were reported as number (percentage). Student’s t, Mann–Whitney U or Fisher’s exact tests were used. All available data was used, and no data imputation was performed for missing values. Statistical analyses were performed with GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA) at significance level of 0.05.
RESULTS
Study population
After introduction of ERP, 35 patients underwent LVRS, of whom 20 (57%) one-stage b-LVRS, 6 (17%) two-stage b-LVRS and 9 (26%) u-LVRS. Baseline demographics between ERP and pre-ERP were similar for most variables apart from oxygen supplementation (44% in pre-ERP vs 20% in ERP, P = 0.031) and BODE-index [6 (5–7) vs 5 [4–6], P = 0.026] (Supplementary Material, Table S3). All procedures were performed through video-assisted thoracoscopic surgery. The time interval between the first and second stage of two-stage bilateral LVRS was 83 days (49–144).
Outcome
Surgical outcome
Duration of the procedure was longer in the ERP cohort compared with the pre-ERP cohort, 104 min (84–127) vs 85 min (70–100). There was a reduction in the incidence of postoperative complications in the ERP compared to pre-ERP (42% vs 83%, P = 0.0002) (Table 2). Fewer minor complications (grade I–II) occurred in ERP compared to pre-ERP (29% vs 61%, P = 0.0065). Following PAL (21%) and subcutaneous emphysema (11%), the most frequent complication in the ERP group was atrial fibrillation (8%). In the pre-ERP cohort infection occurred in 19/41 (46%) patients [pulmonary cause 14/19 (74%), catheter sepsis 4/19 (21%) and urinary tract infection (UTI) 1/19 (5%)] vs 1/38 (3%) UTI in the ERP cohort (P < 0.0001). The ERP cohort presented with shorter chest tube duration (4 vs 12 days, P < 0.0001), a lower incidence of PAL (21% vs 61%, P = 0.005) and 2 ERP patients (5.7%) were discharged with a Heimlich valve versus 6 (15%) patients pre-ERP.
Comparison of surgical outcome between procedures before and after implementation of an enhanced recovery protocol
| Surgical outcome . | Pre-ERP procedures (n = 41) . | ERP procedures (n = 38) . | P-value . |
|---|---|---|---|
| Total postoperative complicationsa | 34 (83%) | 16 (42%) | 0.0002 |
| Minor (grade I–II) a | 25 (61%) | 11 (29%) | 0.0065 |
| Prolonged air leak (>7 days) | 25 (61%) | 8 (21%) | 0.0005 |
| Subcutaneous emphysema | 19 (46%) | 4 (11%) | 0.0005 |
| Infection | 19 (46%) | 1 (3%) | <0.0001 |
| Respiratory infection | 14 (34%) | 0 | <0.0001 |
| Discharge with Heimlich valve | 6 (15%) | 2 (5%) | 0.2660 |
| Atrial fibrillation | 3 (7%) | 3 (8%) | >0.9999 |
| Miscellaneousb | 3 (7%) | 2 (5%) | >0.9999 |
| Major (grade III–IV)a | 7 (17%) | 5 (13%) | 0.7576 |
| Reintervention | 1 (2%) | 1 (3%) | >0.9999 |
