-
PDF
- Split View
-
Views
-
Cite
Cite
Amedeo Anselmi, Alexandre Mansour, Marylou Para, Nicolas Mongardon, Alizée Porto, Julien Guihaire, Marie-Catherine Morgant, Matteo Pozzi, Bernard Cholley, Pierre-Emmanuel Falcoz, Philippe Gaudard, Guillaume Lebreton, François Labaste, Claudio Barbanti, Olivier Fouquet, Sidney Chocron, Nicolas Mottard, Maxime Esvan, Claire Fougerou-Leurent, Erwan Flecher, André Vincentelli, Nicolas Nesseler, ECMOSARS Investigators , Veno-arterial extracorporeal membrane oxygenation for circulatory failure in COVID-19 patients: insights from the ECMOSARS registry, European Journal of Cardio-Thoracic Surgery, Volume 64, Issue 3, September 2023, ezad229, https://doi.org/10.1093/ejcts/ezad229
Close - Share Icon Share
Abstract
The clinical profile and outcomes of patients with Coronavirus Disease 2019 (COVID-19) who require veno-arterial extracorporeal membrane oxygenation (VA-ECMO) or veno-arterial-venous extracorporeal membrane oxygenation (VAV-ECMO) are poorly understood. We aimed to describe the characteristics and outcomes of these patients and to identify predictors of both favourable and unfavourable outcomes.
ECMOSARS is a multicentre, prospective, nationwide French registry enrolling patients who require veno-venous extracorporeal membrane oxygenation (ECMO)/VA-ECMO in the context of COVID-19 infection (652 patients at 41 centres). We focused on 47 patients supported with VA- or VAV-ECMO for refractory cardiogenic shock.
The median age was 49. Fourteen percent of patients had a prior diagnosis of heart failure. The most common aetiologies of cardiogenic shock were acute pulmonary embolism (30%), myocarditis (28%) and acute coronary syndrome (4%). Extracorporeal cardiopulmonary resuscitation (E-CPR) occurred in 38%. In-hospital survival was 28% in the whole cohort, and 43% when E-CPR patients were excluded. ECMO cannulation was associated with significant improvements in pH and FiO2 on day 1, but non-survivors showed significantly more severe acidosis and higher FiO2 than survivors at this point (P = 0.030 and P = 0.006). Other factors associated with death were greater age (P = 0.02), higher body mass index (P = 0.03), E-CPR (P = 0.001), non-myocarditis aetiology (P = 0.02), higher serum lactates (P = 0.004), epinephrine (but not noradrenaline) use before initiation of ECMO (P = 0.003), haemorrhagic complications (P = 0.001), greater transfusion requirements (P = 0.001) and more severe Survival after Veno-Arterial ECMO (SAVE) and Sonographic Assessment of Intravascular Fluid Estimate (SAFE) scores (P = 0.01 and P = 0.03).
We report the largest focused analysis of VA- and VAV-ECMO recipients in COVID-19. Although relatively rare, the need for temporary mechanical circulatory support in these patients is associated with poor prognosis. However, VA-ECMO remains a viable solution to rescue carefully selected patients. We identified factors associated with poor prognosis and suggest that E-CPR is not a reasonable indication for VA-ECMO in this population.
INTRODUCTION
Since the initial months of the Coronavirus Disease 2019 (COVID-19) outbreak, extracorporeal membrane oxygenation (ECMO) has been used in the treatment of severe acute respiratory distress syndrome (ARDS). Research to date has described the outcomes of COVID-19 patients on ECMO, begun to establish criteria for the use of ECMO in COVID and clarified important details of the Intensive Care Unit (ICU) management [1–4]. Published series have demonstrated improved survival with veno-venous extracorporeal membrane oxygenation (VV-ECMO) in selected patients with isolated hypoxemic respiratory failure associated with COVID-19 ARDS compared to similar patients managed without ECMO [5–7]. VV-ECMO is now an accepted therapeutic option in COVID-19-associated ARDS and the parameters of its use have been well described. However, a subset of COVID-19 patients also develop cardiocirculatory failure and require mechanical circulatory support. These patients constitute a particular clinical challenge because of highly variable clinical profiles, ranging from minimal cardiovascular signs to profound cardiogenic shock, and multiple pathophysiologic mechanisms which may independent of the occurrence of COVID-19-related ARDS. While several single-centre studies have begun to describe COVID-19 patients who require veno-arterial or veno-arterio-venous ECMO [8–10], none of the large ECMO studies have specifically addressed this population. The current literature would benefit from further investigation of ECMO for COVID-19-associated cardiogenic shock, along with predictors of survival [3, 11].
Our group previously reported the clinical characteristics and outcomes of VV-ECMO from an ongoing multi-institutional collaboration [1]. The ECMOSARS Registry focuses on the characteristics, management and outcomes of COVID-19 patients requiring extracorporeal support. Here, we provide a sub-analysis of the ECMOSARS Registry focusing on patients supported with veno-arterial extracorporeal membrane oxygenation (VA-ECMO) or veno-arterial-venous extracorporeal membrane oxygenation (VAV) ECMO. Our objectives were to describe the clinical profile of these patients and report outcomes and establish which factors are associated with in-hospital mortality.
METHODS
Ethics statement
Approval by the Ethics Committee of the University Hospital of Rennes (Rennes, France) was obtained under the number 20.43 on 18 April 2020. According to the French law, patients’ written consent was waived due to the observational and non-interventional design of the study. Non-opposition for the use of personal data was obtained from all patients or legal representatives. This study complies with the Declaration of Helsinki.
Design and collection of data
ECMOSARS is a multicentre, nationwide registry launched on 21 April 2020 to investigate the determinants and clinical results of ECMO in patients with COVID-19 in France (ClinicalTrials.gov Identifier: NCT04397588). The registry was closed to new patients on 11 November 2021. A total of 47 centres participated.
Data were obtained from patient records by research assistants and entered in an electronic database via electronic data collection sheets. The database was regularly checked for consistency and completeness. The dataset included 384 variables per patient (baseline features, clinical management of COVID-19-related ARDS, characteristics at the time of ECMO institution, adverse events, outcomes). The main variables and end points are described in Supplementary Material, Table S2. Inclusion in ECMOSARS and collection of data were performed prospectively in all patients who were implanted with ECMO after the launch of the registry. For patients who underwent ECMO implantation before this date, data collection was performed retrospectively.
Inclusion criteria
All adult or paediatric patients with a positive reverse-transcriptase polymerase chain reaction for severe acute respiratory syndrome coronavirus 2 (nasopharyngeal swabs, sputum, endotracheal aspiration, bronchoalveolar lavage or stool sample), and/or with a diagnosis of COVID-19 based on findings at chest CT-scan, and undergoing VV-, VA- or VAV-ECMO were eligible for inclusion in the Registry. The exclusion was based on patients’ or legal representatives’ refusal to provide consent for the use of personal data, or on the adults’ protected legal status.
In the present study, we only considered patients supported with VA-ECMO or VAV-ECMO, and excluded patients supported with VV-ECMO. ECMO was initiated to treat isolated circulatory failure or combined circulatory and pulmonary failure in patients affected by severe acute respiratory syndrome coronavirus 2 and included in ECMOSARS. For the purposes of the present analysis and to improve the reliability of the conclusions, only patients with a minimum follow-up of 28 days after starting ECMO or died within 28 days after starting ECMO were included.
Endpoints
Study end points were as follows: in-hospital survival after the use of veno-arterial ECMO in COVID-19 patients; in-hospital complications after the use of veno-arterial ECMO in COVID-19 patients; and factors associated with death among recipients of veno-arterial ECMO in COVID-19 patients.
Statistical analysis
Continuous data are described as median and interquartile range or as mean ± standard deviation, and categorical data as number and percentages. Comparisons between survivors and non-survivors were performed using independent samples’ t-test or the Mann–Whitney-Wilcoxon test for continuous variables and χ2 test or the Fisher’s exact test for categorical variables. The paired t-test was employed for the comparison of continuous data between day 0 and day 1 on ECMO. Time-to-event distribution was estimated according to the Kaplan–Meier method, and the corresponding survival curve was built. Statistical analyses were computed at a two-sided α level of 5% with SAS version 9.4 software (SAS Institute, Cary, NC, USA).
RESULTS
Patient characteristics at initiation of extracorporeal membrane oxygenation
Of the 652 patients included in the ECMOSARS registry from 47 centres, 47 (8%) received VA- or VAV-ECMO. Of these patients, 7 (15%) initially received VV-ECMO, which was later converted (mostly within 24 h) into a VAV strategy after the development of cardiogenic shock. The remainder received VA-ECMO from the point of initial cannulation. The baseline features of the population at the time of hospital admission are reported in Supplementary Material, Table S1. Of note, the majority of patients were male (77%), with a median age of 49 years (interquartile range: 36.5–61.5) and more than a third were younger than 40. The median body mass index (BMI) was 29.3 kg/m2, with 44% of patients having BMI ≥30. A minority of patients were affected by pre-existing known cardiovascular disease. Fourteen percent had a previous diagnosis of chronic heart failure, 2% presented with stable coronary disease, 27% had diabetes (insulin-dependent in 15%) and 27% had known arterial hypertension. No patients had a history of previous cardiac surgery; only 1 had a history of previous myocardial revascularization; and none had known heart valve disease. The rate of previous venous thromboembolism was 11.6%. While the last known left ventricular ejection fraction (LVEF) before hospitalization did not suggest baseline myocardial dysfunction in this population (median value 60%), at the time of ECMO initiation, the median LVEF was 22%. Patients who survived to at least 28 days were significantly younger, had a significantly lower BMI and presented with significantly lower baseline LVEF. No statistically meaningful differences were observed in the distribution of baseline medications.
