-
PDF
- Split View
-
Views
-
Cite
Cite
Vincent Dahmen, Paul Philipp Heinisch, Helena Staehler, Thibault Schaeffer, Melchior Burri, Christoph Röhlig, Frank Klawonn, Alfred Hager, Peter Ewert, Jürgen Hörer, Masamichi Ono, Longitudinal analysis of systemic ventricular function and atrioventricular valve function after the Fontan procedure, European Journal of Cardio-Thoracic Surgery, Volume 63, Issue 6, June 2023, ezad078, https://doi.org/10.1093/ejcts/ezad078
Close - Share Icon Share
Abstract
This study aimed to determine the longitudinal change of systemic ventricular function and atrioventricular valve (AVV) regurgitation after total cavopulmonary connection (TCPC).
In 620 patients who underwent TCPC between 1994 and 2021, 4219 longitudinal echocardiographic examinations of systemic ventricular function and AVV regurgitation were evaluated retrospectively.
The most frequent primary diagnosis was hypoplastic left heart syndrome in 172, followed by single ventricle in 131, tricuspid atresia in 95 and double inlet left ventricle (LV) in 91 patients. Dominant right ventricle (RV) was observed in 329 (53%) and dominant LV in 291 (47%). The median age at TCPC was 2.3 (1.8–3.4) years. Transplant-free survival at 5, 10 and 15 years after TCPC was 96.3%, 94.7% and 93.6%, respectively, in patients with dominant RV and 97.3%, 94.6% and 94.6%, respectively, in those with dominant LV (P = 0.987). Longitudinal analysis of systemic ventricular function was similar in both groups during the first 10 years postoperatively. Thereafter, systemic ventricular function worsened significantly in patients with dominant RV, compared with those with dominant LV (15 years: P = 0.007, 20 years: P = 0.03). AVV regurgitation was more frequent after TCPC in patients with dominant RV compared with those with dominant LV (P < 0.001 at 3 months, 3 years, 5 years, 10 years and 15 years, P = 0.023 at 20 years). There was a significant correlation between postoperative systemic ventricular dysfunction and AVV regurgitation (P < 0.001).
There were no transplant-free survival difference and no difference in ventricular function between dominant RV and dominant LV for the first 10 years after TCPC. Thereafter, ventricular function in dominant RV was inferior to that in dominant LV. The degree of AVV regurgitation was significantly higher in dominant RV, compared with dominant LV, and it was positively associated with ventricular dysfunction, especially in dominant RV.
INTRODUCTION
Single-ventricle heart disease currently requires staged surgical palliation with bidirectional cavopulmonary shunt and later total cavopulmonary connection (TCPC) as a successful treatment concept [1]. The single ventricle can be distinguished as dominant right ventricle (RV) or dominant left ventricle (LV) morphology, or in rare cases, as indeterminate morphology [2]. Although difference in survival between dominant RV and LV is still a controversy, no difference in transplant-free survival after the Fontan procedure has been reported depending on right or left dominant ventricle morphology in current studies from high-volume centres [3–7]. However, the long-term impact of RV versus LV morphology on systemic ventricular function and atrioventricular valve (AVV) function has not been fully studied. Morphologic RV and LV differ in their embryological origin, anatomy and associated AVV [8, 9]. Furthermore, the RVs and LVs are generally subjected to different afterload conditions. When the RV functions as a systemic ventricle, it is pressure overloaded. It is well known that the systemic RV and tricuspid valve are prone to failure in patients with congenitally corrected transposition of the great arteries (ccTGA) which places the RV in the systemic circulation [10]. Furthermore, regurgitation of tricuspid valve is found in a substantial number of patients with hypoplastic left heart syndrome (HLHS) who have a single RV. Tricuspid valve regurgitation has been attributed to be secondary to annular dilatation and poor function or intrinsic anatomical abnormalities of the tricuspid valve [11, 12]. Therefore, we hypothesized that after TCPC, patients with dominant RV would have worse systemic ventricular function and AVV function compared with patients with dominant LV in the long term. There are few studies that evaluated longitudinally ventricular function and AVV function after the Fontan procedure [13].
To evaluate our hypothesis, we performed a detailed longitudinal analysis of the Fontan experience at our center. We especially evaluated the effect of ventricular morphology on longitudinal ventricular function and AVV function in patients who underwent the Fontan procedure. This study also allowed to perform a secondary analysis of the data to determine the overall long-term outcomes of the TCPC.
METHODS
Ethical statement
This study was approved by the Institutional Review Board of the Technical University of Munich (approved number of 2022-303-S-KH on 27 June 2022). Because of the retrospective nature of the study, the need for individual patient consent was waived.
Patients and data collection
A single-centre retrospective cohort study of 620 consecutive patients who underwent a TCPC at the German Heart Center Munich from May 1994 to December 2021 was performed. Medical records included baseline morphology and demographics as well as pre-, intra- and postoperative data using electrical and paper chart reviews of each patient.
Operative techniques
The operative techniques for TCPC are described in previous reports [7, 14]. Lateral tunnel TCPC was performed in 50 patients in the early era. In January 1999, extra-cardiac TCPC was introduced, and it became our standard procedure since May 2002 [14]. Fenestration was not routinely performed and was only used for high-risk patients [7].
Echocardiography
An experienced echo-cardiographer (Christoph Röhlig) reviewed the archived echocardiogram images from pre- and post-TCPC and assessed the systemic ventricular function and AVV regurgitation. The systemic ventricular function was determined by the ejection fraction (EF), qualitatively graded by eyeballing as normal = 0, slightly (EF < 50%) = 1, mildly (EF < 40%) = 2, moderately (EF < 30%) = 3 or severely reduced (EF < 20%) = 4. M-mode assessment of systemic ventricular function was performed, and the EF was calculated [12, 15]. AVV regurgitation was graded as described in our previous study [12, 15]. The degree of AVV regurgitation was determined by the width and length of the regurgitation jet (none = 0, trivial = 1, mild = 2, moderate = 3 and severe = 4). Significant AVV regurgitation was defined as moderate or severe AVV regurgitation. The time points of data collection were: before TCPC, at the hospital discharge, 3 months postoperatively (first visit of the outpatient clinic) and at the yearly visit to the outpatient clinic.
Survival after total cavopulmonary connection
The patients obtained outpatient follow-up with paediatric cardiologists. The most current vital status and follow-up data were obtained from our institutional single-ventricle database, which is regularly tracked. Follow-up times per patient were defined as the time from the TCPC to the time of the last visit. For patients who died, the end point of the survey was marked at the time of death.
