-
PDF
- Split View
-
Views
-
Cite
Cite
Viktoria Weixler, Julia Gaal, Peter Murin, Peter Kramer, Olga Romanchenko, Mi-Young Cho, Katharina Schmitt, Stanislav Ovroutski, Joachim Photiadis, Repair of complex transposition of great arteries: What is the best technique to avoid outflow tract obstructions?, European Journal of Cardio-Thoracic Surgery, Volume 65, Issue 4, April 2024, ezae094, https://doi.org/10.1093/ejcts/ezae094
Close - Share Icon Share
Abstract
This study aimed to evaluate the short-/mid-term outcome of patients with complex dextro (d)-/levo (l)-transposition of the great arteries (TGA), ventricular septal defect and left ventricular outflow tract obstructions.
A single-centre, retrospective review of all complex dextro-TGA (n = 85) and levo-TGA (n = 22) patients undergoing different surgeries [Arterial switch operation + left ventricular outflow tract obstruction-resection (ASO-R), half-turned truncal switch/Mair (HTTS), Nikaidoh and Rastelli] between May 1990 and September 2022 was performed. Groups were analysed using Kruskal–Wallis test with post hoc pairwise comparison and Kaplan–Meier time-to-event models.
A total of 107 patients [ASO-R (n = 20), HTTS (n = 23), Nikaidoh (n = 21), Rastelli (n = 43)] were included, with a median age of 1.0 year (0.5–2.5) and surgical repair median follow-up was 3.8 years (0.3–10.5). Groups did not differ in respect to early postoperative complications/early mortality. Five-year overall survival curves were comparable: ASO-R 78.9% (53.2–91.5), HTTS 75.3% (46.8–89.9), Nikaidoh 85% (60.4–94.9) and Rastelli 83.9% (67.5–92.5), P = 0.9. Highest rates of right ventricular outflow tract (RVOT) reinterventions [33.3% and 32.6% (P = 0.04)] and reoperations [28.6% and 32.6% (P = 0.02)] occurred after Nikaidoh and Rastelli procedures. However, overall freedom from RVOT reinterventions and RVOT reoperations at 5 years did not differ statistically significantly between the groups (ASO-R, HTTS, Nikaidoh and Rastelli): 94.4% (66.6–99.2), 69.1% (25.4–90.5), 67.8% (34–86.9), 64.4% (44.6–78.7), P = 0.2, and 90.0% (65.6–97.4), 91% (50.8–98.7), 65.3% (32.0–85.3) and 67.0% (47.4–80.6), P = 0.3.
Surgical repair of complex dextro-/levo-TGA can be performed with satisfying early/mid-term survival. RVOT reinterventions/reoperations were frequent, with highest rates after Nikaidoh and Rastelli procedures. Left ventricular outflow tract obstruction reoperations were rare with zero events after Nikaidoh and HTTS procedures.
INTRODUCTION
Complex transposition of the great arteries (TGA) including dextro (d)- and levo (l)-TGA accompanied by a ventricular septal defect (VSD) and significant left ventricular outflow tract obstruction (LVOTO) are rare but heterogeneous congenital heart defects requiring individualized surgical decision-making [1]. While d-TGA and l-TGA are distinct entities, they share the common feature of ventriculo-arterial discordance, requiring biventricular correction for the morphologic left ventricle acting as the systemic ventricle and the morphologic right ventricle as the subpulmonary ventricle.
Several surgical techniques have been introduced for anatomic repair of complex d- and l-TGA/VSD/LVOTO (either at ventricular, conus or arterial level); however, the optimal surgical approach in regard of long-term outcome is still controversial [2, 3].
The degree and type of LVOTO, pulmonary valve (PV) anatomy, coronary artery pattern, and VSD location and morphology play a major role in the decision-making process for the preferable surgical approach in the individual patient [2, 3]. If arterial switch operation with LVOTO resection (ASO-R) is not feasible, decision must be made between a potential tortuous intraventricular tunnel frequently seen after Rastelli technique, often leading to LVOTO reinterventions/reoperations [4] or extensive aortic root mobilization, necessary with Nikaidoh and even aortic and pulmonary root harvesting with half-turned truncal switch/Mair procedure (HTTS) [5, 6], requiring a high level of surgical expertise [7]. Even though several retrospective comparative studies exist, cohorts are often small, follow-up times short and there is a lack on studies comparing the more complex repair techniques (Nikaidoh and HTTS) [2, 7–9].
The aim of this study was to identify the optimal surgical approach for preventing outflow tract obstructions and to determine potential risk factors for reinterventions/reoperations.
PATIENTS AND METHODS
Study design and patient population
A retrospective, single-centre study was conducted after institutional review board approval (Charité—Universitätsmedizin Berlin, EA2/015/23); informed consent for data analysis was waived due to the study’s retrospective nature.
All patients with complex d-/l-TGA + VSD + LVOTO who underwent anatomic repair (ASO-R, HTTS, Nikaidoh or Rastelli procedure) at our institution between May 1990 and September 2022 were included. LVOTO was defined as either PV Z-score ≤–2.0 or LVOTO peak flow gradient ≥20 mmHg with the presence of subvalvular, valvular or supravalvular obstruction diagnosed by preoperative echocardiogram [2]. Patients not meeting the LVOTO definition and/or undergoing other surgeries were excluded.
Medical records were reviewed and preoperative characteristics, procedural data as well as early/mid-term outcome data were extracted from patients’ electronic charts and analysed.
Study end-points and follow-up
Primary study end-points were early/overall mortality and early postoperative outcome measures. Any death occurring during the same admission was defined as early/in-hospital, whereas 30-day mortality only included deaths during the 1st 30 postoperative days. Overall mortality was also included summarizing all deaths. Early major postoperative complications (renal failure requiring dialysis, heart block requiring pacemaker implantation, persisting neurological impairment, postoperative mechanical circulatory support, phrenic nerve injury), intensive care unit/total hospital stay were noted [10].
Secondary end-points were mid-term outcome and five-year overall freedom from left ventricular outflow tract (LVOT)/ right ventricular outflow tract (RVOT) reoperations/reinterventions.
Preoperative and postoperative echocardiograms were reviewed and analysed by a paediatric cardiologist blinded to the performed surgical procedure. All reinterventions/operations were recorded. Patients without recent follow-up data (before 2022) were contacted, and available medical records from referring cardiologists were retrieved. Patients or guardians were questioned about the occurrence of heart failure symptoms, all-cause death and reoperations/interventions outside our institution since their last visit. Patients were considered ‘lost in follow-up’ if no data were available after discharge from our institution or patient could not be contacted.
Surgical decision process
The technique of surgical repair was chosen for each patient individually according to the severity of present LVOTO, PV and coronary artery anatomy. All procedures were part of the usual clinical practice. In general, in patients with mild LVOTO, ASO-R was the preferred strategy. If moderate–severe LVOTO was present, alternative surgical techniques were required. In case of RVOT-crossing coronary arteries, Rastelli procedure was chosen. If coronary anatomy was favourable, either Nikaidoh or HTTS was performed, depending on the suitability of the PV for a valve sparing. See Fig. 1 for the institutional decision-making process.
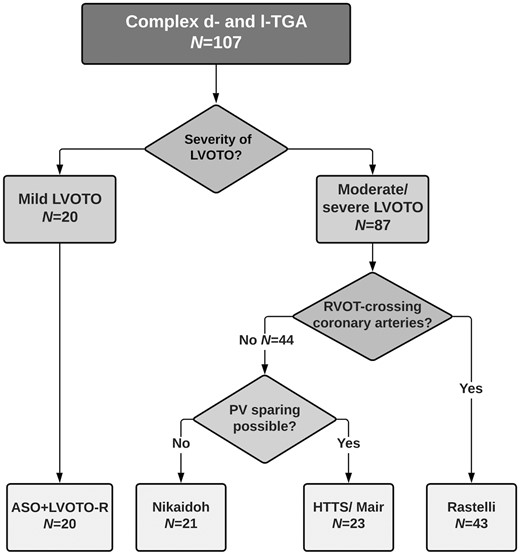
Surgical decision-making algorithm for patients with complex transposition of the great arteries (TGA). Depiction of our institutional decision-making algorithm for patients with complex dextro (d)- and levo (l)-TGA with left ventricular outflow tract obstruction (LVOTO) and ventricular septal defect (VSD). HTTS: half-turned truncal switch; PV: pulmonary valve; RVOT: right ventricular outflow tract.
