-
PDF
- Split View
-
Views
-
Cite
Cite
Mateo Marin-Cuartas, Manuela De La Cuesta, Piroze M Davierwala, Jagdip Kang, Guillermo Stöger, Martin Misfeld, Philipp Kiefer, Sergey Leontyev, Alexander Verevkin, Bettina Pfanmüller, Diyar Saaed, Michael A Borger, Thilo Noack, Mid-term outcomes following the Hemi-Commando procedure for complex infective endocarditis involving the aortomitral junction, European Journal of Cardio-Thoracic Surgery, Volume 64, Issue 1, July 2023, ezad208, https://doi.org/10.1093/ejcts/ezad208
Close - Share Icon Share
Abstract
Perivalvular abscesses with destruction of the aortomitral junction (AMJ) are a severe complication of infective endocarditis (IE) and are associated with high mortality and complex management. The Hemi-Commando procedure is a mitral valve-sparing alternative to the Commando procedure in suitable patients with complex IE and paravalvular destruction. This study reviews the mid-term outcomes in patients undergoing the Hemi-Commando procedure for treating IE with destruction of the AMJ.
The clinical outcomes of patients with IE and AMJ involvement who underwent the Hemi-Commando procedure between 2015 and 2021 at the Leipzig Heart Center were retrospectively analysed. Primary outcomes were 30-day mortality and 1-year survival. Secondary outcome was 1-year freedom from reoperation.
A total of 22 patients underwent the Hemi-Commando procedure during the study period. The patients’ mean age was 59.8 ± 18.3 years. The study population was predominantly male (86.4%). Preoperative sepsis was present in 6 (27.3%) patients, and the median EuroSCORE II was 28.5%. Almost two-thirds (N = 14; 63.6%) of the patients presented with native IE. Streptococci were the most common pathogens (N = 8; 36.4%). Paravalvular abscess was found intraoperatively in 16 (72.7%) patients. The 30-day mortality was 13.6%. The estimated 1- and 3-year survival rates were 77.5% and 66.4%, respectively. The estimated freedom from reoperation at 1 and 3 years was 92.3%.
The Hemi-Commando procedure offers an acceptable mid-term survival chance with low reoperation rates and is, therefore, a reasonable mitral valve-sparing alternative to the Commando procedure in suitable patients with extensive IE and perivalvular involvement.
INTRODUCTION
The development of perivalvular abscesses that lead to the destruction of the aortomitral junction (AMJ) is one of the most dreadful complications of infective endocarditis (IE) since it is associated with high morbidity and mortality rates as well as a complex surgical treatment with prolonged intensive care and hospital stays.
In these cases, a conventional valve operation is not radical enough to prevent further cardiac involvement or recurring infection. Moreover, without prompt surgical treatment, IE-related death is very likely. Therefore, thorough debridement and excision of the infected tissues and/or prosthetic material, together with drainage of all abscess cavities, is needed to provide adequate treatment [1]. This intervention, albeit effective in eradicating the cardiac infection, creates a large cardiac defect that requires complex reconstructive surgery with reconstruction of the AMJ to restore tissue continuity and make valve surgery possible. The Commando procedure, also known as the ‘UFO’ operation, is the most commonly used surgical technique to treat these patients. This procedure involves double valve replacement and patch reconstruction of the AMJ [1–6]. The Commando procedure is associated with high early morbidity and mortality rates [4–8], but acceptable mid-and long-term results among those patients who survive the initial postoperative period [1]. In patients with infective involvement limited to the anterior mitral annulus, without severe damage to the anterior mitral leaflet (AML) and no affectation of the posterior mitral leaflet (PML), the Hemi-Commando procedure is an alternative mitral valve (MV)-sparing surgical technique [9]. Despite being a feasible surgical option in suitable patients, however, this procedure is not frequently performed, and its mid-and long-term outcomes are poorly reported in the literature.
The purpose of this study is to review the early and mid-term outcomes in patients undergoing the Hemi-Commando procedure for treating IE with the destruction of the AMJ, mainly focusing on mid-term survival and freedom from reoperation. We hypothesized that this procedure offers acceptable mid-term survival and low reoperation rates.
METHODS
Ethical statement
This study was approved by the ethics committee of the faculty of medicine at the University of Leipzig (Protocol number 381/19-ek). Individual patient informed consent was waived.