Statistical analysis
Categorical variables are presented as absolute numbers and percentages. A chi-square test was used for categorical data. Continuous variables are expressed as medians with interquartile ranges (IQR). An independent sample t-test was used to compare normally distributed variables. The Mann–Whitney U-test was used for variables that were not normally distributed. Levene's test was used to differentiate between normally and non-normally distributions. Survival after TCPC was the second end point as the post hoc analysis. Transplant-free survival was estimated using the Kaplan–Meier method, and differences between groups were determined using log-rank test. The median follow-up was estimated using the so-called ‘reverse’ Kaplan–Meier estimator (follow-up time distribution based on the Kaplan–Meier method applied to the censored times, reversing the roles of event status and censored). For the statistical analysis of longitudinal systemic ventricular function and AVV regurgitation, cubic splines were used for approximation of the data, to model the potentially non-linear relation of the continuous predictors ‘postoperative period’ and ‘dominant ventricle’ to the outcome ‘ventricular function and AVV regurgitation’. Three knots were used located at the quartiles of the corresponding time values after TCPC. First, a cubic spline was fitted to all data independent of the systemic ventricular morphology and a 95% confidence band was computed. Then, corresponding cubic splines were computed separately for the 2 different ventricular morphologies. Listwise deletion was used for missing data. Data analysis was performed using SPSS version 28.0 for Windows (IBM, Ehningen, Germany) and R-statistical software (state package). Cubic spline approximation and corresponding graphs were generated with R, version 4.1.0 and the splines package.
RESULTS
Patient characteristics and perioperative data
Patient characteristics are presented in Table 1. The median age and median weight at TCPC were 2.3 (IQR: 1.8–3.4) years and 12.0 (10.7–14.0) kg, respectively. The most frequent diagnosis was HLHS in 172 patients, followed by single ventricle in 131, tricuspid atresia in 95, double inlet LV in 91, pulmonary atresia and intact ventricular septum in 32, ccTGA in 29 and unbalanced atrioventricular septal defect in 25. Dominant RV was observed in 329 (53%) and dominant LV in 291 (47%) patients.
| Variables, N (%) or median (IQR) . | Total cases . | RV . | LV . | P-Value . |
|---|---|---|---|---|
| Number of patients | 620 | 329 (53.1) | 291 (46.9) | |
| Male sex | 385 (62.1) | 217 (66.0) | 168 (57.7) | 0.035 |
| Age at TCPC (years) | 2.3 (1.8–3.4) | 2.3 (1.9–3.2) | 2.3 (1.7–3.7) | 0.076 |
| Weight at TCPC (kg) | 12.0 (10.7–14.0) | 11.8 (10.7–14.0) | 12.0 (10.7–14.5) | 0.055 |
| Primary diagnosis | ||||
| HLHS | 172 (27.7) | 172 (52.3) | 0 (0.0) | <0.001 |
| UVH | 131 (21.1) | 97 (29.5) | 34 (11.7) | <0.001 |
| TA | 95 (15.3) | 0 (0.0) | 95 (32.6) | <0.001 |
| DILV | 91 (14.7) | 0 (0.0) | 91 (31.3) | <0.001 |
| PAIVS | 32 (5.2) | 0 (0.0) | 32 (11.0) | <0.001 |
| ccTGA | 29 (4.7) | 8 (2.4) | 21 (7.2) | 0.005 |
| UAVSD | 25 (4.0) | 15 (4.6) | 10 (3.4) | 0.478 |
| Others | 46 (7.4) | 37 (11.2) | 9 (3.1) | <0.001 |
| Associated cardiac anomaly | ||||
| TGA | 208 (33.5) | 78 (23.7) | 130 (44.7) | <0.001 |
| DORV | 81 (13.1) | 72 (21.9) | 9 (3.1) | <0.001 |
| CoA | 79 (12.7) | 37 (11.2) | 42 (14.4) | 0.235 |
| Dextrocardia/situs inversus | 56 (9.0) | 37 (11.2) | 19 (6.5) | 0.041 |
| Heterotaxy | 48 (7.7) | 40 (12.2) | 8 (2.7) | <0.001 |
| TAPVC/PAPVC | 42 (6.8) | 37 (11.2) | 5 (1.7) | <0.001 |
| Systemic venous return anomaly | 58 (9.4) | 43 (13.1) | 15 (5.2) | <0.001 |
| Palliation and pre Fontan condition | ||||
| Norwood/DKS | 264 (42.6) | 193 (73.1) | 71 (24.4) | <0.001 |
| AP shunt | 185 (29.8) | 72 (21.9) | 113 (38.8) | <0.001 |
| PAB | 90 (15.5) | 40 (12.2) | 50 (17.2) | 0.076 |
| Number of palliative surgeries | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.134 |
| Prior BCPS | 571 (92.1) | 317 (96.4) | 256 (87.3) | <0.001 |
| Age at BCPS (months) | 5.4 (3.6–10.5) | 4.7 (3.4–8.4) | 6.1 (4.0–11.6) | 0.202 |
| Weight at BCPS (kg) | 5.8 (4.9–7.5) | 5.5 (4.7–7.0) | 6.3 (5.2–7.9) | 0.009 |
| Variables, N (%) or median (IQR) . | Total cases . | RV . | LV . | P-Value . |
|---|---|---|---|---|
| Number of patients | 620 | 329 (53.1) | 291 (46.9) | |
| Male sex | 385 (62.1) | 217 (66.0) | 168 (57.7) | 0.035 |
| Age at TCPC (years) | 2.3 (1.8–3.4) | 2.3 (1.9–3.2) | 2.3 (1.7–3.7) | 0.076 |
| Weight at TCPC (kg) | 12.0 (10.7–14.0) | 11.8 (10.7–14.0) | 12.0 (10.7–14.5) | 0.055 |
| Primary diagnosis | ||||
| HLHS | 172 (27.7) | 172 (52.3) | 0 (0.0) | <0.001 |
| UVH | 131 (21.1) | 97 (29.5) | 34 (11.7) | <0.001 |
| TA | 95 (15.3) | 0 (0.0) | 95 (32.6) | <0.001 |
| DILV | 91 (14.7) | 0 (0.0) | 91 (31.3) | <0.001 |
| PAIVS | 32 (5.2) | 0 (0.0) | 32 (11.0) | <0.001 |
| ccTGA | 29 (4.7) | 8 (2.4) | 21 (7.2) | 0.005 |
| UAVSD | 25 (4.0) | 15 (4.6) | 10 (3.4) | 0.478 |
| Others | 46 (7.4) | 37 (11.2) | 9 (3.1) | <0.001 |
| Associated cardiac anomaly | ||||
| TGA | 208 (33.5) | 78 (23.7) | 130 (44.7) | <0.001 |
| DORV | 81 (13.1) | 72 (21.9) | 9 (3.1) | <0.001 |
| CoA | 79 (12.7) | 37 (11.2) | 42 (14.4) | 0.235 |
| Dextrocardia/situs inversus | 56 (9.0) | 37 (11.2) | 19 (6.5) | 0.041 |
| Heterotaxy | 48 (7.7) | 40 (12.2) | 8 (2.7) | <0.001 |
| TAPVC/PAPVC | 42 (6.8) | 37 (11.2) | 5 (1.7) | <0.001 |
| Systemic venous return anomaly | 58 (9.4) | 43 (13.1) | 15 (5.2) | <0.001 |
| Palliation and pre Fontan condition | ||||
| Norwood/DKS | 264 (42.6) | 193 (73.1) | 71 (24.4) | <0.001 |
| AP shunt | 185 (29.8) | 72 (21.9) | 113 (38.8) | <0.001 |
| PAB | 90 (15.5) | 40 (12.2) | 50 (17.2) | 0.076 |
| Number of palliative surgeries | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.134 |
| Prior BCPS | 571 (92.1) | 317 (96.4) | 256 (87.3) | <0.001 |
| Age at BCPS (months) | 5.4 (3.6–10.5) | 4.7 (3.4–8.4) | 6.1 (4.0–11.6) | 0.202 |
| Weight at BCPS (kg) | 5.8 (4.9–7.5) | 5.5 (4.7–7.0) | 6.3 (5.2–7.9) | 0.009 |
AP: aorto-pulmonary; BCPS: bidirectional cavopulmonary shunt; ccTGA: congenitally corrected TGA; CoA: coarctation of the aorta; DILV: double inlet left ventricle; DKS: Damus–Kaye–Stansel anastomosis; DORV: double outlet right ventricle; HLHS: hypoplastic left heart syndrome; IQR: interquartile range; LV: left ventricle; PAB: pulmonary artery banding; PAIVS: pulmonary atresia and intact ventricular septum; PAPVC: partial anomalous pulmonary venous connection; RV: right ventricle; SV: single ventricle; TA: tricuspid atresia; TAPVC; Total anomalous pulmonary venous connection; TCPC: total cavopulmonary connection; TGA: transposition of the great arteries; UAVSD: unbalanced atrioventricular septal defect; UVH: univentricular heart.
