-
PDF
- Split View
-
Views
-
Cite
Cite
Mario Gaudino, Marcus Flather, Davide Capodanno, Milan Milojevic, Deepak L Bhatt, Giuseppe Biondi Zoccai, William E Boden, P J Devereaux, Torsten Doenst, Michael Farkouh, Nicholas Freemantle, Stephen Fremes, John Puskas, Giovanni Landoni, Jennifer Lawton, Patrick O Myers, Björn Redfors, Sigrid Sandner, European Association of Cardio-Thoracic Surgery (EACTS) expert consensus statement on perioperative myocardial infarction after cardiac surgery, European Journal of Cardio-Thoracic Surgery, Volume 65, Issue 2, February 2024, ezad415, https://doi.org/10.1093/ejcts/ezad415
Close - Share Icon Share
Abstract
Cardiac surgery may lead to myocardial damage and release of cardiac biomarkers through various mechanisms such as cardiac manipulation, systemic inflammation, myocardial hypoxia, cardioplegic arrest and ischaemia caused by coronary or graft occlusion. Defining perioperative myocardial infarction (PMI) after cardiac surgery presents challenges, and the association between the current PMI definitions and postoperative outcomes remains uncertain. To address these challenges, the European Association of Cardio-Thoracic Surgery (EACTS) facilitated collaboration among a multidisciplinary group to evaluate the existing evidence on the mechanisms, diagnosis and prognostic implications of PMI after cardiac surgery. The review found that the postoperative troponin value thresholds associated with an increased risk of mortality are markedly higher than those proposed by all the current definitions of PMI. Additionally, it was found that large postoperative increases in cardiac biomarkers are prognostically relevant even in absence of additional supportive signs of ischaemia. A new algorithm for PMI detection after cardiac surgery was also proposed, and a consensus was reached within the group that establishing a prognostically relevant definition of PMI is critically needed in the cardiovascular field and that PMI should be included in the primary composite outcome of coronary intervention trials.
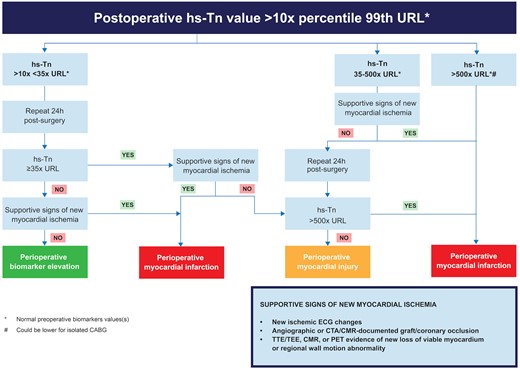
Proposed algorithm for detecting myocardial injury and myocardial infarction by using troponin levels as a key metric determinant. This algorithm requires additional testing and verification before further application in clinical and research settings. From the VISION study, 90% of patients showed a troponin I elevation ≥35 times the upper reference limit after cardiac surgery; hence the threshold on the left of the image. The same study associated levels of troponin 500 times the upper reference limit with an increased risk of death (not specifically related to myocardial infarction) after cardiac surgery; hence the use of this threshold in the algorithm.
INTRODUCTION
Cardiac surgery involves the performance of life-enhancing procedures in patients with severe cardiac conditions, including congenital, coronary, valvular and structural heart disease. Despite the complexity, the risk of major complications for elective procedures is generally below 5% and as low as 1% with careful patient selection [1, 2].
Cardiac surgery is associated with inflammation and varying levels of myocardial damage [3]. Cardiac manipulation, hypoxia and cardioplegia can add to myocardial injury related to ischaemia and lead to release of cardiac biomarkers [4]. In addition, myocardial infarction (MI) may occur due to coronary or graft occlusion, hypoxia or metabolic injury due to inappropriate myocardial protection or suboptimal cardioplegia administration [5]. These may be detected clinically and by biomarker elevation, but electrocardiogram (ECG), imaging or angiographic evidence is often needed to confirm the diagnosis [6]. The attempt to ascertain normal or expected levels of myocardial damage during cardiac surgery has proven challenging because it may vary based on the type and technique of the operation, and there is no explicit agreement on expected levels of biomarker release during uncomplicated operations where MI has been ruled out.
These factors have led to varying definitions of perioperative MI (PMI) after cardiac surgery [7] and to confusion and debate, especially when comparing revascularization methods in randomized clinical trials [8, 9]. Analysis of datasets using different PMI definitions can lead to important changes in the frequency of outcomes and the interpretation of results [10]. Schools of thought vary between applying a single definition of PMI to all clinical scenarios versus using variations of the definition, recognizing that the evidence base for either approach is insufficient.
The challenge of defining PMI after cardiac surgical procedures is understandable, given the multiple potential mechanisms of cardiac damage that can occur during the operation and that can cause varying degrees of myocardial injury or necrosis. However, addressing this challenge is urgent because difficulties in classifying PMI outcomes in clinical trials may led to confusion ambiguous in the interpretation of the available evidence and even hamper the ability to improve patient care. This consensus document brings together experts from different cardiovascular specialities to address this challenge by reviewing the available information to provide new insights into and practical advice on defining and detecting cardiac surgery-related PMI.
METHODOLOGY
To provide guidance and advice for both healthcare practitioners and researchers for diagnosing PMI following cardiac surgery, a task force of internationally recognized experts in the fields of cardiac surgery, clinical and interventional cardiology, anaesthesiology, clinical epidemiology and biostatistics was selected by the governing bodies of the European Association for Cardio-Thoracic Surgery (EACTS) following the processes detailed in the EACTS methodology manual for clinical practice documents [11]. The EACTS strived to ensure diversity in the formation of the writing group and adequate transparency in disclosing any relationships with industry and other entities. The chairperson was entirely free of relevant conflicts of interests (COIs) from 1 year before the task force was assembled until the publication of the document. Disclosure of any COIs was required from the other task force members prior to the start of the project and in the event that a change occurred during the writing period. After the task force members agreed upon the project's scope and approved the final table of contents, sections were allocated to the task force members who had no relevant COIs. A rapid systematic review of the published literature was conducted using Population, Intervention, Comparison, Outcome and Time (PICOT) approach for the synthesis of the most current available data. Key evidence was then summarized in detailed evidence tables. To ensure that clinical practice documents remain fully applicable to modern clinical practice, the synthesis of the evidence was focused on the most current data whenever possible. However, essential publications, irrespective of publication age, were also included. The present document focused on adult cardiac surgery and did not include studies in languages other than English. After the methodological quality was assessed, with attention to study type and quality, prioritizing randomized control trials and prospective studies over observational data, consensus statements and explicative text were written following the process defined by the EACTS Methodology manual for clinical guidelines [11]. The evidence was critically appraised for quality by the members of the writing group with the assistance of a clinical epidemiologist and biostatistician if needed.
All chapters were written in close collaboration among the task force members, and the key statements were developed during the task force meetings. According to the EACTS policies for dealing with COIs, each task force member was asked to emphasize any change in COIs immediately before meeting and voting and was allowed to vote on expert statements only in the absence of relevant COIs for the particular topic. Although the consensus threshold was set at 75%, the average consensus for all statements and proposed algorithms for detecting PMI was 88%. Due to high variability in economic parameters and lack of data on cost-effectiveness, cost analyses were not considered or delivered. The drafted document underwent internal validation and approval by all writing committee members and then external validation by the anonymous reviewers selected by the journal editor.
