-
PDF
- Split View
-
Views
-
Cite
Cite
Wen Zhang, Renjie Hu, Qi Jiang, Hongbin Zhu, Lisheng Qiu, Wei Dong, Haibo Zhang, Mitral intervention for anomalous left coronary artery from the pulmonary artery: midterm outcomes, European Journal of Cardio-Thoracic Surgery, Volume 63, Issue 2, February 2023, ezac521, https://doi.org/10.1093/ejcts/ezac521
Close - Share Icon Share
Abstract
Anomalous left coronary artery from the pulmonary artery (ALCAPA) is frequently associated with significant mitral regurgitation (MR). We aim to identify surgical outcomes in patients with or without concomitant mitral intervention.
All patients with ALCAPA who presented with >mild degree of MR at our institution between January 2008 and June 2020 were included in the retrospective study. MR recovery was defined as ≤mild MR at the last follow-up.
The study cohort included 101 patients. The median age at repair was 7.6 months. The concomitant mitral intervention was performed in 66 patients (65%). MR grade significantly improved at the last follow-up. The cumulative incidence of MR recovery 3 years after ALCAPA repair was 34% [95% confidence interval (CI), 19–50%) in patients with mitral intervention, compared to 59% (95% CI, 41–73%) in patients without mitral intervention (P = 0.050). MR grade on postoperative day 1 was the predictor for MR recovery in patients with mitral intervention (hazard ratio, 0.080; 95% CI, 0.018–0.366; P = 0.001), whereas preoperative mitral annulus diameter z-score was the predictor in patients without mitral intervention (hazard ratio, 0.480; 95% CI, 0.232–0.993; P = 0.048). Freedom from mitral reoperation in patients with mitral intervention was 94% and 88% at 3 and 5 years after surgery, while freedom from mitral reoperation in patients without mitral intervention was 100% at both timepoints (P = 0.177).
Despite significant MR improvement after ALCAPA repair, MR grade may not always return to normal regardless of the initial mitral management strategy, and reoperation for persistent MR is not rare.
INTRODUCTION
Anomalous left coronary artery from the pulmonary artery (ALCAPA) is a rare congenital anomaly, occurring in 1 in 300 000 live births. Mitral regurgitation (MR), secondary to mitral valve annulus (MVA) dilatation or papillary muscle ischaemia, is commonly found in patients with ALCAPA [1, 2].
While the coronary reimplantation technique to rebuild the dual-coronary supply has become the method of choice in the current era, there are conflicting data with regard to the initial management of MR [3–6]. Some believed that postoperative MR would improve along with the recovery of left ventricular function; thus, no concomitant intervention was needed [4]. Other suggested mitral interventions should be considered in patients with moderate or severe MR [5]. However, regardless of the strategy, persistent MR after ALCAPA repair is not uncommon [7]. Therefore, we sought to review the management of MR in patients with ALCAPA at our institution and evaluate its postoperative recovery course.
PATIENTS AND METHODS
Ethics statement
This study was approved by the Institutional Review Board of Shanghai Children’s Medical Center (SCMCIRB-W2021067, 7 December 2021). The need for individual consent was waived due to the retrospective nature of the collected data and review.
Patients
Between January 2008 and June 2020, 143 patients underwent coronary reimplantation technique for ALCAPA repair at Shanghai Children’s Medical Center. Among them, 101 patients (71%) presented with >mild degree of MR and were thus included in our retrospective analysis.
Definitions
Early mortality was defined as death occurring within 30 days after surgery. Severity of the MR was graded as none/trivial (0), mild (1), mild-moderate (1.5), moderate (2), moderate–severe (2.5) and severe (3) based on vena contracta width as well as jet area [8]. Postoperative MR recovery was defined as ≤mild degree of MR at the last follow-up. In these patients, all echocardiographic reports since ALCAPA repair were reviewed to determine the onset of MR recovery.
Surgical technique
The coronary reimplantation technique has been described previously [2]. Briefly, the orifice of the left coronary artery (LCA) was harvested as button or flap from the pulmonary artery and then anastomosed to the LCA orifice, by using direct reimplantation, flap-to-flap or tubular extension technique. The decision on concomitant mitral intervention was made according to preoperative MR grade and surgeons’ preferences (Video 1).
Intraoperative assessment of the mitral valve in one patient with ALCAPA.
Follow-up
Data were collected retrospectively from hospital records and outpatient clinics. A total of 883 postoperative echocardiograms were available for 97 patients (96% of the population). Follow-ups were scheduled 1, 3 and 6 months after discharge, then every 6 months for the next 18 months and then every year. The median follow-up time was 2.8 years [95% confidence interval (CI), 1.9–3.6 years]. Follow-up echocardiograms after discharge were available in 88 patients (87%). Nineteen percentage of the living patients were followed up for >6 years. The number of patients with postoperative echocardiograms at monthly intervals within 6 years after surgery is shown in Supplementary Material, Fig. S1.