Statistical analysis
Patients were grouped by the type of surgery and analysed using Kruskal–Wallis test with post hoc pairwise comparison. Additional subgroup analyses were performed for patients undergoing Rastelli procedure versus patients undergoing one of the other surgical techniques (non-Rastelli group) as well as for d-TGA versus l-TGA patients using the Mann–Whitney U-test.
Data are presented as median (interquartile range) for continuous data and as frequency (%) for categorical data. Survival and freedom from reoperation/catheter reintervention were analysed using Kaplan–Meier time-to-event models; survival was compared using the log-rank test. Multivariable logistic regression was implemented to identify independent associations between risk factors and RVOT/LVOT reoperations/reinterventions. A full traditional multivariable model was fitted to adjust for covariates, and results are reported as adjusted odds ratios, 95% confidence intervals and P-values. The model selection strategy was to include clinically important/relevant confounders/baseline covariates (age, weight, d-TGA versus l-TGA morphology, Rastelli versus all other surgical procedures). Statistical analysis was performed using IBM-SPSS version 24.0 (IBM-SPSS Inc, Armonk, NY, USA) and GraphPad Prism version 8.4.0 (GraphPad Software, Inc., San Diego, CA, USA). P-values <0.05 were considered statistically significant.
RESULTS
Baseline characteristics and preoperative procedures
A total of 107 patients were included in the study undergoing following surgeries: ASO-R (n = 20), HTTS (n = 23), Nikaidoh (n = 21) and Rastelli (n = 43). Out of these 107 patients, 85 (79.4%) had d-TGA and 22 l-TGA (20.6%) morphology (ASO-R n = 13/n = 7, HTTS n = 18/n = 5, Nikaidoh n = 19/n = 2 and Rastelli n = 35/n = 8 d-TGA/l-TGA). Median age and weight were 1.0 year (0.5–2.5) and 9.2 kg (6.6–12.4) at surgery, respectively. Patients in Rastelli group were statistically significantly older (P = 0.05) (Table 1).
| Variable . | ASO-R (n = 20) . | HTTS (n = 23) . | Nikaidoh (n = 21) . | Rastelli (n = 43) . | P-values . |
|---|---|---|---|---|---|
| Male gender, n (%) | 13 (65) | 18 (78.3) | 11 (52.4) | 28 (65.1) | 0.4 |
| Body weight (kg), median (IQR) | 7.6 (5.4–9.4) | 8 (6–10.3) | 9.2 (7.2–13.9) | 10.5 (7.5–18.1) | <0.05 |
| Age at surgery (years), median (IQR) | 0.7 (0.3–1) | 0.7 (0.5–1.6) | 0.8 (0.5–3.4) | 1.8 (0.6–6.7) | <0.01 |
| BSA (m², Boyd formula), median (IQR) | 0.4 (0.3–0.4) | 0.4 (0.3–0.5) | 0.5 (0.4–0.6) | 0.5 (0.4–0.8) | 0.1 |
| Follow-up (years), median (IQR) | 2.1 (1–5.5) | 0.7 (0.1–4.5) | 5.8 (0.3–10.8) | 5.9 (0.5–14.8) | 0.1 |
| l-TGA, n (%) | 7 (35) | 5 (21.7) | 2 (9.5) | 8 (18.6) | 0.3 |
| Preterm birth, n (%) | 0 (0) | 4 (17.4) | 1 (4.8) | 0 (0) | 0.01 |
| Genetic disorder, n (%) | 1 (5) | 1 (4.3) | 1 (4.8) | 2 (4.7) | 0.9 |
| Situs inversus, n (%) | 0 (0) | 2 (8.7) | 1 (4.8) | 6 (14) | 0.3 |
| Right aortic arch, n (%) | 1 (5) | 2 (8.7) | 1 (4.8) | 10 (23.3) | 0.1 |
| Interrupted IVC, n (%) | 0 (0) | 0 (0) | 1 (4.8) | 1 (2.3) | 0.6 |
| Echocardiographic characteristics | |||||
| Level of LVOTO/PV stenosis | |||||
| Supravalvular, n (%) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0.2 |
| Valvular, n (%) | 3 (15) | 2 (8.7) | 0 (0) | 4 (9.3) | 0.6 |
| Infundibular, n (%) | 8 (40) | 3 (13) | 0 (0) | 2 (4.7) | <0.01 |
| Multilevel, n (%) | 8 (40) | 18 (78.3) | 15 (71.4) | 27 (62.7) | 0.04 |
| Bicuspid PV, n (%) | 6 (30) | 7 (30.4) | 7 (33.3) | 7 (16.3) | 0.2 |
| Pulmonary atresia, n (%) | 0 (0) | 0 (0) | 6 (28.6) | 10 (23.3) | <0.01 |
| PV Z-Score, median (IQR) | –1.2 (–2.1 to 0.6) | –3.1 (–4.2 to –2) | –4.3 (–4.7 to –3) | –3.6 (–5 to –2.4) | <0.01 |
| AoV Z-Score, median (IQR) | 3.9 (2.6–5.4) | 3.7 (2.5–6.3) | 4.2 (2.7–5.3) | 4.8 (3–7.2) | 0.5 |
| LVOT peak gradient (mmHg), median (IQR) | 60 (41.5–80) | 70 (61.5–85) | 70 (61–84) | 75 (66–81) | 0.1 |
| RVOT peak gradient (mmHg), median (IQR) | 3.5 (3–6) | 4 (3–5) | 4 (3–6) | 6 (4–7.5) | 0.1 |
| Variable . | ASO-R (n = 20) . | HTTS (n = 23) . | Nikaidoh (n = 21) . | Rastelli (n = 43) . | P-values . |
|---|---|---|---|---|---|
| Male gender, n (%) | 13 (65) | 18 (78.3) | 11 (52.4) | 28 (65.1) | 0.4 |
| Body weight (kg), median (IQR) | 7.6 (5.4–9.4) | 8 (6–10.3) | 9.2 (7.2–13.9) | 10.5 (7.5–18.1) | <0.05 |
| Age at surgery (years), median (IQR) | 0.7 (0.3–1) | 0.7 (0.5–1.6) | 0.8 (0.5–3.4) | 1.8 (0.6–6.7) | <0.01 |
| BSA (m², Boyd formula), median (IQR) | 0.4 (0.3–0.4) | 0.4 (0.3–0.5) | 0.5 (0.4–0.6) | 0.5 (0.4–0.8) | 0.1 |
| Follow-up (years), median (IQR) | 2.1 (1–5.5) | 0.7 (0.1–4.5) | 5.8 (0.3–10.8) | 5.9 (0.5–14.8) | 0.1 |
| l-TGA, n (%) | 7 (35) | 5 (21.7) | 2 (9.5) | 8 (18.6) | 0.3 |
| Preterm birth, n (%) | 0 (0) | 4 (17.4) | 1 (4.8) | 0 (0) | 0.01 |
| Genetic disorder, n (%) | 1 (5) | 1 (4.3) | 1 (4.8) | 2 (4.7) | 0.9 |
| Situs inversus, n (%) | 0 (0) | 2 (8.7) | 1 (4.8) | 6 (14) | 0.3 |
| Right aortic arch, n (%) | 1 (5) | 2 (8.7) | 1 (4.8) | 10 (23.3) | 0.1 |
| Interrupted IVC, n (%) | 0 (0) | 0 (0) | 1 (4.8) | 1 (2.3) | 0.6 |
| Echocardiographic characteristics | |||||
| Level of LVOTO/PV stenosis | |||||
| Supravalvular, n (%) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0.2 |
| Valvular, n (%) | 3 (15) | 2 (8.7) | 0 (0) | 4 (9.3) | 0.6 |
| Infundibular, n (%) | 8 (40) | 3 (13) | 0 (0) | 2 (4.7) | <0.01 |
| Multilevel, n (%) | 8 (40) | 18 (78.3) | 15 (71.4) | 27 (62.7) | 0.04 |
| Bicuspid PV, n (%) | 6 (30) | 7 (30.4) | 7 (33.3) | 7 (16.3) | 0.2 |
| Pulmonary atresia, n (%) | 0 (0) | 0 (0) | 6 (28.6) | 10 (23.3) | <0.01 |
| PV Z-Score, median (IQR) | –1.2 (–2.1 to 0.6) | –3.1 (–4.2 to –2) | –4.3 (–4.7 to –3) | –3.6 (–5 to –2.4) | <0.01 |
| AoV Z-Score, median (IQR) | 3.9 (2.6–5.4) | 3.7 (2.5–6.3) | 4.2 (2.7–5.3) | 4.8 (3–7.2) | 0.5 |
| LVOT peak gradient (mmHg), median (IQR) | 60 (41.5–80) | 70 (61.5–85) | 70 (61–84) | 75 (66–81) | 0.1 |
| RVOT peak gradient (mmHg), median (IQR) | 3.5 (3–6) | 4 (3–5) | 4 (3–6) | 6 (4–7.5) | 0.1 |
AoV: aortic valve; ASO-R: arterial switch operation with resection of LVOTO; BSA: body surface area; HTTS: half-turned truncal switch; IQR: interquartile range; IVC: inferior vena cava; l-TGA: levo-transposition of the great arteries; LVOT: left ventricular outflow tract; LVOTO: left ventricular outflow tract obstruction; PV: pulmonary valve; RVOT: right ventricular outflow tract.