Table 1 reports the characteristics of the population at the time of institution of ECMO as well as the ECMO modality and indications. COVID-associated myocarditis was reported in 28% of cases. Among the indications for VA-ECMO, myocarditis was associated with significantly better survival (P = 0.007). On the other hand, 37.7% of patients required ECMO implantation during extracorporeal cardiopulmonary resuscitation (E-CPR) (Video 1). E-CPR was associated with worse survival (P = 0.002). Non-survivors were also characterized by higher serum lactates (P = 0.004) and use of epinephrine (P = 0.004). No difference was observed in the pre-ECMO serum troponin level.
Characteristics and clinical management features at the time of extracorporeal membrane oxygenation implantation
| Characteristic . | Missing values, N (%) . | Entire cohort (N = 47) . | Vital status . | P-Value . | |
|---|---|---|---|---|---|
| Non-survivors (N = 33) . | Survivors (N = 13) . | ||||
| Time from hospitalization to ICU admission (days), median (IQR) | 3 (6) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.48 |
| SAVE score, median (IQR) | 0 (0) | 0.0 (2.0–4.0) | 0.0 (2.0–2.0) | 9.0 (0.0–12.0) | 0.015 |
| SOFA score, median (IQR) | 9 (19) | 10 (8–13) | 10 (9–13) | 8 (6–11) | 0.031 |
| Mechanical ventilation time before ECMO (days), median (IQR) | 5 (11) | 1 (0.0–5.0) | 2 (0.0–5.0) | 0.5 (0.0–4.0) | 0.31 |
| ARDS (Berlin criteria), n (%) | 3 (6) | 33 (75) | 24 (80) | 9 (69) | 0.46 |
| Cannulation strategy, n (%) | 1 (2) | 0.07 | |||
| 43 (93) | 32 (97) | 10 (83) | ||
| 2 (4) | 0 (0) | 2 (17) | ||
| 1 (2) | 1 (3) | 0 (0) | ||
| Cannulation technique, n (%) | 1 (2) | >0.99 | |||
| 19 (41) | 14 (42) | 5 (42) | ||
| 27 (59) | 19 (58) | 7 (58) | ||
| Site of ECMO initiation, n (%) | 1 (2) | 0.034 | |||
| 11 (24) | 4 (12) | 6 (50) | ||
| 32 (70) | 26 (79) | 6 (50) | ||
| 3 (6) | 3 (9.1) | 0 (0) | ||
| Indications for ECMO, n (%) | |||||
| 0 (0) | 13 (28) | 5 (15) | 7 (54) | 0.021 |
| 0 (0) | 14 (30) | 11 (33) | 3 (23) | 0.72 |
| 0 (0) | 29 (70) | 21 (72) | 6 (54) | 0.45 |
| 0 (0) | 2 (4) | 2 (6) | 0 (0) | >0.99 |
| 0 (0) | 3 (6) | 2 (6) | 0 (0) | >0.99 |
| 1 (2) | 17 (37) | 17 (51) | 0 (0) | 0.001 |
| LVEF (%), median (IQR) | 15 (32) | 22.5 (15–45) | 25 (12.5–45) | 20 (15–50) | 0.90 |
| Serum troponin T (ng/ml), median (IQR) | 14 (30) | 4.9 (0.2–28.0) | 5.2 (0.1–33.0) | 4.9 (0.3–8.6) | 0.72 |
| Serum lactates (mmol/l), median (IQR) | 8 (17.0) | 4.1 (2.3–9.1) | 5.2 (3.6–10.3) | 3.2 (2.3–4.3) | 0.004 |
| Dobutamine, n (%) | 6 (13) | 16 (40) | 9 (31) | 6 (54) | 0.27 |
| Noradrenaline, n (%) | 6 (13) | 34 (83) | 24 (83) | 10 (91) | >0.99 |
| Adrenaline, n (%) | 5 (11) | 15 (36) | 15 (50) | 0 (0) | 0.003 |
| Characteristic . | Missing values, N (%) . | Entire cohort (N = 47) . | Vital status . | P-Value . | |
|---|---|---|---|---|---|
| Non-survivors (N = 33) . | Survivors (N = 13) . | ||||
| Time from hospitalization to ICU admission (days), median (IQR) | 3 (6) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.48 |
| SAVE score, median (IQR) | 0 (0) | 0.0 (2.0–4.0) | 0.0 (2.0–2.0) | 9.0 (0.0–12.0) | 0.015 |
| SOFA score, median (IQR) | 9 (19) | 10 (8–13) | 10 (9–13) | 8 (6–11) | 0.031 |
| Mechanical ventilation time before ECMO (days), median (IQR) | 5 (11) | 1 (0.0–5.0) | 2 (0.0–5.0) | 0.5 (0.0–4.0) | 0.31 |
| ARDS (Berlin criteria), n (%) | 3 (6) | 33 (75) | 24 (80) | 9 (69) | 0.46 |
| Cannulation strategy, n (%) | 1 (2) | 0.07 | |||
| 43 (93) | 32 (97) | 10 (83) | ||
| 2 (4) | 0 (0) | 2 (17) | ||
| 1 (2) | 1 (3) | 0 (0) | ||
| Cannulation technique, n (%) | 1 (2) | >0.99 | |||
| 19 (41) | 14 (42) | 5 (42) | ||
| 27 (59) | 19 (58) | 7 (58) | ||
| Site of ECMO initiation, n (%) | 1 (2) | 0.034 | |||
| 11 (24) | 4 (12) | 6 (50) | ||
| 32 (70) | 26 (79) | 6 (50) | ||
| 3 (6) | 3 (9.1) | 0 (0) | ||
| Indications for ECMO, n (%) | |||||
| 0 (0) | 13 (28) | 5 (15) | 7 (54) | 0.021 |
| 0 (0) | 14 (30) | 11 (33) | 3 (23) | 0.72 |
| 0 (0) | 29 (70) | 21 (72) | 6 (54) | 0.45 |
| 0 (0) | 2 (4) | 2 (6) | 0 (0) | >0.99 |
| 0 (0) | 3 (6) | 2 (6) | 0 (0) | >0.99 |
| 1 (2) | 17 (37) | 17 (51) | 0 (0) | 0.001 |
| LVEF (%), median (IQR) | 15 (32) | 22.5 (15–45) | 25 (12.5–45) | 20 (15–50) | 0.90 |
| Serum troponin T (ng/ml), median (IQR) | 14 (30) | 4.9 (0.2–28.0) | 5.2 (0.1–33.0) | 4.9 (0.3–8.6) | 0.72 |
| Serum lactates (mmol/l), median (IQR) | 8 (17.0) | 4.1 (2.3–9.1) | 5.2 (3.6–10.3) | 3.2 (2.3–4.3) | 0.004 |
| Dobutamine, n (%) | 6 (13) | 16 (40) | 9 (31) | 6 (54) | 0.27 |
| Noradrenaline, n (%) | 6 (13) | 34 (83) | 24 (83) | 10 (91) | >0.99 |
| Adrenaline, n (%) | 5 (11) | 15 (36) | 15 (50) | 0 (0) | 0.003 |
Bold correspond to statistically meaningful p-values.
ECMO: extracorporeal membrane oxygenation; E-CPR: extracorporeal cardiopulmonary resuscitation; ICU: Intensive Care Unit; IQR: interquartile range; LVEF: left ventricular ejection fraction; SAVE: Survival after Veno-Arterial ECMO Score.