| Variables, N (%) or median (IQR) . | Total cases . | RV . | LV . | P-Value . |
|---|---|---|---|---|
| Number of patients | 620 | 329 (53.1) | 291 (46.9) | |
| Male sex | 385 (62.1) | 217 (66.0) | 168 (57.7) | 0.035 |
| Age at TCPC (years) | 2.3 (1.8–3.4) | 2.3 (1.9–3.2) | 2.3 (1.7–3.7) | 0.076 |
| Weight at TCPC (kg) | 12.0 (10.7–14.0) | 11.8 (10.7–14.0) | 12.0 (10.7–14.5) | 0.055 |
| Primary diagnosis | ||||
| HLHS | 172 (27.7) | 172 (52.3) | 0 (0.0) | <0.001 |
| UVH | 131 (21.1) | 97 (29.5) | 34 (11.7) | <0.001 |
| TA | 95 (15.3) | 0 (0.0) | 95 (32.6) | <0.001 |
| DILV | 91 (14.7) | 0 (0.0) | 91 (31.3) | <0.001 |
| PAIVS | 32 (5.2) | 0 (0.0) | 32 (11.0) | <0.001 |
| ccTGA | 29 (4.7) | 8 (2.4) | 21 (7.2) | 0.005 |
| UAVSD | 25 (4.0) | 15 (4.6) | 10 (3.4) | 0.478 |
| Others | 46 (7.4) | 37 (11.2) | 9 (3.1) | <0.001 |
| Associated cardiac anomaly | ||||
| TGA | 208 (33.5) | 78 (23.7) | 130 (44.7) | <0.001 |
| DORV | 81 (13.1) | 72 (21.9) | 9 (3.1) | <0.001 |
| CoA | 79 (12.7) | 37 (11.2) | 42 (14.4) | 0.235 |
| Dextrocardia/situs inversus | 56 (9.0) | 37 (11.2) | 19 (6.5) | 0.041 |
| Heterotaxy | 48 (7.7) | 40 (12.2) | 8 (2.7) | <0.001 |
| TAPVC/PAPVC | 42 (6.8) | 37 (11.2) | 5 (1.7) | <0.001 |
| Systemic venous return anomaly | 58 (9.4) | 43 (13.1) | 15 (5.2) | <0.001 |
| Palliation and pre Fontan condition | ||||
| Norwood/DKS | 264 (42.6) | 193 (73.1) | 71 (24.4) | <0.001 |
| AP shunt | 185 (29.8) | 72 (21.9) | 113 (38.8) | <0.001 |
| PAB | 90 (15.5) | 40 (12.2) | 50 (17.2) | 0.076 |
| Number of palliative surgeries | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.134 |
| Prior BCPS | 571 (92.1) | 317 (96.4) | 256 (87.3) | <0.001 |
| Age at BCPS (months) | 5.4 (3.6–10.5) | 4.7 (3.4–8.4) | 6.1 (4.0–11.6) | 0.202 |
| Weight at BCPS (kg) | 5.8 (4.9–7.5) | 5.5 (4.7–7.0) | 6.3 (5.2–7.9) | 0.009 |
| Variables, N (%) or median (IQR) . | Total cases . | RV . | LV . | P-Value . |
|---|---|---|---|---|
| Number of patients | 620 | 329 (53.1) | 291 (46.9) | |
| Male sex | 385 (62.1) | 217 (66.0) | 168 (57.7) | 0.035 |
| Age at TCPC (years) | 2.3 (1.8–3.4) | 2.3 (1.9–3.2) | 2.3 (1.7–3.7) | 0.076 |
| Weight at TCPC (kg) | 12.0 (10.7–14.0) | 11.8 (10.7–14.0) | 12.0 (10.7–14.5) | 0.055 |
| Primary diagnosis | ||||
| HLHS | 172 (27.7) | 172 (52.3) | 0 (0.0) | <0.001 |
| UVH | 131 (21.1) | 97 (29.5) | 34 (11.7) | <0.001 |
| TA | 95 (15.3) | 0 (0.0) | 95 (32.6) | <0.001 |
| DILV | 91 (14.7) | 0 (0.0) | 91 (31.3) | <0.001 |
| PAIVS | 32 (5.2) | 0 (0.0) | 32 (11.0) | <0.001 |
| ccTGA | 29 (4.7) | 8 (2.4) | 21 (7.2) | 0.005 |
| UAVSD | 25 (4.0) | 15 (4.6) | 10 (3.4) | 0.478 |
| Others | 46 (7.4) | 37 (11.2) | 9 (3.1) | <0.001 |
| Associated cardiac anomaly | ||||
| TGA | 208 (33.5) | 78 (23.7) | 130 (44.7) | <0.001 |
| DORV | 81 (13.1) | 72 (21.9) | 9 (3.1) | <0.001 |
| CoA | 79 (12.7) | 37 (11.2) | 42 (14.4) | 0.235 |
| Dextrocardia/situs inversus | 56 (9.0) | 37 (11.2) | 19 (6.5) | 0.041 |
| Heterotaxy | 48 (7.7) | 40 (12.2) | 8 (2.7) | <0.001 |
| TAPVC/PAPVC | 42 (6.8) | 37 (11.2) | 5 (1.7) | <0.001 |
| Systemic venous return anomaly | 58 (9.4) | 43 (13.1) | 15 (5.2) | <0.001 |
| Palliation and pre Fontan condition | ||||
| Norwood/DKS | 264 (42.6) | 193 (73.1) | 71 (24.4) | <0.001 |
| AP shunt | 185 (29.8) | 72 (21.9) | 113 (38.8) | <0.001 |
| PAB | 90 (15.5) | 40 (12.2) | 50 (17.2) | 0.076 |
| Number of palliative surgeries | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.134 |
| Prior BCPS | 571 (92.1) | 317 (96.4) | 256 (87.3) | <0.001 |
| Age at BCPS (months) | 5.4 (3.6–10.5) | 4.7 (3.4–8.4) | 6.1 (4.0–11.6) | 0.202 |
| Weight at BCPS (kg) | 5.8 (4.9–7.5) | 5.5 (4.7–7.0) | 6.3 (5.2–7.9) | 0.009 |
AP: aorto-pulmonary; BCPS: bidirectional cavopulmonary shunt; ccTGA: congenitally corrected TGA; CoA: coarctation of the aorta; DILV: double inlet left ventricle; DKS: Damus–Kaye–Stansel anastomosis; DORV: double outlet right ventricle; HLHS: hypoplastic left heart syndrome; IQR: interquartile range; LV: left ventricle; PAB: pulmonary artery banding; PAIVS: pulmonary atresia and intact ventricular septum; PAPVC: partial anomalous pulmonary venous connection; RV: right ventricle; SV: single ventricle; TA: tricuspid atresia; TAPVC; Total anomalous pulmonary venous connection; TCPC: total cavopulmonary connection; TGA: transposition of the great arteries; UAVSD: unbalanced atrioventricular septal defect; UVH: univentricular heart.