MECHANISMS OF PERIOPERATIVE MYOCARDIAL INFARCTION AFTER CARDIAC SURGERY
Multiple mechanisms can trigger PMI after cardiac surgery (Table 1), with only partial overlap with non-cardiovascular surgery and the non-perioperative settings. Some of the key mechanisms that may be responsible for PMI are well described in the Universal Definition of Myocardial Infarction (MI) [12]. Type 1 MIs are due to rupture or erosion of atherosclerotic plaques with consequent intraluminal thrombus in one or more of the epicardial coronary arteries [12]. They generally occur in patients with severe coronary artery disease, but on occasion they may occur in patients with non-obstructive coronary artery disease. In type 2 MIs, myocardial injury and necrosis are caused by an imbalance between myocardial oxygen demand and supply. Conditions that may cause type 2 MI are coronary endothelial dysfunction, coronary artery spasm, coronary embolism, tachy-brady arrhythmias, anaemia, respiratory failure, hypotension and hypertension with or without left ventricular hypertrophy, all of which may occur during or after cardiac surgery and may lead to type 2 MI.
| Graft related |
|
| Non-graft related |
|
| Graft related |
|
| Non-graft related |
|
| Graft related |
|
| Non-graft related |
|
| Graft related |
|
| Non-graft related |
|
Other mechanisms of PMI that are unique to cardiac surgery are due to coronary artery bypass graft (CABG) failure or other causes (Table 1) [13]. Graft failure represents the most common cause, accounting for approximately two-thirds of PMIs [14]. In a recent meta-analysis of 9 studies including 1104 patients with PMI after CABG, Biancari et al. [15] found that, among patients submitted to post-CABG angiography, 62% had acute graft failure, 6% had incomplete revascularization and 3.5% developed a new native coronary artery lesion. Other analyses have reported similar findings [16, 17]. Causes of graft failure include thrombosis, kinking or overstretching, competitive coronary flow, anastomotic technical error or graft spasm.
Non-graft-related causes of PMI include ischaemia–reperfusion injury triggered by myocardial ischaemia during cardioplegic arrest (generally due to inefficient myocardial protection or extended aortic cross-clamp time), postoperative systemic inflammatory injury, intraoperative coronary embolization of air or particulates, iatrogenic damage to a coronary artery (e.g. the left circumflex artery during mitral valve repair or the right coronary artery during tricuspid valve repair) [18], intimal flap propagating into the coronary arteries in case of ascending aortic dissection [19] and ostial coronary stenosis following aortic root replacement [18]. In a retrospective review of a consecutive cohort of 5275 patients who underwent cardiac surgery, new native coronary artery occlusion was found in 20% and coronary artery spasm in 13% of those with PMI [20]. Non-graft-related causes are more frequent in patients undergoing combined surgical procedures [14].
Postoperative systemic inflammatory reaction deserves special mention because it occurs with greater frequency in cardiac surgery than in any other type of surgical operation due to the contact of blood with the foreign surfaces of the cardiopulmonary bypass circuit [21]. The components of the inflammatory response include consumptive coagulopathy, cytokines, chemokines, vasoactive substances, cytotoxins, reactive oxygen species and proteases of the coagulation and fibrinolytic systems [22].
BIOMARKERS AND THE CUT-OFF FOR THE DIAGNOSIS OF PERIOPERATIVE MYOCARDIAL INFARCTION
The diagnosis of PMI is based primarily on the elevation of biomarkers suggestive of cardiac injury in the postoperative period, typically defined as the first 48 to 72 h after the operation (Tables 2 and 3). The biomarkers used in contemporary definitions of PMI are creatine kinase-MB (CK-MB) and troponin I or T. CK-MB is less sensitive for detecting myocardial necrosis, and it has been replaced by troponins in most centres [12, 23]. Troponins, however, are not specific for myocardial necrosis, and they may be released even in the setting of non-necrotic myocardial ischaemia [24].
Definition of perioperative myocardial infarction after surgical and percutaneous coronary revascularization
| Definition . | Year . | Time after procedure . | Peak biomarker threshold post-CABG . | Supporting evidence post-CABG . | Peak biomarker threshold post-PCI . | Supporting evidence post-PCI . |
|---|---|---|---|---|---|---|
| Fourth UDMI [12] | 2018 | Within 48 h | cTn >10× 99th percentile URL (or CK-MB >10× 99th percentile URL if cTn unavailable). |
| cTn values >5× the 99th percentile URL |
|
| ARC-2 [63] | 2018 | Within 48 h | Troponin ≥35× URL |
| Troponin ≥35× URL |
|
| Troponin ≥70× URLa | None | |||||
| SIRS [64] | 2015 | Within 72 h | CK-MB (mass) ≥6× URL | None | N/A | |
| CK-MB (activity) ≥40 | None | N/A | ||||
| SCAI [65] | 2013 | Within 48 h | CK-MB ≥ 10× URL (or troponin ≥70× URL) | None | cTn to >5× the 99th percentile of the URL |
|
| CK-MB ≥5× URL (or troponin ≥35× URL) | New pathologic Q waves in 2 contiguous leads or new persistent LBBB | |||||
| Ischaemia [60] | 2012 | Within 48 h | CK-MB >10× URL (or troponin ≥70× URL) |
|
| |
| CK-MB >15× URL (or troponin ≥100× URL) | None | |||||
| Third UDMI [66] | 2012 | Within 48 h | cTn >10× URL (or CK-MB >10× 99th percentile URL if cTn unavailable). |
|
|
|
| CORONARY [67] | 2012 | Within 72 h | CK-MB >5× URL | None | N/A | N/A |
| N/A | N/A | ||||
| EXCEL [68] | 2010 | Within 72 h | CK-MB >10× URL | None | CK-MB >10× URL | None |
| CK-MB >5× URL |
| CK-MB >5× URL |
| |||
| Second UDMI [69] | 2007 | Within 72 h | cTn >5× URL (or CK-MB >5× URL if cTn unavailable) |
| cTn >3× the 99th percentile URL | N/A |
| ARC [70] | 2007 | Within 72 h | Troponin ≥5× URL or CK-MB ≥5× URL |
| Troponin >3 times URL or CK-MB >3 times URL | |
| SYNTAX [71] | 2005 | Within 7 days | Peak CK-MB/peak total CK >10% | New Q waves in ≥2 leads | Peak CK-MB/peak total CK >10% | New Q waves in ≥2 leads |
| CK-MB ≥5× URL | New Q waves in ≥2 leads | CK-MB ≥5× URL | New Q waves in ≥2 leads |
| Definition . | Year . | Time after procedure . | Peak biomarker threshold post-CABG . | Supporting evidence post-CABG . | Peak biomarker threshold post-PCI . | Supporting evidence post-PCI . |
|---|---|---|---|---|---|---|
| Fourth UDMI [12] | 2018 | Within 48 h | cTn >10× 99th percentile URL (or CK-MB >10× 99th percentile URL if cTn unavailable). |
| cTn values >5× the 99th percentile URL |
|
| ARC-2 [63] | 2018 | Within 48 h | Troponin ≥35× URL |
| Troponin ≥35× URL |
|
| Troponin ≥70× URLa | None | |||||
| SIRS [64] | 2015 | Within 72 h | CK-MB (mass) ≥6× URL | None | N/A | |
| CK-MB (activity) ≥40 | None | N/A | ||||
| SCAI [65] | 2013 | Within 48 h | CK-MB ≥ 10× URL (or troponin ≥70× URL) | None | cTn to >5× the 99th percentile of the URL |
|
| CK-MB ≥5× URL (or troponin ≥35× URL) | New pathologic Q waves in 2 contiguous leads or new persistent LBBB | |||||
| Ischaemia [60] | 2012 | Within 48 h | CK-MB >10× URL (or troponin ≥70× URL) |
|
| |
| CK-MB >15× URL (or troponin ≥100× URL) | None | |||||
| Third UDMI [66] | 2012 | Within 48 h | cTn >10× URL (or CK-MB >10× 99th percentile URL if cTn unavailable). |
|
|
|
| CORONARY [67] | 2012 | Within 72 h | CK-MB >5× URL | None | N/A | N/A |
| N/A | N/A | ||||
| EXCEL [68] | 2010 | Within 72 h | CK-MB >10× URL | None | CK-MB >10× URL | None |
| CK-MB >5× URL |
| CK-MB >5× URL |
| |||
| Second UDMI [69] | 2007 | Within 72 h | cTn >5× URL (or CK-MB >5× URL if cTn unavailable) |
| cTn >3× the 99th percentile URL | N/A |
| ARC [70] | 2007 | Within 72 h | Troponin ≥5× URL or CK-MB ≥5× URL |
| Troponin >3 times URL or CK-MB >3 times URL | |
| SYNTAX [71] | 2005 | Within 7 days | Peak CK-MB/peak total CK >10% | New Q waves in ≥2 leads | Peak CK-MB/peak total CK >10% | New Q waves in ≥2 leads |
| CK-MB ≥5× URL | New Q waves in ≥2 leads | CK-MB ≥5× URL | New Q waves in ≥2 leads |
Termed significant periprocedural injury.