| Respiratory failure/reintubation/tracheostomy | 7 (17%) | 0 | 0.0121 |
| Miscellaneousc | 3 (7%) | 4 (11%) | 0.7053 |
| Mortality (grade V)a | 2 (5%) | 0 | 0.4943 |
| Length of stay (days) | 14 (9–21) | 6 (4–10) | <0.0001 |
| ICU admission | 39 (95%) | 2 (5%) | <0.0001 |
| Air leak POD1 | 36 (88%) | 22 (58%) | 0.0044 |
| Chest tube duration (days) | 12 (4–19) | 4 (3–7.25) | <0.0001 |
| Readmission first 30 days | 1 (2%) | 0 | >0.9999 |
| Duration procedure | 85 (70–100) | 104 (87–126) | 0.0019 |
| In-hospital mortality | 4 (10%) | 0 | 0.1165 |
| 90-Day mortality | 4 (10%) | 1 (3%)d | 0.3606 |
| Surgical outcome . | Pre-ERP procedures (n = 41) . | ERP procedures (n = 38) . | P-value . |
|---|---|---|---|
| Total postoperative complicationsa | 34 (83%) | 16 (42%) | 0.0002 |
| Minor (grade I–II) a | 25 (61%) | 11 (29%) | 0.0065 |
| Prolonged air leak (>7 days) | 25 (61%) | 8 (21%) | 0.0005 |
| Subcutaneous emphysema | 19 (46%) | 4 (11%) | 0.0005 |
| Infection | 19 (46%) | 1 (3%) | <0.0001 |
| Respiratory infection | 14 (34%) | 0 | <0.0001 |
| Discharge with Heimlich valve | 6 (15%) | 2 (5%) | 0.2660 |
| Atrial fibrillation | 3 (7%) | 3 (8%) | >0.9999 |
| Miscellaneousb | 3 (7%) | 2 (5%) | >0.9999 |
| Major (grade III–IV)a | 7 (17%) | 5 (13%) | 0.7576 |
| Reintervention | 1 (2%) | 1 (3%) | >0.9999 |
| Respiratory failure/reintubation/tracheostomy | 7 (17%) | 0 | 0.0121 |
| Miscellaneousc | 3 (7%) | 4 (11%) | 0.7053 |
| Mortality (grade V)a | 2 (5%) | 0 | 0.4943 |
| Length of stay (days) | 14 (9–21) | 6 (4–10) | <0.0001 |
| ICU admission | 39 (95%) | 2 (5%) | <0.0001 |
| Air leak POD1 | 36 (88%) | 22 (58%) | 0.0044 |
| Chest tube duration (days) | 12 (4–19) | 4 (3–7.25) | <0.0001 |
| Readmission first 30 days | 1 (2%) | 0 | >0.9999 |
| Duration procedure | 85 (70–100) | 104 (87–126) | 0.0019 |
| In-hospital mortality | 4 (10%) | 0 | 0.1165 |
| 90-Day mortality | 4 (10%) | 1 (3%)d | 0.3606 |
Data are represented as median (IQR) or parameter counts n (%). Bold values represent statistically significant results.
Clavien-Dindo based classification system for thoracic surgery complications, within 30 days or until discharged from the hospital.
Minor complications: atrial arrhythmia (other than atrial fibrillation), anaemia requiring transfusion, syndrome of inappropriate antidiuretic hormone secretion.
Major complications: re-insertion of chest tube, acute kidney injury, severe pulmonary embolism, tension pneumothorax. Patients with multiple complications were classified according to their highest-grade complication.
Coronavirus disease 2019-related mortality.
ERP: enhanced recovery protocol; ICU: intensive care unit; IQR: interquartile range; POD: postoperative day.