Perioperative data are shown in Table 2. Intra-cardiac lateral tunnel TCPC was performed in 50 patients from May 1994 to April 2002. In January 1999, extra-cardiac TCPC was introduced, and this procedure was performed in 570 patients. The median duration of CPB time was 66 (IQR: 47–102) min. Aortic cross-clamp was needed in 161 patients with a median duration of 46 (26–73) min. The main concomitant procedures included 79 AVV procedure, 59 pulmonary artery reconstructions and 29 atrioseptectomies. There were 8 in-hospital deaths (mortality during the postoperative hospital stay). Postoperative median length of stay in the intensive care unit was 6 (4–9) days and the median hospital stay was 20 (14–28) days. Patients with dominant RV had significantly longer intensive care unit stay (7 vs 6 days, P = 0.002), hospital stay (21 vs 18 days, P < 0.001), higher incidence of prolonged pleural effusions (57 vs 40%, P < 0.001), chylothorax (28 vs 14%, P < 0.001) and ascites (24 vs 16%, P = 0.02), compared with those with dominant LV.
| Variables . | Total . | RV . | LV . | P-Value . |
|---|---|---|---|---|
| Operative data | 620 | 329 (53.1) | 291 (46.9) | |
| Type of TCPC | ||||
| Intracardial | 50 (8.1) | 14 (4.3) | 36 (12.4) | <0.001 |
| Extra-cardiac | 570 (91.9) | 315 (95.7) | 255 (87.6) | |
| Conduit diameter (mm) | ||||
| 14 | 1 (0.2) | 0(0) | 1 (0.4) | 0.080 |
| 16 | 9 (1.5) | 3 (1.0) | 6 (2.4) | |
| 18 | 489 (78.9) | 277 (87.9) | 212 (83.1) | |
| 20 | 56 (9.0) | 31 (9.8) | 25 (9.8) | |
| 22 | 15 (2.4) | 4 (1.3) | 11 (4.3) | |
| CPB time (min) | 66 (47–102) | 69 (48–101) | 63 (47–102) | 0.212 |
| Aortic cross-clamp (AXC) | 161 (26.0) | 91 (27.7) | 70 (24.1) | 0.307 |
| AXC time (min) | 46 (26–73) | 39 (23–70) | 49 (33–76) | 0.072 |
| Fenestration | 46 (7.4) | 19 (5.8) | 27 (9.3) | 0.097 |
| Concomitant procedure | 164 (26.5) | 94 (28.6) | 70 (24.1) | 0.203 |
| DKS | 17 (2.7) | 9 (2.7) | 8 (2.7) | 0.992 |
| AVV procedure | 79 (12.7) | 56 (17.0) | 23 (7.9) | <0.001 |
| PA reconstruction | 59 (9.5) | 31 (9.4) | 28 (9.6) | 0.933 |
| Atrioseptectomy | 29 (4.7) | 11 (3.3) | 18 (6.2) | 0.094 |
| SAS/VSD enlargement | 13 (2.1) | 7 (2.1) | 6 (2.1) | 0.954 |
| Pacemaker implant | 12 (1.9) | 6 (1.8) | 6 (2.1) | 0.830 |
| Fenestration at TCPC | 46 (7.4) | 19 (5.8) | 27 (9.3) | 0.097 |
| Postoperative data | ||||
| Thirty-day survival | 8 (1.3) | 4 (1.2) | 4 (1.4) | 0.861 |
| ICU stay (days) | 6 (4–9) | 7 (4–10) | 6 (4–8) | 0.002 |
| Hospital stay (days) | 20 (14–28) | 21 (16–29) | 18 (14–25) | <0.001 |
| Complications | ||||
| Pleural effusion | 285 (48.7) | 178 (56.5) | 107 (39.6) | <0.001 |
| Chylothorax | 125 (21.4) | 87 (27.7) | 38 (14.1) | <0.001 |
| Ascites | 118 (20.1) | 75 (23.7) | 43 (15.9) | 0.018 |
| Secondary fenestration | 11 (1.8) | 11 (3.3) | 0 (0) | 0.002 |
| Variables . | Total . | RV . | LV . | P-Value . |
|---|---|---|---|---|
| Operative data | 620 | 329 (53.1) | 291 (46.9) | |
| Type of TCPC | ||||
| Intracardial | 50 (8.1) | 14 (4.3) | 36 (12.4) | <0.001 |
| Extra-cardiac | 570 (91.9) | 315 (95.7) | 255 (87.6) | |
| Conduit diameter (mm) | ||||
| 14 | 1 (0.2) | 0(0) | 1 (0.4) | 0.080 |
| 16 | 9 (1.5) | 3 (1.0) | 6 (2.4) | |
| 18 | 489 (78.9) | 277 (87.9) | 212 (83.1) | |
| 20 | 56 (9.0) | 31 (9.8) | 25 (9.8) | |
| 22 | 15 (2.4) | 4 (1.3) | 11 (4.3) | |
| CPB time (min) | 66 (47–102) | 69 (48–101) | 63 (47–102) | 0.212 |
| Aortic cross-clamp (AXC) | 161 (26.0) | 91 (27.7) | 70 (24.1) | 0.307 |
| AXC time (min) | 46 (26–73) | 39 (23–70) | 49 (33–76) | 0.072 |
| Fenestration | 46 (7.4) | 19 (5.8) | 27 (9.3) | 0.097 |
| Concomitant procedure | 164 (26.5) | 94 (28.6) | 70 (24.1) | 0.203 |
| DKS | 17 (2.7) | 9 (2.7) | 8 (2.7) | 0.992 |
| AVV procedure | 79 (12.7) | 56 (17.0) | 23 (7.9) | <0.001 |
| PA reconstruction | 59 (9.5) | 31 (9.4) | 28 (9.6) | 0.933 |
| Atrioseptectomy | 29 (4.7) | 11 (3.3) | 18 (6.2) | 0.094 |
| SAS/VSD enlargement | 13 (2.1) | 7 (2.1) | 6 (2.1) | 0.954 |
| Pacemaker implant | 12 (1.9) | 6 (1.8) | 6 (2.1) | 0.830 |
| Fenestration at TCPC | 46 (7.4) | 19 (5.8) | 27 (9.3) | 0.097 |
| Postoperative data | ||||
| Thirty-day survival | 8 (1.3) | 4 (1.2) | 4 (1.4) | 0.861 |
| ICU stay (days) | 6 (4–9) | 7 (4–10) | 6 (4–8) | 0.002 |
| Hospital stay (days) | 20 (14–28) | 21 (16–29) | 18 (14–25) | <0.001 |
| Complications | ||||
| Pleural effusion | 285 (48.7) | 178 (56.5) | 107 (39.6) | <0.001 |
| Chylothorax | 125 (21.4) | 87 (27.7) | 38 (14.1) | <0.001 |
| Ascites | 118 (20.1) | 75 (23.7) | 43 (15.9) | 0.018 |
| Secondary fenestration | 11 (1.8) | 11 (3.3) | 0 (0) | 0.002 |
Variables were presented in N (%) or median (IQR). P-Value <0.05 emphasized in bold.