Statistical analysis
Data were analysed using SPSS software version 22.0 (IBM-SPSS Inc, Armonk, NY, USA) unless noted. Continuous variables were summarized using mean ± standard deviation or median [interquartile range (IQR)] for skewness variables. Shapiro–Wilk test was used to assess the normality of the data. Categorical variables were summarized as frequency and percentage. Comparison between 2 groups was performed by Student’s t-test for normally distributed variables and by Wilcoxon rank-sum test for non-normally distributed variables. Categorical variables were compared using chi-square test, or Fisher’s exact test when sample sizes were <40, or if >20% of expected cell counts were <5. The reverse Kaplan–Meier method was used to estimate the median follow-up time. Competing risk analysis was used to estimate the cumulative incidence of MR recovery after ALCAPAC repair, with follow-up death or reoperation defined as competing risks, and was performed in R version 4.0.2 software (R Foundation, Vienna, Austria). Predictors for postoperative MR recovery were analysed by cox proportional hazard analysis. Variables whose P-value was <0.2 in the univariable analysis were included in the multivariable analysis. Freedom from reoperation was evaluated as time-to-event end points and analysed using Kaplan–Meier curve with a log-rank test. Patients without echocardiographic follow-up after discharge of the index operation were excluded from the analysis. P-values of <0.05 were considered statistically significant.
RESULTS
Patient characteristics
The median age at repair was 7.6 months (IQR, 4.2–20.1 months). The mean preoperative left ventricular ejection fraction (LVEF) was 50 ± 16%. Twenty-three patients (23%) presented with LVEF <35%. The mean preoperative MVA diameter was 2.0 ± 0.4 cm, and the mean MVA diameter z-score was +1.6 ± 1.0. Severe MR was found in 11 patients (11%). The median cardiopulmonary bypass time and the median aortic cross-clamping time were 118 min (IQR, 98–145 min) and 74 min (IQR, 62–87 min), respectively.
Concomitant mitral intervention
Sixty-six patients (65%) underwent concomitant mitral intervention (Table 1). The mitral valve pathology found intraoperatively in these patients was various and combined: MVA dilatation was observed in 34 patients (52%), leaflet prolapse in 18 patients (27%), ischaemia changes of the papillary muscle in 8 patients (12%), tethered cords of the posterior leaflet in 6 patients (9%) and cleft mitral valve in 4 patients (6%). The most common mitral intervention technique performed was posterior annuloplasty [32 patients (48%)], followed by commissure plication annuloplasty [27 patients (41%)] (Supplementary Material, Table S1).
| . | With mitral intervention . | Without mitral intervention . | P-Value . |
|---|---|---|---|
| N, n (%) | 66 (65) | 35 (35) | |
| Age at repair (months), median (IQR) | 7.8 (4.2–22.8) | 7.4 (3.6–11.8) | 0.229 |
| Weight at repair (kg), median (IQR) | 7.0 (5.7–10.8) | 6.5 (5.5–8.5) | 0.253 |
| Female, n (%) | 39 (59) | 20 (57) | 0.850 |
| Preoperative LVEF (%), mean ± SD | 54.6 ± 14.4 | 41.6 ± 15.0 | <0.001 |
| MR grade, n (%) | <0.001 | ||
| Mild–moderate | 9 (14) | 18 (51) | |
| Moderate | 28 (42) | 15 (43) | |
| Moderate–severe | 19 (29) | 1 (3) | |
| Severe | 10 (15) | 1 (3) | |
| Preoperative LVEDD, mean ± SD | 4.1 ± 0.7 | 4.0 ± 0.5 | 0.460 |
| Preoperative LVEDD z-score, mean ± SD | +4.7 ± 1.8 | +5.0 ± 2.1 | 0.483 |
| Preoperative MVA diameter, mean ± SD | 2.0 ± 0.4 | 1.9 ± 0.4 | 0.080 |
| Preoperative MVA diameter z-score, mean ± SD | +1.7 ± 1.1 | +1.3 ± 0.9 | 0.125 |
| . | With mitral intervention . | Without mitral intervention . | P-Value . |
|---|---|---|---|
| N, n (%) | 66 (65) | 35 (35) | |
| Age at repair (months), median (IQR) | 7.8 (4.2–22.8) | 7.4 (3.6–11.8) | 0.229 |
| Weight at repair (kg), median (IQR) | 7.0 (5.7–10.8) | 6.5 (5.5–8.5) | 0.253 |
| Female, n (%) | 39 (59) | 20 (57) | 0.850 |
| Preoperative LVEF (%), mean ± SD | 54.6 ± 14.4 | 41.6 ± 15.0 | <0.001 |
| MR grade, n (%) | <0.001 | ||
| Mild–moderate | 9 (14) | 18 (51) | |
| Moderate | 28 (42) | 15 (43) | |
| Moderate–severe | 19 (29) | 1 (3) | |
| Severe | 10 (15) | 1 (3) | |
| Preoperative LVEDD, mean ± SD | 4.1 ± 0.7 | 4.0 ± 0.5 | 0.460 |
| Preoperative LVEDD z-score, mean ± SD | +4.7 ± 1.8 | +5.0 ± 2.1 | 0.483 |
| Preoperative MVA diameter, mean ± SD | 2.0 ± 0.4 | 1.9 ± 0.4 | 0.080 |
| Preoperative MVA diameter z-score, mean ± SD | +1.7 ± 1.1 | +1.3 ± 0.9 | 0.125 |
IQR: interquartile range; LVEDD: left ventricular end-diastolic dimension; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; MVA: mitral valve annulus; SD: standard deviation.