Characteristics and clinical management features at the time of extracorporeal membrane oxygenation implantation
| Characteristic . | Missing values, N (%) . | Entire cohort (N = 47) . | Vital status . | P-Value . | |
|---|---|---|---|---|---|
| Non-survivors (N = 33) . | Survivors (N = 13) . | ||||
| Time from hospitalization to ICU admission (days), median (IQR) | 3 (6) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.48 |
| SAVE score, median (IQR) | 0 (0) | 0.0 (2.0–4.0) | 0.0 (2.0–2.0) | 9.0 (0.0–12.0) | 0.015 |
| SOFA score, median (IQR) | 9 (19) | 10 (8–13) | 10 (9–13) | 8 (6–11) | 0.031 |
| Mechanical ventilation time before ECMO (days), median (IQR) | 5 (11) | 1 (0.0–5.0) | 2 (0.0–5.0) | 0.5 (0.0–4.0) | 0.31 |
| ARDS (Berlin criteria), n (%) | 3 (6) | 33 (75) | 24 (80) | 9 (69) | 0.46 |
| Cannulation strategy, n (%) | 1 (2) | 0.07 | |||
| 43 (93) | 32 (97) | 10 (83) | ||
| 2 (4) | 0 (0) | 2 (17) | ||
| 1 (2) | 1 (3) | 0 (0) | ||
| Cannulation technique, n (%) | 1 (2) | >0.99 | |||
| 19 (41) | 14 (42) | 5 (42) | ||
| 27 (59) | 19 (58) | 7 (58) | ||
| Site of ECMO initiation, n (%) | 1 (2) | 0.034 | |||
| 11 (24) | 4 (12) | 6 (50) | ||
| 32 (70) | 26 (79) | 6 (50) | ||
| 3 (6) | 3 (9.1) | 0 (0) | ||
| Indications for ECMO, n (%) | |||||
| 0 (0) | 13 (28) | 5 (15) | 7 (54) | 0.021 |
| 0 (0) | 14 (30) | 11 (33) | 3 (23) | 0.72 |
| 0 (0) | 29 (70) | 21 (72) | 6 (54) | 0.45 |
| 0 (0) | 2 (4) | 2 (6) | 0 (0) | >0.99 |
| 0 (0) | 3 (6) | 2 (6) | 0 (0) | >0.99 |
| 1 (2) | 17 (37) | 17 (51) | 0 (0) | 0.001 |
| LVEF (%), median (IQR) | 15 (32) | 22.5 (15–45) | 25 (12.5–45) | 20 (15–50) | 0.90 |
| Serum troponin T (ng/ml), median (IQR) | 14 (30) | 4.9 (0.2–28.0) | 5.2 (0.1–33.0) | 4.9 (0.3–8.6) | 0.72 |
| Serum lactates (mmol/l), median (IQR) | 8 (17.0) | 4.1 (2.3–9.1) | 5.2 (3.6–10.3) | 3.2 (2.3–4.3) | 0.004 |
| Dobutamine, n (%) | 6 (13) | 16 (40) | 9 (31) | 6 (54) | 0.27 |
| Noradrenaline, n (%) | 6 (13) | 34 (83) | 24 (83) | 10 (91) | >0.99 |
| Adrenaline, n (%) | 5 (11) | 15 (36) | 15 (50) | 0 (0) | 0.003 |
| Characteristic . | Missing values, N (%) . | Entire cohort (N = 47) . | Vital status . | P-Value . | |
|---|---|---|---|---|---|
| Non-survivors (N = 33) . | Survivors (N = 13) . | ||||
| Time from hospitalization to ICU admission (days), median (IQR) | 3 (6) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.48 |
| SAVE score, median (IQR) | 0 (0) | 0.0 (2.0–4.0) | 0.0 (2.0–2.0) | 9.0 (0.0–12.0) | 0.015 |
| SOFA score, median (IQR) | 9 (19) | 10 (8–13) | 10 (9–13) | 8 (6–11) | 0.031 |
| Mechanical ventilation time before ECMO (days), median (IQR) | 5 (11) | 1 (0.0–5.0) | 2 (0.0–5.0) | 0.5 (0.0–4.0) | 0.31 |
| ARDS (Berlin criteria), n (%) | 3 (6) | 33 (75) | 24 (80) | 9 (69) | 0.46 |
| Cannulation strategy, n (%) | 1 (2) | 0.07 | |||
| 43 (93) | 32 (97) | 10 (83) | ||
| 2 (4) | 0 (0) | 2 (17) | ||
| 1 (2) | 1 (3) | 0 (0) | ||
| Cannulation technique, n (%) | 1 (2) | >0.99 | |||
| 19 (41) | 14 (42) | 5 (42) | ||
| 27 (59) | 19 (58) | 7 (58) | ||
| Site of ECMO initiation, n (%) | 1 (2) | 0.034 | |||
| 11 (24) | 4 (12) | 6 (50) | ||
| 32 (70) | 26 (79) | 6 (50) | ||
| 3 (6) | 3 (9.1) | 0 (0) | ||
| Indications for ECMO, n (%) | |||||
| 0 (0) | 13 (28) | 5 (15) | 7 (54) | 0.021 |
| 0 (0) | 14 (30) | 11 (33) | 3 (23) | 0.72 |
| 0 (0) | 29 (70) | 21 (72) | 6 (54) | 0.45 |
| 0 (0) | 2 (4) | 2 (6) | 0 (0) | >0.99 |
| 0 (0) | 3 (6) | 2 (6) | 0 (0) | >0.99 |
| 1 (2) | 17 (37) | 17 (51) | 0 (0) | 0.001 |
| LVEF (%), median (IQR) | 15 (32) | 22.5 (15–45) | 25 (12.5–45) | 20 (15–50) | 0.90 |
| Serum troponin T (ng/ml), median (IQR) | 14 (30) | 4.9 (0.2–28.0) | 5.2 (0.1–33.0) | 4.9 (0.3–8.6) | 0.72 |
| Serum lactates (mmol/l), median (IQR) | 8 (17.0) | 4.1 (2.3–9.1) | 5.2 (3.6–10.3) | 3.2 (2.3–4.3) | 0.004 |
| Dobutamine, n (%) | 6 (13) | 16 (40) | 9 (31) | 6 (54) | 0.27 |
| Noradrenaline, n (%) | 6 (13) | 34 (83) | 24 (83) | 10 (91) | >0.99 |
| Adrenaline, n (%) | 5 (11) | 15 (36) | 15 (50) | 0 (0) | 0.003 |
Bold correspond to statistically meaningful p-values.
ECMO: extracorporeal membrane oxygenation; E-CPR: extracorporeal cardiopulmonary resuscitation; ICU: Intensive Care Unit; IQR: interquartile range; LVEF: left ventricular ejection fraction; SAVE: Survival after Veno-Arterial ECMO Score.
Outcomes
Institution of ECMO yielded significant improvement of metabolic parameters on blood gases (increase in blood pH and decrease in FiO2) in the first day on ECMO (Table 2). While there were no significant differences in pre-ECMO arterial blood pH, PaO2, PaCO2 and FiO2 on the ventilator between survivors and non-survivors, on ECMO day 1, the non-survivors showed significantly more severe acidosis and greater FiO2 on the ventilator (P = 0.03 and P = 0.007, respectively).
Blood gas data at the time of extracorporeal membrane oxygenation implantation and day 1
| . | Entire cohort . | P-Value . | Baseline (at ECMO implantation) . | P-Value . | On ECMO day 1 . | P-Value . | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline . | On ECMO day 1 . | Non-survivors (N = 33) . | Survivors (N = 13) . | Non-survivors (N = 33) . | Survivors (N = 13) . | ||||
| pH | 7.28 (7.1–7.43) | 7.37 (7.32–7.43) | 0.006 | 7.27 (7.08–7.31) | 7.42 (7.18–7.46) | 0.11 | 7.33 (7.29–7.41) | 7.42 (7.39–7.44) | 0.03 |
| PaO2 (mmHg) | 72 (58–101) | 79.5 (65–142) | 0.36 | 69 (53.5–85) | 99.5 (79.5–117) | 0.68 | 104.8 ± 66.9 | 111.6 ± 65.2 | 0.99 |
| PaCO2 (mmHg) | 46 (33–62) | 43.1 ± 9.8 | 0.09 | 49.5 (35.5–64) | 36.5 (28.5–56) | 0.19 | 43.4 ± 9.4 | 42.5 ± 11.4 | 0.81 |
| FiO2 (%) | 100 (67.5–100) | 62.4 ± 24.8 | <0.001 | 100 (84–100) | 80 (60–100) | 0.21 | 70.7 ± 25.1 | 48.3 ± 18.1 | 0.007 |
| . | Entire cohort . | P-Value . | Baseline (at ECMO implantation) . | P-Value . | On ECMO day 1 . | P-Value . | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline . | On ECMO day 1 . | Non-survivors (N = 33) . | Survivors (N = 13) . | Non-survivors (N = 33) . | Survivors (N = 13) . | ||||
| pH | 7.28 (7.1–7.43) | 7.37 (7.32–7.43) | 0.006 | 7.27 (7.08–7.31) | 7.42 (7.18–7.46) | 0.11 | 7.33 (7.29–7.41) | 7.42 (7.39–7.44) | 0.03 |
| PaO2 (mmHg) | 72 (58–101) | 79.5 (65–142) | 0.36 | 69 (53.5–85) | 99.5 (79.5–117) | 0.68 | 104.8 ± 66.9 | 111.6 ± 65.2 | 0.99 |
| PaCO2 (mmHg) | 46 (33–62) | 43.1 ± 9.8 | 0.09 | 49.5 (35.5–64) | 36.5 (28.5–56) | 0.19 | 43.4 ± 9.4 | 42.5 ± 11.4 | 0.81 |
| FiO2 (%) | 100 (67.5–100) | 62.4 ± 24.8 | <0.001 | 100 (84–100) | 80 (60–100) | 0.21 | 70.7 ± 25.1 | 48.3 ± 18.1 | 0.007 |
Values are represented as median (IQR) or mean ± SD. Bold correspond to statistically meaningful p-values.