ARC: academic research consortium; CABG: coronary artery bypass grafting; CK-MB: creatine kinase MB; CORONARY: CABG Off or On Pump Revascularization Study; cTn: cardiac troponin; ECG: electrocardiogram; EXCEL: Evaluation of Xience versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization; ISCHEMIA: International Study of Comparative Health Effectiveness with Medical and Invasive Approaches; LBBB: left bundle branch block; PCI: percutaneous coronary intervention; SCAI: Society of Cardiovascular Angiography and Interventions; SIRS: Steroids In cardiac Surgery Trial; SYNTAX: Synergy between Percutaneous Coronary Interventions with Taxus and Cardiac Surgery; UDMI: Universal Definition of Myocardial Infarction; ULN: upper limit of normal: URL: upper reference limit.
Definition of perioperative myocardial infarction after surgical and percutaneous coronary revascularization
| Definition . | Year . | Time after procedure . | Peak biomarker threshold post-CABG . | Supporting evidence post-CABG . | Peak biomarker threshold post-PCI . | Supporting evidence post-PCI . |
|---|---|---|---|---|---|---|
| Fourth UDMI [12] | 2018 | Within 48 h | cTn >10× 99th percentile URL (or CK-MB >10× 99th percentile URL if cTn unavailable). |
| cTn values >5× the 99th percentile URL |
|
| ARC-2 [63] | 2018 | Within 48 h | Troponin ≥35× URL |
| Troponin ≥35× URL |
|
| Troponin ≥70× URLa | None | |||||
| SIRS [64] | 2015 | Within 72 h | CK-MB (mass) ≥6× URL | None | N/A | |
| CK-MB (activity) ≥40 | None | N/A | ||||
| SCAI [65] | 2013 | Within 48 h | CK-MB ≥ 10× URL (or troponin ≥70× URL) | None | cTn to >5× the 99th percentile of the URL |
|
| CK-MB ≥5× URL (or troponin ≥35× URL) | New pathologic Q waves in 2 contiguous leads or new persistent LBBB | |||||
| Ischaemia [60] | 2012 | Within 48 h | CK-MB >10× URL (or troponin ≥70× URL) |
|
| |
| CK-MB >15× URL (or troponin ≥100× URL) | None | |||||
| Third UDMI [66] | 2012 | Within 48 h | cTn >10× URL (or CK-MB >10× 99th percentile URL if cTn unavailable). |
|
|
|
| CORONARY [67] | 2012 | Within 72 h | CK-MB >5× URL | None | N/A | N/A |
| N/A | N/A | ||||
| EXCEL [68] | 2010 | Within 72 h | CK-MB >10× URL | None | CK-MB >10× URL | None |
| CK-MB >5× URL |
| CK-MB >5× URL |
| |||
| Second UDMI [69] | 2007 | Within 72 h | cTn >5× URL (or CK-MB >5× URL if cTn unavailable) |
| cTn >3× the 99th percentile URL | N/A |
| ARC [70] | 2007 | Within 72 h | Troponin ≥5× URL or CK-MB ≥5× URL |
| Troponin >3 times URL or CK-MB >3 times URL | |
| SYNTAX [71] | 2005 | Within 7 days | Peak CK-MB/peak total CK >10% | New Q waves in ≥2 leads | Peak CK-MB/peak total CK >10% | New Q waves in ≥2 leads |
| CK-MB ≥5× URL | New Q waves in ≥2 leads | CK-MB ≥5× URL | New Q waves in ≥2 leads |
| Definition . | Year . | Time after procedure . | Peak biomarker threshold post-CABG . | Supporting evidence post-CABG . | Peak biomarker threshold post-PCI . | Supporting evidence post-PCI . |
|---|---|---|---|---|---|---|
| Fourth UDMI [12] | 2018 | Within 48 h | cTn >10× 99th percentile URL (or CK-MB >10× 99th percentile URL if cTn unavailable). |
| cTn values >5× the 99th percentile URL |
|
| ARC-2 [63] | 2018 | Within 48 h | Troponin ≥35× URL |
| Troponin ≥35× URL |
|
| Troponin ≥70× URLa | None | |||||
| SIRS [64] | 2015 | Within 72 h | CK-MB (mass) ≥6× URL | None | N/A | |
| CK-MB (activity) ≥40 | None | N/A | ||||
| SCAI [65] | 2013 | Within 48 h | CK-MB ≥ 10× URL (or troponin ≥70× URL) | None | cTn to >5× the 99th percentile of the URL |
|
| CK-MB ≥5× URL (or troponin ≥35× URL) | New pathologic Q waves in 2 contiguous leads or new persistent LBBB | |||||
| Ischaemia [60] | 2012 | Within 48 h | CK-MB >10× URL (or troponin ≥70× URL) |
|
| |
| CK-MB >15× URL (or troponin ≥100× URL) | None | |||||
| Third UDMI [66] | 2012 | Within 48 h | cTn >10× URL (or CK-MB >10× 99th percentile URL if cTn unavailable). |
|
|
|
| CORONARY [67] | 2012 | Within 72 h | CK-MB >5× URL | None | N/A | N/A |
| N/A | N/A | ||||
| EXCEL [68] | 2010 | Within 72 h | CK-MB >10× URL | None | CK-MB >10× URL | None |
| CK-MB >5× URL |
| CK-MB >5× URL |
| |||
| Second UDMI [69] | 2007 | Within 72 h | cTn >5× URL (or CK-MB >5× URL if cTn unavailable) |
| cTn >3× the 99th percentile URL | N/A |
| ARC [70] | 2007 | Within 72 h | Troponin ≥5× URL or CK-MB ≥5× URL |
| Troponin >3 times URL or CK-MB >3 times URL | |
| SYNTAX [71] | 2005 | Within 7 days | Peak CK-MB/peak total CK >10% | New Q waves in ≥2 leads | Peak CK-MB/peak total CK >10% | New Q waves in ≥2 leads |
| CK-MB ≥5× URL | New Q waves in ≥2 leads | CK-MB ≥5× URL | New Q waves in ≥2 leads |
Termed significant periprocedural injury.
ARC: academic research consortium; CABG: coronary artery bypass grafting; CK-MB: creatine kinase MB; CORONARY: CABG Off or On Pump Revascularization Study; cTn: cardiac troponin; ECG: electrocardiogram; EXCEL: Evaluation of Xience versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization; ISCHEMIA: International Study of Comparative Health Effectiveness with Medical and Invasive Approaches; LBBB: left bundle branch block; PCI: percutaneous coronary intervention; SCAI: Society of Cardiovascular Angiography and Interventions; SIRS: Steroids In cardiac Surgery Trial; SYNTAX: Synergy between Percutaneous Coronary Interventions with Taxus and Cardiac Surgery; UDMI: Universal Definition of Myocardial Infarction; ULN: upper limit of normal: URL: upper reference limit.