| . | With mitral intervention . | Without mitral intervention . | P-Value . |
|---|---|---|---|
| N, n (%) | 66 (65) | 35 (35) | |
| Age at repair (months), median (IQR) | 7.8 (4.2–22.8) | 7.4 (3.6–11.8) | 0.229 |
| Weight at repair (kg), median (IQR) | 7.0 (5.7–10.8) | 6.5 (5.5–8.5) | 0.253 |
| Female, n (%) | 39 (59) | 20 (57) | 0.850 |
| Preoperative LVEF (%), mean ± SD | 54.6 ± 14.4 | 41.6 ± 15.0 | <0.001 |
| MR grade, n (%) | <0.001 | ||
| Mild–moderate | 9 (14) | 18 (51) | |
| Moderate | 28 (42) | 15 (43) | |
| Moderate–severe | 19 (29) | 1 (3) | |
| Severe | 10 (15) | 1 (3) | |
| Preoperative LVEDD, mean ± SD | 4.1 ± 0.7 | 4.0 ± 0.5 | 0.460 |
| Preoperative LVEDD z-score, mean ± SD | +4.7 ± 1.8 | +5.0 ± 2.1 | 0.483 |
| Preoperative MVA diameter, mean ± SD | 2.0 ± 0.4 | 1.9 ± 0.4 | 0.080 |
| Preoperative MVA diameter z-score, mean ± SD | +1.7 ± 1.1 | +1.3 ± 0.9 | 0.125 |
| . | With mitral intervention . | Without mitral intervention . | P-Value . |
|---|---|---|---|
| N, n (%) | 66 (65) | 35 (35) | |
| Age at repair (months), median (IQR) | 7.8 (4.2–22.8) | 7.4 (3.6–11.8) | 0.229 |
| Weight at repair (kg), median (IQR) | 7.0 (5.7–10.8) | 6.5 (5.5–8.5) | 0.253 |
| Female, n (%) | 39 (59) | 20 (57) | 0.850 |
| Preoperative LVEF (%), mean ± SD | 54.6 ± 14.4 | 41.6 ± 15.0 | <0.001 |
| MR grade, n (%) | <0.001 | ||
| Mild–moderate | 9 (14) | 18 (51) | |
| Moderate | 28 (42) | 15 (43) | |
| Moderate–severe | 19 (29) | 1 (3) | |
| Severe | 10 (15) | 1 (3) | |
| Preoperative LVEDD, mean ± SD | 4.1 ± 0.7 | 4.0 ± 0.5 | 0.460 |
| Preoperative LVEDD z-score, mean ± SD | +4.7 ± 1.8 | +5.0 ± 2.1 | 0.483 |
| Preoperative MVA diameter, mean ± SD | 2.0 ± 0.4 | 1.9 ± 0.4 | 0.080 |
| Preoperative MVA diameter z-score, mean ± SD | +1.7 ± 1.1 | +1.3 ± 0.9 | 0.125 |
IQR: interquartile range; LVEDD: left ventricular end-diastolic dimension; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; MVA: mitral valve annulus; SD: standard deviation.
There were no significant differences in age and weight at repair between patients with and without concomitant mitral intervention. Sixty-one percentage of the infants received mitral intervention compared to 75% in patients beyond infancy (P = 0.165) (Fig. 1). Preoperative LVEF was higher in patients who underwent concomitant mitral intervention (55 ± 14% vs 42 ± 15%, P < 0.001), while preoperative left ventricular end-diastolic dimension and MVA diameter did not show significant difference. Preoperative MR grade was more severe in patients with concomitant mitral intervention than patients without intervention (P < 0.001) (Fig. 2). Specifically, in patients with concomitant mitral intervention, 10 (15%) presented with severe MR before surgery, whereas in patients without mitral intervention only 1 patient (3%) presented with severe MR (Table 1).
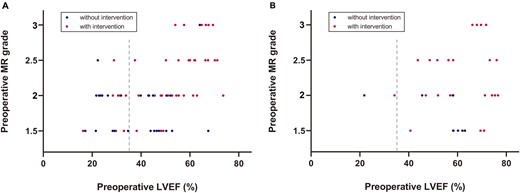
Distribution of patients with or without mitral intervention, stratified by (A) infant or (B) children.
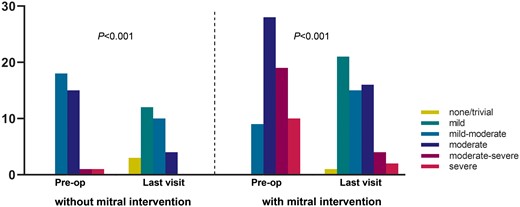
Grade of mitral regurgitation in patients with and without concomitant mitral intervention preoperatively and at the last visit.