ECMO: extracorporeal membrane oxygenation; IQR: interquartile range; SD: standard deviation.
Blood gas data at the time of extracorporeal membrane oxygenation implantation and day 1
| . | Entire cohort . | P-Value . | Baseline (at ECMO implantation) . | P-Value . | On ECMO day 1 . | P-Value . | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline . | On ECMO day 1 . | Non-survivors (N = 33) . | Survivors (N = 13) . | Non-survivors (N = 33) . | Survivors (N = 13) . | ||||
| pH | 7.28 (7.1–7.43) | 7.37 (7.32–7.43) | 0.006 | 7.27 (7.08–7.31) | 7.42 (7.18–7.46) | 0.11 | 7.33 (7.29–7.41) | 7.42 (7.39–7.44) | 0.03 |
| PaO2 (mmHg) | 72 (58–101) | 79.5 (65–142) | 0.36 | 69 (53.5–85) | 99.5 (79.5–117) | 0.68 | 104.8 ± 66.9 | 111.6 ± 65.2 | 0.99 |
| PaCO2 (mmHg) | 46 (33–62) | 43.1 ± 9.8 | 0.09 | 49.5 (35.5–64) | 36.5 (28.5–56) | 0.19 | 43.4 ± 9.4 | 42.5 ± 11.4 | 0.81 |
| FiO2 (%) | 100 (67.5–100) | 62.4 ± 24.8 | <0.001 | 100 (84–100) | 80 (60–100) | 0.21 | 70.7 ± 25.1 | 48.3 ± 18.1 | 0.007 |
| . | Entire cohort . | P-Value . | Baseline (at ECMO implantation) . | P-Value . | On ECMO day 1 . | P-Value . | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline . | On ECMO day 1 . | Non-survivors (N = 33) . | Survivors (N = 13) . | Non-survivors (N = 33) . | Survivors (N = 13) . | ||||
| pH | 7.28 (7.1–7.43) | 7.37 (7.32–7.43) | 0.006 | 7.27 (7.08–7.31) | 7.42 (7.18–7.46) | 0.11 | 7.33 (7.29–7.41) | 7.42 (7.39–7.44) | 0.03 |
| PaO2 (mmHg) | 72 (58–101) | 79.5 (65–142) | 0.36 | 69 (53.5–85) | 99.5 (79.5–117) | 0.68 | 104.8 ± 66.9 | 111.6 ± 65.2 | 0.99 |
| PaCO2 (mmHg) | 46 (33–62) | 43.1 ± 9.8 | 0.09 | 49.5 (35.5–64) | 36.5 (28.5–56) | 0.19 | 43.4 ± 9.4 | 42.5 ± 11.4 | 0.81 |
| FiO2 (%) | 100 (67.5–100) | 62.4 ± 24.8 | <0.001 | 100 (84–100) | 80 (60–100) | 0.21 | 70.7 ± 25.1 | 48.3 ± 18.1 | 0.007 |
Values are represented as median (IQR) or mean ± SD. Bold correspond to statistically meaningful p-values.
ECMO: extracorporeal membrane oxygenation; IQR: interquartile range; SD: standard deviation.
Table 3 summarizes the main outcomes in the study population. Overall in-hospital survival was 28%. When patients who received E-CPR were excluded, in-hospital survival rose to 43%. Patients with cardiogenic shock due to myocarditis had significantly better prognoses than other aetiologies, including pulmonary emboli. Complications in this cohort were common. Acute kidney injury occurred in 69% of patients, and haemorrhagic complications in 47%. Haemorrhagic complications were significantly more frequent among non-survivors (P = 0.004), and non-survivors had higher transfusion volumes compared to survivors. Thrombotic complications on ECMO occurred in 19%, though half of these were clotting of the circuit or oxygenator requiring equipment exchange. Not surprisingly, non-survivors displayed markedly shorter ICU and hospital stay. In fact, death occurred after median 7 days following ICU admission while survivors had, as a consequence, a longer average ICU stay. No subsequent death was observed among survivors at hospital discharge; Fig. 1 reports the Kaplan–Meier survival curve obtained on the basis of a mean 31.7 ± 39.4 days follow-up in the enrolled population (90 days among survivors). In non-survivors, the leading causes of death were multiorgan failure (44%), followed by brain death (14%).
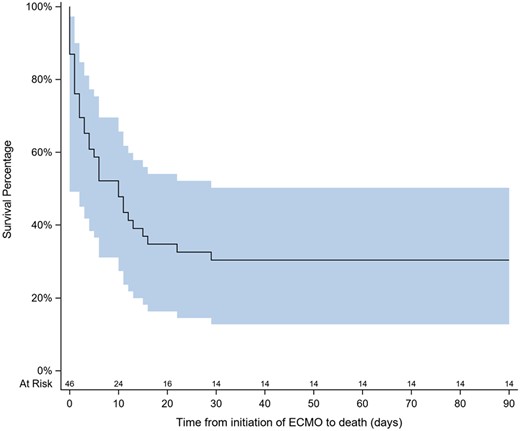
Kaplan–Meier survival curve in the entire patient population. The shaded area represents the confidence interval.
Veno-arterial extracorporeal membrane oxygenation (extracorporeal cardiopulmonary resuscitation) insertion for refractory cardiac arrest in a young patient. The video demonstrates the intended simplification of the surgical and general management protocols to minimize the low-flow time.
| Characteristic . | Missing values, N (%) . | Entire cohort (N = 47) . | Vital status . | P-Value . | |
|---|---|---|---|---|---|
| Non-survivors (N = 33) . | Survivors (N = 13) . | ||||
| ECMO duration (days) | 3 (6.4) | 5 (1.5–10) | 4 (1–10) | 5 (4–10) | 0.13 |
| ECMO-free days on day 28 | 2 (4) | 5.3 ± 9.5 | 1.1 ± 4.4 | 18.4 ± 9.4 | <0.001 |
| Total mechanical ventilation time (days) | 13 (28) | 8 (4–19) | 8 (2–15) | 24 (6–31) | 0.02 |
| Ventilator-free days at day 28 | 1 (2.1) | 2.5 ± 6.6 | 0.1 ± 0.5 | 9.4 ± 10.3 | <0.001 |
| Left heart unload | 6 (13) | 0.50 | |||
| 2 (5) | 2 (7) | 0 (0) | ||
| 1 (2) | 0 (0) | 1 (1) | ||
| 1 (2) | 1 (3) | 0 (0) | ||
| Hemorrhagic complications | 5 (11) | ||||
| 23 (55) | 21 (70) | 2 (17) | 0.002 | |
| 12 (25) | 11 (33) | 1 (8) | 0.13 | |
| 6 (13) | 5 (15) | 1 (8) | 0.66 | |
| 1 (2) | 1 (3) | 0 (0) | >0.99 | |
| 5 (11) | 5 (15) | 0 (0) | 0.30 | |
| 5 (11) | 5 (15) | 0 (0) | 0.30 | |
| Thrombotic complications | 5 (11) | 14 (33) | 10 (33) | 2 (33) | >0.99 |
| Transfusion of blood products,Nof units | |||||
| 6 (13) | 2 (2–9) | 6.5 (2–11) | 0 (0–2) | 0.001 |
| 6 (13) | 0 (0–2) | 0 (0–4) | 0 (0–0) | 0.076 |
| 6 (13) | 0 (0–0) | 0 (0–1) | 0 (0–0) | 0.046 |
| Neurologic complications | 5 (11) | >0.99 | |||
| 3 (7) | 2 (7) | 1 (8) | ||
| 3 (7) | 3 (10) | 0 (0) | ||
| Lower limb ischemia | 5 (11) | 5 (12) | 4 (13) | 1 (8) | >0.99 |
| Acute kidney injury | 5 (11) | 29 (69) | 23 (77) | 6 (50) | 0.14 |
| Infectious complications | 6 (13) | 7 (17) | 4 (14) | 3 (25) | 0.40 |
| ICU stay (days) | 5 (11) | 11 (4–18) | 7 (2–15) | 15 (12–33) | 0.003 |
| Hospital stay (days) | 5 (11) | 14.5 (5–29) | 8.5 (3–18) | 41.5 (27–51) | <0.001 |
| Characteristic . | Missing values, N (%) . | Entire cohort (N = 47) . | Vital status . | P-Value . | |
|---|---|---|---|---|---|
| Non-survivors (N = 33) . | Survivors (N = 13) . | ||||
| ECMO duration (days) | 3 (6.4) | 5 (1.5–10) | 4 (1–10) | 5 (4–10) | 0.13 |
| ECMO-free days on day 28 | 2 (4) | 5.3 ± 9.5 | 1.1 ± 4.4 | 18.4 ± 9.4 | <0.001 |
| Total mechanical ventilation time (days) | 13 (28) | 8 (4–19) | 8 (2–15) | 24 (6–31) | 0.02 |
| Ventilator-free days at day 28 | 1 (2.1) | 2.5 ± 6.6 | 0.1 ± 0.5 | 9.4 ± 10.3 | <0.001 |
| Left heart unload | 6 (13) | 0.50 | |||
| 2 (5) | 2 (7) | 0 (0) | ||
| 1 (2) | 0 (0) | 1 (1) | ||
| 1 (2) | 1 (3) | 0 (0) | ||
| Hemorrhagic complications | 5 (11) | ||||
| 23 (55) | 21 (70) | 2 (17) | 0.002 | |
| 12 (25) | 11 (33) | 1 (8) | 0.13 | |
| 6 (13) | 5 (15) | 1 (8) | 0.66 | |
| 1 (2) | 1 (3) | 0 (0) | >0.99 | |
| 5 (11) | 5 (15) | 0 (0) | 0.30 | |
| 5 (11) | 5 (15) | 0 (0) | 0.30 | |
| Thrombotic complications | 5 (11) | 14 (33) | 10 (33) | 2 (33) | >0.99 |
| Transfusion of blood products,Nof units | |||||
| 6 (13) | 2 (2–9) | 6.5 (2–11) | 0 (0–2) | 0.001 |
| 6 (13) | 0 (0–2) | 0 (0–4) | 0 (0–0) | 0.076 |
| 6 (13) | 0 (0–0) | 0 (0–1) | 0 (0–0) | 0.046 |
| Neurologic complications | 5 (11) | >0.99 | |||
| 3 (7) | 2 (7) | 1 (8) | ||
| 3 (7) | 3 (10) | 0 (0) | ||
| Lower limb ischemia | 5 (11) | 5 (12) | 4 (13) | 1 (8) | >0.99 |
| Acute kidney injury | 5 (11) | 29 (69) | 23 (77) | 6 (50) | 0.14 |
| Infectious complications | 6 (13) | 7 (17) | 4 (14) | 3 (25) | 0.40 |
| ICU stay (days) | 5 (11) | 11 (4–18) | 7 (2–15) | 15 (12–33) | 0.003 |
| Hospital stay (days) | 5 (11) | 14.5 (5–29) | 8.5 (3–18) | 41.5 (27–51) | <0.001 |
Values are represented as n (%), median (IQR) or mean ± SD. Bold correspond to statistically meaningful p-values.