AVV: atrioventricular valve; AXC: aortic cross-clamp; CPB: cardiopulmonary bypass; DKS: Damus–Kaye–Stansel anastomosis; ICU: intensive care unit; IQR: interquartile range; LV: left ventricle; PA: pulmonary artery; RV: right ventricle; SAS: subaortic stenosis; TCPC: total cavopulmonary connection; VSD: ventricular septal defect.
| Variables . | Total . | RV . | LV . | P-Value . |
|---|---|---|---|---|
| Operative data | 620 | 329 (53.1) | 291 (46.9) | |
| Type of TCPC | ||||
| Intracardial | 50 (8.1) | 14 (4.3) | 36 (12.4) | <0.001 |
| Extra-cardiac | 570 (91.9) | 315 (95.7) | 255 (87.6) | |
| Conduit diameter (mm) | ||||
| 14 | 1 (0.2) | 0(0) | 1 (0.4) | 0.080 |
| 16 | 9 (1.5) | 3 (1.0) | 6 (2.4) | |
| 18 | 489 (78.9) | 277 (87.9) | 212 (83.1) | |
| 20 | 56 (9.0) | 31 (9.8) | 25 (9.8) | |
| 22 | 15 (2.4) | 4 (1.3) | 11 (4.3) | |
| CPB time (min) | 66 (47–102) | 69 (48–101) | 63 (47–102) | 0.212 |
| Aortic cross-clamp (AXC) | 161 (26.0) | 91 (27.7) | 70 (24.1) | 0.307 |
| AXC time (min) | 46 (26–73) | 39 (23–70) | 49 (33–76) | 0.072 |
| Fenestration | 46 (7.4) | 19 (5.8) | 27 (9.3) | 0.097 |
| Concomitant procedure | 164 (26.5) | 94 (28.6) | 70 (24.1) | 0.203 |
| DKS | 17 (2.7) | 9 (2.7) | 8 (2.7) | 0.992 |
| AVV procedure | 79 (12.7) | 56 (17.0) | 23 (7.9) | <0.001 |
| PA reconstruction | 59 (9.5) | 31 (9.4) | 28 (9.6) | 0.933 |
| Atrioseptectomy | 29 (4.7) | 11 (3.3) | 18 (6.2) | 0.094 |
| SAS/VSD enlargement | 13 (2.1) | 7 (2.1) | 6 (2.1) | 0.954 |
| Pacemaker implant | 12 (1.9) | 6 (1.8) | 6 (2.1) | 0.830 |
| Fenestration at TCPC | 46 (7.4) | 19 (5.8) | 27 (9.3) | 0.097 |
| Postoperative data | ||||
| Thirty-day survival | 8 (1.3) | 4 (1.2) | 4 (1.4) | 0.861 |
| ICU stay (days) | 6 (4–9) | 7 (4–10) | 6 (4–8) | 0.002 |
| Hospital stay (days) | 20 (14–28) | 21 (16–29) | 18 (14–25) | <0.001 |
| Complications | ||||
| Pleural effusion | 285 (48.7) | 178 (56.5) | 107 (39.6) | <0.001 |
| Chylothorax | 125 (21.4) | 87 (27.7) | 38 (14.1) | <0.001 |
| Ascites | 118 (20.1) | 75 (23.7) | 43 (15.9) | 0.018 |
| Secondary fenestration | 11 (1.8) | 11 (3.3) | 0 (0) | 0.002 |
| Variables . | Total . | RV . | LV . | P-Value . |
|---|---|---|---|---|
| Operative data | 620 | 329 (53.1) | 291 (46.9) | |
| Type of TCPC | ||||
| Intracardial | 50 (8.1) | 14 (4.3) | 36 (12.4) | <0.001 |
| Extra-cardiac | 570 (91.9) | 315 (95.7) | 255 (87.6) | |
| Conduit diameter (mm) | ||||
| 14 | 1 (0.2) | 0(0) | 1 (0.4) | 0.080 |
| 16 | 9 (1.5) | 3 (1.0) | 6 (2.4) | |
| 18 | 489 (78.9) | 277 (87.9) | 212 (83.1) | |
| 20 | 56 (9.0) | 31 (9.8) | 25 (9.8) | |
| 22 | 15 (2.4) | 4 (1.3) | 11 (4.3) | |
| CPB time (min) | 66 (47–102) | 69 (48–101) | 63 (47–102) | 0.212 |
| Aortic cross-clamp (AXC) | 161 (26.0) | 91 (27.7) | 70 (24.1) | 0.307 |
| AXC time (min) | 46 (26–73) | 39 (23–70) | 49 (33–76) | 0.072 |
| Fenestration | 46 (7.4) | 19 (5.8) | 27 (9.3) | 0.097 |
| Concomitant procedure | 164 (26.5) | 94 (28.6) | 70 (24.1) | 0.203 |
| DKS | 17 (2.7) | 9 (2.7) | 8 (2.7) | 0.992 |
| AVV procedure | 79 (12.7) | 56 (17.0) | 23 (7.9) | <0.001 |
| PA reconstruction | 59 (9.5) | 31 (9.4) | 28 (9.6) | 0.933 |
| Atrioseptectomy | 29 (4.7) | 11 (3.3) | 18 (6.2) | 0.094 |
| SAS/VSD enlargement | 13 (2.1) | 7 (2.1) | 6 (2.1) | 0.954 |
| Pacemaker implant | 12 (1.9) | 6 (1.8) | 6 (2.1) | 0.830 |
| Fenestration at TCPC | 46 (7.4) | 19 (5.8) | 27 (9.3) | 0.097 |
| Postoperative data | ||||
| Thirty-day survival | 8 (1.3) | 4 (1.2) | 4 (1.4) | 0.861 |
| ICU stay (days) | 6 (4–9) | 7 (4–10) | 6 (4–8) | 0.002 |
| Hospital stay (days) | 20 (14–28) | 21 (16–29) | 18 (14–25) | <0.001 |
| Complications | ||||
| Pleural effusion | 285 (48.7) | 178 (56.5) | 107 (39.6) | <0.001 |
| Chylothorax | 125 (21.4) | 87 (27.7) | 38 (14.1) | <0.001 |
| Ascites | 118 (20.1) | 75 (23.7) | 43 (15.9) | 0.018 |
| Secondary fenestration | 11 (1.8) | 11 (3.3) | 0 (0) | 0.002 |
Variables were presented in N (%) or median (IQR). P-Value <0.05 emphasized in bold.