Significant P-values in bold.
| Variable . | ASO-R (n = 20) . | HTTS (n = 23) . | Nikaidoh (n = 21) . | Rastelli (n = 43) . | P-values . |
|---|---|---|---|---|---|
| Male gender, n (%) | 13 (65) | 18 (78.3) | 11 (52.4) | 28 (65.1) | 0.4 |
| Body weight (kg), median (IQR) | 7.6 (5.4–9.4) | 8 (6–10.3) | 9.2 (7.2–13.9) | 10.5 (7.5–18.1) | <0.05 |
| Age at surgery (years), median (IQR) | 0.7 (0.3–1) | 0.7 (0.5–1.6) | 0.8 (0.5–3.4) | 1.8 (0.6–6.7) | <0.01 |
| BSA (m², Boyd formula), median (IQR) | 0.4 (0.3–0.4) | 0.4 (0.3–0.5) | 0.5 (0.4–0.6) | 0.5 (0.4–0.8) | 0.1 |
| Follow-up (years), median (IQR) | 2.1 (1–5.5) | 0.7 (0.1–4.5) | 5.8 (0.3–10.8) | 5.9 (0.5–14.8) | 0.1 |
| l-TGA, n (%) | 7 (35) | 5 (21.7) | 2 (9.5) | 8 (18.6) | 0.3 |
| Preterm birth, n (%) | 0 (0) | 4 (17.4) | 1 (4.8) | 0 (0) | 0.01 |
| Genetic disorder, n (%) | 1 (5) | 1 (4.3) | 1 (4.8) | 2 (4.7) | 0.9 |
| Situs inversus, n (%) | 0 (0) | 2 (8.7) | 1 (4.8) | 6 (14) | 0.3 |
| Right aortic arch, n (%) | 1 (5) | 2 (8.7) | 1 (4.8) | 10 (23.3) | 0.1 |
| Interrupted IVC, n (%) | 0 (0) | 0 (0) | 1 (4.8) | 1 (2.3) | 0.6 |
| Echocardiographic characteristics | |||||
| Level of LVOTO/PV stenosis | |||||
| Supravalvular, n (%) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0.2 |
| Valvular, n (%) | 3 (15) | 2 (8.7) | 0 (0) | 4 (9.3) | 0.6 |
| Infundibular, n (%) | 8 (40) | 3 (13) | 0 (0) | 2 (4.7) | <0.01 |
| Multilevel, n (%) | 8 (40) | 18 (78.3) | 15 (71.4) | 27 (62.7) | 0.04 |
| Bicuspid PV, n (%) | 6 (30) | 7 (30.4) | 7 (33.3) | 7 (16.3) | 0.2 |
| Pulmonary atresia, n (%) | 0 (0) | 0 (0) | 6 (28.6) | 10 (23.3) | <0.01 |
| PV Z-Score, median (IQR) | –1.2 (–2.1 to 0.6) | –3.1 (–4.2 to –2) | –4.3 (–4.7 to –3) | –3.6 (–5 to –2.4) | <0.01 |
| AoV Z-Score, median (IQR) | 3.9 (2.6–5.4) | 3.7 (2.5–6.3) | 4.2 (2.7–5.3) | 4.8 (3–7.2) | 0.5 |
| LVOT peak gradient (mmHg), median (IQR) | 60 (41.5–80) | 70 (61.5–85) | 70 (61–84) | 75 (66–81) | 0.1 |
| RVOT peak gradient (mmHg), median (IQR) | 3.5 (3–6) | 4 (3–5) | 4 (3–6) | 6 (4–7.5) | 0.1 |
| Variable . | ASO-R (n = 20) . | HTTS (n = 23) . | Nikaidoh (n = 21) . | Rastelli (n = 43) . | P-values . |
|---|---|---|---|---|---|
| Male gender, n (%) | 13 (65) | 18 (78.3) | 11 (52.4) | 28 (65.1) | 0.4 |
| Body weight (kg), median (IQR) | 7.6 (5.4–9.4) | 8 (6–10.3) | 9.2 (7.2–13.9) | 10.5 (7.5–18.1) | <0.05 |
| Age at surgery (years), median (IQR) | 0.7 (0.3–1) | 0.7 (0.5–1.6) | 0.8 (0.5–3.4) | 1.8 (0.6–6.7) | <0.01 |
| BSA (m², Boyd formula), median (IQR) | 0.4 (0.3–0.4) | 0.4 (0.3–0.5) | 0.5 (0.4–0.6) | 0.5 (0.4–0.8) | 0.1 |
| Follow-up (years), median (IQR) | 2.1 (1–5.5) | 0.7 (0.1–4.5) | 5.8 (0.3–10.8) | 5.9 (0.5–14.8) | 0.1 |
| l-TGA, n (%) | 7 (35) | 5 (21.7) | 2 (9.5) | 8 (18.6) | 0.3 |
| Preterm birth, n (%) | 0 (0) | 4 (17.4) | 1 (4.8) | 0 (0) | 0.01 |
| Genetic disorder, n (%) | 1 (5) | 1 (4.3) | 1 (4.8) | 2 (4.7) | 0.9 |
| Situs inversus, n (%) | 0 (0) | 2 (8.7) | 1 (4.8) | 6 (14) | 0.3 |
| Right aortic arch, n (%) | 1 (5) | 2 (8.7) | 1 (4.8) | 10 (23.3) | 0.1 |
| Interrupted IVC, n (%) | 0 (0) | 0 (0) | 1 (4.8) | 1 (2.3) | 0.6 |
| Echocardiographic characteristics | |||||
| Level of LVOTO/PV stenosis | |||||
| Supravalvular, n (%) | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 0.2 |
| Valvular, n (%) | 3 (15) | 2 (8.7) | 0 (0) | 4 (9.3) | 0.6 |
| Infundibular, n (%) | 8 (40) | 3 (13) | 0 (0) | 2 (4.7) | <0.01 |
| Multilevel, n (%) | 8 (40) | 18 (78.3) | 15 (71.4) | 27 (62.7) | 0.04 |
| Bicuspid PV, n (%) | 6 (30) | 7 (30.4) | 7 (33.3) | 7 (16.3) | 0.2 |
| Pulmonary atresia, n (%) | 0 (0) | 0 (0) | 6 (28.6) | 10 (23.3) | <0.01 |
| PV Z-Score, median (IQR) | –1.2 (–2.1 to 0.6) | –3.1 (–4.2 to –2) | –4.3 (–4.7 to –3) | –3.6 (–5 to –2.4) | <0.01 |
| AoV Z-Score, median (IQR) | 3.9 (2.6–5.4) | 3.7 (2.5–6.3) | 4.2 (2.7–5.3) | 4.8 (3–7.2) | 0.5 |
| LVOT peak gradient (mmHg), median (IQR) | 60 (41.5–80) | 70 (61.5–85) | 70 (61–84) | 75 (66–81) | 0.1 |
| RVOT peak gradient (mmHg), median (IQR) | 3.5 (3–6) | 4 (3–5) | 4 (3–6) | 6 (4–7.5) | 0.1 |
AoV: aortic valve; ASO-R: arterial switch operation with resection of LVOTO; BSA: body surface area; HTTS: half-turned truncal switch; IQR: interquartile range; IVC: inferior vena cava; l-TGA: levo-transposition of the great arteries; LVOT: left ventricular outflow tract; LVOTO: left ventricular outflow tract obstruction; PV: pulmonary valve; RVOT: right ventricular outflow tract.