Study design
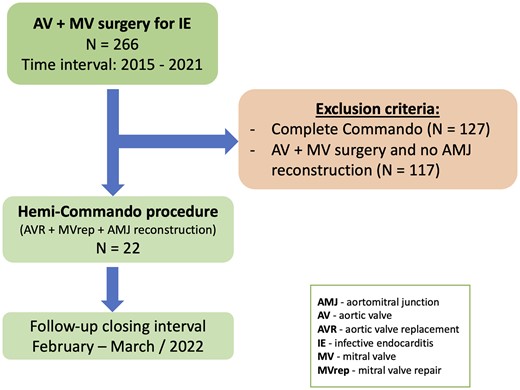
Between January 2015 and December 2021, 266 patients underwent combined aortic and MV surgery for the treatment of IE at our institution. Amongst them, 22 (8.3%) patients underwent the Hemi-Commando procedure (Fig. 1). Patients who underwent a complete Commando procedure (N = 127) and patients who underwent combined aortic valve and MV surgery without AMJ reconstruction (N = 117) were excluded from the analysis. Primary outcomes were 30-day mortality and 1-year survival. Freedom from reoperation at 1 year was regarded as a secondary outcome.
Surgical technique
Our technique to perform the Hemi-Commando procedure is based on a modification of the full Commando (UFO) procedure, which we have previously described [1, 4]. Briefly, the operation was performed through a median sternotomy and cardiopulmonary bypass. Standard cardiotomy incisions included an oblique aortotomy extending through the non-coronary aortic sinus and annulus and a longitudinal incision in the left atrial roof extending towards the right superior pulmonary vein ostium. Following the excision of the native/prosthetic aortic valve, en bloc resection of the area of confluence of the aortic root, the left atrial roof, the AMJ and the base of the AML was performed. The base (annular/atrial aspect) of the AML was resected, whereas the free edge of the AML (coaptation zone), including primary and select secondary chordae, was preserved (Fig. 2A). The extent of resection depended upon the macroscopic spread of the infection and should include an ‘infection-free’ margin of at least 3 mm. Therefore, thorough resection of all infected tissue and prosthetic material results in a common left ventricular orifice instead of the normally observed in- and outflow tracts.
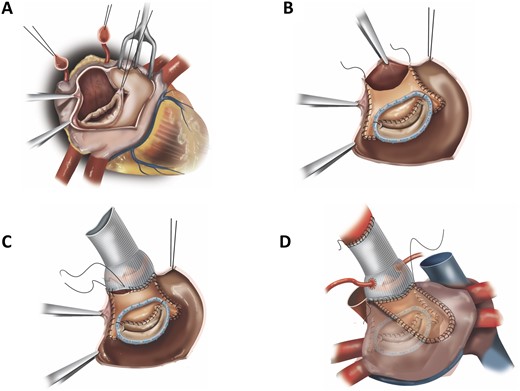
(A) En bloc resection of the area of confluence of the aortic root, the left atrial roof, the aorta mitral junction and the base of the anterior mitral leaflet with preservation of the free edge of the anterior mitral leaflet. (B) Reconstructed anterior mitral annulus and leaflet with a bovine pericardial patch folded upon itself. (C) Reconstructed left ventricular outflow tract after root replacement. The prosthetic aortic valve/root graft is anchored to the aortic annulus and neo-left ventricular outflow tract, as well as to the anterior limb of the pericardial patch. (D) Left atrial roof closure with a single pericardial patch.
Thereafter, the anterior mitral annulus, which forms the margin of the AMJ, was reconstructed with a bovine pericardial patch that was folded upon itself. The folded margin of the patch was sewn to the free edge of the native AML base, thus forming a new anterior mitral annulus. In cases where significant mitral annular dilation was present, a mitral annuloplasty ring was implanted by securing it to the posterior mitral annulus, the lateral and medial fibrous trigones and the new anterior mitral annulus (in cases of complete annuloplasty ring insertion) (Fig. 2B). Intraoperative transoesophageal echocardiographic measurements were used to determine the size of the mitral annuloplasty ring.
Subsequently, the posterior limb of the patch was sewn to the free margins of the resected left atrial roof. The anterior limb of the patch was then sutured to the margins of the left ventricular outflow tract and superiorly to the incised margins of the ascending aortic wall, thus forming the new AMJ/subaortic curtain and the non-coronary sinus. Aortic valve replacement was then performed. The usual extensive involvement of the aortic root and its surrounding structures by the infectious process requires drainage of all abscess cavities and radical debridement of all infected tissue and prosthetic material, leading to a large defect that makes additional aortic root replacement necessary. The aortic valve/root prostheses are anchored to the aortic annulus and neo-left ventricular outflow tract, as well as to the anterior limb of the pericardial patch (Fig. 2C). In the case of aortic valve replacement without root replacement, the patch is used to close the right side of the aortotomy.
In select cases based on operator preference and homograft availability, a homograft was used to perform the procedure. Aortic root replacement was performed with the homograft, and the AMJ/subaortic curtain was reconstructed by sewing the AML of the homograft to the free edge of the native AML base and, when a complete annuloplasty ring was inserted to the anterior third of the sewing cuff of the mitral annuloplasty ring. A single pericardial patch was then used to close the left atrial roof (Fig. 2D).