Comparison of surgical outcome between procedures before and after implementation of an enhanced recovery protocol
| Surgical outcome . | Pre-ERP procedures (n = 41) . | ERP procedures (n = 38) . | P-value . |
|---|---|---|---|
| Total postoperative complicationsa | 34 (83%) | 16 (42%) | 0.0002 |
| Minor (grade I–II) a | 25 (61%) | 11 (29%) | 0.0065 |
| Prolonged air leak (>7 days) | 25 (61%) | 8 (21%) | 0.0005 |
| Subcutaneous emphysema | 19 (46%) | 4 (11%) | 0.0005 |
| Infection | 19 (46%) | 1 (3%) | <0.0001 |
| Respiratory infection | 14 (34%) | 0 | <0.0001 |
| Discharge with Heimlich valve | 6 (15%) | 2 (5%) | 0.2660 |
| Atrial fibrillation | 3 (7%) | 3 (8%) | >0.9999 |
| Miscellaneousb | 3 (7%) | 2 (5%) | >0.9999 |
| Major (grade III–IV)a | 7 (17%) | 5 (13%) | 0.7576 |
| Reintervention | 1 (2%) | 1 (3%) | >0.9999 |
| Respiratory failure/reintubation/tracheostomy | 7 (17%) | 0 | 0.0121 |
| Miscellaneousc | 3 (7%) | 4 (11%) | 0.7053 |
| Mortality (grade V)a | 2 (5%) | 0 | 0.4943 |
| Length of stay (days) | 14 (9–21) | 6 (4–10) | <0.0001 |
| ICU admission | 39 (95%) | 2 (5%) | <0.0001 |
| Air leak POD1 | 36 (88%) | 22 (58%) | 0.0044 |
| Chest tube duration (days) | 12 (4–19) | 4 (3–7.25) | <0.0001 |
| Readmission first 30 days | 1 (2%) | 0 | >0.9999 |
| Duration procedure | 85 (70–100) | 104 (87–126) | 0.0019 |
| In-hospital mortality | 4 (10%) | 0 | 0.1165 |
| 90-Day mortality | 4 (10%) | 1 (3%)d | 0.3606 |
| Surgical outcome . | Pre-ERP procedures (n = 41) . | ERP procedures (n = 38) . | P-value . |
|---|---|---|---|
| Total postoperative complicationsa | 34 (83%) | 16 (42%) | 0.0002 |
| Minor (grade I–II) a | 25 (61%) | 11 (29%) | 0.0065 |
| Prolonged air leak (>7 days) | 25 (61%) | 8 (21%) | 0.0005 |
| Subcutaneous emphysema | 19 (46%) | 4 (11%) | 0.0005 |
| Infection | 19 (46%) | 1 (3%) | <0.0001 |
| Respiratory infection | 14 (34%) | 0 | <0.0001 |
| Discharge with Heimlich valve | 6 (15%) | 2 (5%) | 0.2660 |
| Atrial fibrillation | 3 (7%) | 3 (8%) | >0.9999 |
| Miscellaneousb | 3 (7%) | 2 (5%) | >0.9999 |
| Major (grade III–IV)a | 7 (17%) | 5 (13%) | 0.7576 |
| Reintervention | 1 (2%) | 1 (3%) | >0.9999 |
| Respiratory failure/reintubation/tracheostomy | 7 (17%) | 0 | 0.0121 |
| Miscellaneousc | 3 (7%) | 4 (11%) | 0.7053 |
| Mortality (grade V)a | 2 (5%) | 0 | 0.4943 |
| Length of stay (days) | 14 (9–21) | 6 (4–10) | <0.0001 |
| ICU admission | 39 (95%) | 2 (5%) | <0.0001 |
| Air leak POD1 | 36 (88%) | 22 (58%) | 0.0044 |
| Chest tube duration (days) | 12 (4–19) | 4 (3–7.25) | <0.0001 |
| Readmission first 30 days | 1 (2%) | 0 | >0.9999 |
| Duration procedure | 85 (70–100) | 104 (87–126) | 0.0019 |
| In-hospital mortality | 4 (10%) | 0 | 0.1165 |
| 90-Day mortality | 4 (10%) | 1 (3%)d | 0.3606 |
Data are represented as median (IQR) or parameter counts n (%). Bold values represent statistically significant results.
Clavien-Dindo based classification system for thoracic surgery complications, within 30 days or until discharged from the hospital.
Minor complications: atrial arrhythmia (other than atrial fibrillation), anaemia requiring transfusion, syndrome of inappropriate antidiuretic hormone secretion.
Major complications: re-insertion of chest tube, acute kidney injury, severe pulmonary embolism, tension pneumothorax. Patients with multiple complications were classified according to their highest-grade complication.