Significant P-values in bold.
A total of 91 previous surgeries were performed in 59 patients (59/107; 55.1%) as well as 76 prior catheter interventions in 53 patients (53/107; 49.5%). Both, previous surgeries and catheter interventions were performed in 31 patients (31/107; 29%). No statistically significant differences in the frequency of prior surgeries were found between the surgical groups (P = 0.3, Supplementary Material, Table S1). However, systemic-to-pulmonary shunt operations were more frequent in Nikaidoh and Rastelli groups (n = 14; 66.7%, n = 20; 46,5%, P < 0.01), and pulmonary artery banding (PAB) was performed more often in the ASO-R group (n = 6; 30%, P = 0.01). Prior PAB (n = 9) was mainly performed in l-TGA patients (5/9; 55.6%) to train the morphological left ventricle to undergo double-switch procedure. Preoperative echocardiographic data can be found in Table 1.
Intraoperative and early postoperative outcome
There was no difference in total operating time between the 4 surgeries (P = 0.4); however, cardiopulmonary bypass/cross-clamp times were statistically significantly longer in ASO-R and HTTS (P = 0.03 and <0.01). The differences were still noticeable after excluding l-TGA patients receiving additional atrial switch operation. Concomitant procedures were frequent with no statistically significant differences between groups (P > 0.1). RVOT reconstruction was performed with a valved heterograft conduit in various sizes (12–22 mm) in all Nikaidoh and Rastelli patients. In the HTTS group, RVOT reconstruction was performed by PV commissurotomy (n = 11), monocusp creation (n = 2), transannular patch (n = 7) or relief of infundibular stenosis (n = 3). Among the entire study population, 30-day mortality was 6.5% (7/107) with no differences between techniques (P = 0.9). In-hospital mortality was 11.2% (12/107) and did not differ among groups (P = 0.9). Intensive care unit/total hospital stay, ventilation time and major postoperative complications did also not differ among techniques (P > 0.3) (Table 2, Supplementary Material, Table S2).
| Variable . | ASO-R (n = 20) . | HTTS (n = 23) . | Nikaidoh (n = 21) . | Rastelli (n = 43) . | P-value . |
|---|---|---|---|---|---|
| Total op. time (min), median (IQR) | 436 (330–633) | 433 (367–580) | 380 (290.5–490.5) | 405 (295–515) | 0.4 |
| CPB time (min), median (IQR) | 271 (231–371) | 267 (234–370) | 228 (184.5–287) | 234 (173–285) | 0.03 |
| CC time (min), median (IQR) | 175 (127–213) | 171 (151–222) | 147 (129.5–177) | 116 (97–141) | <0.01 |
| Reperfusion time (min), median (IQR) | 77.5 (59.8–101) | 80 (71–100) | 73 (45–100) | 88 (52–120) | 0.5 |
| Temperature (°C), median (IQR) | 28 (24–30) | 28 (24–28) | 24 (19–28) | 28 (24–28) | 0.1 |
| Extubation (POD), median (IQR) | 4 (3–6) | 6 (1.5–9) | 1 (1–5) | 3 (1–8) | 0.3 |
| ICU stay (days), median (IQR) | 6 (4–8) | 8 (4–19) | 7 (4–12) | 6 (3–14) | 0.3 |
| Hospital stay (days), median (IQR) | 12 (9–15) | 14 (9–26) | 11 (9–21) | 15 (9.5–21) | 0.6 |
| 30-Days mortality, n (%) | 1 (5.0) | 1 (4.3) | 2 (9.5) | 3 (7.0) | 0.9 |
| In-hospital mortality, n (%) | 2 (10.0) | 3 (13.0) | 3 (14.3) | 4 (9.3) | 0.9 |
| Concomitant procedures, n (%) | |||||
| Atrial switch | 7 (35.0) | 5 (21.7) | 2 (9.5) | 8 (18.6) | 0.3 |
| MV repair | 0 (0.0) | 2 (8.7) | 1 (4.8) | 4 (9.3) | 0.5 |
| TV repair | 5 (25.0) | 2 (8.7) | 1 (4.8) | 3 (7.0) | 0.1 |
| PA enlargement | 6 (30.0) | 4 (17.4) | 4 (19.0) | 16 (37.2) | 0.3 |
| CoA repair | 1 (5.0) | 0 (0.0) | 0 (0.0) | 1 (2.3) | 0.6 |
| Variable . | ASO-R (n = 20) . | HTTS (n = 23) . | Nikaidoh (n = 21) . | Rastelli (n = 43) . | P-value . |
|---|---|---|---|---|---|
| Total op. time (min), median (IQR) | 436 (330–633) | 433 (367–580) | 380 (290.5–490.5) | 405 (295–515) | 0.4 |
| CPB time (min), median (IQR) | 271 (231–371) | 267 (234–370) | 228 (184.5–287) | 234 (173–285) | 0.03 |
| CC time (min), median (IQR) | 175 (127–213) | 171 (151–222) | 147 (129.5–177) | 116 (97–141) | <0.01 |
| Reperfusion time (min), median (IQR) | 77.5 (59.8–101) | 80 (71–100) | 73 (45–100) | 88 (52–120) | 0.5 |
| Temperature (°C), median (IQR) | 28 (24–30) | 28 (24–28) | 24 (19–28) | 28 (24–28) | 0.1 |
| Extubation (POD), median (IQR) | 4 (3–6) | 6 (1.5–9) | 1 (1–5) | 3 (1–8) | 0.3 |
| ICU stay (days), median (IQR) | 6 (4–8) | 8 (4–19) | 7 (4–12) | 6 (3–14) | 0.3 |
| Hospital stay (days), median (IQR) | 12 (9–15) | 14 (9–26) | 11 (9–21) | 15 (9.5–21) | 0.6 |
| 30-Days mortality, n (%) | 1 (5.0) | 1 (4.3) | 2 (9.5) | 3 (7.0) | 0.9 |
| In-hospital mortality, n (%) | 2 (10.0) | 3 (13.0) | 3 (14.3) | 4 (9.3) | 0.9 |
| Concomitant procedures, n (%) | |||||
| Atrial switch | 7 (35.0) | 5 (21.7) | 2 (9.5) | 8 (18.6) | 0.3 |
| MV repair | 0 (0.0) | 2 (8.7) | 1 (4.8) | 4 (9.3) | 0.5 |
| TV repair | 5 (25.0) | 2 (8.7) | 1 (4.8) | 3 (7.0) | 0.1 |
| PA enlargement | 6 (30.0) | 4 (17.4) | 4 (19.0) | 16 (37.2) | 0.3 |
| CoA repair | 1 (5.0) | 0 (0.0) | 0 (0.0) | 1 (2.3) | 0.6 |
ASO-R: arterial switch operation with resection of LVOTO; CC: cross-clamp; CoA: aortic coarctation; CPB: cardiopulmonary bypass; HTTS: half-turned truncal switch; ICU: intensive care unit; IQR: interquartile range; MV: mitral valve; PA: pulmonary artery; POD: postoperative day; TV: tricuspid valve.
Significant P-values in bold.