ECMO: extracorporeal membrane oxygenation; FFP: Fresh Frozen Plasma; IABP: Intra Aortic Balloon Pump; ICU: Intensive Care Unit; IQR: interquartile range; PLT: Platelets; PRBc: Packed red Blood Cells; SD: standard deviation.
| Characteristic . | Missing values, N (%) . | Entire cohort (N = 47) . | Vital status . | P-Value . | |
|---|---|---|---|---|---|
| Non-survivors (N = 33) . | Survivors (N = 13) . | ||||
| ECMO duration (days) | 3 (6.4) | 5 (1.5–10) | 4 (1–10) | 5 (4–10) | 0.13 |
| ECMO-free days on day 28 | 2 (4) | 5.3 ± 9.5 | 1.1 ± 4.4 | 18.4 ± 9.4 | <0.001 |
| Total mechanical ventilation time (days) | 13 (28) | 8 (4–19) | 8 (2–15) | 24 (6–31) | 0.02 |
| Ventilator-free days at day 28 | 1 (2.1) | 2.5 ± 6.6 | 0.1 ± 0.5 | 9.4 ± 10.3 | <0.001 |
| Left heart unload | 6 (13) | 0.50 | |||
| 2 (5) | 2 (7) | 0 (0) | ||
| 1 (2) | 0 (0) | 1 (1) | ||
| 1 (2) | 1 (3) | 0 (0) | ||
| Hemorrhagic complications | 5 (11) | ||||
| 23 (55) | 21 (70) | 2 (17) | 0.002 | |
| 12 (25) | 11 (33) | 1 (8) | 0.13 | |
| 6 (13) | 5 (15) | 1 (8) | 0.66 | |
| 1 (2) | 1 (3) | 0 (0) | >0.99 | |
| 5 (11) | 5 (15) | 0 (0) | 0.30 | |
| 5 (11) | 5 (15) | 0 (0) | 0.30 | |
| Thrombotic complications | 5 (11) | 14 (33) | 10 (33) | 2 (33) | >0.99 |
| Transfusion of blood products,Nof units | |||||
| 6 (13) | 2 (2–9) | 6.5 (2–11) | 0 (0–2) | 0.001 |
| 6 (13) | 0 (0–2) | 0 (0–4) | 0 (0–0) | 0.076 |
| 6 (13) | 0 (0–0) | 0 (0–1) | 0 (0–0) | 0.046 |
| Neurologic complications | 5 (11) | >0.99 | |||
| 3 (7) | 2 (7) | 1 (8) | ||
| 3 (7) | 3 (10) | 0 (0) | ||
| Lower limb ischemia | 5 (11) | 5 (12) | 4 (13) | 1 (8) | >0.99 |
| Acute kidney injury | 5 (11) | 29 (69) | 23 (77) | 6 (50) | 0.14 |
| Infectious complications | 6 (13) | 7 (17) | 4 (14) | 3 (25) | 0.40 |
| ICU stay (days) | 5 (11) | 11 (4–18) | 7 (2–15) | 15 (12–33) | 0.003 |
| Hospital stay (days) | 5 (11) | 14.5 (5–29) | 8.5 (3–18) | 41.5 (27–51) | <0.001 |
| Characteristic . | Missing values, N (%) . | Entire cohort (N = 47) . | Vital status . | P-Value . | |
|---|---|---|---|---|---|
| Non-survivors (N = 33) . | Survivors (N = 13) . | ||||
| ECMO duration (days) | 3 (6.4) | 5 (1.5–10) | 4 (1–10) | 5 (4–10) | 0.13 |
| ECMO-free days on day 28 | 2 (4) | 5.3 ± 9.5 | 1.1 ± 4.4 | 18.4 ± 9.4 | <0.001 |
| Total mechanical ventilation time (days) | 13 (28) | 8 (4–19) | 8 (2–15) | 24 (6–31) | 0.02 |
| Ventilator-free days at day 28 | 1 (2.1) | 2.5 ± 6.6 | 0.1 ± 0.5 | 9.4 ± 10.3 | <0.001 |
| Left heart unload | 6 (13) | 0.50 | |||
| 2 (5) | 2 (7) | 0 (0) | ||
| 1 (2) | 0 (0) | 1 (1) | ||
| 1 (2) | 1 (3) | 0 (0) | ||
| Hemorrhagic complications | 5 (11) | ||||
| 23 (55) | 21 (70) | 2 (17) | 0.002 | |
| 12 (25) | 11 (33) | 1 (8) | 0.13 | |
| 6 (13) | 5 (15) | 1 (8) | 0.66 | |
| 1 (2) | 1 (3) | 0 (0) | >0.99 | |
| 5 (11) | 5 (15) | 0 (0) | 0.30 | |
| 5 (11) | 5 (15) | 0 (0) | 0.30 | |
| Thrombotic complications | 5 (11) | 14 (33) | 10 (33) | 2 (33) | >0.99 |
| Transfusion of blood products,Nof units | |||||
| 6 (13) | 2 (2–9) | 6.5 (2–11) | 0 (0–2) | 0.001 |
| 6 (13) | 0 (0–2) | 0 (0–4) | 0 (0–0) | 0.076 |
| 6 (13) | 0 (0–0) | 0 (0–1) | 0 (0–0) | 0.046 |
| Neurologic complications | 5 (11) | >0.99 | |||
| 3 (7) | 2 (7) | 1 (8) | ||
| 3 (7) | 3 (10) | 0 (0) | ||
| Lower limb ischemia | 5 (11) | 5 (12) | 4 (13) | 1 (8) | >0.99 |
| Acute kidney injury | 5 (11) | 29 (69) | 23 (77) | 6 (50) | 0.14 |
| Infectious complications | 6 (13) | 7 (17) | 4 (14) | 3 (25) | 0.40 |
| ICU stay (days) | 5 (11) | 11 (4–18) | 7 (2–15) | 15 (12–33) | 0.003 |
| Hospital stay (days) | 5 (11) | 14.5 (5–29) | 8.5 (3–18) | 41.5 (27–51) | <0.001 |
Values are represented as n (%), median (IQR) or mean ± SD. Bold correspond to statistically meaningful p-values.
ECMO: extracorporeal membrane oxygenation; FFP: Fresh Frozen Plasma; IABP: Intra Aortic Balloon Pump; ICU: Intensive Care Unit; IQR: interquartile range; PLT: Platelets; PRBc: Packed red Blood Cells; SD: standard deviation.
DISCUSSION
Cardiovascular complications associated with COVID-19 infection cover a wide spectrum, potentially involving all of the components of the cardiovascular system [12]. Of the 2 most common aetiologies in our cohort, myocarditis has been described both as the consequence of a direct viral infiltrate of the myocardium and as part of a systemic inflammatory syndrome [13–15]. Pulmonary embolism has also been closely associated with COVID-19 pneumonia [16]. Other cardiac manifestations of COVID-19, such as acute coronary syndrome, are less common but occur with a discrete frequency [12].