Coronavirus disease 2019-related mortality.
ERP: enhanced recovery protocol; ICU: intensive care unit; IQR: interquartile range; POD: postoperative day.
The incidence of major complications (grade III–IV) was similar between both cohorts (16% vs 13%). No acute respiratory failure or need for reintubation was required in ERP in contrast to 17% pre-ERP (P = 0.01). In both cohorts the need for a reintervention was similar. There were no complications leading to death (grade V) in ERP compared to 2 pre-ERP. One patient died in the first 90 days due to coronavirus disease 2019 (before vaccination was available) in the ERP cohort versus 4 pre-ERP due to respiratory insufficiency (in 2 patients) and pulmonary sepsis.
Compared to the historical non-ERP, the ERP group was found to have a shorter postoperative LOS (6 vs 14 days, P < 0.0001) with lower ICU-admissions (6% vs 95%, P < 0.001). Two ICU-admissions in ERP were due to coronavirus disease 2019-induced lack of capacity on the recovery unit and one following a tension pneumothorax at POD8 who was readmitted to the ward the same day. There were no hospital readmissions in the ERP group.
Functional outcome
At 3 months postoperatively, RV and TLC were lower in the ERP group compared to the pre-ERP group (152%pred vs 191%pred and 109%pred vs 122%pred, respectively). The ratio of RV to TLC was 0.57 in the pre-ERP cohort vs 0.49 in the ERP cohort (Table 3). Postoperative FEV1 was higher after implementation of ERP at 3 (1.34 vs 1.02 l) and at 6 months (1.31 vs 1.0 l), respectively. Dropout was larger in the pre-ERP cohort, compared to the ERP group 37% (15/41) vs 22% (8/35). No other differences were observed.
Lung function and exercise capacity in patients at 3- and 6-month follow-up, in the pre-enhanced recovery protocol and enhanced recovery protocol cohorts
| Functional outcomes . | 3 months . | 6 months . | ||||
|---|---|---|---|---|---|---|
| . | Pre-ERP, n = 27 . | ERP, n = 31 . | P-value . | Pre-ERP, n = 26 . | ERP, n = 27 . | P-value . |
| FEV1 (l) | 1.02 (0.90–1.20) | 1.35 (0.96–1.71) | 0.0201 | 1.01 (0.80–1.26) | 1.31 (0.94–1.77) | 0.0301 |
| FEV1 (%pred) | 39 (31–46) | 43 (35–56) | 0.1390 | 40 (32–48) | 39 (33–49) | 0.9102 |
| RV (l) | 3.84 (3.40–4.59) | 3.44 (2.62–4.30) | 0.0836 | 3.54 (3.04–5.08) | 3.55 (3.06–4.34) | 0.7148 |
| RV (%pred) | 191 (166–209) | 152 (123–182) | 0.0013 | 193 (159–220) | 163 (134–184) | 0.0519 |
| TLC (l) | 6.84 (5.90–7.87) | 7.40 (5.60–8.23) | 0.8249 | 6.80 (5.85–8.59) | 7.34 (5.52–8.06) | 0.6994 |
| TLC (%pred) | 122 (109–137) | 109 (101–121) | 0.0109 | 123 (111–136) | 113 (103–129) | 0.0856 |
| RV/TLC | 0.57 (0.50–0.61) | 0.50 (0.42–0.60) | 0.0271 | 0.55 (0.49–0.62) | 0.55 (0.47–0.61) | 0.7106 |
| DLCO (%pred) | 39 (33–44) | 45 (34–52) | 0.1342 | 42 (32–49) | 38 (31–50) | 0.8573 |
| 6MWD (m) | 406 (364–441) | 426 (379–475) | 0.3282 | 400 (363–513) | 421 (376–505) | 0.6843 |
