-
PDF
- Split View
-
Views
-
Cite
Cite
Helga Bachmann, Sandrine V C Dackam, Aljaz Hojski, Jelena Jankovic, Deborah R Vogt, Mark N Wiese, Didier Lardinois, Neoveil versus TachoSil in the treatment of pulmonary air leak following open lung surgery: a prospective randomized trial, European Journal of Cardio-Thoracic Surgery, Volume 63, Issue 1, January 2023, ezad003, https://doi.org/10.1093/ejcts/ezad003
Close - Share Icon Share
Abstract
Prolonged air leak (PAL) is often associated with pain and immobilization and is a major limiting factor for discharge from the hospital. The efficacy of 2 surgical patches was investigated in the treatment of air leak following open surgery.
Forty-five patients were randomized in a 1:1 ratio either to treatment with Neoveil (polyglycolic acid) (n = 22) or TachoSil (collagen sponge) (n = 23). Air leak was monitored at 2, 4, 8, 12 and 24 h after surgery and then daily at 8 am and 6 pm, using a digital recording system. The primary outcome was the time to air leak closure. Secondary outcomes were incidence, air leak intensity, incidence of PAL and incidence of pneumonia.
Air leak 2 h after surgery was observed in 11/22 (50%) vs 14/23 (61%) patients treated with polyglycolic acid, respectively, with collagen sponge. On average, air loss within the first 24 h after surgery was lower and declined faster in patients treated with polyglycolic acid. Time to pulmonary air leak closure was somewhat shorter with polyglycolic acid (median [interquartile range] 10 [2, 52] h) compared to collagen sponge (19 [2, 141] h). However, the difference was not statistically significant (P = 0.35, Wilcoxon rank-sum test). PAL occurred in 3/22 (14%) vs 6/23 (26%) patients, and pneumonia occurred in 2/22 (9%) vs 3/23 (13%) patients treated with polyglycolic acid, respectively, collagen sponge.
Both systems are effective in the treatment of air leak. Our results suggest a possible superiority of Neoveil over TachoSil in post-surgery air leak control.
NCT04065880
INTRODUCTION
Prolonged air leak (PAL), defined as an air leak lasting >5–7 days, is often associated with pain owing to chest tubes and immobilization and is a major limiting factor for discharge from the hospital [1–5]. Various techniques for minimizing this complication have been tried with mixed results, but the overall incidence of air leaks remains higher than 60% in the early postoperative period [4, 6–9]. However, most air leaks are benign and resolve within a few days with appropriate drain management [1, 7]. Failure of the air leak to close within 5–7 days is generally an indication for resurgery, as spontaneous closure is not to be expected.
A variety of biologic and synthetic materials including fibrin sealants, collagen fleece and synthetic glues are commercially available to decrease the occurrence of postoperative air leaks after pulmonary resection [1, 3, 6–8, 10].
This prospective, randomized-controlled, monocentric, single-blinded trial has investigated the efficacy of 2 bioabsorbable surgical patches in the treatment of pulmonary air leaks following open lung resection. The 2 different products Neoveil™ (Gunze Ltd., Japan) and TachoSil® (Corza Medical, Switzerland) are routinely used in our department as medical devices in the treatment of air leaks; they are commercially available and have been certified for years [11, 12]. Several studies have shown the effectiveness of both patches for air leak control but also as haemostatic agents [13]. So far no clinical research data are available to indicate which of these 2 materials produces a better result in closing air leaks following open lung surgery.
A potential advantage of the Neoveil sheet compared with TachoSil lies in its elastic and malleable structure, making it easy to apply not only on the lateral surface of the lungs but also in less flat regions like the hilum or near the fissures. This might result in faster closure and lower intensity and incidence of air leaks. For that reason, we hypothesized Neoveil’s superiority to TachoSil in the treatment of air leaks.
PATIENTS AND METHODS
Ethical statement
This study was approved by the Ethikkommission Nordwest- und Zentralschweiz on 12 February 2020 with Registration No. 2019-01505. A written informed individual patient consent was required.