Early outcomes
Patients who had concomitant mitral intervention required longer time for cardiopulmonary bypass [124 min (IQR, 102–152 min) vs 112 min (IQR, 89–134 min), P = 0.009] and aortic cross-clamping [78 min (IQR, 67–90 min) vs 62 min (IQR, 52–74 min), P < 0.001] than patients who did not. Assessment of the MR grade on postoperative day 1 by echocardiography was available in 98 patients (97%). Twenty-eight of 65 patients (43%) with concomitant mitral intervention had ≤mild MR, whereas 10 of 33 (30%) without mitral intervention had ≤mild MR. Duration of mechanical ventilation and intensive care unit stay after surgery tended to be shorter in patients with mitral intervention, although no significant differences were achieved. The need for mechanical circulatory support was similar between the 2 groups of patients, as well as early mortality (Table 2).
| . | With mitral intervention . | Without mitral intervention . | P-Value . |
|---|---|---|---|
| Cardiopulmonary bypass time (min), median (IQR) | 124 (102–152) | 112 (89–134) | 0.009 |
| Aortic cross-clamping time (min), median (IQR) | 78 (67–90) | 62 (52–74) | <0.001 |
| MR grade on postoperative day 1, n (%) | 0.589 | ||
| ≤Mild | 28 (43) | 10 (30) | |
| Mild–moderate | 21 (32) | 13 (40) | |
| Moderate | 14 (22) | 8 (24) | |
| Moderate–severe | 2 (3) | 2 (6) | |
| Mechanical circulatory support, n (%) | 7 (11) | 6 (17) | 0.534 |
| Intubation time (h), median (IQR) | 94 (48–156) | 120 (48–206) | 0.392 |
| Intensive care unit stay (days), median (IQR) | 7 (5–12) | 10 (7–16) | 0.106 |
| Early mortality, n (%) | 5 (8) | 5 (14) | 0.469 |
| . | With mitral intervention . | Without mitral intervention . | P-Value . |
|---|---|---|---|
| Cardiopulmonary bypass time (min), median (IQR) | 124 (102–152) | 112 (89–134) | 0.009 |
| Aortic cross-clamping time (min), median (IQR) | 78 (67–90) | 62 (52–74) | <0.001 |
| MR grade on postoperative day 1, n (%) | 0.589 | ||
| ≤Mild | 28 (43) | 10 (30) | |
| Mild–moderate | 21 (32) | 13 (40) | |
| Moderate | 14 (22) | 8 (24) | |
| Moderate–severe | 2 (3) | 2 (6) | |
| Mechanical circulatory support, n (%) | 7 (11) | 6 (17) | 0.534 |
| Intubation time (h), median (IQR) | 94 (48–156) | 120 (48–206) | 0.392 |
| Intensive care unit stay (days), median (IQR) | 7 (5–12) | 10 (7–16) | 0.106 |
| Early mortality, n (%) | 5 (8) | 5 (14) | 0.469 |
IQR: interquartile range; MR: mitral regurgitation.
| . | With mitral intervention . | Without mitral intervention . | P-Value . |
|---|---|---|---|
| Cardiopulmonary bypass time (min), median (IQR) | 124 (102–152) | 112 (89–134) | 0.009 |
| Aortic cross-clamping time (min), median (IQR) | 78 (67–90) | 62 (52–74) | <0.001 |
| MR grade on postoperative day 1, n (%) | 0.589 | ||
| ≤Mild | 28 (43) | 10 (30) | |
| Mild–moderate | 21 (32) | 13 (40) | |
| Moderate | 14 (22) | 8 (24) | |
| Moderate–severe | 2 (3) | 2 (6) | |
| Mechanical circulatory support, n (%) | 7 (11) | 6 (17) | 0.534 |
| Intubation time (h), median (IQR) | 94 (48–156) | 120 (48–206) | 0.392 |
| Intensive care unit stay (days), median (IQR) | 7 (5–12) | 10 (7–16) | 0.106 |
| Early mortality, n (%) | 5 (8) | 5 (14) | 0.469 |
| . | With mitral intervention . | Without mitral intervention . | P-Value . |
|---|---|---|---|
| Cardiopulmonary bypass time (min), median (IQR) | 124 (102–152) | 112 (89–134) | 0.009 |
| Aortic cross-clamping time (min), median (IQR) | 78 (67–90) | 62 (52–74) | <0.001 |
| MR grade on postoperative day 1, n (%) | 0.589 | ||
| ≤Mild | 28 (43) | 10 (30) | |
| Mild–moderate | 21 (32) | 13 (40) | |
| Moderate | 14 (22) | 8 (24) | |
| Moderate–severe | 2 (3) | 2 (6) | |
| Mechanical circulatory support, n (%) | 7 (11) | 6 (17) | 0.534 |
| Intubation time (h), median (IQR) | 94 (48–156) | 120 (48–206) | 0.392 |
| Intensive care unit stay (days), median (IQR) | 7 (5–12) | 10 (7–16) | 0.106 |
| Early mortality, n (%) | 5 (8) | 5 (14) | 0.469 |
IQR: interquartile range; MR: mitral regurgitation.
Patients with severe MR
Of the 10 patients presenting with severe MR before surgery who underwent concomitant mitral intervention, MR grade at the last follow-up was severe in 2 patients, moderate–severe in 1 patient, moderate in 5 patients, mild–moderate in 1 patient and mild in 1 patient. The follow-up of the only patient presenting with severe MR but without concomitant mitral intervention was lost. Three patients required mitral reoperations as stated below.
Patients with severe left ventricular dysfunction
Of the 23 patients with preoperative LVEF <35%, concomitant mitral intervention was performed in 9 patients (39%). There were no significant differences in preoperative characteristics between patients with and without mitral intervention, despite that LVEF tended to be lower in patients without mitral intervention (P = 0.109) (Supplementary Material, Table S2). MR recovery was achieved on postoperative day 1 in 63% of patients with concomitant mitral intervention, compared to 15% in patients without mitral intervention (P = 0.059). At the last follow-up, MR recovery was achieved in 5 (83%) of those with concomitant mitral intervention, compared to 6 (55%) of those without mitral intervention (P = 0.333). No reoperations were performed in both groups.