| Functional outcomes . | 3 months . | 6 months . | ||||
|---|---|---|---|---|---|---|
| . | Pre-ERP, n = 27 . | ERP, n = 31 . | P-value . | Pre-ERP, n = 26 . | ERP, n = 27 . | P-value . |
| FEV1 (l) | 1.02 (0.90–1.20) | 1.35 (0.96–1.71) | 0.0201 | 1.01 (0.80–1.26) | 1.31 (0.94–1.77) | 0.0301 |
| FEV1 (%pred) | 39 (31–46) | 43 (35–56) | 0.1390 | 40 (32–48) | 39 (33–49) | 0.9102 |
| RV (l) | 3.84 (3.40–4.59) | 3.44 (2.62–4.30) | 0.0836 | 3.54 (3.04–5.08) | 3.55 (3.06–4.34) | 0.7148 |
| RV (%pred) | 191 (166–209) | 152 (123–182) | 0.0013 | 193 (159–220) | 163 (134–184) | 0.0519 |
| TLC (l) | 6.84 (5.90–7.87) | 7.40 (5.60–8.23) | 0.8249 | 6.80 (5.85–8.59) | 7.34 (5.52–8.06) | 0.6994 |
| TLC (%pred) | 122 (109–137) | 109 (101–121) | 0.0109 | 123 (111–136) | 113 (103–129) | 0.0856 |
| RV/TLC | 0.57 (0.50–0.61) | 0.50 (0.42–0.60) | 0.0271 | 0.55 (0.49–0.62) | 0.55 (0.47–0.61) | 0.7106 |
| DLCO (%pred) | 39 (33–44) | 45 (34–52) | 0.1342 | 42 (32–49) | 38 (31–50) | 0.8573 |
| 6MWD (m) | 406 (364–441) | 426 (379–475) | 0.3282 | 400 (363–513) | 421 (376–505) | 0.6843 |
Data presented as median (IQR) based on the available data in patient reports. Loss of follow-up is based on absolute values in FEV1 (l). Bold values represent statistically significant results.
DLCO: diffusing capacity for carbon monoxide; ERP: enhanced recovery protocol; FEV1: forced expiratory volume in 1 s; IQR: interquartile range; 6MWD: 6-min walk distance; RV: residual volume; TLC: total lung capacity.
Lung function and exercise capacity in patients at 3- and 6-month follow-up, in the pre-enhanced recovery protocol and enhanced recovery protocol cohorts
| Functional outcomes . | 3 months . | 6 months . | ||||
|---|---|---|---|---|---|---|
| . | Pre-ERP, n = 27 . | ERP, n = 31 . | P-value . | Pre-ERP, n = 26 . | ERP, n = 27 . | P-value . |
| FEV1 (l) | 1.02 (0.90–1.20) | 1.35 (0.96–1.71) | 0.0201 | 1.01 (0.80–1.26) | 1.31 (0.94–1.77) | 0.0301 |
| FEV1 (%pred) | 39 (31–46) | 43 (35–56) | 0.1390 | 40 (32–48) | 39 (33–49) | 0.9102 |
| RV (l) | 3.84 (3.40–4.59) | 3.44 (2.62–4.30) | 0.0836 | 3.54 (3.04–5.08) | 3.55 (3.06–4.34) | 0.7148 |
| RV (%pred) | 191 (166–209) | 152 (123–182) | 0.0013 | 193 (159–220) | 163 (134–184) | 0.0519 |
| TLC (l) | 6.84 (5.90–7.87) | 7.40 (5.60–8.23) | 0.8249 | 6.80 (5.85–8.59) | 7.34 (5.52–8.06) | 0.6994 |
| TLC (%pred) | 122 (109–137) | 109 (101–121) | 0.0109 | 123 (111–136) | 113 (103–129) | 0.0856 |
| RV/TLC | 0.57 (0.50–0.61) | 0.50 (0.42–0.60) | 0.0271 | 0.55 (0.49–0.62) | 0.55 (0.47–0.61) | 0.7106 |
| DLCO (%pred) | 39 (33–44) | 45 (34–52) | 0.1342 | 42 (32–49) | 38 (31–50) | 0.8573 |
| 6MWD (m) | 406 (364–441) | 426 (379–475) | 0.3282 | 400 (363–513) | 421 (376–505) | 0.6843 |
| Functional outcomes . | 3 months . | 6 months . | ||||
|---|---|---|---|---|---|---|
| . | Pre-ERP, n = 27 . | ERP, n = 31 . | P-value . | Pre-ERP, n = 26 . | ERP, n = 27 . | P-value . |