Definition of perioperative myocardial infarction after cardiac valve surgery
| Definition . | Year . | Time after procedure . | Peak biomarker threshold . | Supporting evidence . |
|---|---|---|---|---|
| VARC 3 [72] | 2013 | Within 48 h | CK-MB ≥10× URL (or troponin ≥70× URL) | None |
| CK-MB ≥5× URL (or Troponin ≥35× URL) |
| |||
| MVARC [73] | 2012 | Within 48 h | CK-MB >10× URL | ECG: New ST-segment elevation or depression of ≥1 mm in ≥2 contiguous leads (measured 80 ms after the J-point) |
| CK-MB >15× URL | ECG: New Q-waves or new persistent LBBB |
| Definition . | Year . | Time after procedure . | Peak biomarker threshold . | Supporting evidence . |
|---|---|---|---|---|
| VARC 3 [72] | 2013 | Within 48 h | CK-MB ≥10× URL (or troponin ≥70× URL) | None |
| CK-MB ≥5× URL (or Troponin ≥35× URL) |
| |||
| MVARC [73] | 2012 | Within 48 h | CK-MB >10× URL | ECG: New ST-segment elevation or depression of ≥1 mm in ≥2 contiguous leads (measured 80 ms after the J-point) |
| CK-MB >15× URL | ECG: New Q-waves or new persistent LBBB |
CK-MB: creatine kinase MB; ECG: electrocardiogram; LBBB: left bundle branch block; MVARC: Mitral Valve Academic Research Consortium; URL: upper reference limit; VARC-3: Valve Academic Research Consortium-3.
Definition of perioperative myocardial infarction after cardiac valve surgery
| Definition . | Year . | Time after procedure . | Peak biomarker threshold . | Supporting evidence . |
|---|---|---|---|---|
| VARC 3 [72] | 2013 | Within 48 h | CK-MB ≥10× URL (or troponin ≥70× URL) | None |
| CK-MB ≥5× URL (or Troponin ≥35× URL) |
| |||
| MVARC [73] | 2012 | Within 48 h | CK-MB >10× URL | ECG: New ST-segment elevation or depression of ≥1 mm in ≥2 contiguous leads (measured 80 ms after the J-point) |
| CK-MB >15× URL | ECG: New Q-waves or new persistent LBBB |
| Definition . | Year . | Time after procedure . | Peak biomarker threshold . | Supporting evidence . |
|---|---|---|---|---|
| VARC 3 [72] | 2013 | Within 48 h | CK-MB ≥10× URL (or troponin ≥70× URL) | None |
| CK-MB ≥5× URL (or Troponin ≥35× URL) |
| |||
| MVARC [73] | 2012 | Within 48 h | CK-MB >10× URL | ECG: New ST-segment elevation or depression of ≥1 mm in ≥2 contiguous leads (measured 80 ms after the J-point) |
| CK-MB >15× URL | ECG: New Q-waves or new persistent LBBB |
CK-MB: creatine kinase MB; ECG: electrocardiogram; LBBB: left bundle branch block; MVARC: Mitral Valve Academic Research Consortium; URL: upper reference limit; VARC-3: Valve Academic Research Consortium-3.
Numerous studies have reported an independent association between elevated levels of CK-MB and troponin I and T after cardiac surgery and an increased risk of death (Tables 4 and 5). Importantly, this association holds true even in the absence of an ECG or imaging evidence of ischaemia [25–29]. Yet, definitions of PMI relying solely on the release of biomarkers release have generated substantial controversy, and there is no general consensus on cut-off values to be used [30]. In addition, there is high variability in postoperative biomarker levels across the different assays and the types of cardiac surgery operations [31] (Table 5).
Reported associations of different biomarker cut-offs with clinical outcomes after cardiac surgery in recent studies
| Year . | Author . | Study design, N . | Type of surgery . | Biomarker . | Timinga . | Key findings . |
|---|---|---|---|---|---|---|
| 2022 | Devereaux [34] | Prospective cohort, 13 862 | Isolated CABG (46.9%), isolated AVR (12.5%), other (40.6%) | TnI | 3–12, 24, 48 and 72 h |
|
| 2022 | Pölzl [58] | Consecutive registry, 2829 | CABG (on-pump in all patients except 1) | TnI | 1, 6, 12, 24, 48 and 72 h |
|
| 2020 | Belley-Cote [55] | RCT, 4752 | CABG (on-pump = 2377; off-pump = 2375) | CK-MB | 24, 48 h |
|
| 2019 | Hara [56] | RCT, 795 | Isolated CABG (84.4% vs 15.6% on- vs off-pump) | CK-MB | 6 h, 12 h and at discharge |
|
| 2019 | Ben-Yehuda [32] | RCT, 923 | Isolated CABG (71% vs 29% on- vs off-pump) | CK-MB | 12 h, 24 h |
|
| 2018 | Gahl [74] | Prospective registry, 1722 | Isolated CABG | TnT | 6–12 h |
|
| 2016 | Hueb [75] | Prospective cohort, 136 | Isolated CABG (51% on- vs 49% off-pump) |
| 6, 12, 24, 36, 48, 72 h |
|
| 2014 | Jorgensen [76] | Prospective cohort, 99 | On-pump isolated CABG | TnI | 0, 2, 4, 6, 12, 24, 48 and 72 h |
|
| 2013 | Farooq [77] | RCT, 802 (474 with CK-MB data) | Isolated CABG (84.4% vs 15.6% on- vs off-pump) |
| 6 and 12 h and discharge |
|
| 2011 | Domanski [27] | META-analysis, 18 908 | CABG |
| Varied across studies |
|
| 2011 | Pegg [78] | RCT, 40 | CABG (conventional vs beating heart on-pump CABG) |
| 1, 6, 24, 48, 120 h and 6 months |
|
| 2009 | Mohammed [79] | Prospective registry, 847 | Isolated CABG (10% off-pump or beating heart) | TnT | 6–8 and 18–24 h |
|
| 2009 | Muehlschlaegel [80] | Prospective registry, 1013/545 (validation/test cohorts) | Isolated on-pump CABG |
| Morning time postoperative days 1, 2, 3, 4 and 5 |
|
| 2009 | Petäjä [81] | Meta-analysis | Variable across studies | CK-MB | Varied across studies |
|
| Year . | Author . | Study design, N . | Type of surgery . | Biomarker . | Timinga . | Key findings . |
|---|---|---|---|---|---|---|
| 2022 | Devereaux [34] | Prospective cohort, 13 862 | Isolated CABG (46.9%), isolated AVR (12.5%), other (40.6%) | TnI | 3–12, 24, 48 and 72 h |
|
| 2022 | Pölzl [58] | Consecutive registry, 2829 | CABG (on-pump in all patients except 1) | TnI | 1, 6, 12, 24, 48 and 72 h |
|
| 2020 | Belley-Cote [55] | RCT, 4752 | CABG (on-pump = 2377; off-pump = 2375) | CK-MB | 24, 48 h |
|
| 2019 | Hara [56] | RCT, 795 | Isolated CABG (84.4% vs 15.6% on- vs off-pump) | CK-MB | 6 h, 12 h and at discharge |
|
| 2019 | Ben-Yehuda [32] | RCT, 923 | Isolated CABG (71% vs 29% on- vs off-pump) | CK-MB | 12 h, 24 h |
|
| 2018 | Gahl [74] | Prospective registry, 1722 | Isolated CABG | TnT | 6–12 h |
|
| 2016 | Hueb [75] | Prospective cohort, 136 | Isolated CABG (51% on- vs 49% off-pump) |
| 6, 12, 24, 36, 48, 72 h |
|
| 2014 | Jorgensen [76] | Prospective cohort, 99 | On-pump isolated CABG | TnI | 0, 2, 4, 6, 12, 24, 48 and 72 h |
|
| 2013 | Farooq [77] | RCT, 802 (474 with CK-MB data) | Isolated CABG (84.4% vs 15.6% on- vs off-pump) |
| 6 and 12 h and discharge |
|
| 2011 | Domanski [27] | META-analysis, 18 908 | CABG |
| Varied across studies |
|
| 2011 | Pegg [78] | RCT, 40 | CABG (conventional vs beating heart on-pump CABG) |
| 1, 6, 24, 48, 120 h and 6 months |
|
| 2009 | Mohammed [79] | Prospective registry, 847 | Isolated CABG (10% off-pump or beating heart) | TnT | 6–8 and 18–24 h |
|
| 2009 | Muehlschlaegel [80] | Prospective registry, 1013/545 (validation/test cohorts) | Isolated on-pump CABG |
| Morning time postoperative days 1, 2, 3, 4 and 5 |
|
| 2009 | Petäjä [81] | Meta-analysis | Variable across studies | CK-MB | Varied across studies |
|
Time from cardiac surgery.
adjHR: adjusted hazard ratio; ARC-2: Academic Research Consortium-2; AUC: area under the curve; AVR: aortic valve replacement; CABG: coronary artery bypass graft; CI: confidence interval; CK: creatine; CK-MB: creatine kinase MB; CMR: cardiac magnetic resonance; CORONARY: CABG Off or On Pump Revascularization Study; HR: hazard ratio; IQR: interquartile range; MI: myocardial infarction; PMI: procedural myocardial infarction; RCT: randomized clinical trial; RTC: randomized controlled trial; SCAI: Society of Cardiovascular Angiography and Interventions; SIRS: Steroids In cardiac Surgery Trial; Tn: troponin; TnI: troponin I; TnT: troponin T; UDMI: Universal Definition of Myocardial Infarction; URL: upper reference limit.