Indications for the Hemi-Commando versus the commando operation
The extension of MV involvement in the infectious process was decisive in choosing between the Hemi-Commando procedure and the Commando procedure. The pre- and intraoperative echocardiography provided clues about the extent of damage and required debridement. However, the ultimate decision on the surgical technique was finally made based on the intraoperative findings after radical debridement and careful intraoperative MV inspection. The Hemi-Commando procedure is only feasible if sufficient coaptation surface and adequate mobility of the AML after radical debridement can be preserved. Hence, mitral regurgitation due to sole AML damage, without the involvement of the free edge of the AML or the PML and posterior mitral annulus, is mandatory for the Hemi-Commando procedure. Contrarily, extensive damage to the AML, the fibrous trigones, the subvalvular apparatus and/or the PML or posterior mitral annulus likely would require a full Commando operation. Patients with root abscesses and/or communication to other heart chambers requiring root replacement and simultaneous involvement of the AMJ and AML are the ideal candidates for the Hemi-Commando procedure. However, the need for root replacement is not decisive in choosing the Hemi-Commando procedure.
Data collection and follow-up
Patient information, including demographic characteristics, intraoperative data and postoperative outcomes, was retrospectively collected into a computerized institutional database and prospectively analysed. Follow-up was conducted by phone communication with patients and/or close family members. The closing interval for this study was between February and March 2022.
Statistical analysis
Categorical variables are expressed as frequencies and percentages, while continuous variables are expressed as mean and standard deviation for normally distributed variables and median and interquartile range for non-normally distributed variables throughout the manuscript. Comparisons were performed using the chi-squared and Wilcoxon rank-sum tests for categorical and continuous variables, respectively. Survival and freedom from reoperation were estimated with Kaplan–Meier survival methods. Survival estimates for the entire cohort were presented at 1- and 3-year intervals. Competing risk analysis to calculate reoperation-free survival was performed using the Fine and Gray’s test.
All statistical analyses were performed using SPSS Version 25.0 (Chicago, IL, USA).
RESULTS
Preoperative characteristics
Patient demographic characteristics are shown in Table 1. The patients’ mean age was 59.8 years (±18.3 years). Only 3 patients (13.6%) were female. Preoperative sepsis occurred in 6 (27.3%) patients. Splenic, renal and/or cerebral septic embolism was observed in computed tomography scan images of 7 (31.8%) patients. One (4.5%) patient presented with cardiogenic shock. A total of 9 (40.9%) patients had previous cardiac surgery. Most cases were native valve IE (n = 14, 63.6%). Half (N = 11, 50.0%) of the procedures were classified as urgent, while 8 (36.4%) were considered emergent.
| Age (years) | 59.8 ± 18.3 |
| Male gender | 19 (86.4) |
| Body mass index (kg/m2) | 26.3 ± 6.4 |
| Arterial hypertension | 14 (63.6) |
| Diabetes mellitus | 6 (27.3) |
| Dyslipidemia | 8 (36.4) |
| COPD | 4 (18.2) |
| Creatinine (mg/dl) | 1.2 ± 0.8 |
| Dialysis | 2 (9.1) |
| Peripheral vascular disease | 0 (0) |
| Stroke | 0 (0) |
| Sepsis | 6 (27.3) |
| Septic embolisma | 7 (31.8) |
| NYHA class | |
| I | 11 (50) |
| II | 3 (13.6) |
| III | 5 (22.7) |
| IV | 3 (13.6) |
| Left ventricular ejection fraction (%) | 50 (40–59) |
| Atrial fibrillation | 9 (40.9) |
| Pacemaker | 3 (13.6) |
| Cardiogenic shock | 1 (4.5) |
| Use of inotropes or vasopressors | 2 (9.1) |
| Mechanical ventilation | 1 (4.5) |
| Coronary artery disease | 5 (22.7) |
| Native valve endocarditis | 14 (63.6) |
| Prosthetic valve endocarditis | 8 (36.4) |