Forty-five patients were enrolled in the study, consisting of 30 men and 15 women with a mean age of 65 years (range 28–86 years). All patients had undergone open lung resection between April 2020 and May 2021. Characteristics of the patient population and type of resections are described in Table 1 and in Table 2.
| . | Neoveil (n = 22) . | TachoSil (n = 23) . |
|---|---|---|
| Demographics | ||
| Gender female/male, n (%) | 7 (32)/15 (68) | 8 (35)/15 (65) |
| Age in years, mean (SD) | 63.3 (13.6) | 66.9 (10.3) |
| Medical history, n (%) | ||
| COPD | 10 (45) | 11 (48) |
| Smoker or former smoker | 17 (77) | 21 (91) |
| Steroid use >10 mg/day oral or inhalative | 5 (23) | 7 (30) |
| Previous operation on the same side | 7 (32) | 9 (39) |
| Characteristics of surgery, n (%) | ||
| Indication | ||
| Tumour | 18 (82) | 17 (74) |
| Empyema | 2 (9) | 5 (22) |
| Others | 2 (9) | 1 (4) |
| Side right/left | 15 (68)/7 (32) | 14 (61)/9 (39) |
| Adhesions | 10 (45) | 13 (57) |
| . | Neoveil (n = 22) . | TachoSil (n = 23) . |
|---|---|---|
| Demographics | ||
| Gender female/male, n (%) | 7 (32)/15 (68) | 8 (35)/15 (65) |
| Age in years, mean (SD) | 63.3 (13.6) | 66.9 (10.3) |
| Medical history, n (%) | ||
| COPD | 10 (45) | 11 (48) |
| Smoker or former smoker | 17 (77) | 21 (91) |
| Steroid use >10 mg/day oral or inhalative | 5 (23) | 7 (30) |
| Previous operation on the same side | 7 (32) | 9 (39) |
| Characteristics of surgery, n (%) | ||
| Indication | ||
| Tumour | 18 (82) | 17 (74) |
| Empyema | 2 (9) | 5 (22) |
| Others | 2 (9) | 1 (4) |
| Side right/left | 15 (68)/7 (32) | 14 (61)/9 (39) |
| Adhesions | 10 (45) | 13 (57) |
COPD: chronic obstructive pulmonary disease; SD: standard deviation.
| . | Neoveil (n = 22) . | TachoSil (n = 23) . |
|---|---|---|
| Demographics | ||
| Gender female/male, n (%) | 7 (32)/15 (68) | 8 (35)/15 (65) |
| Age in years, mean (SD) | 63.3 (13.6) | 66.9 (10.3) |
| Medical history, n (%) | ||
| COPD | 10 (45) | 11 (48) |
| Smoker or former smoker | 17 (77) | 21 (91) |
| Steroid use >10 mg/day oral or inhalative | 5 (23) | 7 (30) |
| Previous operation on the same side | 7 (32) | 9 (39) |
| Characteristics of surgery, n (%) | ||
| Indication | ||
| Tumour | 18 (82) | 17 (74) |
| Empyema | 2 (9) | 5 (22) |
| Others | 2 (9) | 1 (4) |
| Side right/left | 15 (68)/7 (32) | 14 (61)/9 (39) |
| Adhesions | 10 (45) | 13 (57) |
| . | Neoveil (n = 22) . | TachoSil (n = 23) . |
|---|---|---|
| Demographics | ||
| Gender female/male, n (%) | 7 (32)/15 (68) | 8 (35)/15 (65) |
| Age in years, mean (SD) | 63.3 (13.6) | 66.9 (10.3) |
| Medical history, n (%) | ||
| COPD | 10 (45) | 11 (48) |
| Smoker or former smoker | 17 (77) | 21 (91) |
| Steroid use >10 mg/day oral or inhalative | 5 (23) | 7 (30) |
| Previous operation on the same side | 7 (32) | 9 (39) |
| Characteristics of surgery, n (%) | ||
| Indication | ||
| Tumour | 18 (82) | 17 (74) |
| Empyema | 2 (9) | 5 (22) |
| Others | 2 (9) | 1 (4) |
| Side right/left | 15 (68)/7 (32) | 14 (61)/9 (39) |
| Adhesions | 10 (45) | 13 (57) |
COPD: chronic obstructive pulmonary disease; SD: standard deviation.