AVV: atrioventricular valve; AXC: aortic cross-clamp; CPB: cardiopulmonary bypass; DKS: Damus–Kaye–Stansel anastomosis; ICU: intensive care unit; IQR: interquartile range; LV: left ventricle; PA: pulmonary artery; RV: right ventricle; SAS: subaortic stenosis; TCPC: total cavopulmonary connection; VSD: ventricular septal defect.
Follow-up and survival
The median follow-up was 6.1 (IQR: 1.4–13.7) years. There were 15 late deaths after hospital discharge and 4 heart transplantations. No patient underwent Fontan takedown. Transplant-free survival at 5, 10, 15 and 20 years after TCPC was 96.8%, 94.6%, 94.2% and 91.3%, respectively. For patients after TCPC with dominant RV, transplant-free survival at 5, 10, 15 and 20 years was 96.3%, 94.7%, 93.6% and 93.6%, and for patients with dominant LV, it was 97.3%, 94.6%, 94.6% and 90.7%, respectively (Fig. 1). There was no significant difference in transplant-free survival between dominant RV and dominant LV (P = 0.987). During the follow-up period, a total of 11 patients required repair or replacement of AVV. The procedures included 7 tricuspid valve replacements, 2 common AVV repair, 1 mitral valve repair and 1 mitral valve replacement.
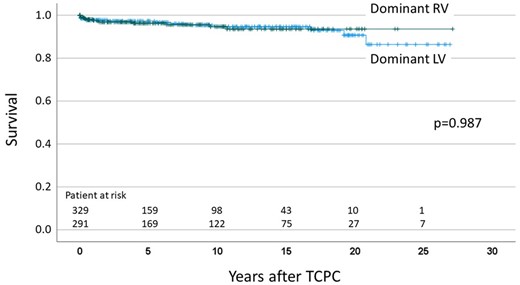
Transplant-free survival after total cavopulmonary connection.
Longitudinal analysis of systemic ventricular function and atrioventricular valve regurgitation
We collected a total of 4219 echocardiograms (2088 with dominant RV and 2131 with dominant LV). The median interval between the last recorded postoperative echocardiographic assessment and the time of TCPC was 1525 (IQR 195–3614) days. The proportions of ventricular function and AVV regurgitation severity over time since TCPC are illustrated in Figs 2 and 3 and listed in Supplementary Material, Tables S1 and S2. The morphological comparison was performed. Systemic ventricular function during the first 10 years following TCPC did not differ significantly between dominant RV and dominant LV. Thereafter, ventricular function in dominant RV patients was inferior to those with dominant LV (15 years: P = 0.007, 20 years: P = 0.03). As for the AVV regurgitation, patients with dominant RV demonstrated significantly inferior function soon after the TCPC, compared with patients with a dominant LV (3 months to 15 years: P < 0.001, 20 years: P = 0.023) (Fig. 3 and Supplementary Material, Tables S1 and S2). When we compare the proportions of ventricular dysfunction and severity of AVV regurgitation between early era (1994–2008) and late era (2009–2021), ventricular function was better in the late era compared to the early era (pre-TCPC: P < 0.001, post 3 months: P < 0.001, post 3 years: P = 0.032 and post 10 years: P = 0.015). However, significant AVV regurgitation was more frequently observed in the late era compared to the early era (pre-TCPC: P = 0.013, post 3 months: P = 0.008, post 3 years: P = 0.002, post 5 years: P = 0.06 and post 10 years: P < 0.001) (Supplementary Material, Table S3). When we compare the proportions of ventricular function between PAB patients (n = 90) and APS patients (n = 185), postoperative ventricular function was worse in PAB patients compared to APS patients (post 3 months: P = 0.028 and post 10 years: P < 0.001) (Supplementary Material, Table S4).
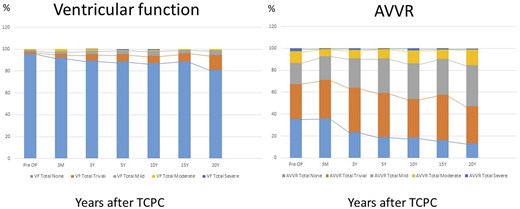
Histograms illustrating the proportions of ventricular dysfunction and AVVR after total cavopulmonary connection over time.
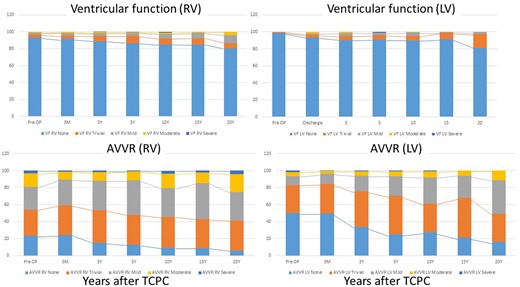
Progression of ventricular dysfunction and atrioventricular valve regurgitation after total cavopulmonary connection by serial echocardiogram: comparison between dominant right ventricle and dominant left ventricle.
To identify differences in the temporal behaviour of dominant RV and dominant LV, cubic B-splines were used to approximate the data. For testing whether dominant RV and dominant LV deviated from the general course of the joint data, the corresponding subgroups were approximated separately by B-splines shown in blue and red. The longitudinal degrees of ventricular function and AVV regurgitation after TCPC are shown in Fig. 4.
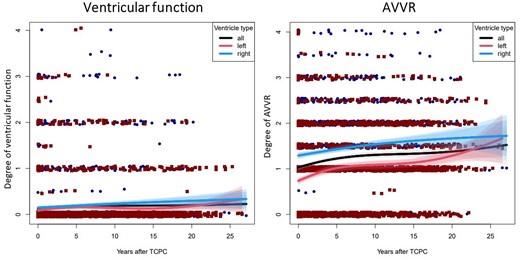
Longitudinal postoperative ventricular dysfunction and atrioventricular valve regurgitation in patients with dominant right ventricle and dominant left ventricle. Degree of ventricular dysfunction (left) and degree of atrioventricular valve regurgitation (right) for all patients (black curve), for patients with dominant right ventricle (blue curve with 95% confidence interval and 99% confidence interval) and dominant left ventricle (red curve with 95% confidence interval and 99% confidence interval), are shown.