| Early (<1 year) | 4 (18.2) |
| Late (>1 year) | 4 (18.2) |
| Previous open cardiac surgery | 9 (40.9) |
| Timing of surgery | |
| Elective | 3 (13.6) |
| Urgent | 11 (50) |
| Emergent | 8 (36.4) |
| EuroSCORE II (%) | 28.5 (13.1–51.9) |
| Age (years) | 59.8 ± 18.3 |
| Male gender | 19 (86.4) |
| Body mass index (kg/m2) | 26.3 ± 6.4 |
| Arterial hypertension | 14 (63.6) |
| Diabetes mellitus | 6 (27.3) |
| Dyslipidemia | 8 (36.4) |
| COPD | 4 (18.2) |
| Creatinine (mg/dl) | 1.2 ± 0.8 |
| Dialysis | 2 (9.1) |
| Peripheral vascular disease | 0 (0) |
| Stroke | 0 (0) |
| Sepsis | 6 (27.3) |
| Septic embolisma | 7 (31.8) |
| NYHA class | |
| I | 11 (50) |
| II | 3 (13.6) |
| III | 5 (22.7) |
| IV | 3 (13.6) |
| Left ventricular ejection fraction (%) | 50 (40–59) |
| Atrial fibrillation | 9 (40.9) |
| Pacemaker | 3 (13.6) |
| Cardiogenic shock | 1 (4.5) |
| Use of inotropes or vasopressors | 2 (9.1) |
| Mechanical ventilation | 1 (4.5) |
| Coronary artery disease | 5 (22.7) |
| Native valve endocarditis | 14 (63.6) |
| Prosthetic valve endocarditis | 8 (36.4) |
| Early (<1 year) | 4 (18.2) |
| Late (>1 year) | 4 (18.2) |
| Previous open cardiac surgery | 9 (40.9) |
| Timing of surgery | |
| Elective | 3 (13.6) |
| Urgent | 11 (50) |
| Emergent | 8 (36.4) |
| EuroSCORE II (%) | 28.5 (13.1–51.9) |
Continuous variables are expressed as mean ± standard deviation or median and interquartile range in parentheses. Categorical variables are expressed in numbers (n) and percentages in parentheses.
Splenic, renal and/or cerebral embolism observed on computed tomography scan images.
COPD: chronic obstructive pulmonary disease; NYHA: New York Heart Association.
| Age (years) | 59.8 ± 18.3 |
| Male gender | 19 (86.4) |
| Body mass index (kg/m2) | 26.3 ± 6.4 |
| Arterial hypertension | 14 (63.6) |
| Diabetes mellitus | 6 (27.3) |
| Dyslipidemia | 8 (36.4) |
| COPD | 4 (18.2) |
| Creatinine (mg/dl) | 1.2 ± 0.8 |
| Dialysis | 2 (9.1) |
| Peripheral vascular disease | 0 (0) |
| Stroke | 0 (0) |
| Sepsis | 6 (27.3) |
| Septic embolisma | 7 (31.8) |
| NYHA class | |
| I | 11 (50) |
| II | 3 (13.6) |
| III | 5 (22.7) |
| IV | 3 (13.6) |
| Left ventricular ejection fraction (%) | 50 (40–59) |
| Atrial fibrillation | 9 (40.9) |
| Pacemaker | 3 (13.6) |
| Cardiogenic shock | 1 (4.5) |
| Use of inotropes or vasopressors | 2 (9.1) |
| Mechanical ventilation | 1 (4.5) |
| Coronary artery disease | 5 (22.7) |
| Native valve endocarditis | 14 (63.6) |
| Prosthetic valve endocarditis | 8 (36.4) |
| Early (<1 year) | 4 (18.2) |
| Late (>1 year) | 4 (18.2) |
| Previous open cardiac surgery | 9 (40.9) |
| Timing of surgery | |
| Elective | 3 (13.6) |
| Urgent | 11 (50) |
| Emergent | 8 (36.4) |
| EuroSCORE II (%) | 28.5 (13.1–51.9) |
| Age (years) | 59.8 ± 18.3 |
| Male gender | 19 (86.4) |
| Body mass index (kg/m2) | 26.3 ± 6.4 |
| Arterial hypertension | 14 (63.6) |
| Diabetes mellitus | 6 (27.3) |
| Dyslipidemia | 8 (36.4) |
| COPD | 4 (18.2) |
| Creatinine (mg/dl) | 1.2 ± 0.8 |
| Dialysis | 2 (9.1) |
| Peripheral vascular disease | 0 (0) |
| Stroke | 0 (0) |
| Sepsis | 6 (27.3) |
| Septic embolisma | 7 (31.8) |
| NYHA class | |
| I | 11 (50) |
| II | 3 (13.6) |
| III | 5 (22.7) |
| IV | 3 (13.6) |
| Left ventricular ejection fraction (%) | 50 (40–59) |
| Atrial fibrillation | 9 (40.9) |
| Pacemaker | 3 (13.6) |
| Cardiogenic shock | 1 (4.5) |
| Use of inotropes or vasopressors | 2 (9.1) |
| Mechanical ventilation | 1 (4.5) |
| Coronary artery disease | 5 (22.7) |
| Native valve endocarditis | 14 (63.6) |
| Prosthetic valve endocarditis | 8 (36.4) |
| Early (<1 year) | 4 (18.2) |
| Late (>1 year) | 4 (18.2) |
| Previous open cardiac surgery | 9 (40.9) |
| Timing of surgery | |
| Elective | 3 (13.6) |
| Urgent | 11 (50) |
| Emergent | 8 (36.4) |
| EuroSCORE II (%) | 28.5 (13.1–51.9) |
Continuous variables are expressed as mean ± standard deviation or median and interquartile range in parentheses. Categorical variables are expressed in numbers (n) and percentages in parentheses.