Left coronary artery patency
LCA was patent in all patients after surgery as detected by echocardiography except one, which was later confirmed by cardiac catheterization as severe LCA stenosis. This patient presented with persistent moderate MR despite normal left ventricular function during the follow-up period.
Mitral regurgitation recovery
Regardless of the mitral intervention strategy used, MR grade at the last visit improved significantly in both groups compared to the preoperative grade (P < 0.001) (Fig. 2). The cumulative incidence of MR recovery 3 years after ALCAPA repair was 59% (95% CI, 41–73%) in patients without mitral intervention, compared to 34% (95% CI, 19–50%) in patients with mitral intervention (P = 0.050) (Fig. 3). Predictors for postoperative MR recovery in the 2 groups are displayed in Tables 3 and 4, respectively. Of note, MR grade on postoperative day 1 was the only independent predictor for MR recovery in patients with mitral intervention (hazard ratio, 0.080; 95% CI, 0.018–0.366; P = 0.001), whereas preoperative MVA diameter z-score was the only independent predictor in patients without mitral intervention (hazard ratio, 0.480; 95% CI, 0.232–0.993; P = 0.048). Using a receiver operating characteristic curve (Supplementary Material, Fig. S2), preoperative MVA diameter z-score had an area under the curve of 0.738 for predicting MR recovery in patients without concomitant mitral intervention. The cut point of +1.7 was 85% sensitive and 73% specific for the prediction.
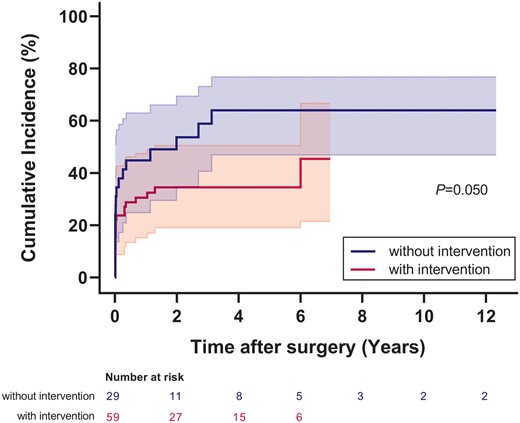
Cumulative incidence of postoperative mitral regurgitation recovery in patients with and without mitral intervention. The shaded area indicates 95% confidence limits.
Predictors of postoperative mitral regurgitation recovery in patients without mitral intervention
| Variables . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-Value . | HR (95% CI) . | P-Value . | |
| Age | 1.007 (0.990–1.025) | 0.408 | ||
| Weight | 1.039 (0.961–1.123) | 0.341 | ||
| Female | 1.573 (0.602–4.108) | 0.355 | ||
| Preoperative MR grade | 0.263 (0.037–1.875) | 0.183 | 0.413 (0.036–4.721) | 0.477 |
| Preoperative LVEF | 1.012 (0.978–1.048) | 0.476 | ||
| Preoperative LVEDD z-score | 0.809 (0.638–1.025) | 0.079 | 1.062 (0.736–1.532) | 0.749 |
| Preoperative MVA diameter z-score | 0.474 (0.248–0.905) | 0.024 | 0.480 (0.232–0.993) | 0.048 |
| Cardiopulmonary bypass time | 0.993 (0.980–1.006) | 0.308 | ||
| Aortic cross-clamping time | 0.990 (0.969–1.012) | 0.375 | ||
| MR grade on postoperative day 1 | 0.490 (0.151–1.587) | 0.234 | ||
| Variables . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-Value . | HR (95% CI) . | P-Value . | |
| Age | 1.007 (0.990–1.025) | 0.408 | ||
| Weight | 1.039 (0.961–1.123) | 0.341 | ||
| Female | 1.573 (0.602–4.108) | 0.355 | ||
| Preoperative MR grade | 0.263 (0.037–1.875) | 0.183 | 0.413 (0.036–4.721) | 0.477 |
| Preoperative LVEF | 1.012 (0.978–1.048) | 0.476 | ||
| Preoperative LVEDD z-score | 0.809 (0.638–1.025) | 0.079 | 1.062 (0.736–1.532) | 0.749 |
| Preoperative MVA diameter z-score | 0.474 (0.248–0.905) | 0.024 | 0.480 (0.232–0.993) | 0.048 |
| Cardiopulmonary bypass time | 0.993 (0.980–1.006) | 0.308 | ||
| Aortic cross-clamping time | 0.990 (0.969–1.012) | 0.375 | ||
| MR grade on postoperative day 1 | 0.490 (0.151–1.587) | 0.234 | ||
CI: confidence interval; HR: hazard ratio; LVEDD: left ventricular end-diastolic dimension; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; MVA: mitral valve annulus.