| Variable . | ASO-R (n = 20) . | HTTS (n = 23) . | Nikaidoh (n = 21) . | Rastelli (n = 43) . | P-value . |
|---|---|---|---|---|---|
| Total op. time (min), median (IQR) | 436 (330–633) | 433 (367–580) | 380 (290.5–490.5) | 405 (295–515) | 0.4 |
| CPB time (min), median (IQR) | 271 (231–371) | 267 (234–370) | 228 (184.5–287) | 234 (173–285) | 0.03 |
| CC time (min), median (IQR) | 175 (127–213) | 171 (151–222) | 147 (129.5–177) | 116 (97–141) | <0.01 |
| Reperfusion time (min), median (IQR) | 77.5 (59.8–101) | 80 (71–100) | 73 (45–100) | 88 (52–120) | 0.5 |
| Temperature (°C), median (IQR) | 28 (24–30) | 28 (24–28) | 24 (19–28) | 28 (24–28) | 0.1 |
| Extubation (POD), median (IQR) | 4 (3–6) | 6 (1.5–9) | 1 (1–5) | 3 (1–8) | 0.3 |
| ICU stay (days), median (IQR) | 6 (4–8) | 8 (4–19) | 7 (4–12) | 6 (3–14) | 0.3 |
| Hospital stay (days), median (IQR) | 12 (9–15) | 14 (9–26) | 11 (9–21) | 15 (9.5–21) | 0.6 |
| 30-Days mortality, n (%) | 1 (5.0) | 1 (4.3) | 2 (9.5) | 3 (7.0) | 0.9 |
| In-hospital mortality, n (%) | 2 (10.0) | 3 (13.0) | 3 (14.3) | 4 (9.3) | 0.9 |
| Concomitant procedures, n (%) | |||||
| Atrial switch | 7 (35.0) | 5 (21.7) | 2 (9.5) | 8 (18.6) | 0.3 |
| MV repair | 0 (0.0) | 2 (8.7) | 1 (4.8) | 4 (9.3) | 0.5 |
| TV repair | 5 (25.0) | 2 (8.7) | 1 (4.8) | 3 (7.0) | 0.1 |
| PA enlargement | 6 (30.0) | 4 (17.4) | 4 (19.0) | 16 (37.2) | 0.3 |
| CoA repair | 1 (5.0) | 0 (0.0) | 0 (0.0) | 1 (2.3) | 0.6 |
| Variable . | ASO-R (n = 20) . | HTTS (n = 23) . | Nikaidoh (n = 21) . | Rastelli (n = 43) . | P-value . |
|---|---|---|---|---|---|
| Total op. time (min), median (IQR) | 436 (330–633) | 433 (367–580) | 380 (290.5–490.5) | 405 (295–515) | 0.4 |
| CPB time (min), median (IQR) | 271 (231–371) | 267 (234–370) | 228 (184.5–287) | 234 (173–285) | 0.03 |
| CC time (min), median (IQR) | 175 (127–213) | 171 (151–222) | 147 (129.5–177) | 116 (97–141) | <0.01 |
| Reperfusion time (min), median (IQR) | 77.5 (59.8–101) | 80 (71–100) | 73 (45–100) | 88 (52–120) | 0.5 |
| Temperature (°C), median (IQR) | 28 (24–30) | 28 (24–28) | 24 (19–28) | 28 (24–28) | 0.1 |
| Extubation (POD), median (IQR) | 4 (3–6) | 6 (1.5–9) | 1 (1–5) | 3 (1–8) | 0.3 |
| ICU stay (days), median (IQR) | 6 (4–8) | 8 (4–19) | 7 (4–12) | 6 (3–14) | 0.3 |
| Hospital stay (days), median (IQR) | 12 (9–15) | 14 (9–26) | 11 (9–21) | 15 (9.5–21) | 0.6 |
| 30-Days mortality, n (%) | 1 (5.0) | 1 (4.3) | 2 (9.5) | 3 (7.0) | 0.9 |
| In-hospital mortality, n (%) | 2 (10.0) | 3 (13.0) | 3 (14.3) | 4 (9.3) | 0.9 |
| Concomitant procedures, n (%) | |||||
| Atrial switch | 7 (35.0) | 5 (21.7) | 2 (9.5) | 8 (18.6) | 0.3 |
| MV repair | 0 (0.0) | 2 (8.7) | 1 (4.8) | 4 (9.3) | 0.5 |
| TV repair | 5 (25.0) | 2 (8.7) | 1 (4.8) | 3 (7.0) | 0.1 |
| PA enlargement | 6 (30.0) | 4 (17.4) | 4 (19.0) | 16 (37.2) | 0.3 |
| CoA repair | 1 (5.0) | 0 (0.0) | 0 (0.0) | 1 (2.3) | 0.6 |
ASO-R: arterial switch operation with resection of LVOTO; CC: cross-clamp; CoA: aortic coarctation; CPB: cardiopulmonary bypass; HTTS: half-turned truncal switch; ICU: intensive care unit; IQR: interquartile range; MV: mitral valve; PA: pulmonary artery; POD: postoperative day; TV: tricuspid valve.
Significant P-values in bold.
Follow-up
Clinical follow-up after discharge was available for 92 patients (overall data-completeness 85.9%) with 15 patients lost in follow-up, mostly foreign patients returning to their home countries (9/15; 60%). Median follow-up was 3.8 years (0.3–10.5) in the entire cohort without statistically significant differences between groups (P = 0.06, Table 1).
Midterm outcome
Survival
Overall mortality was 20.6% (22/107) with 6 late deaths, occurring >1 year post initially surgery. Causes of late death were chronic heart failure (n = 3), reoperation mortality (n = 2) and out-of-hospital unknown death (n = 1) (details are provided in Supplementary Material, Table S3).
Overall survival rates at 5 years were 78.9% (53.2–91.5) after ASO-R, 75.3% (46.8–89.9) after HTTS, 85% (60.4–94.9) after Nikaidoh and 83.9% (67.5–92.5) after Rastelli procedure (P = 0.9), respectively. Comparing d- versus l-TGA patients as well as Rastelli versus non-Rastelli patients, there was no survival difference (Fig. 2A and B).
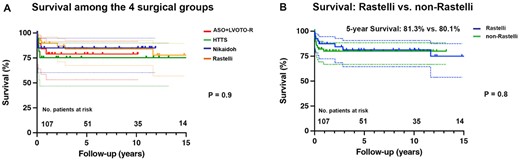
Survival of patients with complex transposition of the great arteries (TGA). (A) Survival of patients with complex dextro (d)- and levo (l)-TGA after different surgical repair techniques: arterial switch operation with resection of left ventricular outflow tract obstruction (ASO-R), half-turned truncal switch (HTTS), Nikaidoh and Rastelli procedure. (B) Survival after Rastelli versus non-Rastelli procedures. No.: number.
Right ventricular outflow tract/left ventricular outflow tract reinterventions/reoperations
Catheter reinterventions were frequent with a total of 27/107 patients (25.2%) receiving 87 RVOT reinterventions (Rastelli 17/43, 39.5%, Nikaidoh 5/21, 23.8%, HTTS 4/23, 17.4% and ASO-R 1/20, 5%, P = 0.02; Table 3). Overall freedom from RVOT reintervention at 5 years was highest in the ASO-R group [94.4% (66.6–99.2) versus 69.1% (25.4–90.5) in HTTS, 67.8% (34–86.9) in Nikaidoh and 64.4% (44.6–78.7) in Rastelli group, respectively); however, not statistically significant (P = 0.2, Fig. 3A). Also comparing Rastelli versus non-Rastelli or d-TGA versus l-TGA, five-year overall freedom from RVOT reintervention was not different [64.4% (44.6–78.7) vs 76.9% (57.5–88.3), P = 0.2 and 67.7% (53.2–78.8) vs 89.3% (63.2–97.2), P = 0.2, respectively].
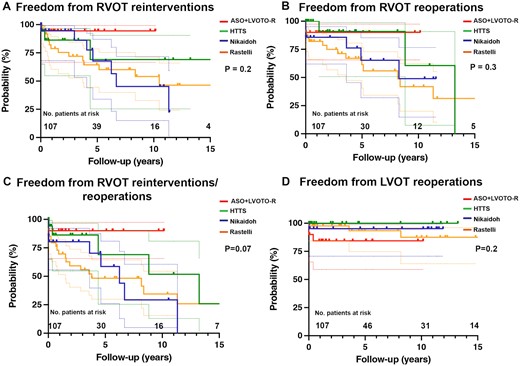
Freedom from right/left outflow tract (RVOT/LVOT) reinterventions/reoperations in patients with complex transposition of the great arteries (TGA). (A) Freedom from RVOT reinterventions of complex dextro (d)- and levo (l)-TGA patients after different surgical repair techniques: arterial switch operation with resection of left ventricular outflow tract obstruction (ASO-R), half-turned truncal switch (HTTS), Nikaidoh and Rastelli procedure. (B) Freedom from RVOT reoperations of complex d-/l-TGA patients after different surgical repair. (C) Freedom from RVOT reinterventions/reoperations of complex d-/l-TGA patients. (D) Freedom from LVOT reoperations of complex d-/l-TGA patients. No.: number.