| . | Neoveil (n = 22) . | TachoSil (n = 23) . |
|---|---|---|
| Lobectomy, bilobectomy | 9 (41) | 10 (43) |
| Segmentectomy | 0 | 1 (4) |
| Atypical lung resection | 5 (23) | 4 (17) |
| Lobectomy and segmentectomy | 0 | 1 (4) |
| Lobectomy and atypical lung resection | 3 (14) | 2 (9) |
| Segmentectomy and atypical lung resection | 1 (5) | 0 |
| Lung decortication | 2 (9) | 3 (13) |
| Other | 2 (9) | 2 (9) |
| . | Neoveil (n = 22) . | TachoSil (n = 23) . |
|---|---|---|
| Lobectomy, bilobectomy | 9 (41) | 10 (43) |
| Segmentectomy | 0 | 1 (4) |
| Atypical lung resection | 5 (23) | 4 (17) |
| Lobectomy and segmentectomy | 0 | 1 (4) |
| Lobectomy and atypical lung resection | 3 (14) | 2 (9) |
| Segmentectomy and atypical lung resection | 1 (5) | 0 |
| Lung decortication | 2 (9) | 3 (13) |
| Other | 2 (9) | 2 (9) |
| . | Neoveil (n = 22) . | TachoSil (n = 23) . |
|---|---|---|
| Lobectomy, bilobectomy | 9 (41) | 10 (43) |
| Segmentectomy | 0 | 1 (4) |
| Atypical lung resection | 5 (23) | 4 (17) |
| Lobectomy and segmentectomy | 0 | 1 (4) |
| Lobectomy and atypical lung resection | 3 (14) | 2 (9) |
| Segmentectomy and atypical lung resection | 1 (5) | 0 |
| Lung decortication | 2 (9) | 3 (13) |
| Other | 2 (9) | 2 (9) |
| . | Neoveil (n = 22) . | TachoSil (n = 23) . |
|---|---|---|
| Lobectomy, bilobectomy | 9 (41) | 10 (43) |
| Segmentectomy | 0 | 1 (4) |
| Atypical lung resection | 5 (23) | 4 (17) |
| Lobectomy and segmentectomy | 0 | 1 (4) |
| Lobectomy and atypical lung resection | 3 (14) | 2 (9) |
| Segmentectomy and atypical lung resection | 1 (5) | 0 |
| Lung decortication | 2 (9) | 3 (13) |
| Other | 2 (9) | 2 (9) |
Neoveil is a sheet made of polyglycolic acid. It is a biodegradable, thermoplastic and non-antigenic polymer. The sheet is placed into a small container filled with NaCl and left to soak for about 30 s. The sheet is then placed in 1 layer onto the area to be covered. The sheet is kept on the surface of the lung for 2 min with slight pressure using wet pads.
TachoSil is a collagen sponge coated with human fibrinogen and thrombin. The yellow side becomes active when the fleece comes into contact with blood, other body fluids or saline to form a clot that glues it to the tissue surface. The patch can be cut to the appropriate size and is kept under slight pressure with wet pads on the surface with air leaks for 3 min.
For both products, it was very important for the lung to be slightly re-ventilated during this phase to allow the patch to adapt to the shape of the lung. No additional fibrin glue or other substances were used.
Design
At the end of the surgery, a water test was performed and, if an air leak was identified, the lung surface was covered either with Neoveil or TachoSil depending on randomization. Patients were randomized in a 1:1 ratio via a web-based electronic data capture system (secuTrial®). Randomization was not stratified according to the type of surgery or any other patient characteristics. A multifocal air leak was not an exclusion criterion. The degree of intraoperative air leak was not routinely measured, since it would have been impossible to receive precise information on the degree of each air leak separately. Therefore, we have decided to only describe the number of air leaks. At the end of the surgery, 2 chest tubes were inserted in the chest cavity, 1 apically (24 CH) and 1 basally (28 CH), to facilitate re-expansion of the lung and to drain any air and fluid. At our division, 2 chest tubes are routinely inserted after open surgery. It means that chest tube management was the same as usual, independently of the study. Immediately after surgery, the chest tubes were connected to a Thopaz+™ digital drainage system (Medela, Switzerland) with -15 cm H2O suction. This system has integrated suction and quantifies the air leak. Chest tube removal was possible as soon as the air leak stopped. To avoid an overestimation of air leak duration, the exact time point of an air leak closure was determined on the basis of the digital recordings by the drainage system.
Primary outcome
The primary outcome was the time to pulmonary air leak closure (h) defined as the time from the end of surgery to the first point in time when air loss was <30 ml for (at least) 2 consecutive measurements on the digital recordings. The observation period for air leaks was 7 days; for that reason, air leak measurement was censored at 168 h after surgery. For patients with a persistent air leak at 168 h after surgery, PAL was defined, and the primary outcome was set to 168 h.
Secondary outcomes
These outcomes consisted of intensity of air leak (ml) during the observation period, area under the curve (AUC) of air leak evolution within the first 24 h postoperative, incidence of air leak (binary variable: air leak ˃30 ml) during the observation period, PAL and pneumonia during hospitalization. Pneumonia was defined as a combination of clinical signs, laboratory and radiological findings.
Air leak was recorded at 2, 4, 8, 12 and 24 h after surgery and then daily at 8 am and 6 pm from the first day post-surgery until removal of the chest tubes or until day 7 in the case of PAL. The air leak trajectory, AUC was calculated based on the data taken at 2, 4, 8, 12 and 24 h after surgery using interpolation (linear trapezoidal method).