Correlation of ventricular dysfunction and AVVR
We found a significant correlation between ventricular function and AVV regurgitation (Table 3). In patients with AVV regurgitation ≥ moderate, 8.1% of the patients had associated significant ventricular dysfunction (≥ moderate) (P < 0.001). On the other hand, only 0.6% of patients with AVV regurgitation < moderate developed significant ventricular dysfunction (P < 0.001). Importantly, AVV regurgitation ≥ moderate and ventricular dysfunction was observed only in patients with dominant RV. In all patients with dominant LV who presented with ventricular dysfunction, AVV regurgitation remained less than moderate. When we performed the same analysis using the cut-off value of mild ventricular dysfunction and mild AVV regurgitation, we obtained the significant correlation between ventricular function and AVV regurgitation both in dominant RV and dominant LV (Supplementary Material, Table S5).
| Variables . | AVVR < moderate . | AVVR ≥ moderate . | ||||
|---|---|---|---|---|---|---|
| Total . | RV . | LV . | Total . | RV . | LV . | |
| Total period | ||||||
| Dysfunction < moderate | 3975 | 1925 | 2050 | 193 | 125 | 68 |
| Dysfunction ≥ moderate | 26 | 14 | 12 | 17 | 17 | 0 |
| % incidence of dysfunction ≥ moderate | 0.6* | 0.7* | 0.6 | 8.1* | 12.0* | 0.0 |
| <5 years | ||||||
| Dysfunction < moderate | 2411 | 1264 | 1147 | 125 | 81 | 44 |
| Dysfunction ≥ moderate | 17 | 7 | 10 | 9 | 9 | 0 |
| % incidence of dysfunction ≥ moderate | 0.7* | 0.6* | 0.9 | 6.7* | 10.0* | 0.0 |
| 5–10 years | ||||||
| Dysfunction < moderate | 716 | 347 | 369 | 35 | 24 | 11 |
| Dysfunction ≥ moderate | 8 | 6 | 2 | 5 | 5 | 0 |
| % incidence of dysfunction ≥ moderate | 1.1* | 1.7* | 0.5 | 12.5* | 17.2* | 0.0 |
| >10 years | ||||||
| Dysfunction < moderate | 848 | 314 | 534 | 33 | 20 | 13 |
| Dysfunction ≥ moderate | 1 | 1 | 0 | 3 | 3 | 0 |
| % incidence of dysfunction ≥ moderate | 0.1* | 0.3* | 0.0 | 8.3* | 13.0* | 0.0 |
| Variables . | AVVR < moderate . | AVVR ≥ moderate . | ||||
|---|---|---|---|---|---|---|
| Total . | RV . | LV . | Total . | RV . | LV . | |
| Total period | ||||||
| Dysfunction < moderate | 3975 | 1925 | 2050 | 193 | 125 | 68 |
| Dysfunction ≥ moderate | 26 | 14 | 12 | 17 | 17 | 0 |
| % incidence of dysfunction ≥ moderate | 0.6* | 0.7* | 0.6 | 8.1* | 12.0* | 0.0 |
| <5 years | ||||||
| Dysfunction < moderate | 2411 | 1264 | 1147 | 125 | 81 | 44 |
| Dysfunction ≥ moderate | 17 | 7 | 10 | 9 | 9 | 0 |
| % incidence of dysfunction ≥ moderate | 0.7* | 0.6* | 0.9 | 6.7* | 10.0* | 0.0 |
| 5–10 years | ||||||
| Dysfunction < moderate | 716 | 347 | 369 | 35 | 24 | 11 |
| Dysfunction ≥ moderate | 8 | 6 | 2 | 5 | 5 | 0 |
| % incidence of dysfunction ≥ moderate | 1.1* | 1.7* | 0.5 | 12.5* | 17.2* | 0.0 |
| >10 years | ||||||
| Dysfunction < moderate | 848 | 314 | 534 | 33 | 20 | 13 |
| Dysfunction ≥ moderate | 1 | 1 | 0 | 3 | 3 | 0 |
| % incidence of dysfunction ≥ moderate | 0.1* | 0.3* | 0.0 | 8.3* | 13.0* | 0.0 |
P < 0.001.
AVVR: atrioventricular valve regurgitation; LV: left ventricle; RV: right ventricle.
| Variables . | AVVR < moderate . | AVVR ≥ moderate . | ||||
|---|---|---|---|---|---|---|
| Total . | RV . | LV . | Total . | RV . | LV . | |
| Total period | ||||||
| Dysfunction < moderate | 3975 | 1925 | 2050 | 193 | 125 | 68 |
| Dysfunction ≥ moderate | 26 | 14 | 12 | 17 | 17 | 0 |
| % incidence of dysfunction ≥ moderate | 0.6* | 0.7* | 0.6 | 8.1* | 12.0* | 0.0 |
| <5 years | ||||||
| Dysfunction < moderate | 2411 | 1264 | 1147 | 125 | 81 | 44 |
| Dysfunction ≥ moderate | 17 | 7 | 10 | 9 | 9 | 0 |
| % incidence of dysfunction ≥ moderate | 0.7* | 0.6* | 0.9 | 6.7* | 10.0* | 0.0 |
| 5–10 years | ||||||
| Dysfunction < moderate | 716 | 347 | 369 | 35 | 24 | 11 |
| Dysfunction ≥ moderate | 8 | 6 | 2 | 5 | 5 | 0 |
| % incidence of dysfunction ≥ moderate | 1.1* | 1.7* | 0.5 | 12.5* | 17.2* | 0.0 |
| >10 years | ||||||
| Dysfunction < moderate | 848 | 314 | 534 | 33 | 20 | 13 |
| Dysfunction ≥ moderate | 1 | 1 | 0 | 3 | 3 | 0 |
| % incidence of dysfunction ≥ moderate | 0.1* | 0.3* | 0.0 | 8.3* | 13.0* | 0.0 |
| Variables . | AVVR < moderate . | AVVR ≥ moderate . | ||||
|---|---|---|---|---|---|---|
| Total . | RV . | LV . | Total . | RV . | LV . | |
| Total period | ||||||
| Dysfunction < moderate | 3975 | 1925 | 2050 | 193 | 125 | 68 |
| Dysfunction ≥ moderate | 26 | 14 | 12 | 17 | 17 | 0 |
| % incidence of dysfunction ≥ moderate | 0.6* | 0.7* | 0.6 | 8.1* | 12.0* | 0.0 |
| <5 years | ||||||
| Dysfunction < moderate | 2411 | 1264 | 1147 | 125 | 81 | 44 |
| Dysfunction ≥ moderate | 17 | 7 | 10 | 9 | 9 | 0 |
| % incidence of dysfunction ≥ moderate | 0.7* | 0.6* | 0.9 | 6.7* | 10.0* | 0.0 |
| 5–10 years | ||||||
| Dysfunction < moderate | 716 | 347 | 369 | 35 | 24 | 11 |
| Dysfunction ≥ moderate | 8 | 6 | 2 | 5 | 5 | 0 |
| % incidence of dysfunction ≥ moderate | 1.1* | 1.7* | 0.5 | 12.5* | 17.2* | 0.0 |
| >10 years | ||||||
| Dysfunction < moderate | 848 | 314 | 534 | 33 | 20 | 13 |
| Dysfunction ≥ moderate | 1 | 1 | 0 | 3 | 3 | 0 |
| % incidence of dysfunction ≥ moderate | 0.1* | 0.3* | 0.0 | 8.3* | 13.0* | 0.0 |
P < 0.001.
AVVR: atrioventricular valve regurgitation; LV: left ventricle; RV: right ventricle.
DISCUSSION
The present study evaluated the long-term serial change of systemic ventricular function and AVV regurgitation in patients after the TCPC at our center over 25 years of follow-up. Although transplant-free survival was similar between patients with dominant RV and dominant LV, late systemic ventricular function decreased 10 years after TCPC in patients with dominant RV, compared with those with dominant LV. AVV function was inferior in patients with dominant RV, compared with those with dominant LV starting 3 months after the TCPC.