Splenic, renal and/or cerebral embolism observed on computed tomography scan images.
COPD: chronic obstructive pulmonary disease; NYHA: New York Heart Association.
Intraoperative data and early postoperative outcomes
Cardiac abscesses were identified in 16 (72.7%) patients. Causative microorganisms were isolated in 17 (77.3%) blood samples. Over a third of all samples (N = 8, 36.4%) were Streptococci positive. Other causative organisms consisted of Staphylococci in 4 (18.2%) patients, Enterococcus faecalis in 3 (13.6%) patients and Cutibacterium acnes in 2 (9.1%) patients. A total of 5 patients (22.7%) had culture-negative IE. Concomitant mitral annuloplasty was required in 10 (45.4%) patients. Additional tricuspid valve repair was performed in 5 (22.7%) patients, and 8 (36.4%) patients needed concomitant aortic surgery. Further intraoperative details are depicted in Table 2.
| Aortic valve prosthesis type | 22 (100) |
| Mechanical valve | 6 (27.3) |
| Biological valve | 11 (50.0) |
| Aortic homograft | 5 (22.7) |
| Mitral valve annuloplasty | 10 (45.4) |
| Concomitant procedures | |
| Root replacement | 5 (22.7) |
| Tricuspid valve repair | 5 (22.7) |
| Ascending aorta replacement | 6(27.3) |
| Ascending aorta + arch replacement | 2 (9.1) |
| Cardiopulmonary bypass time (min) | 153 (134–192) |
| Aortic cross-clamp time (min) | 125 (110–143) |
| Paravalvular abscesses | 16 (72.7) |
| Aortic valve prosthesis type | 22 (100) |
| Mechanical valve | 6 (27.3) |
| Biological valve | 11 (50.0) |
| Aortic homograft | 5 (22.7) |
| Mitral valve annuloplasty | 10 (45.4) |
| Concomitant procedures | |
| Root replacement | 5 (22.7) |
| Tricuspid valve repair | 5 (22.7) |
| Ascending aorta replacement | 6(27.3) |
| Ascending aorta + arch replacement | 2 (9.1) |
| Cardiopulmonary bypass time (min) | 153 (134–192) |
| Aortic cross-clamp time (min) | 125 (110–143) |
| Paravalvular abscesses | 16 (72.7) |
Continuous variables are expressed as median and interquartile range in parentheses. Categorical variables are expressed in numbers (n) and percentages in parentheses.
| Aortic valve prosthesis type | 22 (100) |
| Mechanical valve | 6 (27.3) |
| Biological valve | 11 (50.0) |
| Aortic homograft | 5 (22.7) |
| Mitral valve annuloplasty | 10 (45.4) |
| Concomitant procedures | |
| Root replacement | 5 (22.7) |
| Tricuspid valve repair | 5 (22.7) |
| Ascending aorta replacement | 6(27.3) |
| Ascending aorta + arch replacement | 2 (9.1) |
| Cardiopulmonary bypass time (min) | 153 (134–192) |
| Aortic cross-clamp time (min) | 125 (110–143) |
| Paravalvular abscesses | 16 (72.7) |
| Aortic valve prosthesis type | 22 (100) |
| Mechanical valve | 6 (27.3) |
| Biological valve | 11 (50.0) |
| Aortic homograft | 5 (22.7) |
| Mitral valve annuloplasty | 10 (45.4) |
| Concomitant procedures | |
| Root replacement | 5 (22.7) |
| Tricuspid valve repair | 5 (22.7) |
| Ascending aorta replacement | 6(27.3) |
| Ascending aorta + arch replacement | 2 (9.1) |
| Cardiopulmonary bypass time (min) | 153 (134–192) |
| Aortic cross-clamp time (min) | 125 (110–143) |
| Paravalvular abscesses | 16 (72.7) |
Continuous variables are expressed as median and interquartile range in parentheses. Categorical variables are expressed in numbers (n) and percentages in parentheses.