Reported associations of different biomarker cut-offs with clinical outcomes after cardiac surgery in recent studies
| Year . | Author . | Study design, N . | Type of surgery . | Biomarker . | Timinga . | Key findings . |
|---|---|---|---|---|---|---|
| 2022 | Devereaux [34] | Prospective cohort, 13 862 | Isolated CABG (46.9%), isolated AVR (12.5%), other (40.6%) | TnI | 3–12, 24, 48 and 72 h |
|
| 2022 | Pölzl [58] | Consecutive registry, 2829 | CABG (on-pump in all patients except 1) | TnI | 1, 6, 12, 24, 48 and 72 h |
|
| 2020 | Belley-Cote [55] | RCT, 4752 | CABG (on-pump = 2377; off-pump = 2375) | CK-MB | 24, 48 h |
|
| 2019 | Hara [56] | RCT, 795 | Isolated CABG (84.4% vs 15.6% on- vs off-pump) | CK-MB | 6 h, 12 h and at discharge |
|
| 2019 | Ben-Yehuda [32] | RCT, 923 | Isolated CABG (71% vs 29% on- vs off-pump) | CK-MB | 12 h, 24 h |
|
| 2018 | Gahl [74] | Prospective registry, 1722 | Isolated CABG | TnT | 6–12 h |
|
| 2016 | Hueb [75] | Prospective cohort, 136 | Isolated CABG (51% on- vs 49% off-pump) |
| 6, 12, 24, 36, 48, 72 h |
|
| 2014 | Jorgensen [76] | Prospective cohort, 99 | On-pump isolated CABG | TnI | 0, 2, 4, 6, 12, 24, 48 and 72 h |
|
| 2013 | Farooq [77] | RCT, 802 (474 with CK-MB data) | Isolated CABG (84.4% vs 15.6% on- vs off-pump) |
| 6 and 12 h and discharge |
|
| 2011 | Domanski [27] | META-analysis, 18 908 | CABG |
| Varied across studies |
|
| 2011 | Pegg [78] | RCT, 40 | CABG (conventional vs beating heart on-pump CABG) |
| 1, 6, 24, 48, 120 h and 6 months |
|
| 2009 | Mohammed [79] | Prospective registry, 847 | Isolated CABG (10% off-pump or beating heart) | TnT | 6–8 and 18–24 h |
|
| 2009 | Muehlschlaegel [80] | Prospective registry, 1013/545 (validation/test cohorts) | Isolated on-pump CABG |
| Morning time postoperative days 1, 2, 3, 4 and 5 |
|
| 2009 | Petäjä [81] | Meta-analysis | Variable across studies | CK-MB | Varied across studies |
|
| Year . | Author . | Study design, N . | Type of surgery . | Biomarker . | Timinga . | Key findings . |
|---|---|---|---|---|---|---|
| 2022 | Devereaux [34] | Prospective cohort, 13 862 | Isolated CABG (46.9%), isolated AVR (12.5%), other (40.6%) | TnI | 3–12, 24, 48 and 72 h |
|
| 2022 | Pölzl [58] | Consecutive registry, 2829 | CABG (on-pump in all patients except 1) | TnI | 1, 6, 12, 24, 48 and 72 h |
|
| 2020 | Belley-Cote [55] | RCT, 4752 | CABG (on-pump = 2377; off-pump = 2375) | CK-MB | 24, 48 h |
|
| 2019 | Hara [56] | RCT, 795 | Isolated CABG (84.4% vs 15.6% on- vs off-pump) | CK-MB | 6 h, 12 h and at discharge |
|
| 2019 | Ben-Yehuda [32] | RCT, 923 | Isolated CABG (71% vs 29% on- vs off-pump) | CK-MB | 12 h, 24 h |
|
| 2018 | Gahl [74] | Prospective registry, 1722 | Isolated CABG | TnT | 6–12 h |
|
| 2016 | Hueb [75] | Prospective cohort, 136 | Isolated CABG (51% on- vs 49% off-pump) |
| 6, 12, 24, 36, 48, 72 h |
|
| 2014 | Jorgensen [76] | Prospective cohort, 99 | On-pump isolated CABG | TnI | 0, 2, 4, 6, 12, 24, 48 and 72 h |
|
| 2013 | Farooq [77] | RCT, 802 (474 with CK-MB data) | Isolated CABG (84.4% vs 15.6% on- vs off-pump) |
| 6 and 12 h and discharge |
|
| 2011 | Domanski [27] | META-analysis, 18 908 | CABG |
| Varied across studies |
|
| 2011 | Pegg [78] | RCT, 40 | CABG (conventional vs beating heart on-pump CABG) |
| 1, 6, 24, 48, 120 h and 6 months |
|
| 2009 | Mohammed [79] | Prospective registry, 847 | Isolated CABG (10% off-pump or beating heart) | TnT | 6–8 and 18–24 h |
|
| 2009 | Muehlschlaegel [80] | Prospective registry, 1013/545 (validation/test cohorts) | Isolated on-pump CABG |
| Morning time postoperative days 1, 2, 3, 4 and 5 |
|
| 2009 | Petäjä [81] | Meta-analysis | Variable across studies | CK-MB | Varied across studies |
|
Time from cardiac surgery.
adjHR: adjusted hazard ratio; ARC-2: Academic Research Consortium-2; AUC: area under the curve; AVR: aortic valve replacement; CABG: coronary artery bypass graft; CI: confidence interval; CK: creatine; CK-MB: creatine kinase MB; CMR: cardiac magnetic resonance; CORONARY: CABG Off or On Pump Revascularization Study; HR: hazard ratio; IQR: interquartile range; MI: myocardial infarction; PMI: procedural myocardial infarction; RCT: randomized clinical trial; RTC: randomized controlled trial; SCAI: Society of Cardiovascular Angiography and Interventions; SIRS: Steroids In cardiac Surgery Trial; Tn: troponin; TnI: troponin I; TnT: troponin T; UDMI: Universal Definition of Myocardial Infarction; URL: upper reference limit.
Procedure-specific associations between cardiac injury biomarkers and outcomes
| Year . | Author . | Study design, N . | Biomarker . | Outcome . | Findings . |
|---|---|---|---|---|---|
| 2022 | Devereaux [34] | Prospective registry, 13 862 | TnI | Biomarker elevations after different surgical procedures |
|
| 2022 | Niclauss [82] | Retrospective, 400 |
| Biomarker elevations after different surgical procedures |
|
| 2022 | Zhou [83] | Registry, 10 253 | TnT |
|
|
| 2015 | Mastro [84] | Registry, 200 |
| Magnitude and release pattern of cardiac injury biomarkers |
|
| 2014 | Paparella [85] | Registry, 965 | TnI |
|
|
| Year . | Author . | Study design, N . | Biomarker . | Outcome . | Findings . |
|---|---|---|---|---|---|
| 2022 | Devereaux [34] | Prospective registry, 13 862 | TnI | Biomarker elevations after different surgical procedures |
|
| 2022 | Niclauss [82] | Retrospective, 400 |
| Biomarker elevations after different surgical procedures |
|
| 2022 | Zhou [83] | Registry, 10 253 | TnT |
|
|
| 2015 | Mastro [84] | Registry, 200 |
| Magnitude and release pattern of cardiac injury biomarkers |
|
| 2014 | Paparella [85] | Registry, 965 | TnI |
|
|
AV: aortic valve; AVR: aortic valve replacement; CABG: coronary artery bypass graft; CK-MB: creatine kinase MB; LCOS: low cardiac output syndrome; MV: mitral valve; MVR: mitral valve regurgitation; TnI: troponin I; TnT: troponin.