Predictors of postoperative mitral regurgitation recovery in patients without mitral intervention
| Variables . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-Value . | HR (95% CI) . | P-Value . | |
| Age | 1.007 (0.990–1.025) | 0.408 | ||
| Weight | 1.039 (0.961–1.123) | 0.341 | ||
| Female | 1.573 (0.602–4.108) | 0.355 | ||
| Preoperative MR grade | 0.263 (0.037–1.875) | 0.183 | 0.413 (0.036–4.721) | 0.477 |
| Preoperative LVEF | 1.012 (0.978–1.048) | 0.476 | ||
| Preoperative LVEDD z-score | 0.809 (0.638–1.025) | 0.079 | 1.062 (0.736–1.532) | 0.749 |
| Preoperative MVA diameter z-score | 0.474 (0.248–0.905) | 0.024 | 0.480 (0.232–0.993) | 0.048 |
| Cardiopulmonary bypass time | 0.993 (0.980–1.006) | 0.308 | ||
| Aortic cross-clamping time | 0.990 (0.969–1.012) | 0.375 | ||
| MR grade on postoperative day 1 | 0.490 (0.151–1.587) | 0.234 | ||
| Variables . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-Value . | HR (95% CI) . | P-Value . | |
| Age | 1.007 (0.990–1.025) | 0.408 | ||
| Weight | 1.039 (0.961–1.123) | 0.341 | ||
| Female | 1.573 (0.602–4.108) | 0.355 | ||
| Preoperative MR grade | 0.263 (0.037–1.875) | 0.183 | 0.413 (0.036–4.721) | 0.477 |
| Preoperative LVEF | 1.012 (0.978–1.048) | 0.476 | ||
| Preoperative LVEDD z-score | 0.809 (0.638–1.025) | 0.079 | 1.062 (0.736–1.532) | 0.749 |
| Preoperative MVA diameter z-score | 0.474 (0.248–0.905) | 0.024 | 0.480 (0.232–0.993) | 0.048 |
| Cardiopulmonary bypass time | 0.993 (0.980–1.006) | 0.308 | ||
| Aortic cross-clamping time | 0.990 (0.969–1.012) | 0.375 | ||
| MR grade on postoperative day 1 | 0.490 (0.151–1.587) | 0.234 | ||
CI: confidence interval; HR: hazard ratio; LVEDD: left ventricular end-diastolic dimension; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; MVA: mitral valve annulus.
Predictors of postoperative MR recovery in patients with mitral intervention
| Variables . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-Value . | HR (95% CI) . | P-Value . | |
| Age | 0.997 (0.974–1.020) | 0.809 | ||
| Weight | 0.987 (0.906–1.075) | 0.757 | ||
| Female | 0.912 (0.378–2.205) | 0.839 | ||
| Preoperative MR grade | 0.162 (0.049–0.539) | 0.003 | 0.548 (0.110–2.715) | 0.461 |
| Preoperative LVEF | 0.971 (0.938–1.004) | 0.082 | 0.975 (0.939–1.012) | 0.182 |
| Preoperative LVEDD z-score | 1.032 (0.783–1.360) | 0.825 | ||
| Preoperative MVA diameter z-score | 0.678 (0.453–1.016) | 0.060 | 0.793 (0.438–1.435) | 0.443 |
| Cardiopulmonary bypass time | 0.997 (0.986–1.007) | 0.544 | ||
| Aortic cross-clamping time | 0.985 (0.963–1.008) | 0.199 | 0.983 (0.956–1.010) | 0.212 |
| MR grade on postoperative day 1 | 0.110 (0.041–0.293) | <0.001 | 0.080 (0.018–0.366) | 0.001 |
| Variables . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-Value . | HR (95% CI) . | P-Value . | |
| Age | 0.997 (0.974–1.020) | 0.809 | ||
| Weight | 0.987 (0.906–1.075) | 0.757 | ||
| Female | 0.912 (0.378–2.205) | 0.839 | ||
| Preoperative MR grade | 0.162 (0.049–0.539) | 0.003 | 0.548 (0.110–2.715) | 0.461 |
| Preoperative LVEF | 0.971 (0.938–1.004) | 0.082 | 0.975 (0.939–1.012) | 0.182 |
| Preoperative LVEDD z-score | 1.032 (0.783–1.360) | 0.825 | ||
| Preoperative MVA diameter z-score | 0.678 (0.453–1.016) | 0.060 | 0.793 (0.438–1.435) | 0.443 |
| Cardiopulmonary bypass time | 0.997 (0.986–1.007) | 0.544 | ||
| Aortic cross-clamping time | 0.985 (0.963–1.008) | 0.199 | 0.983 (0.956–1.010) | 0.212 |
| MR grade on postoperative day 1 | 0.110 (0.041–0.293) | <0.001 | 0.080 (0.018–0.366) | 0.001 |
CI: confidence interval; HR: hazard ratio; LVEDD: left ventricular end-diastolic dimension; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; MVA: mitral valve annulus.