| FEV1 (l) | 1.02 (0.90–1.20) | 1.35 (0.96–1.71) | 0.0201 | 1.01 (0.80–1.26) | 1.31 (0.94–1.77) | 0.0301 |
| FEV1 (%pred) | 39 (31–46) | 43 (35–56) | 0.1390 | 40 (32–48) | 39 (33–49) | 0.9102 |
| RV (l) | 3.84 (3.40–4.59) | 3.44 (2.62–4.30) | 0.0836 | 3.54 (3.04–5.08) | 3.55 (3.06–4.34) | 0.7148 |
| RV (%pred) | 191 (166–209) | 152 (123–182) | 0.0013 | 193 (159–220) | 163 (134–184) | 0.0519 |
| TLC (l) | 6.84 (5.90–7.87) | 7.40 (5.60–8.23) | 0.8249 | 6.80 (5.85–8.59) | 7.34 (5.52–8.06) | 0.6994 |
| TLC (%pred) | 122 (109–137) | 109 (101–121) | 0.0109 | 123 (111–136) | 113 (103–129) | 0.0856 |
| RV/TLC | 0.57 (0.50–0.61) | 0.50 (0.42–0.60) | 0.0271 | 0.55 (0.49–0.62) | 0.55 (0.47–0.61) | 0.7106 |
| DLCO (%pred) | 39 (33–44) | 45 (34–52) | 0.1342 | 42 (32–49) | 38 (31–50) | 0.8573 |
| 6MWD (m) | 406 (364–441) | 426 (379–475) | 0.3282 | 400 (363–513) | 421 (376–505) | 0.6843 |
Data presented as median (IQR) based on the available data in patient reports. Loss of follow-up is based on absolute values in FEV1 (l). Bold values represent statistically significant results.
DLCO: diffusing capacity for carbon monoxide; ERP: enhanced recovery protocol; FEV1: forced expiratory volume in 1 s; IQR: interquartile range; 6MWD: 6-min walk distance; RV: residual volume; TLC: total lung capacity.
DISCUSSION
This single-centre cohort study demonstrates that implementation of an ERP for LVRS is feasible, despite that these patients are often fragile and functionally impaired. Furthermore, postoperative short-term outcomes are improved by decreased postoperative complications, reduced incidence and duration of air leak and shorter LOS. Excellent 3- and 6-month functional results were observed. Despite these promising results and improved minimally invasive surgical techniques, LVRS is still one of the most underutilized [22] and undervalued procedures in thoracic surgery. This is mainly due to misinterpretation of the National Emphysema Treatment Trial [3], which reported a high mortality risk of LVRS compared to medical treatment (7.9% vs 1.3%) but included a high-risk population that should not have been selected for this procedure: patients with an FEV1 ≤ 20%pred and homogeneous emphysema or DLCO ≤ 20%pred.
Based on previous ERP reports in thoracic surgery [18, 19], our experience (ERP for oesophagectomy, lobectomy) complemented by insights from W. Weder, a multidisciplinary ERP for LVRS has been developed including pre-and postoperative care pathways and optimisation of perioperative care. Our median LOS of 6 days compares favourably to a recent study by Seadler et al. of one-stage video-assisted thoracoscopic surgery b-LVRS (n = 123) [7], where a median LOS of 10 days was reported. This is mainly due to a shorter chest drainage time of 4 days and limited PAL (21%) in our ERP cohort, compared to 9 days with PAL occurring in 59% of patients in the study by Seadler et al. [7].