Postoperative outcomes are summarized in Table 3. Re-exploration for bleeding was required in 6 (27.3%) patients. The median hospital stay was 21.5 days (12.5–25.0). Two (9.1%) patients died within 30 days following surgery. One died due to septic shock, and the other due to cardiogenic shock. No patient presented with clinically relevant residual mitral regurgitation in the transthoracic echocardiography at discharge (Table 3).
| 30-Day mortality (%) | 13.6 |
| Low cardiac output | 2 (9.1) |
| ECMO | 1 (4.5) |
| Sepsis | 6 (27.3) |
| Re-exploration for bleeding | 6 (27.3) |
| New-onset atrial fibrillation | 0 (0) |
| Pacemaker implantation | 5 (22.7) |
| Stroke | 1 (4.5) |
| AKI requiring dialysis | 5 (22.7) |
| Respiratory failure requiring reintubation | 4 (18.2) |
| Tracheotomy | 3 (13.6) |
| ICU stay (days) | 3 (1–11) |
| Hospital stay (days) | 21 (12–25) |
| Residual MR at discharge | |
| Mild MR | 2 (9.1) |
| Moderate/severe MR | 0 (0) |
| 30-Day mortality (%) | 13.6 |
| Low cardiac output | 2 (9.1) |
| ECMO | 1 (4.5) |
| Sepsis | 6 (27.3) |
| Re-exploration for bleeding | 6 (27.3) |
| New-onset atrial fibrillation | 0 (0) |
| Pacemaker implantation | 5 (22.7) |
| Stroke | 1 (4.5) |
| AKI requiring dialysis | 5 (22.7) |
| Respiratory failure requiring reintubation | 4 (18.2) |
| Tracheotomy | 3 (13.6) |
| ICU stay (days) | 3 (1–11) |
| Hospital stay (days) | 21 (12–25) |
| Residual MR at discharge | |
| Mild MR | 2 (9.1) |
| Moderate/severe MR | 0 (0) |
Continuous variables are expressed as median and interquartile range in parentheses. Categorical variables are expressed in numbers (n) and percentages in parentheses.
AKI: acute kidney injury; ECMO: extracorporeal membrane oxygenation; ICU: intensive care unit; MR: mitral regurgitation.
| 30-Day mortality (%) | 13.6 |
| Low cardiac output | 2 (9.1) |
| ECMO | 1 (4.5) |
| Sepsis | 6 (27.3) |
| Re-exploration for bleeding | 6 (27.3) |
| New-onset atrial fibrillation | 0 (0) |
| Pacemaker implantation | 5 (22.7) |
| Stroke | 1 (4.5) |
| AKI requiring dialysis | 5 (22.7) |
| Respiratory failure requiring reintubation | 4 (18.2) |
| Tracheotomy | 3 (13.6) |
| ICU stay (days) | 3 (1–11) |
| Hospital stay (days) | 21 (12–25) |
| Residual MR at discharge | |
| Mild MR | 2 (9.1) |
| Moderate/severe MR | 0 (0) |
| 30-Day mortality (%) | 13.6 |
| Low cardiac output | 2 (9.1) |
| ECMO | 1 (4.5) |
| Sepsis | 6 (27.3) |
| Re-exploration for bleeding | 6 (27.3) |
| New-onset atrial fibrillation | 0 (0) |
| Pacemaker implantation | 5 (22.7) |
| Stroke | 1 (4.5) |
| AKI requiring dialysis | 5 (22.7) |
| Respiratory failure requiring reintubation | 4 (18.2) |
| Tracheotomy | 3 (13.6) |
| ICU stay (days) | 3 (1–11) |
| Hospital stay (days) | 21 (12–25) |
| Residual MR at discharge | |
| Mild MR | 2 (9.1) |
| Moderate/severe MR | 0 (0) |
Continuous variables are expressed as median and interquartile range in parentheses. Categorical variables are expressed in numbers (n) and percentages in parentheses.
AKI: acute kidney injury; ECMO: extracorporeal membrane oxygenation; ICU: intensive care unit; MR: mitral regurgitation.
Follow-up
Follow-up was 77.3% complete at 1 year. The median follow-up was 12.0 (0.8–36.8) months. Two (9.1%) patients died between 30 days and 1 year following surgery. In both cases, the death was of non-cardiovascular origin and was not related to recurrent IE or the initial Hemi-Commando procedure. Two more patients (9.1%) died during follow-up after the 1-year mark: 1 (4.5%) patient had a cardiac death and the other died from a non-cardiovascular cause. The estimated 1- and 3-year survival rates were 77.5% and 66.4%, respectively (Fig. 3). One (4.5%) patient required reoperation after 178 days due to recurrent IE. The estimated 1- and 3-year freedom from reoperation was 92.3% (Fig. 4). Competing risk analysis yielded a 93.5% reoperation-free survival at 1 and 3 years.
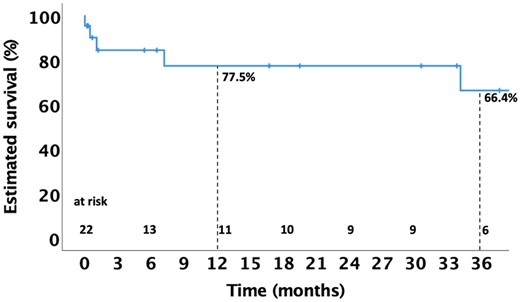
Kaplan–Meier curve depicting estimated survival up to 36 months following the Hemi-Commando procedure.