Procedure-specific associations between cardiac injury biomarkers and outcomes
| Year . | Author . | Study design, N . | Biomarker . | Outcome . | Findings . |
|---|---|---|---|---|---|
| 2022 | Devereaux [34] | Prospective registry, 13 862 | TnI | Biomarker elevations after different surgical procedures |
|
| 2022 | Niclauss [82] | Retrospective, 400 |
| Biomarker elevations after different surgical procedures |
|
| 2022 | Zhou [83] | Registry, 10 253 | TnT |
|
|
| 2015 | Mastro [84] | Registry, 200 |
| Magnitude and release pattern of cardiac injury biomarkers |
|
| 2014 | Paparella [85] | Registry, 965 | TnI |
|
|
| Year . | Author . | Study design, N . | Biomarker . | Outcome . | Findings . |
|---|---|---|---|---|---|
| 2022 | Devereaux [34] | Prospective registry, 13 862 | TnI | Biomarker elevations after different surgical procedures |
|
| 2022 | Niclauss [82] | Retrospective, 400 |
| Biomarker elevations after different surgical procedures |
|
| 2022 | Zhou [83] | Registry, 10 253 | TnT |
|
|
| 2015 | Mastro [84] | Registry, 200 |
| Magnitude and release pattern of cardiac injury biomarkers |
|
| 2014 | Paparella [85] | Registry, 965 | TnI |
|
|
AV: aortic valve; AVR: aortic valve replacement; CABG: coronary artery bypass graft; CK-MB: creatine kinase MB; LCOS: low cardiac output syndrome; MV: mitral valve; MVR: mitral valve regurgitation; TnI: troponin I; TnT: troponin.
Biomarker thresholds for diagnosing perioperative myocardial infarction after cardiac surgery
Most contemporary definitions of PMI require CK-MB elevations of >10× the upper reference limit (URL) or troponin elevations of ≥10× or 35× the upper URL to define PMI in the presence of ischaemia on ECG, non-invasive imaging or coronary angiography (Tables 2 and 3). Most studies have reported an independent association between CK-MB ≥10× URL and postoperative mortality [32]. However, at least some of this risk may be driven by the most severe cases, and a substantial proportion of patients with biomarker elevations above these thresholds may not have significant myocardial infarction on cardiac magnetic resonance (CMR) images and do not have increased risk of mortality [33].
As for troponin levels, recent data suggest that the biomarker thresholds to define PMI after cardiac surgery should be substantially higher than those proposed in the current PMI definitions (Table 4). Among 13 862 patients undergoing cardiac surgery in the recent Vascular Events In Surgery Patients Cohort Evaluation (VISION Cardiac Surgery study), the recommended troponin thresholds in the most recent PMI definitions (>10×, ≥35× and ≥70× URL) were exceeded within the first day after surgery in 97.5%, 89.4% and 74.7% of patients, respectively. Among patients who underwent isolated CABG or aortic valve replacement, the threshold troponin value associated with increased risk of 30-day mortality was 5670 ng/l (>210× the upper reference limit) within 1 day after surgery and 1522 ng/l (>55× the upper reference limit) on postoperative day 2 or 3. Corresponding levels were higher for patients who underwent other cardiac operations (almost 500 times the upper reference limit within 1 day after surgery). The lowest troponin I threshold associated with increased 30-day mortality risk greatly exceeded all the recommended thresholds [34]. Similar results were reported in another large single institution study [35].
Biomarker release kinetics and the time window for biomarker elevations after cardiac surgery
The release kinetics after a myocardial injury differ among the biomarkers, with important differences observed between troponin T and troponin I and some variability across different troponin I assays [36, 37]. Whereas all biomarkers reach peak levels in plasma within a similar time frame after myocardial injury, plasma troponin T levels decrease at a slower rate than the other biomarkers. In VISION Cardiac Surgery, the threshold for a prognostically significant biomarker elevation after cardiac surgery was several-fold higher for troponin I values obtained within 1 day after surgery than for troponin I values obtained on postoperative day 2 or 3 [34] (Fig. 1).
![Median high-sensitivity cardiac troponin I measurements during the first 3 days following cardiac surgery. Reproduced with permission from [34] Copyright Massachusetts Medical Society. High-sensitivity troponin I after cardiac surgery and 30-day mortality. AVR: aortic valve replacement; CABG: coronary artery bypass graft; CS: cardiac surgeries; hs-cTnI: high-sensitive cardiac troponin I; postop: postoperative.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ejcts/65/2/10.1093_ejcts_ezad415/1/m_ezad415f1.jpeg?Expires=1749148653&Signature=VMnu8CzJMAsjiq026lMeCU9XRxaOi-vI6~qeONWMUCtKqphRk455aWOACqoY3c6i9Uv1CZbw65FAP31zn44rXEO4PubKNy3lYVqYS7HMQ5Fm3XJP9vT5YBo73HMR8NXQ5r8zG~WMXNNpnL6UySuJtqEt1WO-AM2TN5AwNO0y8fPdOwDbJ0iXaxilO~E~MWfTnkLSg-cJoAhpVLLZkWIwl3sFj5H4ZIvNamQdRU2CQbMFkjsI3zaKFs~7dIWinLedRl46Sa8HEyk6lO6mlx2cwmgvEIjOGBP--JuOIu6-PMUFxRHrXsdkFG0qvnJl1f7-hbIii4d2L84qA94DERZJ~Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Median high-sensitivity cardiac troponin I measurements during the first 3 days following cardiac surgery. Reproduced with permission from [34] Copyright Massachusetts Medical Society. High-sensitivity troponin I after cardiac surgery and 30-day mortality. AVR: aortic valve replacement; CABG: coronary artery bypass graft; CS: cardiac surgeries; hs-cTnI: high-sensitive cardiac troponin I; postop: postoperative.
Differences in biomarker release according to type and complexity of the surgery
The extent of biomarker elevation also differs across different cardiac surgical procedures. Studies report the highest levels after more extensive surgery, such as combined valve and CABG surgery and isolated mitral valve surgery, and the lowest levels after isolated aortic valve replacement and isolated CABG (Table 5).
ELECTROCARDIOGRAM AND IMAGING TECHNIQUES IN THE EVALUATION OF PERIOPERATIVE MYOCARDIAL INFARCTION
Diagnosing PMI can be challenging due to pre-existing anomalies (such as left bundle branch block), perioperative sedative and analgesic drugs that may mask symptoms. Twelve-lead ECG and imaging techniques can provide important information that, in combination with biomarker levels, refines the diagnosis of PMI. However, each currently available imaging modality has unique strengths and limitations.
Electrocardiogram
ST-segment deviations and conduction disturbances are common after CABG, with a reported incidence ranging between 3.4% and 55.8% based on data from 30 studies [38]. ECG changes can result from inadequate myocardial preservation during aortic cross-clamping, epicardial and pericardial inflammation and ischaemic or traumatic myocardial injury. Most of the rhythm abnormalities are transient and benign after CABG and are unreliable indicators of myocardial ischaemia in the early postoperative setting. New isolated Q waves after surgery are not associated with adverse cardiac events and are not diagnostic for PMI [25, 39, 40].