Predictors of postoperative MR recovery in patients with mitral intervention
| Variables . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-Value . | HR (95% CI) . | P-Value . | |
| Age | 0.997 (0.974–1.020) | 0.809 | ||
| Weight | 0.987 (0.906–1.075) | 0.757 | ||
| Female | 0.912 (0.378–2.205) | 0.839 | ||
| Preoperative MR grade | 0.162 (0.049–0.539) | 0.003 | 0.548 (0.110–2.715) | 0.461 |
| Preoperative LVEF | 0.971 (0.938–1.004) | 0.082 | 0.975 (0.939–1.012) | 0.182 |
| Preoperative LVEDD z-score | 1.032 (0.783–1.360) | 0.825 | ||
| Preoperative MVA diameter z-score | 0.678 (0.453–1.016) | 0.060 | 0.793 (0.438–1.435) | 0.443 |
| Cardiopulmonary bypass time | 0.997 (0.986–1.007) | 0.544 | ||
| Aortic cross-clamping time | 0.985 (0.963–1.008) | 0.199 | 0.983 (0.956–1.010) | 0.212 |
| MR grade on postoperative day 1 | 0.110 (0.041–0.293) | <0.001 | 0.080 (0.018–0.366) | 0.001 |
| Variables . | Univariable . | Multivariable . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-Value . | HR (95% CI) . | P-Value . | |
| Age | 0.997 (0.974–1.020) | 0.809 | ||
| Weight | 0.987 (0.906–1.075) | 0.757 | ||
| Female | 0.912 (0.378–2.205) | 0.839 | ||
| Preoperative MR grade | 0.162 (0.049–0.539) | 0.003 | 0.548 (0.110–2.715) | 0.461 |
| Preoperative LVEF | 0.971 (0.938–1.004) | 0.082 | 0.975 (0.939–1.012) | 0.182 |
| Preoperative LVEDD z-score | 1.032 (0.783–1.360) | 0.825 | ||
| Preoperative MVA diameter z-score | 0.678 (0.453–1.016) | 0.060 | 0.793 (0.438–1.435) | 0.443 |
| Cardiopulmonary bypass time | 0.997 (0.986–1.007) | 0.544 | ||
| Aortic cross-clamping time | 0.985 (0.963–1.008) | 0.199 | 0.983 (0.956–1.010) | 0.212 |
| MR grade on postoperative day 1 | 0.110 (0.041–0.293) | <0.001 | 0.080 (0.018–0.366) | 0.001 |
CI: confidence interval; HR: hazard ratio; LVEDD: left ventricular end-diastolic dimension; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; MVA: mitral valve annulus.
Mitral reintervention
Four patients developed mitral stenosis after surgery, 3 after posterior annuloplasty and 1 after commissure plication annuloplasty. Four patients required mitral reoperation for persistent MR at the range of 1–6 years after initial concomitant mitral repair. Three of them had severe MR and 1 had moderate–severe MR at initial presentation. Reoperation was done by posterior mitral annuloplasty in 3 and by commissure plication annuloplasty in 1. Freedom from mitral reoperation for patients with the concomitant mitral intervention was 94% and 88% at 3 and 5 years after initial surgery, while freedom from mitral reoperation for patients without concomitant mitral intervention was 100% at both timepoints (P = 0.177) (Fig. 4).
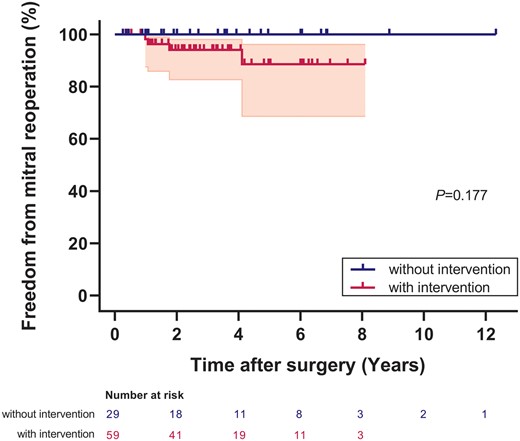
Freedom from mitral reoperation in patients with and without mitral intervention. The shaded area indicates 95% confidence limits.
DISCUSSION
To date, no consensus has been achieved yet about the strategy for the management of MR at the time of ALCAPA repair, and the evidence about the benefit of mitral intervention has been mixed [3, 9]. From our study, we found that regardless of the initial mitral management strategy, MR grade could improve significantly after surgery. However, residual persistent MR was common during the follow-up. Preoperative MVA diameter z-score was the predictor for MR recovery in patients without concomitant mitral intervention, whereas MR grade during the early postoperative period was the predictor in patients with mitral intervention.
About two-thirds of patients with ALCAPA have more than mild degree of MR [2]. Wesselhoeft et al. [10] in 1968 reviewed the pathological findings of the mitral valve in patients with ALCAPA. The causes of MR were various and combined: papillary muscle fibrosis, calcification or extensive scarring; endocardial fibroelastosis involving chordae tendineae, papillary muscle and mitral leaflets; left ventricular dilatation causing MVA dilatation and/or papillary muscle displacement; and left ventricular free wall dyskinesia [10, 11]. Recently, Weixler et al. [6] found that half of patients requiring mitral intervention had MVA dilatation, 32% had prolapse of anterior leaflet and 21% had prolapse of posterior leaflet. Similar findings were observed in our cohort.