Pre-existing cardiovascular disease is a predictor of worse outcomes in COVID-19 pneumonia, and myocardial injury during COVID infection is a marker of increased in-hospital mortality [17]. However, limited data are available about the clinical features and outcomes in COVID-19 patients who require advanced cardiopulmonary support with VA-ECMO due to cardiocirculatory failure. After scattered case reports [8, 9], a recent series of 37 cases highlighted the poor prognosis of patients with COVID-19 who require VA- or VAV-ECMO [18]. Another recent series has focused on COVID-19-associated myocarditis but did not focus on mechanical circulatory support or predictors of outcome [19]. To our knowledge, the current study is the largest on this topic, is based on a multi-institutional registry and is the first to thoroughly evaluate predictors of survival to guide the treatment and decision-making.
Our main findings are as follows. First, the requirement for veno-arterial cannulation is relatively rare in the context of COVID-19 infection [20]. However, the patients who received VA-ECMO in our cohort were relatively young and few had cardiovascular disease or chronic heart failure. Second, our data suggest that patients requiring VA-ECMO in the context of COVID-19 infection have a distinctively worse prognosis (27.6% survival in the current series) compared to patients who require VV-ECMO for COVID-19 ARDS (33–64% survival in major investigations [1, 6, 21–23]). In the ECMOSARS VV-ECMO cohort, in-hospital survival was 49%. Historical series of VA-ECMO patients reported mortality rates of 44% in all-comers with severe cardiogenic shock [24], ranging from 38% in patients with post-cardiotomy syndrome to 60% in patients with early graft failure. The high mortality in our cohort was driven in part by the relatively large proportion of patients (37%) that received E-CPR, which is known to have increased mortality despite in-hospital settings [25]. A similar observation has been made among recipients of VV-ECMO for COVID-19-related ARDS in the EuroELSO registry, where the previous cardiac arrest was a strong predictor of mortality [26]. It can be hypothesized that in the management of refractory cardiogenic shock requiring ECMO, reticence to offer advanced heart failure therapies (such as heart transplantation or long-term mechanical circulatory assistance) in the context of COVID-19 infection and ARDS might have potentially contributed to worsened mortality outcomes. Nonetheless, refractory cardiac arrest seems to be a particularly ominous sign in the current population and might represent a contraindication to ECMO given the concomitant extracardiac organ dysfunction.
Third, despite considerable mortality, the current study also suggests that VA-ECMO is a feasible therapeutic strategy in selected COVID-19 patients with no other options. Although the overall in-hospital survival was 28% in the current series, if E-CPR recipients are excluded, we observed a 43% in-hospital survival among VA-ECMO recipients for COVID-19-associated cardiogenic shock. Such results are similar or better than those observed in previous series of VA-ECMO recipients for generic indications (i.e. primary cardiogenic shock, post-cardiotomy myocardial failure, etc.) [24]. We suggest that ECMO should be instituted as early as possible, before the appearance of end-organ dysfunction.
Fourth, although our statistical analyses were limited by the cohort size, it was possible to identify profile features of the non-surviving patients. Non-survivors were significantly older and had greater BMI and lower LVEF before hospitalization. Non-survivors also had significantly higher serum lactate levels before ECMO and were more likely to have received epinephrine injection and more likely to have received E-CPR. Both the Survival after Veno-Arterial ECMO (SAVE) and Sepsis-related Organ Failure Assessment (SOFA) scores were significantly higher among non-survivors. Conversely, the diagnosis of myocarditis seemed to be a marker of better outcome. The association between lower LVEF at ECMO implantation and better survival should be ascribed to the average lower LVEF among patients with myocarditis, the latter being in turn associated with better survival (possibly due to the potentially reversible nature of myocarditis-associated ventricular dysfunction). Interestingly, although there were no significant baseline differences in blood gas data among survivors and non-survivors, after the institution of VA-ECMO non-survivors displayed significantly lower arterial pH and significantly greater FiO2 than survivors (Table 2). This may suggest a greater severity of underlying ARDS in non-survivors, limited improvement in tissue perfusion under VA-ECMO or possibly ECMO-related complications such as pulmonary oedema. This observation warrants further study as it might be useful to support decision-making in individual cases about the continuation of ECMO. These findings are in line with the observation that more acidic pH, FiO2 = 100% and lower SaO2 (but not greater PaCO2) were independent predictors of worse outcomes among recipients of VV-ECMO in the EuroELSO Registry [26].
Haemorrhagic complications were common in this cohort (affecting 47% of patients) and were the only type of ECMO complication, which was statistically associated with worse outcomes (P = 0.004). Similarly, non-survivors received significantly higher volumes of packed red blood cells and platelets. While increased transfusion requirement is a known feature of VA-ECMO compared to VV-ECMO [27], the occurrence of massive and/or multisite bleeding is an ominous sign in these patients and should prompt a discussion about whether an individual patient is likely to tolerate massive transfusion in a context of ARDS, systemic inflammatory syndrome and, potentially, COVID-19-associated coagulopathy [28]. As cannulation site bleeding is the most frequent site of bleeding in the population, and bleeding under ECMO is associated with increased mortality, we suggest inserting ECMO cannulas in the operative room whenever possible.
Limitations
The current study has several limitations. Positive reverse-transcriptase polymerase chain reaction was required at the time of inclusion; therefore, we might have inappropriately excluded some cases of COVID-19-associated myocarditis or multisystemic inflammatory syndrome. In fact, serologic diagnosis was not considered at the time of design of this registry. This is an observational registry based on the collection of data from medical records, which is potentially prone to incompleteness and information bias. As there were no recommendations for initiation or weaning of ECMO, for anticoagulation under ECMO or for specific cannulation techniques, different protocols may have been used across the participating centres. Similarly, criteria for VA-ECMO implantation may have varied among centres and as the COVID-19 pandemic evolved. Nonetheless, VA-ECMO is practiced only in tertiary-level institutions with experience in the advanced management of heart failure. The sample size was insufficient to perform a comparative analysis among recipients of VA-ECMO and VAV-ECMO. Despite these limitations, the current study represents the largest cohort of patients supported by veno-arterial ECMO with a focused analysis. The multicentre nature and high participation among French ECMO centres allowed a reliable description on a nationwide scale.
CONCLUSIONS
Refractory cardiogenic shock requiring VA-ECMO is relatively rare in patients with COVID-19 infection but does occur, even in the absence of pre-existing cardiovascular disease. VA-ECMO recipients with COVID-19 have a worse prognosis than those who only require VV-ECMO, a finding driven by the very poor outcomes in patients who receive E-CPR. Nonetheless, VA-ECMO is a viable solution to rescue very selected COVID-19 patients with cardiocirculatory failure. However, we suggest that E-CPR is not a reasonable indication for VA-ECMO in this population. We present the largest series of VA-ECMO in COVID-19 patients and describe clinical features that may guide clinical decision-making in this challenging patient population.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
ACKNOWLEDGEMENTS
ECMOSARS was endorsed by the French Society of Thoracic and Cardiovascular Surgery (Société Française de Chirurgie Thoracique et Cardio-Vasculaire, SFCTCV), by the French Society of Thoracic and Cardiovascular Critical Care and Anesthesia (Anesthésie-Réanimation Coeur-Thorax-Vaisseaux, ARCOTHOVA) and by the French Society of Anesthesiology and Critical Care Medicine (Société Française d’Anesthésie-Réanimation, SFAR).
Funding
This work was supported by a grant from the university hospital of Rennes (Appel à projets CFTR2) and by a grant from the French society of thoracic and cardiovascular surgery (Société française de chirurgie thoracique et cardio-vasculaire, Bourse Marc Laskar).
Conflict of interest: none declared.
DATA AVAILABILITY
Raw data are available from the corresponding Author under reasonable request.
Author contributions
Amedeo Anselmi: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Writing—original draft; Writing—review & editing. Alexandre Mansour: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Writing—review & editing. Marylou Para: Data curation; Formal analysis; Resources; Supervision; Validation; Writing—review & editing. Nicolas Mongardon: Data curation; Investigation; Writing—review & editing. Alizée Porto: Data curation; Investigation; Writing—review & editing. Julien Guihaire: Data curation; Investigation; Supervision; Validation; Writing—review & editing. Marie-Catherine Morgant: Data curation; Investigation; Writing—review & editing. Matteo Pozzi: Data curation; Investigation; Writing—review & editing. Bernard Cholley: Data curation; Investigation; Writing—review & editing. Pierre-Emmanuel Falcoz: Data curation; Investigation; Writing—review & editing. Philippe Gaudard: Data curation; Investigation; Writing—review & editing. Guillaume Lebreton: Data curation; Investigation; Writing—review & editing. François Labaste: Data curation; Investigation; Writing—review & editing. Claudio Barbanti: Data curation; Investigation; Writing—review & editing. Olivier Fouquet: Data curation; Investigation; Writing—review & editing. Sidney Chocron: Data curation; Investigation; Writing—review & editing. Nicolas Mottard: Data curation; Investigation; Writing—review & editing. Maxime Esvan: Data curation; Formal analysis; Investigation; Methodology; Software; Writing—review & editing. Claire Fougerou-Leurent: Data curation; Investigation; Writing—review & editing. Erwan Flecher: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Project administration; Supervision; Writing—review & editing. André Vincentelli: Conceptualization; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Writing—review & editing. Nicolas Nesseler: Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Lars Nölke, Daniel H. Drake, Barbara Wilkey and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
APPENDIX. LIST OF ECMOSARS INVESTIGATORS
ECMOSARS Investigators (list of collaborators) are listed as follows:
Marc Pierrot, M.D., University Hospital of Angers, collected data, provided and cared for study patients.