Survival after total cavopulmonary connection
The early mortality after the Fontan procedure has dramatically improved with early staged surgical palliations as well as advances in perioperative and intraoperative management. Mortality after the Fontan procedure has been reported as low as <2% in modern series [16, 17]. In-hospital mortality in our study was 1.2%. Most of these in-hospital deaths occurred in the early era, and we have had no in-hospital mortality during the past 6 years. As early mortality after Fontan appears to be a historical challenge, attention is now being directed towards the late outcomes. As for long-term transplant-free survival, several recent investigators have reported long-term transplant-free survival rates of 90–95% at 10 years and 61–90% at 20 years [4, 16–18]. Our transplant-free survival rates at 10 and 20 years were 94% and 90%, respectively, which were in the upper ranges reported in the aforementioned studies. As for the ventricle morphology, current studies demonstrated no transplant-free survival difference with right or left dominant ventricle after the Fontan procedure [3–7], and our results are consistent with this observation.
Impact of ventricular morphology on late ventricular function and atrioventricular valve regurgitation
However, it is quite questionable whether long-term ventricular function and AVV function is comparable in patients with dominant RV compared to those with dominant LV, because morphological appearances were quite different in dominant RV and dominant LV. The impact of single-ventricle morphology on the late outcomes after Fontan has been controversial, with short- and mid-term studies demonstrating no effect [5, 6], whereas a recent longer-term study demonstrated worse outcomes with dominant RV [13, 19]. Giardini et al. [20] demonstrated that percentage of predicted peak oxygen uptake with cardiopulmonary exercise tests was significantly inferior in patients with dominant RV, compared to those with dominant LV, and the decline of exercise capacity seems to be slower in patients with dominant LV than those with dominant RV. Our results demonstrated that the systemic ventricular function was comparable until 10 years postoperatively between the dominant RV and dominant LV. However, over 10 years postoperatively, an inferior ventricular function was observed in dominant RV, compared with dominant LV. Given the potential development and anatomic differences as well as the pressure-overloaded state that make the dominant RV prone to failure, we hypothesized that dysfunction of the dominant RV increases over time and adversely affects the clinical outcomes of Fontan. Our analysis demonstrated an inferior late ventricle function in patients with a dominant RV as compared with those with dominant LV, and we found a significant correlation between ventricle function and AVV function in patients with dominant RV.
Atrioventricular valve regurgitation
Regurgitation of the systemic tricuspid valve is relatively common in patients with HLHS [21], and it has also been attributed to worsening RV function [22]. However, recent studies have also indicated that these valves may have intrinsic anatomic abnormalities predisposing them to failure [11, 12]. Our results demonstrated a significant difference in AVV function after the TCPC between the dominant RV and LV in the late postoperative period and even in the early postoperative period, although in most of the patients AVV regurgitation remains mild or less than mild. To address this controversy, we ascertained the temporal relationship between AVV regurgitation and ventricular dysfunction to provide insight into possible causative relationships. We found that AVV regurgitation and ventricular dysfunction progressively worsened with time, and were significantly worse in patients with a dominant RV. Moon et al. [13] demonstrated that systemic ventricle function tends to be preserved for a substantial amount of time in the presence of AVV regurgitation. However, our assessment of systemic ventricle function in the presence of AVV regurgitation might underestimate the degree of ventricular dysfunction, as it has been well described in the estimation of LV function in the presence of mitral regurgitation [23]. They speculated that the development of AVV regurgitation in dominant RV might precede ventricular dysfunction, indicating that not only the annular dilatation but also multiple additional factors might affect AVV function, such as pre-existing anatomic abnormalities of valve morphology, abnormal ventricular septum or mechanics due to a diminutive LV. Indeed, tricuspid valve abnormalities have been identified in patients with HLHS in previous studies [11, 12]. Therefore, regurgitation of AVV in patients with dominant RV might be progressive in the long-term after the Fontan procedure and might cause the systemic ventricular dysfunction. In this study, about half of the patients with dominant RV had the diagnosis of HLHS. RV in the setting of an unbalanced AV valve has long been known to be a bad Fontan [24]. We found that the results after TCPC in patients with HLHS were not much worse than other patients with dominant RV. The function of systemic tricuspid vale was relatively stable in the mid-term. Careful observation for systemic ventricular function and AVV regurgitation are mandatory especially in patients with dominant RV. As for the analysis regarding the influence of preoperative variables, including preoperative AVV regurgitation, to be a contributing factor for systemic ventricular dysfunction, further analysis will be needed in future.
Limitations
This study was limited by its retrospective and single-centre design. Because the definitions for indeterminate ventricle are not standardized, we assumed RV morphology in these few patients. In patients with ccTGA and unbalanced atrioventricular septal defect, the larger ventricle was determined to be the dominant ventricle, even though these are not single ventricles by definition. Although the evaluation of the systemic ventricular function was performed by experienced paediatric cardiologist, there still remain valid questions raised about the methodology of portraying RV dysfunction and its impact on the analysis presented. Missing data are likely linked to the outcome and could really introduce bias. The second end point of survival after TCPC was added post hoc, which is certainly also a limitation of this study. Surgical and medical management may have changed during the study period, probably influencing the long-term outcomes. Not all patients with a single ventricle have achieved Fontan completion. Given the incidence of inter-stage death and failing Glenn physiology, there might be a selection bias for this cohort.
CONCLUSIONS
There was no statistical difference observed in ventricular function between dominant RV type and dominant LV type for the first 10 years postoperatively. Thereafter, ventricular function in dominant RV type was inferior to that in dominant LV type. The degree of AVV regurgitation gradually increased after TCPC over time in both dominant RV type and dominant LV type. However, the degree of regurgitation was significantly higher in dominant RV type, compared with dominant LV type, which might precede the development of ventricular dysfunction in dominant RV.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Funding
This study was not supported by any grants.
Conflict of interest: none declared.
DATA AVAILABILITY
The data underlying this article will be shared by the corresponding author on reasonable request.
Author contributions
Vincent Dahmen: Data curation; Writing—original draft. Paul Philipp Heinisch: Writing—review & editing. Helena Staehler: Data curation; Investigation; Visualization; Writing—review & editing. Thibault Schaeffer: Writing—review & editing. Melchior Burri: Writing—review & editing. Christoph Röhlig: Writing—review & editing. Frank Klawonn: Formal analysis; Supervision; Validation; Visualization. Alfred Hager: Conceptualization; Supervision; Validation; Writing—review & editing. Peter Ewert: Conceptualization; Supervision; Validation; Writing—review & editing. Jürgen Hörer: Conceptualization; Project administration; Supervision; Validation; Writing—review & editing. Masamichi Ono: Conceptualization; Data curation; Investigation; Project administration; Supervision; Visualization; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Ujjwal K. Chowdhury, Pedro J. Del Nido, Yves d'Udekem and the other anonymous reviewer(s) for their contribution to the peer review process of this article.
Presented at the 36th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Milan, Italy, 5–8 October 2022.
REFERENCES
ABBREVIATIONS
- AVV
Atrioventricular valve
- ccTGA
Congenitally corrected transposition of the great arteries
- EF
Ejection fraction
- HLHS
Hypoplastic left heart syndrome
- IQR
Interquartile ranges
- LV
Left ventricle
- RV
Right ventricle
- TCPC
Total cavopulmonary connection