| Type of procedure . | ASO-R (n = 20) . | HTTS (n = 23) . | Nikaidoh (n = 21) . | Rastelli (n = 43) . | P-value . | |
|---|---|---|---|---|---|---|
| Reoperations, n (%) | ||||||
| All | 3 (15.0) | 3 (13.0) | 7 (33.3) | 16 (37.2) | 0.1 | |
| LVOTO resection | 2 (10.0) | 0 (0.0) | 0 (0.0) | 3 (7.0) | 0.3 | |
| AoV replacement | 1 (5.0) | 0 (0.0) | 1 (4.8) | 0 (0.0) | 0.4 | |
| RVOTO resection | 0 (0.0) | 0 (0.0) | 3 (14.3) | 4 (9.3) | 0.1 | |
| PV replacement | 0 (0.0) | 3 (13.0) | 6 (28.6) | 14 (32.6) | 0.02 | |
| PA enlargement | 2 (10.0) | 1 (4.3) | 2 (9.5) | 6 (14.0) | 0.7 | |
| Catheter reinterventions, n (%) | ||||||
| RVOT BD | 1 (5.0) | 3 (13.0) | 7 (33.3) | 14 (32.6) | 0.04 | |
| RVOT stent | 0 (0.0) | 2 (8.7) | 3 (14.3) | 6 (14.0) | 0.4 | |
| Percutaneous PV implantation | 0 (0.0) | 2 (8.7) | 4 (19.0) | 5 (11.6) | 0.3 | |
| MPA/LPA/RPA BD | 1 (5.0) | 3 (13.0) | 3 (14.3) | 18 (41.9) | <0.01 | |
| MPA/LPA/RPA stent | 0 (0.0) | 2 (8.7) | 2 (9.5) | 11 (25.6) | 0.03 | |
| Type of procedure . | ASO-R (n = 20) . | HTTS (n = 23) . | Nikaidoh (n = 21) . | Rastelli (n = 43) . | P-value . | |
|---|---|---|---|---|---|---|
| Reoperations, n (%) | ||||||
| All | 3 (15.0) | 3 (13.0) | 7 (33.3) | 16 (37.2) | 0.1 | |
| LVOTO resection | 2 (10.0) | 0 (0.0) | 0 (0.0) | 3 (7.0) | 0.3 | |
| AoV replacement | 1 (5.0) | 0 (0.0) | 1 (4.8) | 0 (0.0) | 0.4 | |
| RVOTO resection | 0 (0.0) | 0 (0.0) | 3 (14.3) | 4 (9.3) | 0.1 | |
| PV replacement | 0 (0.0) | 3 (13.0) | 6 (28.6) | 14 (32.6) | 0.02 | |
| PA enlargement | 2 (10.0) | 1 (4.3) | 2 (9.5) | 6 (14.0) | 0.7 | |
| Catheter reinterventions, n (%) | ||||||
| RVOT BD | 1 (5.0) | 3 (13.0) | 7 (33.3) | 14 (32.6) | 0.04 | |
| RVOT stent | 0 (0.0) | 2 (8.7) | 3 (14.3) | 6 (14.0) | 0.4 | |
| Percutaneous PV implantation | 0 (0.0) | 2 (8.7) | 4 (19.0) | 5 (11.6) | 0.3 | |
| MPA/LPA/RPA BD | 1 (5.0) | 3 (13.0) | 3 (14.3) | 18 (41.9) | <0.01 | |
| MPA/LPA/RPA stent | 0 (0.0) | 2 (8.7) | 2 (9.5) | 11 (25.6) | 0.03 | |
AoV: aortic valve; ASO-R: arterial switch operation with resection of LVOTO; BD: balloon dilatation; HTTS: half-turned truncal switch; LVOT: left ventricular outflow tract; LVOTO: left ventricular outflow tract obstruction; MPA/LPA/RPA: main pulmonary/left/right artery; PA: pulmonary artery; PV: pulmonary valve; RVOT: right ventricular outflow tract; RVOTO: right ventricular outflow tract/obstruction.
Significant P-values in bold.
| Type of procedure . | ASO-R (n = 20) . | HTTS (n = 23) . | Nikaidoh (n = 21) . | Rastelli (n = 43) . | P-value . | |
|---|---|---|---|---|---|---|
| Reoperations, n (%) | ||||||
| All | 3 (15.0) | 3 (13.0) | 7 (33.3) | 16 (37.2) | 0.1 | |
| LVOTO resection | 2 (10.0) | 0 (0.0) | 0 (0.0) | 3 (7.0) | 0.3 | |
| AoV replacement | 1 (5.0) | 0 (0.0) | 1 (4.8) | 0 (0.0) | 0.4 | |
| RVOTO resection | 0 (0.0) | 0 (0.0) | 3 (14.3) | 4 (9.3) | 0.1 | |
| PV replacement | 0 (0.0) | 3 (13.0) | 6 (28.6) | 14 (32.6) | 0.02 | |
| PA enlargement | 2 (10.0) | 1 (4.3) | 2 (9.5) | 6 (14.0) | 0.7 | |
| Catheter reinterventions, n (%) | ||||||
| RVOT BD | 1 (5.0) | 3 (13.0) | 7 (33.3) | 14 (32.6) | 0.04 | |
| RVOT stent | 0 (0.0) | 2 (8.7) | 3 (14.3) | 6 (14.0) | 0.4 | |
| Percutaneous PV implantation | 0 (0.0) | 2 (8.7) | 4 (19.0) | 5 (11.6) | 0.3 | |
| MPA/LPA/RPA BD | 1 (5.0) | 3 (13.0) | 3 (14.3) | 18 (41.9) | <0.01 | |
| MPA/LPA/RPA stent | 0 (0.0) | 2 (8.7) | 2 (9.5) | 11 (25.6) | 0.03 | |
| Type of procedure . | ASO-R (n = 20) . | HTTS (n = 23) . | Nikaidoh (n = 21) . | Rastelli (n = 43) . | P-value . | |
|---|---|---|---|---|---|---|
| Reoperations, n (%) | ||||||
| All | 3 (15.0) | 3 (13.0) | 7 (33.3) | 16 (37.2) | 0.1 | |
| LVOTO resection | 2 (10.0) | 0 (0.0) | 0 (0.0) | 3 (7.0) | 0.3 | |
| AoV replacement | 1 (5.0) | 0 (0.0) | 1 (4.8) | 0 (0.0) | 0.4 | |
| RVOTO resection | 0 (0.0) | 0 (0.0) | 3 (14.3) | 4 (9.3) | 0.1 | |
| PV replacement | 0 (0.0) | 3 (13.0) | 6 (28.6) | 14 (32.6) | 0.02 | |
| PA enlargement | 2 (10.0) | 1 (4.3) | 2 (9.5) | 6 (14.0) | 0.7 | |
| Catheter reinterventions, n (%) | ||||||
| RVOT BD | 1 (5.0) | 3 (13.0) | 7 (33.3) | 14 (32.6) | 0.04 | |
| RVOT stent | 0 (0.0) | 2 (8.7) | 3 (14.3) | 6 (14.0) | 0.4 | |
| Percutaneous PV implantation | 0 (0.0) | 2 (8.7) | 4 (19.0) | 5 (11.6) | 0.3 | |
| MPA/LPA/RPA BD | 1 (5.0) | 3 (13.0) | 3 (14.3) | 18 (41.9) | <0.01 | |
| MPA/LPA/RPA stent | 0 (0.0) | 2 (8.7) | 2 (9.5) | 11 (25.6) | 0.03 | |
AoV: aortic valve; ASO-R: arterial switch operation with resection of LVOTO; BD: balloon dilatation; HTTS: half-turned truncal switch; LVOT: left ventricular outflow tract; LVOTO: left ventricular outflow tract obstruction; MPA/LPA/RPA: main pulmonary/left/right artery; PA: pulmonary artery; PV: pulmonary valve; RVOT: right ventricular outflow tract; RVOTO: right ventricular outflow tract/obstruction.
Significant P-values in bold.
Reoperations occurred in a total of 30/107 patients (28.0%) receiving 41 RVOT reoperations and 7 LVOT reoperations (Table 3). Looking at five-year overall freedom from RVOT reoperations, highest rates were observed in HTTS and ASO-R with 91% (50.8–98.7) and 90.0% (65.6–97.4) compared to Nikaidoh and Rastelli: 65.3% (32.0–85.3) and 67.0% (47.4–80.6); however, this was also statistically not significant (P = 0.3, Fig. 3B).
In addition, combined five-year overall freedom from RVOT reintervention and/or reoperation did not differ statistically significantly (Fig. 3C, P = 0.07) between groups.
Overall freedom from LVOT reoperations at 5 years was 100% in HTTS, 95.2% (70.7–99.3) in Nikaidoh, 93.4% (74.8–98.4) in Rastelli and 84.4% (38.9–94.7) in ASO-R; however, differences were statistically not significant (P = 0.2, Fig. 3D).