The benefits of an ERP result from multidisciplinary and multimodal efforts to improve patient care, rather than depending solely on the impact of individual components. Together with this ERP, a paradigm shift on safety evaluation (optional two-staged approach) and surgical optimisation (volume-based reshaping of the lung and no-touch surgery) was implemented, resulting in a reduction of surgical complications. Staged b-LVRS has previously been introduced as a safe strategy with lower postoperative morbidity attaining a prolonged benefit after the second reduction [23]. In our ERP cohort u-LVRS is performed when intraoperative limitations arise during an intention-to-treat one-stage b-LVRS, in addition to patients with only unilateral disease.
Regarding the actual parenchymal resection, we focused in our ERP cohort on the more peripheral target areas in contrast to a more central resection in the pre-ERP cohort. This results in a reduction of the postoperative residual pleural space with better pleural approximation which enables sealing of potential superficial air leaks. According to literature, use of buttressed staplers, decreases the incidence of air leaks and chest tube duration [24]. Furthermore, the use of polyglycolic acid sheets (Neoveil®) and polymeric biodegradable hydrogel sealant (Progel™), might further augment pleural adhesion and heal air leaks faster by inducing local inflammation and have been proven beneficial in several surgical pulmonary procedures [25–27]. These factors may also have contributed to the favourable outcome data including shorter drainage time, PAL and LOS.
Early extubation, immediately after surgery, is required to reduce the time that the delicate lungs are exposed to positive pressure ventilation [28]. Early postoperative mobilisation is a key factor to minimize complications and reduce the length of hospital stay and is recommended in enhanced recovery guidelines in different surgical fields [13, 15]. In emphysema patients, acute exacerbations following lung resection are frequent and companied with pulmonary complications [29]. The first line treatment for acute exacerbations, when no penicillin allergy is present, is amoxicillin-clavulanic acid [30] and earlier research in 1006 patients has demonstrated the protective effect of a 5-day treatment after lung resection with lower incidence of pneumonia [29]. This study demonstrated that compared with the pre-ERP cohort, the ERP group had a lower incidence of infections (3% vs 46%, P < 0.0001). We attribute the reduction in infections to the following factors in our ERP cohort: a 5-day course of antibiotic therapy instead of single-shot antibiotic prophylaxis (pre-ERP), avoidance of ICU admission and early, pain-free ambulation. In our ERP experience, only 2 patients were admitted to ICU (non-intubated, due to incapacity problems at the recovery room).
In the ERP group, despite earlier discharge, we observed no increase in readmission rate or surgical morbidity. Regarding functional improvement after LVRS, the ERP cohort demonstrated less hyperinflation at 3 months and a higher FEV1 (l) at 3 and 6 months compared to the pre-ERP cohort.
To our knowledge, this is the first cohort study for ERP in LVRS. However, there are some limitations in this study. First, this study was limited by its retrospective and single-centre design. Second, due to the 2020 SARS-CoV-2 pandemic, dozens of LVRS operations were postponed. The health crisis also impeded patient follow-up and participation rates in post-discharge rehabilitation programs. Third, no causality could be attributed to the implementation of this ERP and improved patient outcomes nor LOS. The multimodal reconceptualisation of LVRS introduced unmeasured confounding factors that may have influenced outcomes. For example, the learning curve in the ERP cohort was inevitable and was simultaneously followed by the lead surgeon and all involved disciplines. Since this ERP was not designed as a fixed care program, ongoing improvements to the ERP will undoubtedly continue to evolve as we garner more data regarding the individual components of our protocol. Additionally, pre-ERP and ERP procedures were performed at different time periods, and the observed results might be influenced by better patient selection in the ERP era, where systematic multidisciplinary assessment with centralized investigations and standard incorporation of emphysema quantification software were integrated. Finally, caution must be implied interpreting functional results, since a more than 20% loss of follow-up was present at 6 months. This may be attributed to increased surgical morbidity and mortality, regional variations or the absence of routine follow-up during this period. Moreover, relatively more loss to follow-up occurred in pre-ERP compared to ERP, which resulted probably in an overestimation regarding functional outcomes in the pre-ERP cohort. No corrections for time-related effects nor missing values were conducted due to the descriptive character of this study.