Analysis sets
The full analysis set included all randomized patients (n = 45) with all patients analysed as randomized (intention-to-treat principle). There were no resurgeries or other manipulations (partial retrieval of the drainage) prior to day 7 in the set of analysis. The per-protocol set included a total of 42 patients without any protocol violations.
Sample size
Sample size was estimated to be able to show a difference in time to pulmonary air leak closure between both products with at least 90% power (1 − β = 0.9) at a significance level of α = 5% using a Wilcoxon rank-sum test. Assuming a clinically relevant reduction in time to pulmonary air leak closure of 1.5 days for polyglycolic acid as compared to collagen sponge, a total of 40 patients were required. Accounting for an expected dropout rate of 10%, a total of 45 patients were recruited.
Statistical analysis
All statistical analyses were predefined in a statistical analysis plan and performed using the statistical software package R version 4.1.2 (2021-11-01) R Core Team 2021.
Primary outcome
The time to pulmonary air leak closure was tested using a Wilcoxon rank-sum test to account for the anticipated right skewed data distribution. The median difference is provided with 95% bootstrap confidence interval (CI).
Secondary outcomes
The intensity of air loss was summarized by treatment arm at each measurement time. The 24-h AUC was derived and assessed for a difference between both products, using a Wilcoxon rank-sum test to account for the anticipated right skewed data distribution.
Binary outcomes were analysed descriptively only. No hypothesis testing, i.e. comparison between both sealants had been planned a priori since the sample size does not provide sensible power for binary outcomes.
RESULTS
The majority of patients had multifocal air leaks (68% in the polyglycolic acid and 78% in the collagen sponge group). Characteristics and localization of air leaks are given in Table 3. The mean hospitalization duration was 11.9 (standard deviation 4.8) nights in the polyglycolic acid group and 16.3 (standard deviation 8.9) after treatment with collagen sponge.
| . | Total (n = 45) . | Neoveil (n = 22) . | TachoSil (n = 23) . |
|---|---|---|---|
| Air leak characteristics | |||
| Number of air leaks, n (median) | 113 (2.0) | 47 (2.0) | 66 (2.0) |
| Patients with a single air leak, n (%) | 12 (27) | 7 (32) | 5 (22) |
| Localization of air leaks | |||
| Localization corresponding to lung anatomy (n) | 61 | 30 | 31 |
| Fissure, n (%) | 4 (7) | 3 (10) | 1 (3) |
| Upper lobe, n (%) | 19 (31) | 11 (37) | 8 (26) |
| Middle lobe, n (%) | 13 (21) | 6 (20) | 7 (23) |
| Lower lobe, n (%) | 25 (41) | 10 (33) | 15 (48) |
| . | Total (n = 45) . | Neoveil (n = 22) . | TachoSil (n = 23) . |
|---|---|---|---|
| Air leak characteristics | |||
| Number of air leaks, n (median) | 113 (2.0) | 47 (2.0) | 66 (2.0) |
| Patients with a single air leak, n (%) | 12 (27) | 7 (32) | 5 (22) |
| Localization of air leaks | |||
| Localization corresponding to lung anatomy (n) | 61 | 30 | 31 |
| Fissure, n (%) | 4 (7) | 3 (10) | 1 (3) |
| Upper lobe, n (%) | 19 (31) | 11 (37) | 8 (26) |
| Middle lobe, n (%) | 13 (21) | 6 (20) | 7 (23) |
| Lower lobe, n (%) | 25 (41) | 10 (33) | 15 (48) |