Echocardiography
Echocardiography is the most commonly used imaging modality in cardiac surgery. Transthoracic echocardiographic (TTE) examination of segmental function and global left ventricular performance provides prognostic information and is essential when PMI is suspected based on biomarker criteria or haemodynamic deterioration. New wall motion abnormalities on TTE are commonly used as supportive criteria for defining PMI. However, pericardial effusion, inflammation and mechanical ventilation could compromise the imaging quality of postoperative TTE. Transoesophageal echocardiography (TEE) provides superior imaging quality with minimal risk complications [41]. TEE should be used in patients with poor-quality TTE images or when TTE does not provide conclusive results [42]. Although persistent wall motion abnormalities on TTE or TEE may appear indicative of PMI in patients with elevated biomarkers [43, 44], the ability of TTE and TEE to detect moderate ischaemic myocardial injury (i.e. subendocardial infarcts) is limited [45].
Computed tomography
Multidetector computed tomography (CT) angiography with a minimum of 64 slices can noninvasively assess bypass grafts with a sensitivity similar to that of invasive coronary angiography in identifying graft failure [46]. However, postoperative graft failure does not necessarily lead to PMI, and CT assessment of myocardial perfusion is still evolving and not yet validated as a diagnostic tool in the diagnosis of PMI [47]. The transport of recently operated on or haemodynamically unstable patients is challenging and remains a limit of this technique.
Cardiac magnetic resonance
CMR can detect new loss of viable myocardium with high sensitivity and specificity (100% and 98%, respectively) [48]. Moreover, CMR can systematically detect subendocardial infarcts missed by CT [48] and myocardial infarction in the territory of non-obstructed coronary arteries [49]. In contrast to echocardiography, the image quality of CMR is unaffected by pericardial effusions, adhesions, obesity or pulmonary emphysema, thereby allowing for a more precise examination of cardiovascular morphology and functionality [50]. Despite decades of accruing evidence supporting its clinical utility, the adoption of CMR in routine practice remains limited due to its uncertain added clinical value beyond echocardiography, challenges in transporting recently operated patients and economic concerns.
Nuclear imaging
Nuclear cardiac imaging techniques allow the assessment of myocardial perfusion and viability [51]. However, the need to transport postoperative patients and the associated costs limit their usefulness in routine clinical practice. Cardiac radionuclide imaging is usually restricted to situations where the patient’s serum marker measurements, ECG and echocardiographic findings are inconclusive.
Angiography
Coronary angiography is the gold standard for diagnosing graft or native coronary occlusion, although in isolation those finding are not diagnostic of PMI. Coronary angiography shares the logistic and transport issue described for CT and CMR, but offers the key advantage of allowing expedited treatment in case a coronary lesion is identified. As noted previously, in a meta-analysis of 9 studies that included 1104 patients who had CABG and who underwent postoperative angiography for PMI, 31.7% had a negative finding and 62.1% had an acute graft failure [15]. Similar results were observed in 2 subsequent reports that were not included in this meta-analysis [52, 53]. Invasive coronary angiography is unsuitable for routine bypass graft assessment due to its small but non-negligible risk of complications and costs.
Figure 2, along with the Graphical Abstract, presents proposed algorithms for detecting myocardial injury and myocardial infarction, using troponin levels and CK-MB as separate, crucial indicators. However, before these algorithms can be widely adopted, further testing and validation are necessary.
![Proposed algorithm for detecting myocardial injury and myocardial infarction, utilizing CK-MB as the primary measure. The Academic Research Consortium [61] suggests a CK-MB threshold of ≥10 as the upper reference limit for perioperative myocardial infarction. A threshold of >20 has been included to enhance the certainty of perioperative myocardial infarction detection [27]. CK-MB: creatine kinase MB; CMR: cardiac magnetic resonance; CTA: computed tomography angiography; ECG: electrocardiogram; PET: positron emission tomography; transoesophageal echocardiography; TTE: transthoracic echocardiography; URL: upper reference limit.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ejcts/65/2/10.1093_ejcts_ezad415/1/m_ezad415f2.jpeg?Expires=1749148653&Signature=A7hWkhht2BRtq~WTa5qH8iO1nv68AO7Gt1UaK1uq7z5H2ofXFt1DgnR0vxYFzoULZb99YaeONM~VarOrxzjrsceLLaWYHthd0JFy2DeK72SsQFRHpnOUmf0B0P1xMBVA20E0rOR0APUvAunPT6n1eq43ijrg3xWAI-3LDGAr6rgdAzSx8SFxzxav5PwAWEkF5nPnZAi9T3UgZkhI5wArp6yaNL3zIJltZqxZXZn-1aROpkjP7fZbDguTY1p3wLs3I7Db~cGSA8Sz6we7PSM~s287Q3sQM8mti8g~aeOfffgDB6EGnzA4wQiyzWa5297JucuxOW3Vyybss-dglaQCHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Proposed algorithm for detecting myocardial injury and myocardial infarction, utilizing CK-MB as the primary measure. The Academic Research Consortium [61] suggests a CK-MB threshold of ≥10 as the upper reference limit for perioperative myocardial infarction. A threshold of >20 has been included to enhance the certainty of perioperative myocardial infarction detection [27]. CK-MB: creatine kinase MB; CMR: cardiac magnetic resonance; CTA: computed tomography angiography; ECG: electrocardiogram; PET: positron emission tomography; transoesophageal echocardiography; TTE: transthoracic echocardiography; URL: upper reference limit.
ASSOCIATION OF THE CURRENT PERIOPERATIVE MYOCARDIAL INFARCTION DEFINITIONS WITH PROGNOSIS
Cho et al. [54] investigated the association of the second Universal Definition of Myocardial Infarction (UDMI), the third UDMI and the Society of Cardiovascular Angiography and Interventions (SCAI) definitions of PMI with the outcomes of 7679 patients with multivessel disease undergoing percutaneous coronary intervention (PCI) or CABG. Compared with CABG, the incidence of PMI was higher with PCI using the second UDMI (18.7% vs 2.9%), similar when using the third UDMI (3.2% and 1.9%), and lower using the SCAI definition (18.3% vs 5.5%). The authors reported significant correlations of PMI with 5-year major adverse cardiovascular events in patients undergoing PCI and CABG regardless of the definition used.
In a post hoc analysis of the CORONARY (CABG Off or On Pump Revascularization Study) trial, different thresholds for defining PMI were applied to over 4700 patients undergoing either on- or off-pump CABG [55]. In 46% of patients, the troponin levels were more than tenfold the upper limit of normal, and the fraction of PMI ranged between 0.6% and 19% depending on the definition used. A statistically significant association with 30-day mortality was seen only for troponin values several times higher than those suggested by current definitions (>130-fold).
The SYNTAX Extended Survival (SYNTAXES) study investigators stratified the 10-year outcomes of patients undergoing PCI or CABG using the PMI definitions used in the Synergy between Percutaneous Coronary Interventions with Taxus and Cardiac Surgery (SYNTAX) study, the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA), the Evaluation of Xience versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL), the fourth UDMI or the SCAI definitions of PMI [56]. The incidences of PMI varied largely depending on the definition. When PMI was defined only on the basis of elevated levels of biomarkers, its incidence was significantly higher than when additional signs of ischaemia were also requested. Although the associations with 10-year mortality were significant in the PCI arm regardless of the definition adopted, the associations in the CABG arm were significant only when definitions requiring additional signs of ischaemia were applied (e.g. SYNTAX, fourth UDMI).
In a similar analysis based on the EXCEL trial, the authors stratified the outcomes using the EXCEL or the third UDMI definitions of PMI [57]. The EXCEL definition (which does not require additional signs of ischaemia if biomarker elevations are substantial) resulted in higher proportions of PMI compared to the third UDMI; the association between PMI after CABG and 5-year mortality was stronger when the third UDMI was used.
Finally, in a cohort study of 2829 patients who had CABG, clinical outcomes were analysed based on 5 different definitions of PMI [SCAI, fourth UDMI, Academic Research Consortium (ARC)–myocardial infarction, ARC–myocardial injury or ischaemic ECG changes] [58]. An association with survival was seen only for the definitions that required additional signs of ischaemia (fourth UDMI or ARC–myocardial infarction).
In summary, the available data suggest that the use of different definitions results in varying proportions of PMI and that the highest rates are observed when definitions that require only biomarker release are used. In addition, PMI based on definitions that require additional signs of ischaemia correlate more closely with mortality.