Guillaume Flicoteaux, M.D., University Hospital of Besançon, collected data, provided and cared for study patients.
Philippe Mauriat, M.D., University Hospital of Bordeaux, critically reviewed the study proposal.
Alexandre Ouattara, M.D., Ph.D., University Hospital of Bordeaux, collected data, provided and cared for study patients.
Hadrien Roze, M.D., University Hospital of Bordeaux, collected data, provided and cared for study patients.
Olivier Huet, M.D., Ph.D., University Hospital of Brest, Professor, collected data, provided and cared for study patients.
Marc-Olivier Fischer, M.D., Ph.D., University Hospital of Caen, Professor, collected data, provided and cared for study patients.
Claire Alessandri, M.D., APHP University Hospital Henri Mondor, Créteil, provided and cared for study patients.
Raphel Bellaïche, M.D., APHP University Hospital Henri Mondor, Créteil, provided and cared for study patients.
Ophélie Constant, M.D., APHP University Hospital Henri Mondor, Créteil, provided and cared for study patients.
Quentin De Roux, M.D., APHP University Hospital Henri Mondor, Créteil, provided and cared for study patients.
André Ly, M.D., APHP University Hospital Henri Mondor, Créteil, provided and cared for study patients.
Arnaud Meffert, M.D., APHP University Hospital Henri Mondor, Créteil, provided and cared for study patients.
Jean-Claude Merle, M.D., APHP University Hospital Henri Mondor, Créteil, provided and cared for study patients.
Lucile Picard, M.D., APHP University Hospital Henri Mondor, Créteil, provided and cared for study patients.
Elena Skripkina, M.D, APHP University Hospital Henri Mondor, Créteil, provided and cared for study patients.
Thierry Folliguet, M.D., Ph.D., APHP University Hospital Henri Mondor, Créteil, Professor, provided and cared for study patients.
Antonio Fiore, M.D., APHP University Hospital Henri Mondor, Créteil, provided and cared for study patients.
Nicolas D'Ostrevy, M.D., University Hospital of Clermont-Ferrand, collected data, provided and cared for study patients.
Marie-Catherine Morgan, M.D., University Hospital of Dijon, collected data, provided and cared for study patients.
Pierre-Grégoire Guinot, M.D., Ph.D., University Hospital of Dijon, collected data, provided and cared for study patients.
Maxime Nguyen, M.D., University Hospital of Dijon, collected data, provided and cared for study patients.
Lucie Gaide-Chevronnay, M.D., University Hospital of Grenoble, collected data, provided and cared for study patients.
Nicolas Terzi, M.D., Ph.D., University Hospital of Grenoble, Professor, collected data, provided and cared for study patients.
Gwenhaël Colin, M.D., Vendée Hospital, La Roche-sur-Yon, collected data, provided and cared for study patients.
Olivier Fabre, M.D., Hospital of Lens, collected data, provided and cared for study patients.
Arash Astaneh, M.D., Marie-Lannelongue Hospital, Le Plessis-Robinson, collected data, provided and cared for study patients.
Justin Issard, M.D., Marie-Lannelongue Hospital, Le Plessis-Robinson, collected data, provided and cared for study patients.
Elie Fadel, M.D., Ph.D., Marie-Lannelongue Hospital, Le Plessis-Robinson, Professor, collected data, provided and cared for study patients.
Dominique Fabre, M.D., Marie-Lannelongue Hospital, Le Plessis-Robinson, collected data, provided and cared for study patients.
Antoine Girault, M.D., Marie-Lannelongue Hospital, Le Plessis-Robinson, collected data, provided and cared for study patients.
Iolande Ion, M.D., Marie-Lannelongue Hospital, Le Plessis-Robinson, collected data, provided and cared for study patients.
Jean Baptiste Menager, M.D., Marie-Lannelongue Hospital, Le Plessis-Robinson, collected data, provided and cared for study patients.
Delphine Mitilian, M.D., Marie-Lannelongue Hospital, Le Plessis-Robinson, collected data, provided and cared for study patients.
Olaf Mercier, M.D., Ph.D., Marie-Lannelongue Hospital, Le Plessis-Robinson, Professor, collected data, provided and cared for study patients.
François Stephan, M.D., Marie-Lannelongue Hospital, Le Plessis-Robinson, Professor, collected data, provided and cared for study patients.
Jacques Thes, M.D., Marie-Lannelongue Hospital, Le Plessis-Robinson, collected data, provided and cared for study patients.
Jerôme Jouan, M.D., University Hospital of Limoges, collected data, provided and cared for study patients.
Thibault Duburcq, M.D., University Hospital of Lille, collected data, provided and cared for study patients.
Valentin Loobuyck, M.D., University Hospital of Lille, collected data, provided and cared for study patients.
Mouhammed Moussa, M.D., University Hospital of Lille, collected data, provided and cared for study patients.
Agnes Mugnier, M.D., University Hospital of Lille, collected data, provided and cared for study patients.
Natacha Rousse, M.D., University Hospital of Lille, collected data, provided and cared for study patients.
Sabrina Manganiello, M.D., University Hospital of Lille, collected data, provided and cared for study patients.
Olivier Desebbe, M.D., Clinique de la Sauvegarde, Lyon, collected data, provided and cared for study patients.
Jean-Luc Fellahi, M.D., Ph.D., Hospices civils de Lyon, Professor, critically reviewed the study proposal, provided and cared for study patients.
Roland Henaine, M.D., Ph.D., Hospices civils de Lyon, Professor, critically reviewed the study proposal, provided and cared for study patients.
Matteo Pozzi, M.D, Hospices civils de Lyon, collected data, provided and cared for study patients.
Jean-Christophe Richard, M.D., Ph.D., Hospices civils de Lyon, Professor, collected data, provided and cared for study patients.
Zakaria Riad, M.D., Hospices civils de Lyon, collected data, provided and cared for study patients.
Christophe Guervilly, M.D., North Hospital, APHM, Marseille, collected data, provided and cared for study patients.
Sami Hraiech, M.D., North Hospital, APHM, Marseille, collected data, provided and cared for study patients.
Laurent Papazian, M.D., Ph.D., North Hospital, APHM, Marseille, Professor, collected data, provided and cared for study patients.
Matthias Castanier, M.D., European Hospital, Marseille, collected data, provided and cared for study patients.
Charles Chanavaz, M.D., Clairval Hospital, Marseille, collected data, provided and cared for study patients.
Cyril Cadoz, M.D., Regional Hospital of Metz-Thionville, provided and cared for study patients.
Sebastien Gette, M.D., Regional Hospital of Metz-Thionville, provided and cared for study patients.
Guillaume Louis, M.D., Regional Hospital of Metz-Thionville, provided and cared for study patients.
Erick Portocarrero, M.D., Regional Hospital of Metz-Thionville, provided and cared for study patients.
Kais Brini, M.D., Institut Mutualiste Montsouris, Paris, collected data, provided and cared for study patients.
Nicolas Bischoff, M.D., Emile Muller Hospital, Mulhouse, collected data, provided and cared for study patients.
Bruno Levy, M.D., Ph.D., University Hospital of Nancy, Professor, collected data, provided and cared for study patients.
Antoine Kimmoun, M.D., Ph.D., University Hospital of Nancy, Professor, collected data, provided and cared for study patients.
Mathieu Mattei, M.D., University Hospital of Nancy, collected data, provided and cared for study patients.
Pierre Perez, M.D., University Hospital of Nancy, collected data, provided and cared for study patients.
Alexandre Bourdiol, M.D., University Hospital of Nantes, collected data, provided and cared for study patients.
Yannick Hourmant, M.D., University Hospital of Nantes, collected data, provided and cared for study patients.
Pierre-Joachim Mahé, M.D., University Hospital of Nantes, collected data, provided and cared for study patients.
Bertrand Rozec, M.D., Ph.D., University Hospital of Nantes, collected data, provided and cared for study patients.
Mickaël Vourc'h, M.D., University Hospital of Nantes, collected data, provided and cared for study patients.
Stéphane Aubert, M.D., Ambroise Paré Hospital, Neuilly-sur-Seine, collected data, provided and cared for study patients.
Florian Bazalgette, M.D., University Hospital of Nîmes, collected data, provided and cared for study patients.