| . | Total (n = 45) . | Neoveil (n = 22) . | TachoSil (n = 23) . |
|---|---|---|---|
| Air leak characteristics | |||
| Number of air leaks, n (median) | 113 (2.0) | 47 (2.0) | 66 (2.0) |
| Patients with a single air leak, n (%) | 12 (27) | 7 (32) | 5 (22) |
| Localization of air leaks | |||
| Localization corresponding to lung anatomy (n) | 61 | 30 | 31 |
| Fissure, n (%) | 4 (7) | 3 (10) | 1 (3) |
| Upper lobe, n (%) | 19 (31) | 11 (37) | 8 (26) |
| Middle lobe, n (%) | 13 (21) | 6 (20) | 7 (23) |
| Lower lobe, n (%) | 25 (41) | 10 (33) | 15 (48) |
| . | Total (n = 45) . | Neoveil (n = 22) . | TachoSil (n = 23) . |
|---|---|---|---|
| Air leak characteristics | |||
| Number of air leaks, n (median) | 113 (2.0) | 47 (2.0) | 66 (2.0) |
| Patients with a single air leak, n (%) | 12 (27) | 7 (32) | 5 (22) |
| Localization of air leaks | |||
| Localization corresponding to lung anatomy (n) | 61 | 30 | 31 |
| Fissure, n (%) | 4 (7) | 3 (10) | 1 (3) |
| Upper lobe, n (%) | 19 (31) | 11 (37) | 8 (26) |
| Middle lobe, n (%) | 13 (21) | 6 (20) | 7 (23) |
| Lower lobe, n (%) | 25 (41) | 10 (33) | 15 (48) |
Time to pulmonary air leak closure
On average, time to pulmonary air leak closure was shorter in patients treated with polyglycolic acid (median [interquartile range]: 10 [2, 52] h) as compared to 19 [2, 141] h (Fig. 1). The median difference [95% CI] was −9 [−52, 20] h (P-value 0.35) in the intention-to-treat analysis. This observed difference is much smaller than the assumed clinically relevant difference (1.5 days) and the time to pulmonary air leak closure varied substantially between patients under both treatments. Consequently, the evidence provided by our data is not strong enough to reject the null hypothesis of no difference between both patches. At the first measurement 2 h after surgery, 50% of the patients with polyglycolic acid and 39% of the patients with collagen sponge had no air leak. Seventy-five percent of the patients treated with polyglycolic acid no longer had an air leak after 2 days, whereas this took 6 days in patients treated with the other products.
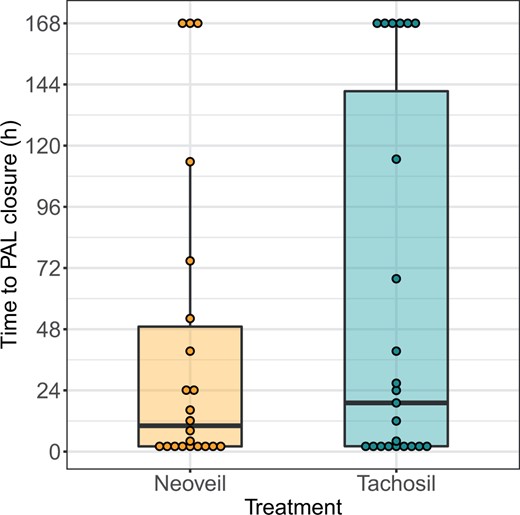
Time to closure of pulmonary air leak in hours from the end of surgery, with dots representing the measures of individual patients.
Air leak intensity
Related to the primary outcome, air leaks within the first 24 h after surgery were on average lower and declined faster in patients treated with polyglycolic acid as compared with collagen sponge (Fig. 2 and Table 4). This is also reflected in the 24-h AUC, which was on average smaller in the polyglycolic acid group (median [interquartile range] 668 [110, 4510] ml/24 h vs 1894 [130, 13 161] ml/24 h; Fig. 3). However, in both treatment groups, there were patients with persistently high air leak. Altogether, the evidence for this difference was not strong enough to reject the null hypothesis of no difference between both treatment modalities: median difference [bootstrap 95% CI]: −1226 [−10 956, 476] ml/24 h, P = 0.45 (Figs. 2 and 3).
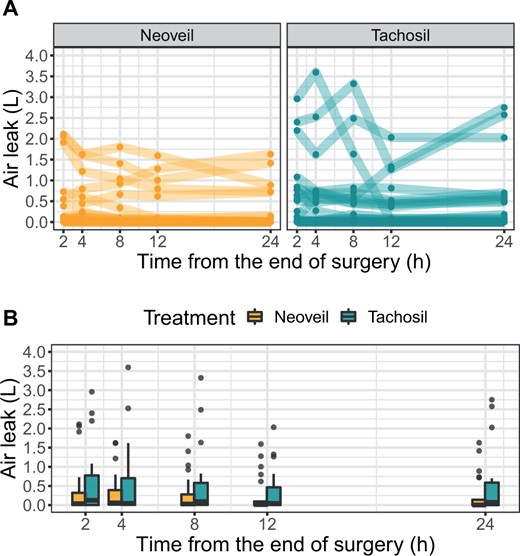
Air leaks (l) within the first 24 h after surgery. Upper panel: individual courses; each line represents 1 patient. Lower panel: summarized data at each measurement time.