PERIOPERATIVE MYOCARDIAL INFARCTION AS A COMPONENT OF PRIMARY COMPOSITE OUTCOMES IN TRIALS OF CORONARY REVASCULARIZATION
Because of the described lack of agreement on a general definition of PMI and its wide variability in incidence according to the definition used, whether PMI should be included in the primary composite outcome of myocardial revascularization trials is controversial. In addition, when comparing different revascularization methods that may be associated with different levels of the perioperative release of cardiac biomarkers (such as PCI and CABG), it is unclear whether the PMI definitions should differ between the 2 treatments.
Recently, there have been numerous examples of large coronary revascularization trials in which the primary outcome results have been largely dependent on the PMI definition used, generating confusion ambiguity in the interpretation of the findings and controversy in the cardiovascular community [10, 59–61].
In some trials, this uncertainty has been avoided by removing PMI from the composite primary end-point and focusing solely on non-procedural MIs [62]. However, excluding PMI may mask important safety concerns; in comparative trials of non-invasive or less invasive versus invasive management of coronary artery disease, removing PMI introduces bias by ignoring the potential periprocedural risk and artificially inflating the potential late benefits of the invasive treatments.
An alternative approach would be to include PMI in the primary endpoint using a definition that is balanced between treatment strategies, has prognostic significance and is universally accepted in the cardiovascular community. However, such a definition does not currently exist.
FUTURE DIRECTIONS AND GAPS IN KNOWLEDGE
Although it is likely that the definition of PMI and its inclusion in the primary composite outcome of coronary revascularization trials will remain controversial for some time, it is crucial that more evidence is generated on this important topic. The databases of existing trials and registries represent a formidable source of information, and data sharing and re-analyses by independent groups inclusive of all the necessary content experts (e.g. methodologists, statisticians, invasive and non-invasive cardiologists, cardiac surgeons, intensive care physicians, experts in imaging and biomarkers) as well as patient representatives should be encouraged. In addition, improvement in technology may potentially change the current landscape with the introduction of new imaging techniques or refinement of the current ones.
It is accepted by the authors of this document that the development of a definition of PMI that is prognostically important, equally applicable to all treatment modalities and accepted and endorsed by the entire cardiovascular community is an urgent priority.
EXPERT STATEMENTS
The development of a cardiac surgery-specific PMI definition that can be easily applied in clinical practice, has strong prognostic validity and is broadly accepted by the cardiovascular community is an urgent priority.
PMI using such a broadly accepted definition should be preferentially included in the primary composite outcome of cardiac surgery trials.
The postoperative threshold troponin value associated with increased mortality risk is substantially higher than in current PMI definitions. Current thresholds should be revised.
Large biomarker elevations after cardiac surgery are prognostically relevant even in the absence of additional signs of ischaemia.
ACKNOWLEDGEMENTS
The authors thank Stephanie Halksworth, Giulia Zuodar and Monika Wilusz for valuable assistance.
FUNDING
This article was produced by and is the sole responsibility of the European Association for Cardio-Thoracic Surgery (EACTS).
Conflict of interest: Mario Gaudino has no conflicts of interest. Marcus Flather has no conflicts of interest. Davide Capodanno has received fees for consultancy or lectures from Amgen, Chiesi, Daiichi Sankyo, Sanofi, Terumo, Medtronic, Biotronik and MedAlliance all unrelated to the present work. Milan Milojevic has received fee for presentation from Medtronic unrelated to the present work. Deepak L. Bhatt has received grants from Abbott, Acesion Pharma, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera. Cereno Scientific, Chiesi, Cincor, Cleerly, CSL Behringer, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Labaratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer Inc, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, 89bio, royalties from Elsevier, consulting fees from Broadview Ventures, Hims, McKinsey, honoraria from American College of Cardiology, Baim Institute for Clinical Research, Belvoir Publications, Boston Scientific, Cleveland Clinic, Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Novartis, Population Health Research Institute, Rutgers University, Canadian Medical and Surgical Knowledge Translation Research Group, Cowen and Company, HMP Global, Journal of the American College of Cardiology, K2P, Level Ex, Medtelligence/ReachMD, MJH Life Sciences, Oakstone CME, Piper Sandler, Population Health Research Institute, Slack Publications, WebMD, Wiley, Society of Cardiovascular Patient Care, payment for expert testimony from Arnold and Porter law firm, support for attending meetings from American College of Cardiology, Society of Cardiovascular Patient Care, American Heart Association, patent for sotagliflozin, fees from advisory board of Acesion Pharma, Assistance Publique-Hôpitaux de Paris, AngioWave, Baim Institute, Bayer, Boehringer Ingelheim, Boston Scientific, Cardax, CellProthera, Cereno Scientific, Cleveland Clinic, Contego Medical, Duke Clinical Research Institute, Elsevier Practice Update Cardiology, Janssen, Level Ex, Mayo Clinic, Medscape Cardiology, Merck, Mount Sinai School of Medicine, MyoKardia, NirvaMed, Novartis, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Population Health Research Institute, Stasys, fees for leadership or fiduciary role from American College of Cardiology, AngioWave, Boston VA Research Institute, Bristol Myers Squibb, DRS.LINQ, High Enroll, Society of Cardiovascular Patient Care, TobeSoft, stocks from AngioWave, Bristol Myers Squibb, DRS.LINQ, High Enroll, Other financial or non-financial interests from Clinical Cardiology, NCDR-ACTION Registry Steering Committee, Conducts unfunded research with FlowCo, Contego Medical, American Heart Association Quality Oversight Committee, Inaugural Chair, VA CART Research and Publications Committee, Site co-investigator for Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Phillips SpectraWAVE, Svelte and Vascular Solutions. Giuseppe Biondi Zoccai has received consultancy fees from Amarin, Balmed, Cardionovum, Crannmedical, Endocore Lab, Eukon, Guidotti, Innovheart, Meditrial, Micropor, Opsens Medical, Terumo, Translumina. William Boden has no conflicts of interest. P.J. Devereaux has received grants from Abbott Diagnostics, Roche Diagnostics, Siemens, receipt of equipment from CloudDX, Philips Healthcare, and other financial or non-financial interests from Abbott Diagnostics, Roche Diagnostics, Trimedic Canada. Torsten Doenst has no conflicts of interest. Michael Farkouh has received grants from Novartis, Amgen, Nova Nordisk, consultancy fees from Otitopic, speaker fees from Sanofi, fees for participation on advisory board from Astra Zeneca. Nicholas Freemantle has received institutional support for present manuscript from EACTS, has received grants from NIHR, MRC, Cure Parkinson’s Trust, EU, consulting fees from ALK, Sanofi Aventis, Gedeon Richter, Abbot, Galderma, Astra Zeneka, Ipsen, Vertex, Thea, Novo Nordisk, Aimmune, Ipsen, speakear fees from Abbott Singapore, fees for participation on advisory board from Orion. Stephen Fremes has received grants from NPI CIHR funded ROMA and STICJ3C, CoA CIHR funded ODIN, VISION, TRICS, CoA NIH funded ROMA QofL and ROMA Cog, Site co-PI Medtronic SURTAVI and Low Risk Evolut Trial, Boston Scientific NeoAccurate II IDE and NEWTON trials, participation on advisory board from Polypid DSMB. John Puskas has received fees for consultancy from Medtronic for OPCAB training, and fees from lectures/teaching from Medistim, Atricure, Artivion and Edwards. Giovanni Landoni has no conflicts of interest. Jennifer Lawton has no conflicts of interest. Patrick O. Myers has no conflicts of interest. Björn Redfors has received grants from European Research Council, Swedish Scientific Council, Swedish Heart and Lung foundation, consultancy fees from Boehringer Ingelheim, fees for participation on advisory board from DSMB for the TARGET study (tricuspid valve intervention), patents issued on pharmacological treatment of ST-elevation myocardial infarction. Sigrid Sandner has no conflicts of interest.
DATA AVAILABILITY
The data underlying this article will be shared on reasonable request to the corresponding author.