Claire Roger, M.D., University Hospital of Nîmes, collected data, provided and cared for study patients,
Pierre Jaquet, M.D., APHP, Hôpital Bichat-Claude Bernard, Paris University Hospital, provided and cared for study patients.
Brice Lortat-Jacob, M.D., APHP, Hôpital Bichat-Claude Bernard, Paris University Hospital, provided and cared for study patients.
Pierre Mordant, M.D., Ph.D., APHP, Hôpital Bichat-Claude Bernard, Paris University Hospital, Professor, provided and cared for study patients.
Patrick Nataf, M.D., Ph.D., APHP, Hôpital Bichat-Claude Bernard, Paris University Hospital, Professor, provided and cared for study patients.
Juliette Patrier, M.D., Hôpital Bichat-Claude Bernard, Paris University Hospital, provided and cared for study patients.
Sophie Provenchere, M.D., Ph.D., Hôpital Bichat-Claude Bernard, Paris University Hospital, provided and cared for study patients.
Morgan Roué, M.D., APHP, Hôpital Bichat-Claude Bernard, Paris University Hospital, provided and cared for study patients.
Romain Sonneville, M.D., Ph.D., APHP, Hôpital Bichat-Claude Bernard, Paris University Hospital, Professor, provided and cared for study patients.
Alexy Tran-Dinh, M.D., Hôpital Bichat-Claude Bernard, Paris University Hospital, provided and cared for study patients.
Paul-Henri Wicky, M.D., APHP, Hôpital Bichat-Claude Bernard, Paris University Hospital, provided and cared for study patients.
Charles Al Zreibi, M.D., APHP Hôpital Européen Georges Pompidou—Paris University Hospital, collected data, provided and cared for study patients.
Yannis Guyonvarch, M.D., APHP Hôpital Européen Georges Pompidou—Paris University Hospital, collected data, provided and cared for study patients.
Sophie Hamada, M.D., APHP Hôpital Européen Georges Pompidou—Paris University Hospital, collected data, provided and cared for study patients.
Astrid Bertier, M.D., APHP Le Kremlin-Bicêtre, Paris University Hospital, collected data, provided and cared for study patients.
Anatole Harrois, M.D., APHP Le Kremlin-Bicêtre, Paris University Hospital, collected data, provided and cared for study patients.
Jordi Matiello, M.D., APHP Le Kremlin-Bicêtre, Paris University Hospital, collected data, provided and cared for study patients.
Thomas Kerforne, M.D., University Hospital of Poitiers, collected data, provided and cared for study patients.
Corentin Lacroix, M.D., University Hospital of Poitiers, collected data, provided and cared for study patients.
Nicolas Brechot, M.D., APHP, Sorbonne Université, Hôpital Pitié–Salpêtrière, Paris, France, collected data, provided and cared for study patients.
Alain Combes, M.D., Ph.D., APHP, Sorbonne Université, Hôpital Pitié–Salpêtrière, Paris, France, Professor, collected data, provided and cared for study patients.
Matthieu Schmidt, M.D., Ph.D., APHP, Sorbonne Université, Hôpital Pitié–Salpêtrière, Paris, France, Professor, collected data, provided and cared for study patients.
Juliette Chommeloux, M.D., APHP, Sorbonne Université, Hôpital Pitié–Salpêtrière, Paris, France, collected data, provided and cared for study patients.
Jean Michel Constantin, M.D., Ph.D., APHP, Sorbonne Université, Hôpital Pitié–Salpêtrière, Paris, France, Professor, collected data, provided and cared for study patients.
Cosimo D’Alessandro, M.D., APHP, Sorbonne Université, Hôpital Pitié–Salpêtrière, Paris, France, collected data, provided and cared for study patients.
Pierre Demondion, M.D., APHP, Sorbonne Université, Hôpital Pitié–Salpêtrière, Paris, France, collected data, provided and cared for study patients.
Alexandre Demoule, M.D., APHP, Sorbonne Université, Hôpital Pitié–Salpêtrière, Paris, France, collected data, provided and cared for study patients.
Martin Dres, M.D., APHP, Sorbonne Université, Hôpital Pitié–Salpêtrière, Paris, France, collected data, provided and cared for study patients.
Guillaume Fadel, M.D., APHP, Sorbonne Université, Hôpital Pitié–Salpêtrière, Paris, France, collected data, provided and cared for study patients.
Muriel Fartoukh, M.D., APHP, Sorbonne Université, Hôpital Pitié–Salpêtrière, Paris, France, collected data, provided and cared for study patients.
Guillaume Hekimian, M.D., APHP, Sorbonne Université, Hôpital Pitié–Salpêtrière, Paris, France, collected data, provided and cared for study patients.
Charles Juvin, M.D., APHP, Sorbonne Université, Hôpital Pitié–Salpêtrière, Paris, France, collected data, provided and cared for study patients.
Pascal Leprince, M.D., Ph.D., APHP, Sorbonne Université, Hôpital Pitié–Salpêtrière, Paris, France, Professor, collected data, provided and cared for study patients.
David Levy, M.D., APHP, Sorbonne Université, Hôpital Pitié–Salpêtrière, Paris, France, collected data, provided and cared for study patients.
Charles Edouard Luyt, M.D., Ph.D., APHP, Sorbonne Université, Hôpital Pitié–Salpêtrière, Paris, France, Professor, collected data, provided and cared for study patients.
Marc Pineton De Chambrun, M.D., APHP, Sorbonne Université, Hôpital Pitié–Salpêtrière, Paris, France, collected data, provided and cared for study patients.
Thibaut Schoell, M.D., APHP, Sorbonne Université, Hôpital Pitié–Salpêtrière, Paris, France, collected data, provided and cared for study patients.
Pierre Fillâtre, M.D., Ph.D., Hospital of Saint-Brieuc, collected data, provided and cared for study patients.
Nicolas Massart, M.D., Hospital of Saint-Brieuc, collected data, provided and cared for study patients.
Roxane Nicolas, M.D., University Hospital of Saint-Etienne, collected data, provided and cared for study patients.
Maud Jonas, M.D., Saint-Nazaire Hospital, collected data, provided and cared for study patients.
Charles Vidal, M.D., University Hospital of Saint-Denis, La Réunion, collected data, provided and cared for study patients.
Nicolas Allou, M.D., University Hospital of Saint-Denis, La Réunion, collected data, provided and cared for study patients.
Salvatore Muccio, M.D., University Hospital of Reims, collected data, provided and cared for study patients.
Dario Di Perna, M.D., University Hospital of Reims, collected data, provided and cared for study patients.
Vito-Giovanni Ruggieri, M.D., Ph.D., University Hospital of Reims, collected data, provided and cared for study patients.
Bruno Mourvillier, M.D., Ph.D., University Hospital of Reims, Professor, collected data, provided and cared for study patients.
Karl Bounader, M.D., University Hospital of Rennes, provided and cared for study patients.
Yoann Launey, M.D., Ph.D., University Hospital of Rennes, provided and cared for study patients.
Thomas Lebouvier, M.D., University Hospital of Rennes, provided and cared for study patients.
Alessandro Parasido, University Hospital of Rennes, provided and cared for study patients.
Florian Reizine, M.D., University Hospital of Rennes, provided and cared for study patients.
Philippe Seguin, M.D., Ph.D., University Hospital of Rennes, Professor, provided and cared for study patients.
Emmanuel Besnier, M.D., University Hospital of Rouen, collected data, provided and cared for study patients.
Dorothée Carpentier, M.D., University Hospital of Rouen, collected data, provided and cared for study patients.
Thomas Clavier, M.D., University Hospital of Rouen, collected data, provided and cared for study patients.
Anne Olland, M.D., Ph.D., University Hospital of Strasbourg, Professor, collected data, provided and cared for study patients.
Marion Villard, M.D., University Hospital of Strasbourg, collected data, provided and cared for study patients.
Fanny Bounes, M.D., University Hospital of Toulouse, collected data, provided and cared for study patients.
Vincent Minville, M.D., Ph.D., University Hospital of Toulouse, Professor, collected data, provided and cared for study patients.
Antoine Guillon, M.D., University Hospital of Tours, collected data, provided and cared for study patients.
Yannick Fedun, M.D., Bretagne Atlantique Hospital, Vannes, collected data, provided and cared for study patients.
James T. Ross, M.D., Department of Surgery, University of California Davis, Sacramento, USA, participated in writing of the manuscript.
ABBREVIATIONS
- ARDS
Acute respiratory distress syndrome
- BMI
Body mass index
- ECMO
Extracorporeal membrane oxygenation
- E-CPR
Extracorporeal cardiopulmonary resuscitation
- LVEF
Left ventricular ejection fraction
- VA-ECMO
Veno-arterial extracorporeal membrane oxygenation
- VAV-ECMO
Veno-arterial-venous extracorporeal membrane oxygenation
- VV-ECMO
Veno-venous extracorporeal membrane oxygenation
Author notes
ECMOSARS Investigators are listed in the Appendix.