Patients after Rastelli technique had lower overall five-year freedom from RVOT/LVOT reoperations compared to non-Rastelli patients, although not statistically significant: 63.4% (43.5–77.8) vs 77.8% (59.8–88.5), P = 0.2. When comparing overall five-year freedom from RVOT/LVOT reoperations between d-TGA patients and l-TGA patients, no statically significant differences (P = 0.8) were found: 70.4% (55.6–81.1) vs 77.3% (53.7–89.8).
Echocardiographic measurements and clinical status at last visit
Echocardiography at last follow-up demonstrated overall well-preserved left ventricular ejection fraction in all groups without statistically significant differences (P = 0.4). Groups did not differ in any echocardiographic parameters except for right ventricular outflow tract obstruction (RVOTO) peak gradient, which was statistically significantly higher after Rastelli (30 mmHg [16–45]) and Nikaidoh (25 mmHg [18–33]) procedures, P = 0.03. No relevant LVOTO or aortic valve regurgitation greater than moderate was found after aortic root translocation (Nikaidoh and HTTS) (Table 4). At last follow-up among all survivors, 1 patient was classified as New York Heart Association functional class III, all other patients were in class I–II.
| Measures . | ASO-R (n = 20) . | HTTS (n = 23) . | Nikaidoh (n = 21) . | Rastelli (n = 43) . | P-value . |
|---|---|---|---|---|---|
| LVOT/AoV | |||||
| LVOT/AoV Pmax (mmHg), median (IQR) | 7.5 (5–16) | 5 (3–9.8) | 7 (4–9) | 5 (4–8.8) | 0.02 |
| LVOT/AoV Vmax (m/s), median (IQR) | 1.3 (1–1.7) | 0.9 (0.8–1) | 1.3 (0.9–1.5) | 1 (0.9–1.3) | 0.02 |
| AR > moderate, n (%) | 2 (10) | 1 (4.3) | 0 (0.0) | 4 (9.3) | 0.5 |
| RVOT/PV | |||||
| PV/RVOT Pmax (mmHg), median (IQR) | 10 (6.5–19.5) | 22 (12.3–32.8) | 24 (14.5–28.5) | 29 (17–45) | <0.01 |
| PV/RVOT Vmax (m/s), median (IQR) | 1.7 (1.3–2.1) | 2.5 (1.9–2.9) | 2.4 (1.9–2.9) | 2.7 (2–3.3) | <0.01 |
| PR > moderate, n (%) | 1 (5) | 4 (17.4) | 2 (9.5) | 5 (11.6) | 0.1 |
| EF (%), median (IQR) | 58.5 (54.3–67.8) | 56 (53.5–61) | 56 (51.8–65.3) | 57 (50–64) | 0.8 |
| Measures . | ASO-R (n = 20) . | HTTS (n = 23) . | Nikaidoh (n = 21) . | Rastelli (n = 43) . | P-value . |
|---|---|---|---|---|---|
| LVOT/AoV | |||||
| LVOT/AoV Pmax (mmHg), median (IQR) | 7.5 (5–16) | 5 (3–9.8) | 7 (4–9) | 5 (4–8.8) | 0.02 |
| LVOT/AoV Vmax (m/s), median (IQR) | 1.3 (1–1.7) | 0.9 (0.8–1) | 1.3 (0.9–1.5) | 1 (0.9–1.3) | 0.02 |
| AR > moderate, n (%) | 2 (10) | 1 (4.3) | 0 (0.0) | 4 (9.3) | 0.5 |
| RVOT/PV | |||||
| PV/RVOT Pmax (mmHg), median (IQR) | 10 (6.5–19.5) | 22 (12.3–32.8) | 24 (14.5–28.5) | 29 (17–45) | <0.01 |
| PV/RVOT Vmax (m/s), median (IQR) | 1.7 (1.3–2.1) | 2.5 (1.9–2.9) | 2.4 (1.9–2.9) | 2.7 (2–3.3) | <0.01 |
| PR > moderate, n (%) | 1 (5) | 4 (17.4) | 2 (9.5) | 5 (11.6) | 0.1 |
| EF (%), median (IQR) | 58.5 (54.3–67.8) | 56 (53.5–61) | 56 (51.8–65.3) | 57 (50–64) | 0.8 |
AoV: aortic valve; ASO-R: arterial switch operation with resection of LVOTO; AR: aortic valve regurgitation; EF: ejection fraction in %; IQR: interquartile range; LVOT: left ventricular outflow tract; LVOTO: left ventricular outflow tract obstruction; HTTS: half-turned truncal switch; Pmax: peak gradient; PR: pulmonary valve regurgitation; PV: pulmonary valve; RVOT: right ventricular outflow tract; Vmax: peak velocity.
Significant P-values in bold.
| Measures . | ASO-R (n = 20) . | HTTS (n = 23) . | Nikaidoh (n = 21) . | Rastelli (n = 43) . | P-value . |
|---|---|---|---|---|---|
| LVOT/AoV | |||||
| LVOT/AoV Pmax (mmHg), median (IQR) | 7.5 (5–16) | 5 (3–9.8) | 7 (4–9) | 5 (4–8.8) | 0.02 |
| LVOT/AoV Vmax (m/s), median (IQR) | 1.3 (1–1.7) | 0.9 (0.8–1) | 1.3 (0.9–1.5) | 1 (0.9–1.3) | 0.02 |
| AR > moderate, n (%) | 2 (10) | 1 (4.3) | 0 (0.0) | 4 (9.3) | 0.5 |
| RVOT/PV | |||||
| PV/RVOT Pmax (mmHg), median (IQR) | 10 (6.5–19.5) | 22 (12.3–32.8) | 24 (14.5–28.5) | 29 (17–45) | <0.01 |
| PV/RVOT Vmax (m/s), median (IQR) | 1.7 (1.3–2.1) | 2.5 (1.9–2.9) | 2.4 (1.9–2.9) | 2.7 (2–3.3) | <0.01 |
| PR > moderate, n (%) | 1 (5) | 4 (17.4) | 2 (9.5) | 5 (11.6) | 0.1 |
| EF (%), median (IQR) | 58.5 (54.3–67.8) | 56 (53.5–61) | 56 (51.8–65.3) | 57 (50–64) | 0.8 |
| Measures . | ASO-R (n = 20) . | HTTS (n = 23) . | Nikaidoh (n = 21) . | Rastelli (n = 43) . | P-value . |
|---|---|---|---|---|---|
| LVOT/AoV | |||||
| LVOT/AoV Pmax (mmHg), median (IQR) | 7.5 (5–16) | 5 (3–9.8) | 7 (4–9) | 5 (4–8.8) | 0.02 |
| LVOT/AoV Vmax (m/s), median (IQR) | 1.3 (1–1.7) | 0.9 (0.8–1) | 1.3 (0.9–1.5) | 1 (0.9–1.3) | 0.02 |
| AR > moderate, n (%) | 2 (10) | 1 (4.3) | 0 (0.0) | 4 (9.3) | 0.5 |
| RVOT/PV | |||||
| PV/RVOT Pmax (mmHg), median (IQR) | 10 (6.5–19.5) | 22 (12.3–32.8) | 24 (14.5–28.5) | 29 (17–45) | <0.01 |
| PV/RVOT Vmax (m/s), median (IQR) | 1.7 (1.3–2.1) | 2.5 (1.9–2.9) | 2.4 (1.9–2.9) | 2.7 (2–3.3) | <0.01 |
| PR > moderate, n (%) | 1 (5) | 4 (17.4) | 2 (9.5) | 5 (11.6) | 0.1 |
| EF (%), median (IQR) | 58.5 (54.3–67.8) | 56 (53.5–61) | 56 (51.8–65.3) | 57 (50–64) | 0.8 |
AoV: aortic valve; ASO-R: arterial switch operation with resection of LVOTO; AR: aortic valve regurgitation; EF: ejection fraction in %; IQR: interquartile range; LVOT: left ventricular outflow tract; LVOTO: left ventricular outflow tract obstruction; HTTS: half-turned truncal switch; Pmax: peak gradient; PR: pulmonary valve regurgitation; PV: pulmonary valve; RVOT: right ventricular outflow tract; Vmax: peak velocity.
Significant P-values in bold.
Risk factor analysis
Binary logistic regression analysis revealed that Rastelli procedure versus all other surgical techniques was the only independent risk factor (odds ratio = 2.8, 95% confidence interval 1.2–6.7, P = 0.02) for combined RVOT/LVOT reinterventions and reoperations in the follow-up period.