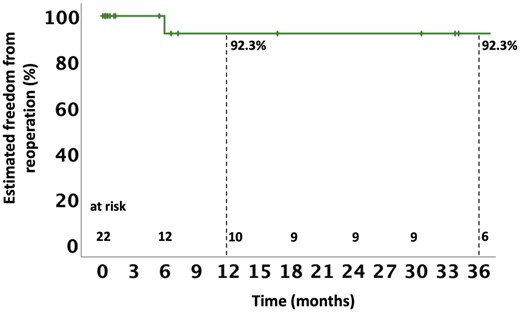
Kaplan–Meier curve depicting estimated freedom from reoperation up to 36 months following the Hemi-Commando procedure.
DISCUSSION
The current study analyses the early and mid-term outcomes following the Hemi-Commando procedure in patients with complex invasive paravalvular IE and the involvement of the AMJ. This procedure is an MV-sparing surgical alternative to the full Commando procedure.
Even though the Hemi-Commando procedure is rarely performed, an increasing number of patients may present with destructive IE requiring complex surgical treatment in the near future. Staphylococcus and Streptococcus species are the leading cause of IE [10], and Staphylococcus species, in particular, are known for their high virulence and capacity to invade and destroy tissue [11, 12]. The increase in cardiac device implantations (especially transcatheter valves) is leading to a shift in the causative organisms of IE, namely an increase of IE caused by gram-positive bacteria, especially Staphylococcus species. Staphylococcus IE is associated with a more severe clinical presentation [10–12]. Moreover, IE is becoming the leading indication for the explant of transcatheter heart valves (THV) [13]. THV infection can lead to severe destructive IE adjacent to the THV stent frame [14]. It is, therefore, possible that more IE patients will be presenting with aortic root and periannular complications requiring complex surgical reconstruction. However, in the current series, no patient had a THV-IE. Yet, the number of patients with THV presenting with destructive IE and paravalvular involvement requiring complex surgical procedures will likely increase due to the shift towards more pathogenic and destructive bacteria [10–12]. This might be particularly true in the current era of low-risk transcatheter aortic valve implantation (TAVI), in which the number of younger TAVI patients with longer life expectancy presenting with IE is rapidly increasing [13, 15, 16].
The ideal management of invasive IE with paravalvular destruction and involvement of the AMJ is still controversial due to the lethality of this dreaded disease. Hence, a multidisciplinary ‘Endocarditis Team’ approach in an experienced high-volume ‘Heart Valve Center’ is recommended to improve the outcomes of these complex patients [17]. Although technically challenging, corrective surgery remains the only option for this high-risk group of patients since conservative therapy is rarely successful. The surgical approach is the only way to restore the heart’s integrity and achieve radical eradication of infected tissue, which is the cornerstone in achieving satisfactory long-term results [1, 4, 5]. The most frequently used technique for patients with double valve endocarditis involving the AMJ is the Commando procedure (also known as ‘UFO operation’). One of the disadvantages of this procedure is the need to replace both the aortic and MV. However, in selected patients with involvement of the AMJ and base of the AML but intact PML, free edge of the AML and subvalvular apparatus, the so-called Hemi-Commando procedure can be performed as an MV-sparing alternative to the full Commando procedure. Sparing the MV may lower the risk of subsequent prosthetic valve endocarditis since IE rates are lower for MV repair than replacement [18, 19].
In addition to the valve-sparing aspect of the Hemi-Commando procedure, its relatively lower technical complexity compared to the complete Commando operation allows shorter operative times. For example, the aortic cross-clamp time observed in our Hemi-Commando series was shorter compared to the available reports on the Commando operation [1–5]. Compared to our own Commando series [1], the Hemi-Commando procedure was associated with an average 37-min reduction in myocardial ischaemic time. However, despite the advantages of the Hemi-Commando procedure, it is advisable to refer patients with IE and involvement of the AMJ to large high-volume ‘Heart Valve Centers’ with experience performing this sort of procedure and managing the perioperative complications.
One possible disadvantage of the Hemi-Commando procedure may be the risk of recurrent IE due to the MV-sparing nature of the procedure. However, this procedure still allows extensive debridement of infected tissues and materials. Another criticism of this technique is the need for a homograft [9], which is not easily available at every institution, thus limiting the wide adoption of this procedure. However, we do not necessarily use homografts to perform this technique while using bovine pericardium to reconstruct the AMJ. Although there is evidence of higher resistance to recurrent infection of homografts compared to other prosthetic materials [20–22], recurrent IE was rare in our series. Moreover, a bovine pericardial patch is used to perform the full Commando operation, which has low IE recurrence rates. Of remark, Tirone David described his initial experience performing the full Commando operation using Dacron to reconstruct the AMJ [5], also with excellent long-term outcomes [3].
