-
PDF
- Split View
-
Views
-
Cite
Cite
David Reineke, Vladimir Makaloski, Florian Schoenhoff, Matthias Siepe, Treatment of infected hybrid arch prosthesis with self-assembled bovine elephant trunk grafts, European Journal of Cardio-Thoracic Surgery, Volume 63, Issue 2, February 2023, ezad025, https://doi.org/10.1093/ejcts/ezad025
Close - Share Icon Share
Abstract
Graft infections are associated with severe morbidity and mortality. The widespread use of the frozen elephant technique increases the incidence of complex aortic patients to suffer from graft infections. Surgery of these patients is challenging. Removal of the stent graft portion of the frozen elephant technique prosthesis via sternotomy carries the risk of irreparable damage to the descending aorta. There is currently no single-stage surgical strategy that allows for the removal of all infected material apart from a hemi-clamshell approach. This approach is technically demanding and associated with significant morbidity and mortality. This results in conservative treatment in a substantial number of patients. Pericardial tube grafts have shown to be an excellent option in treating graft infections in various aortic segments with promising results concerning freedom of re-infection and survival. We report a single-stage, trans-sternal approach to remove all infected materials and simultaneously treat the descending aorta to prevent aortic catastrophe in 2 consecutive cases.
CASE 1
A 56-year-old male patient with type A dissection underwent aortic root replacement with a mechanical conduit and total arch repair using the frozen elephant technique (FET). Recovery was uneventful.
One year later, the patient presented himself in our emergency department and was diagnosed with an endocarditis (Streptococcus oralis) and a PET-positive infection (Fig. 1A) of his prostheses. Echocardiography revealed vegetations on the aortic valve prosthesis. CT scanning showed abscess formation around the graft.
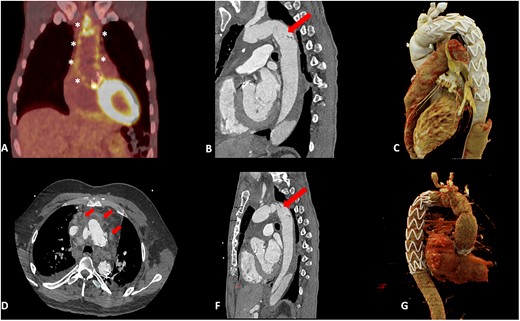
(A) PET-positive result along the aortic prostheses (*). (B and F) CT showing intimal damage of the descending aorta (arrow). (C and G) CT scan post TEVAR. (D) CT showing fluid collection (arrow).
The patient was re-operated 7 days after the start of antibiotic treatment. Both the composite graft and the FET prosthesis were replaced with an intraoperatively constructed valved conduit and a conventional elephant trunk made of bovine pericardium (Fig. 2D). The removal of the stent graft caused considerable intimal damage, which was confirmed in the first postoperative CT (Fig. 1B).
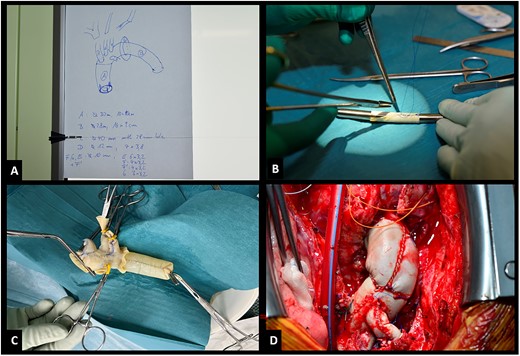
(A) Blueprint of bovine prosthesis. (B) Construction of side branch. (C) Pressurized prosthesis. (D) Implanted prosthesis.
Eight weeks later the patient received a TEVAR (Cook ZTA, 32–28 mm) (Fig. 1C) with a favourable post-interventional CT scan. At 4 months, the patient is free from infection without antibiotic treatment.
CASE 2
A 39-year-old male patient presented with mediastinitis 2 years after receiving a mechanical conduit and FET following type A dissection. Blood cultures were positive for Staphylococcus aureus. The CT scan showed fluid collections and possible abscess formation around the graft (Fig. 1D). Transoesophageal echocardiography showed no signs of vegetations, but cMRI demonstrated multiple emboli.
The patient was re-operated. The root and the FET were replaced as described in the first case with an intraoperatively constructed valved conduit and conventional elephant trunk. The removal of the stent graft again caused intimal damage in the proximal descending aorta, necessitating TEVAR 1 week later (Medtronic Valiant Thoracic, 28 mm) (Fig. 1F and G). The patient could be discharged a week after the intervention on the descending aorta. At 6 months, there are no signs of infections and antibiotic treatment has been discontinued.
TECHNICAL NOTE
The size of the constructed bovine graft should orientate itself on previously implanted prosthesis dimensions. Mechanical valves were replaced with biological valves due to the morbidity of this operation. The operation should be performed in a two-team setting: 1 for constructing the bovine graft (Supple-Peri-Guard®) according to an exact blueprint (Fig. 2A) and a second for re-opening the chest. For sizing the prosthesis 2 options exist: using Hegar dilators (Fig. 2B) or calculating dimensions by multiplicating the desired diameter by π. A 28-mm graft needs a 9-cm patch multiplicated by the length. The composite graft is constructed separately and should be 3 mm larger than the valve. The graft should be pressurized to check for leaks (Fig. 2C). Suture lines must face cranial (Fig. 2D) so that remaining leaks can be easily repaired and do not interfere with the placement of the coronary buttons.
DISCUSSION
Apart from a short technical guide, our cases should convey 2 messages:
First message: While the use of pericardial tubes has been well documented in the ascending and downstream aorta, a combined treatment of infected graft material in the root and arch with a self-assembled branched prosthesis has not been shown before and proved successful in these 2 cases [1–3].
Second message: Only during the procedure itself, we discovered the extensive intimal damage caused by removing the prosthesis. Therefore, it is of paramount importance to not only replace the complete FET prosthesis for infection control but also prepare an adequate landing zone with a conventional elephant trunk. Timing of TEVAR should take into account the lesion morphology in the descending aorta and the risk of ongoing bacteraemia with subsequent re-infection. In case 2, the lesion in the descending aorta originating from the FET-stent part removal was at high risk of rupture. In this case, TEVAR was already done 1 week after arch repair with negative blood cultures and on effective antibiotic treatment. In the other case, the lesion was less severe and we waited until antibiotic treatment was terminated.
The operation should be performed in a hybrid room to have all necessary options at hand in case of substantial damage in the descending aorta that necessitates immediate realigning. The team and patient should be prepared to proceed to a left-sided hemi-clamshell for additional open replacement of the descending aorta using a bovine extension of the elephant trunk part of the arch graft.
ACKNOWLEDGEMENTS
We thank Christoph Gräni, MD, for preparing images.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Tim Berger and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.