The strategy of mitral management at ALCAPA repair is mainly based on institutional preferences. Reports from 2 large multicentre studies suggested only 8% of patients underwent concomitant mitral intervention [12, 13]. In our series, 65% of patients with more than mild degree of MR underwent concomitant mitral intervention, a proportion that is larger than most cardiac centres worldwide [5]. Some believe that concomitant mitral intervention is generally not necessary as MR grade improved in nearly all patients and that mitral intervention during ALCAPA repair would inevitably prolong the ischaemic insult to the sick ventricle [14]. Even if significant residual MR is present in some patients, they would be well-tolerated and could be managed surgically at a later date [4, 9, 15]. In contrast, others advocate concomitant mitral intervention in a specific group of patients with various indications, such as patients with moderate or severe MR [5, 6, 11] or with structural mitral valve abnormalities [14, 16, 17]. It is reasonable and safe and does not increase hospital mortality [13]. In our experience of patients with preoperative severe LV dysfunction, no differences were found in the use of mechanical circulatory support or early mortality between patients with or without mitral intervention, suggesting that mitral repair at the time of ALCAPA repair can be performed safely.
Mitral valve surgery in paediatric patients remains a challenging issue [18]. It is associated with a high risk of mortality and a high rate of recurrence and reintervention, particularly in neonates and infants [18]. This is also the case in patients with ALCAPA [5]. In a multicentre study focusing on ALCAPA patients older than 1 year of age, MR recovery was not achieved in 33% with concomitant mitral intervention at 3-year follow-up [7]. Naimo et al. [5] reported their experiences of 9 patients with concomitant mitral intervention. Two of these patients required mitral reoperation for persisting MR. Freedom from mitral reoperation was 71% at 10 years after the initial repair. In our study, MR recovery was achieved in 34% of patients with mitral intervention at 3 years after surgery, and 4 patients required mitral reoperation. MR grade on postoperative day 1 was the only predictor for MR recovery. The adequacy of the mitral repair during the early postoperative period, measured by the technical performance score, has been established as the strong predictor of mitral reintervention after discharge [19]. It seems that simple techniques such as annuloplasty may not be sufficient for a complete MR recovery. However, complex mitral intervention procedures, although may improve early technical performance, would definitely require much longer aortic cross-clamping time. Structural mitral valve abnormality such as cleft mitral valve was reported to be the reason for mitral reoperation [20] and thus should be addressed at initial surgery.
Although improvement of MR can be observed in patients without concomitant mitral intervention, MR may not fully recover during the follow-up period. MR recovery was seen in only 40% of the children without concomitant mitral intervention at 3-year follow-up [7]. Similarly, 35% of the infants had moderate or greater MR at the hospital discharge, and the degree of MR remained unchanged or worsening in several patients afterwards [21]. In our study, we found that preoperative MVA diameter z-score was predictive of postoperative MR recovery if no mitral intervention was performed at ALCAPA repair. Contrary to patients with mitral intervention, MR grade during the early postoperative period was not the predictor for MR recovery in patients without mitral intervention. A more dilated MVA may indicate a more depressed left ventricular function, which may be associated with the extend of left ventricular myocardial ischaemia. Consistent with Lange et al. [17], we found that MR recovery was not dependent on the age at the time of surgery. Some believed that the low incidence of MR recovery may be attributed to the irreversible preoperative ischaemia and thus successful surgery would not necessarily alter the course [21]. Following coronary reimplantation, some segments supplied by the ALCAPA may remain abnormal [22], and the posterolateral papillary muscle was constantly involved [23]. In addition, coronary artery stenosis must be ruled out for postoperative persistent MR [24], as is the case in 1 patient in our series.
Limitations
This study is a retrospective review from a single centre with a relatively small sample size and short follow-up time. The single-centre nature reduces the external validity of our findings. The small sample size decreases the power of the study. The selection of mitral management strategy at the time of ALCAPA repair was based on preoperative MR grade and surgeon’s preference. Individual experience may have influenced the decision. The majority of our patients had a short period of follow-up; therefore, both the findings and the conclusions of this study could be significantly changed depending on the late outcomes of these patients. In addition, magnetic resonance imaging would be very helpful in understanding the mechanism of MR, which has not been widely performed in patients with ALCAPA in our centre.
CONCLUSIONS
Postoperative MR improvement could be achieved regardless of the mitral management strategy used at ALCAPA repair. However, MR grade may not always return to normal after surgery and reintervention on MR is not rare. MR grade during the early postoperative period is the predictor for MR recovery in patients with concomitant mitral intervention, whereas preoperative MVA diameter z-score is the predictor in patients with ALCAPA repair alone.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Funding
This work was supported by Shanghai Municipal Science and Technology Commission Research Project (19411950200) and Shanghai Shenkang Hospital Developing Center (SHDC12018128).
Conflict of interest: none declared.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Author contributions
Wen Zhang: Data curation; Formal analysis; Methodology; Writing—original draft. Renjie Hu: Data curation; Formal analysis; Methodology; Writing—original draft. Qi Jiang: Data curation; Formal analysis. Hongbin Zhu: Conceptualization; Writing—review & editing. Lisheng Qiu: Methodology; Writing—review & editing. Wei Dong: Conceptualization; Methodology; Supervision; Writing—review & editing. Haibo Zhang: Conceptualization; Methodology; Supervision; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Emre Belli, Francois G. Lacour-Gayet and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
ABBREVIATIONS
- ALCAPA
Anomalous left coronary artery from the pulmonary artery
- CI
Confidence interval
- IQR
Interquartile range
- MR
Mitral regurgitation
- MVA
Mitral valve annulus
- LCA
Left coronary artery
- LVEF
Left ventricular ejection fraction
Author notes
Wen Zhang and RenjieHu contributed equally to this work.