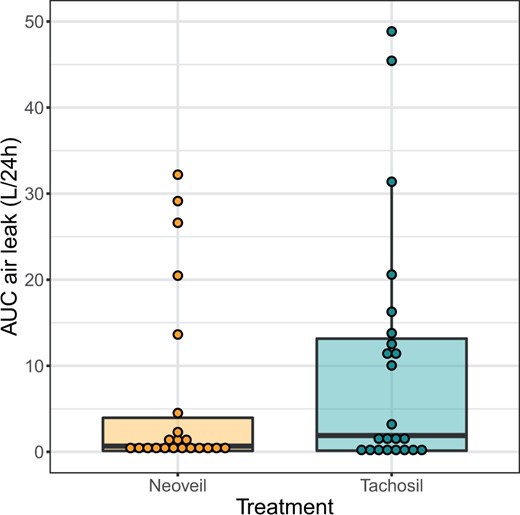
Area under the curve (AUC) of air leaks (l) over the first 24 h after surgery. Dots represent the measures of individual patients.
Summary statistics for air leak (ml) within the first 24 h after surgery according to treatment
| Hours from the end of surgery . | Neoveil (n = 22) . | TachoSil (n = 23) . |
|---|---|---|
| 2 | 38 [4, 322] (1–2112) | 127 [5, 776] (1–2957) |
| 4 | 61 [13, 394] (1–1624) | 46 [8, 702] (1–3595) |
| 8 | 30 [3, 281] (1–1801) | 92 [6, 579] (0–3322) |
| 12 | 14 [2, 96] (1–1594) | 46 [2, 465] (0–2033) |
| 24 | 4 [1, 139] (0–1629) | 74 [1, 588] (0–2751) |
| Hours from the end of surgery . | Neoveil (n = 22) . | TachoSil (n = 23) . |
|---|---|---|
| 2 | 38 [4, 322] (1–2112) | 127 [5, 776] (1–2957) |
| 4 | 61 [13, 394] (1–1624) | 46 [8, 702] (1–3595) |
| 8 | 30 [3, 281] (1–1801) | 92 [6, 579] (0–3322) |
| 12 | 14 [2, 96] (1–1594) | 46 [2, 465] (0–2033) |
| 24 | 4 [1, 139] (0–1629) | 74 [1, 588] (0–2751) |
Numbers indicate median [interquartile range] (minimum–maximum).
Summary statistics for air leak (ml) within the first 24 h after surgery according to treatment
| Hours from the end of surgery . | Neoveil (n = 22) . | TachoSil (n = 23) . |
|---|---|---|
| 2 | 38 [4, 322] (1–2112) | 127 [5, 776] (1–2957) |
| 4 | 61 [13, 394] (1–1624) | 46 [8, 702] (1–3595) |
| 8 | 30 [3, 281] (1–1801) | 92 [6, 579] (0–3322) |
| 12 | 14 [2, 96] (1–1594) | 46 [2, 465] (0–2033) |
| 24 | 4 [1, 139] (0–1629) | 74 [1, 588] (0–2751) |
| Hours from the end of surgery . | Neoveil (n = 22) . | TachoSil (n = 23) . |
|---|---|---|
| 2 | 38 [4, 322] (1–2112) | 127 [5, 776] (1–2957) |
| 4 | 61 [13, 394] (1–1624) | 46 [8, 702] (1–3595) |
| 8 | 30 [3, 281] (1–1801) | 92 [6, 579] (0–3322) |
| 12 | 14 [2, 96] (1–1594) | 46 [2, 465] (0–2033) |
| 24 | 4 [1, 139] (0–1629) | 74 [1, 588] (0–2751) |
Numbers indicate median [interquartile range] (minimum–maximum).
Incidence of postoperative pulmonary air leak
The cumulative incidence of patients with air leak closure over time is shown in Fig. 4. Twenty-four hours after surgery, air leaks were still present in only 7/22 (32%) patients with polyglycolic acid as compared to 13/23 (57%) patients with collagen sponge.
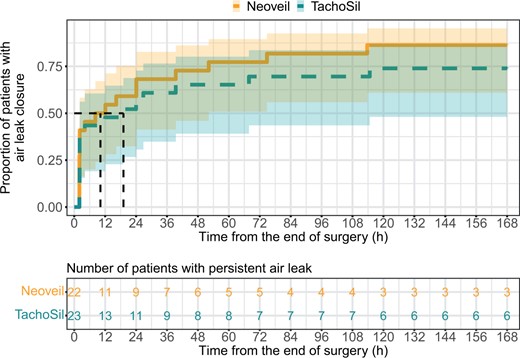
Time to air leak closure. Cumulative event curves with 95% confidence bands. The Y-axis shows the proportion of patients with air leak closure, i.e. air leak ˂30 ml. The vertical dashed lines indicate the time at which half of the patients reached air leak closure for each treatment group (median time to air leak closure). The numbers in the table below indicate the number of patients with a persistent air leak in each treatment group for the respective time point.