The development of invasive IE requiring surgical management is often preceded by failed attempts at medical therapy, which may lead to a delay that contributed to the high EuroSCORE predicted mortality risk observed in our study. The patients’ unfavourable clinical condition, in addition to their baseline comorbidities, results in a particularly morbid perioperative period. We observed similar rates of postoperative complications compared to previous reports of the Commando procedure. Of note, our observed 30-day mortality (13.6%) was less than half the expected (median EuroSCORE II of 28.5%). Our observed 3-year survival estimate showed a sharp decline around 6 months postoperatively, after which survival stabilized (at 1 year 77.5% and at 3 years 66.4%). In comparison, a 2017 publication from the Cleveland Clinic group reported an 8% in-hospital mortality and a 91% 1-year survival for patients who underwent the Hemi-Commando operation [9]. These findings are comparable to the reported mid-term outcomes of the Commando procedure [1–3]. In the Cleveland Clinic cohort, only 1 patient presented with recurrent IE after 1.5 years of follow-up [9], whereas we observed 1 reoperation in the first year due to recurrent IE.
One of the most important theoretical advantages of the Hemi-Commando procedure is the preservation of the MV subvalvular apparatus and, therefore, the conservation of the left ventricular geometry and function. This may be beneficial for these high-risk multimorbid patients undergoing extensive and prolonged cardiac surgery. In addition, echocardiographic assessment of the mitral function showed satisfactory results at discharge. At 3 years postoperatively, no patient required reintervention due to recurrent mitral regurgitation.
Limitations
This study is subject to the inherent biases of single-centre retrospective studies. The follow-up of this cohort is limited to the mid-term. Moreover, the study cohort is small, restricting the interpretation of various statistical analyses. In addition, due to Germany’s decentralized health information system, we have a considerable loss to follow-up of the study patients. However, given the rarity of this disease and procedure, our experience would be one of the largest published to date. Furthermore, as a tertiary referral centre that performs large volumes of surgeries in patients with IE, our current results may not apply to all institutions, especially those with very limited experience managing IE. Finally, we do not have long-term data regarding recurrent mitral regurgitation due to the lack of echocardiographic follow-up.
CONCLUSIONS
Although the Hemi-Commando procedure is a complex and technically challenging operation with high postoperative mortality and morbidity, it offers an acceptable mid-term survival chance with low reoperation rates. This procedure is, therefore, a reasonable MV-sparing alternative to the Commando procedure in suitable patients with extensive IE and perivalvular involvement, who otherwise face a low likelihood of survival. The highest probability of death exists within the first 6 months following surgery, after which the survival and freedom from reoperation are good, considering the type of disease and complexity of the operation required to treat it.
Funding
None.
Conflict of interest: MAB discloses that his hospital receives speakers’ honoraria and/or consulting fees on his behalf from Edwards Lifesciences, Medtronic, Abbott and CryoLife. The remaining authors have no conflicts of interest or financial relationships with the industry to disclose.
DATA AVAILABILITY
The data underlying this article will be shared upon request to the corresponding author.
Author contributions
Mateo Marin-Cuartas: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing. Manuela De La Cuesta: Data curation; Formal analysis; Investigation; Writing—original draft; Writing—review & editing. Piroze M. Davierwala: Conceptualization; Investigation. Jagdip Kang: Conceptualization; Investigation. Guillermo Stöger: Conceptualization; Investigation. Martin Misfeld: Conceptualization; Investigation. Philipp Kiefer: Conceptualization; Investigation. Sergey Leontyev: Conceptualization; Investigation. Alexander Verevkin: Conceptualization; Investigation. Bettina Pfanmüller: Conceptualization; Investigation. Diyar Saaed: Conceptualization; Investigation. Michael A. Borger: Conceptualization; Investigation; Resources; Supervision; Validation; Writing—review & editing. Thilo Noack: Conceptualization; Methodology; Resources; Supervision; Validation; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Ilaria Giambuzzi, Tohru Asai, Valentina Mescola and the other anonymous reviewer(s) for their contribution to the peer review process of this article.
Presented at the 36th EACTS Annual Meeting, Milan, Italy, 5–8 October 2022.
REFERENCES
ABBREVIATIONS
- AMJ
Aortomitral junction
- AML
Anterior mitral leaflet
- IE
Infective endocarditis
- MV
Mitral valve
- PML
Posterior mitral leaflet
- THV
Transcatheter heart valve
Author notes
Mateo Marin-Cuartas and Manuela De La Cuesta authors contributed equally to this work.