DISCUSSION
Anatomic repair of complex d-/l-TGA remains challenging and selecting the most appropriate surgical technique for the individual patient can be difficult. There is no consensus on the preferable technique, and available data on outcomes and reoccurring RVOTO/LVOTO is heterogeneous. Many surgeons therefore still favour the Rastelli procedure with thorough enlargement of the VSD, conjoined with less surgical complexity leaving the aortic root in place and favourable midterm outcomes for both RVOTO and LVOTO. Moreover long-term outcome of the more recent, but at the same time technically more demanding techniques such as Nikaidoh and HTTS are still somewhat uncertain [2, 7, 9]. This study therefore aimed to investigate which surgical repair technique has the lowest rates of reoccurring outflow tract obstructions and if there are certain risk factors associated with higher rates of reinterventions/reoperations.
Comparing baseline characteristics in our cohort, patients receiving Rastelli procedure were statistically significantly older, which was also reported in previous studies [2, 9]. Older age may be associated with lower perioperative risks [11]; however, in our cohort, higher complexity of Nikaidoh, HTTS or even the addition of the Senning procedure in l-TGA patients was not related with differences (P > 0.3) in early postoperative outcome in respect to 30-day mortality, ventilation time, ICU stay or any of the common early postoperative complications.
Systemic-to-pulmonary shunt operations were more often performed prior to Nikaidoh and Rastelli procedures (P < 0.01), while PAB was more frequent prior to ASO-R (P = 0.01). This is not surprising since prior shunt operations were generally performed in cases of pronounced cyanosis due to pulmonary atresia/severe pulmonary stenosis present in those patients among the Nikaidoh and Rastelli groups, in which the PV could not be preserved. Similar to previous reports, we favoured postponing RV–PA conduit placement to a later age [9]. PAB was done prior to the definitive surgery in 9 patients, even though these patients met our inclusions criteria for relevant LVOTO. This may seem contradictory; however, in most of these patients (5/9) with l-TGA and intact ventricle septum, we aimed for sufficient training for the morphologic left ventricle before undergoing the double-switch operation. The fact that prior PAB occurred more often in ASO-R group (6/9) is also intuitive, as the LVOTO was mild enough to be resected during surgery. PAB prior to complex TGA repair was also described by other studies [12].
Looking at bypass durations, we found statistically significantly longer cardiopulmonary bypass and cross-clamp times in ASO-R and HTTS compared to the other groups (P = 0.03 and <0.01). One explanation for the longer durations in ASO-R could be the amount of concomitated procedures such as atrial switch (7/20; 35%) and tricuspid valve repair (5/20; 25%). Not surprisingly, the more complex HTTS took longer [13].
Concerning 30-day/in-hospital/late mortality in our study population, we observed similar survival rates as previous studies [13]. Five-year survival did not differ among the groups (P = 0.8), which is consistent with results from previous reports [9, 14]. Late deaths only occurred within Rastelli group, mainly due to chronic heart failure and redo surgeries. Given the fact that this group had the longest follow-up times, we cannot conclude inferior long-term survival.
Looking at reoccurring outflow tract obstructions, numerically higher rates of RVOT reinterventions/reoperations were found after Nikaidoh and Rastelli procedures. However, when studying five-year overall freedom from RVOT reinterventions and/or reoperations, trends were seen towards lower freedom rates in Nikaidoh and Rastelli groups, which were not statistically significant. HTTS aims to improve both, freedom of RVOT and LVOT reoperations/reinterventions by 1st creating a straight rather than curved LVOT and 2nd avoiding pericardial patch/prothesis material (different to Nikaidoh and Rastelli procedures) by a direct anastomosis of native tissue at posterior wall of autologous PV with the RVOT [15, 16]. A trend towards lower rates of RVOT reinterventions and reoperations could be found in our study cohort, although not statistically significant (P > 0.05).
LVOTO reoperations were in general rare in our study population and occurred in ASO-R (n = 2; 10%) and Rastelli groups (n = 3; 7%), while no LVOTO reoperations were found in Nikaidoh and HTTS groups. This is consistent with previous results [2, 17] and supports the theory that a straight LVOT avoids the reoccurrence of LVOTO. Reoccurring LVOTO in ASO-R group may be a result from insufficient LVOT enlargement at the initial surgery or residual/reoccurring stenosis from the neo-aortic valve.
Echocardiography at last follow-up revealed a statistically significantly higher RVOT/PV peak gradient in Rastelli and Nikaidoh groups, which is again consistent with data from previous studies [9].
Investigating potential risk factors associated with reoccurrence of LVOTO/RVOTO, Rastelli technique was the only independent risk factor. Even though these results should be taken with caution, Rastelli procedure is known for creating 2 potential ‘construction sites’: 1st, a long and potentially curved intracardiac LVOT and 2nd an extra-anatomic prosthetic RVOT almost always exposed to compression by the sternum. Both can cause re-obstructions, leading to reinterventions/reoperations. Therefore, even though Rastelli operation might be less invasive and conjoined with acceptable results midterm, the more complex HTTS might be the better solution in the long run.
Larger comparative studies and longer follow-up periods are necessary to demonstrate a clear advantage of HTTS over other techniques.
Limitations
This study has several limitations based on its single-centre retrospective uncontrolled design. We report a limited number of cases with varying follow-up times. Considering the rather small number of patients per group (n = 20 at the smallest), we have 80% power to detect differences of 27% in mortality/complication rates, assuming a two-tailed 5%-alpha, which is for sure a significant limitation of this study. As the Rastelli group reports the longest follow-up times, late complications are more likely to occur in this group. Due to the single-centre nature of the study, our results cannot be generalized. Additionally, our study may include biases related to changes in surgical decision-making and postoperative management over different surgical eras. Including complex TGA patients with mild LVOTO undergoing ASO-R demonstrates another potential confounder, as these patients probably had more favourable baseline characteristics compared to patients with moderate–severe LVOTO requiring Nikaidoh/HTTS or Rastelli procedures. For all these reasons, results must be interpreted with caution and no definitive conclusions can be drawn.
CONCLUSION
Surgical repair of complex d-/l-TGA is challenging but can be performed with satisfying early/intermediate-term survival. RVOT reinterventions/reoperations were frequent after Nikaidoh and Rastelli repair. HTTS showed promising results with RVOT/LVOT reintervention/reoperation rates but larger-sized studies with longer follow-up are required for confident conclusions.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
FUNDING
None declared.
Conflict of interest: none declared.
DATA AVAILABILITY
Derived data supporting the findings of the study are available from the corresponding author on request.
Author contributions
Viktoria Weixler: Conceptualization; Data curation; Formal analysis; Writing—original draft; Writing—review and editing. Julia Gaal: Data curation; Investigation; Writing—original draft; Writing—review and editing. Peter Murin: Data curation; Methodology; Writing—review and editing. Peter Kramer: Data curation; Formal analysis; Methodology; Writing—review and editing. Olga Romanchenko: Data curation; Writing—review and editing. Mi-Young Cho: Methodology; Writing—review and editing. Katharina Schmitt: Writing—review and editing. Stanislav Ovroutski: Writing—review and editing. Joachim Photiadis: Conceptualization; Project administration; Supervision; Validation; Writing—original draft; Writing—review and editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Supreet P. Marathe and the other anonymous reviewers for their contribution to the peer review process of this article.
Presented at the 37th EACTS Annual, Vienna, Austria, 5–7 October 2023.
REFERENCES
ABBREVIATIONS
- ASO-R
Arterial switch operation + resection of left outflow tract obstruction
- d-/l-TGA
Dextro-/levo-transposition of the great arteries
- HTTS
Half-turned truncal switch
- LVOT
Left ventricular outflow tract
- LVOTO
Left ventricular outflow tract obstruction
- PAB
Pulmonary artery banding
- PV
Pulmonary valve
- RVOT
Right ventricular outflow tract
- RVOTO
Right ventricular outflow tract obstruction
- VSD
Ventricular septal defect
Author notes
Viktoria Weixler and Julia Gaal authors contributed equally to this manuscript.
- left ventricular outflow obstruction
- transposition of great vessels
- congenitally corrected transposition of the great arteries
- arterial switch operation
- ventricular septal defect
- postoperative complications
- follow-up
- repeat surgery
- surgical procedures, operative
- mortality
- left ventricular outflow tract
- right ventricular outflow tract