Prolonged air leak
PAL occurred in 3/22 (14%) patients treated with polyglycolic acid and in 6/23 (26%) patients treated with collagen sponge (post hoc Chi-squared test: P = 0.50). In 1 patient treated with polgyglycolic acid, the air leak stopped spontaneously 1 day later, and the drainages could be removed. The other 2 patients underwent resurgery with closure of the fistula, and their recovery was uneventful. In the collagen sponge group, the air leak stopped spontaneously in 1 patient and after the partial retraction of the drainage in 1 patient. Three patients underwent resurgery, and the last patient developed pneumonia and died from multiorgan failure. All resurgeries were successful, and recovery was uneventful. Patients with PAL had more air leaks than patients without PAL in both treatment groups (2.7 vs 1.7 in the polyglycolic acid and 3.5 vs 1.8 in the collagen sponge group). There was no association between the prevalence of PAL and the severity of emphysema or the use of steroids, previous surgery, the extent of resection, the gender, the side of surgery and complete lung re-expansion on first chest X-ray postoperative. However, extended lung adhesions were observed in 7/9 (78%) patients (100% in the polyglycolic acid group and 67% in the collagen sponge group). The mean body mass index (BMI) of the patients with PAL was ˂25 (21 in the polyglycolic acid group and 19.7 in the collagen sponge one). There was no predominance of air leak according to the lobe. All PAL patients were active or former smokers.
Pneumonia
During hospitalization, pneumonia was observed in 2/22 (9%) in the polyglycolic acid group in comparison to 3/23 (13%) in the collagen sponge group. Only 1 of these 5 patients (1 collagen sponge) had a PAL and died from multiorgan failure after reintubation due to respiratory failure. Four other patients no longer had an air leak when they developed pneumonia. Two of them underwent reintubation and died from multiorgan failure; 2 other presented with an uneventful recovery after repeated bronchial toilette, antibiotic therapy and mobilization. We have no clear explanation for the relatively high rate of pneumonia. A possibility could be a more extended resection in patients with worse general health condition and/or more pain postoperative.
DISCUSSION
PAL is a frequent complication of pulmonary resection and can cause prolonged hospitalization. It also increases overall costs and is associated with complications such as empyema and pneumonia [1–3, 6, 9]. In our study, we have not observed any association between the incidence of PAL and the occurrence of pneumonia postoperatively. Brunelli et al. [14] showed that the relative postoperative costs were 50% higher in PAL patients compared to non-PAL patients. In our study, the global incidence of PAL was 20%, which corresponds to the data in the literature (15–25%) [4, 6–8]. In the study by Brunelli et al. [14], the postoperative stay was 4 days longer in patients with PAL than without PAL (9.6 vs 5.7). We could confirm the prolonged hospitalization duration in PAL patients with a mean value of 19.2 nights compared with 12.9 nights in patients without PAL. There have been several risk scores published to stratify the risk of developing PAL and to select patients in whom to apply intraoperative preventive measures in the most cost-effective way [15–17]. Frequently described risk factors for PAL after logistic regression are BMI ≤25, lobectomy or bilobectomy, forced expiratory volume ˂70% or ˂80%, male gender, right upper lobe procedure, pleural adhesions, smoking history, induction therapy, emphysema, immune suppression and corticosteroid use [3, 5, 9, 14–17]. In our analysis on a small number of patients, we were able to correlate the occurrence of PAL with extended lung adhesions, low BMI, chronic obstructive pulmonary disease and smoking habit. Some authors have suggested that open resections were associated with a higher risk for PAL than video-assisted thoracoscopic surgery (VATS). In the study by Orsini et al. [9], the incidence of PAL was 8% after VATS resections, which was lower than after open procedure. The authors considered a possible explanation that many VATS surgeons promote fissureless techniques, in particular for upper lobectomies [9, 18]. Fissureless techniques have been described and reported as associated with a substantial reduction in the incidence of PAL, median tube time and in-hospital stay [9]. Brunelli et al. [7, 14] have analysed the incidence of PAL in 982 patients. They found that a thoracotomy approach was significantly associated with PAL in a multivariate regression analysis. Although fissureless technique can also be used in open surgery, it can be technically more demanding and is not routinely used. To avoid any influence of the approach used on the incidence of air leak, we have decided to consider open resections only. Various additional techniques, including the use of a variety of sealants, such as fibrin glue, synthetic materials and collagen patches coated with fibrinogen and thrombin, have been employed to minimize air leak intensity and duration [1, 3, 6–8, 10]. The evidence base on the use of sealants in lung surgery is inconsistent. There is clearly a lack of consensus