CONCLUSION
The implementation of ERP as part of a comprehensive reconceptualisation towards LVRS and thereby standardising perioperative patient care in a multidisciplinary expert centre, is observed with fewer postoperative complications which results in a decrease of LOS without affecting readmission or mortality.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
ACKNOWLEDGEMENTS
The authors wish to express their gratitude to the surgeon (Yanina Jansen), pneumologists (Robin Vos, Iwein Gyselinck and Geert Verleden), physiotherapists (Marianne Fontaine, Astrid Blondeel), anaesthesiologists, nurses and the coordinator (Hannelore Geysen) involved in our LVRS program.
FUNDING
The research led by Laurens J. Ceulemans is supported by a Medtronic named chair at the KU Leuven, philanthropic grant by Gunze, senior-research-fellowship funded by the University Hospitals Leuven (KOOR) and research Foundation Flanders (FWO) (G090922N and 18E2B24N).
Conflict of interest: Laurens J. Ceulemans is a consultant and a proctor for Medtronic. The other authors have no conflicts of interest to declare in relation to this manuscript.
DATA AVAILABILITY
All relevant data are available on request to the authors.
Author contributions
Christelle M. Vandervelde: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Validation; Visualization; Writing—original draft; Writing—review & editing; final approval. Stephanie Everaerts: Conceptualization; Investigation; Validation; Writing—review & editing; final approval. Walter Weder: Conceptualization; Visualization; Writing—review & editing; final approval. Siebe Orolé: Conceptualization; Methodology; Validation; Writing—original draft; final approval. Pieter-Jan Hermans: Conceptualization; Writing—review & editing; final approval. Paul De Leyn: Conceptualization; Writing—review & editing; final approval. Philippe Nafteux: Conceptualization; Writing—review & editing; final approval. Herbert Decaluwé: Conceptualization; Writing—review & editing; final approval. Hans Van Veer: Conceptualization; Writing—review & editing; final approval. Lieven Depypere: Conceptualization; Writing—review & editing; final approval. Steve Coppens: Conceptualization; Writing—review & editing; final approval. Arne P. Neyrinck: Conceptualization; Writing—review & editing; final approval. Sofian Bouneb: Conceptualization; Writing—review & editing; final approval. Johan De Coster: Conceptualization; Writing—review & editing; final approval. Johan Coolen: Conceptualization; Writing—review & editing; final approval. Christophe Dooms: Conceptualization; Investigation; Writing—review & editing; final approval. Dirk E. Van Raemdonck: Conceptualization; Investigation; Writing—review & editing; final approval. Wim Janssens: Conceptualization; Investigation; Writing—review & editing; final approval. Laurens J. Ceulemans: Conceptualization; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks John R Benfield, Ilies Bouabdallah and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Presented at the ESTS Annual (virtual) Meeting, 2021.
REFERENCES
ABBREVIATIONS
- b-LVRS
Bilateral lung volume reduction surgery
- DLCO
Diffusion capacity for carbon monoxide
- ERP
Enhanced recovery protocol
- FEV1
Forced expiratory volume in one second
- LOS
Length of stay
- LVRS
Lung volume reduction surgery
- PAL
Prolonged air leak
- POD
Postoperative day
- RV
Residual volume
- TLC
Total lung capacity
- u-LVRS
Unilateral lung volume reduction surgery