regarding the optimal use of sealants in air leak management. In our study, we have not observed any complications due to the use of either sealant. TachoSil is a routinely used sealant not limited to thoracic surgery [19, 20]. The product is frequently used as a haemostatic in liver surgery [13]. A multicentre trial reported a significant reduction in the duration of postoperative air leaks (9.5 vs 35.8 h), in the intensity of intraoperative air leakage and in the percentage of patients with air leak at wound closure (81.1 vs 100%); however, no difference in time to chest drain removal and in hospitalization was observed [21]. In our study, the median time to air leak closure was longer than 9.5 h with a value of 19 h. Other studies using this surgical patch showed inconclusive results [22]. Studies with Neoveil showed no postoperative PAL (>7 days) in patients with non-small-cell lung cancer who had undergone thoracoscopic lobectomies [23]. In our study, all the end points we have considered were in favour of Neoveil (incidence and intensity of air leaks, faster closure of air leaks, incidence of PAL), although the evidence is not strong enough to reject the corresponding null hypotheses of no difference between both treatment modalities. This could be due to the fact that only 2 h after surgery, 40–50% of the patients no longer presented with an air leak, showing that both sealants are effective. As a consequence, only about 50% of the included patients (with measured air leak at 2 h after surgery) were effectively available for comparison. In addition, time to pulmonary air leak closure varied substantially between patients under both treatments. On day 1 after surgery, less than one-third of the polyglycolic acid group thus still presented with an air leak compared to ∼60% in the collagen sponge group. Although it took 4 days longer in the collagen sponge group to reach 75% air leak closure, the evidence provided by our data is not strong enough to reject the null hypothesis of no difference between both products. The inclusion of more patients in the study might have led to stronger results. The incidence of PAL was 14% in the Neoveil group, which corresponds with the literature (∼15%) [3, 6, 8, 9]. In the TachoSil group, this incidence was twice as high (26%). It also means that after the application of collagen sponge, 43% (6/14) of the patients without immediate air closure postoperative will develop PAL in comparison to 27% (3/11) in the polyglycolic acid group. This is probably a consequence of the properties of Neoveil: it is more elastic and can be easier adapted to the shape of the surface to cover than TachoSil. Optimal suction management is a topic of continued debate. The Thopaz+ system incorporates electronic components for the digital quantification of air through chest tubes. Such systems are more accurate in measuring postoperative air leakage, and the goal is to objectify the measurement of air leak with the ultimate intention of reducing drainage duration and length of hospital stay [22]. In our study, the duration of chest tube drainage was not an end point since drainage was also used for the management of pleural effusions. Our policy of chest tube management was uniform. Removal of the first drainage was considered possible only when an air leak ˂30 ml was observed at 2 consecutive observation times, to neutralize the effects of possible fluctuations of the measured air loss in the management of the chest tubes.
CONCLUSION
In conclusion, Neoveil and TachoSil are both effective in the treatment of air leaks following open lung procedures. Our results suggest that Neoveil might be slightly superior in terms of the incidence and the intensity of postoperative air leaks, the duration of air leaks and the incidence of PAL.
ACKNOWLEDGEMENT
We thank Ruth Schweizer-Christen and her team for their excellent assistance in the operating theatre. We also thank Mirjam Stranzl and Marielle Rutquist from the Department of Clinical Research for their competent trial monitoring and secuTrial management.
Funding
This work was supported and funded by the University Hospital Basel, Department of Thoracic Surgery.
Conflict of interest: none declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Author contributions
Helga Bachmann: Formal analysis; Investigation; Methodology; Project administration; Validation; Visualization; Writing—review & editing. Sandrine V.C. Dackam: Data curation; Investigation; Methodology; Project administration; Visualization. Aljaz Hojski: Investigation; Visualization. Jelena Jankovic: Data curation; Visualization. Deborah R. Vogt: Formal analysis; Methodology; Validation; Visualization; Writing—review & editing. Mark N. Wiese: Investigation; Visualization. Didier Lardinois: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Jalal Assouad, Hitoshi Igai, Muhammet Sayan and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Presented at the 30th ESTS Meeting, The Hague, The Netherlands, 19-21 June 2022.
REFERENCES
ABBREVIATIONS
- AUC
Area under the curve
- BMI
Body mass index
- CI
Confidence interval
- PAL
Prolonged air leak
- VATS
Video-assisted thoracoscopic surgery
Author notes
Helga Bachmann and Sandrine V C Dackam contributed equally to this work.





