-
PDF
- Split View
-
Views
-
Cite
Cite
Yoshimasa Seike, Tetsuya Fukuda, Koki Yokawa, Shigeki Koizumi, Kenta Masada, Yosuke Inoue, Hitoshi Matsuda, Aggressive use of prophylactic cerebrospinal fluid drainage to prevent spinal cord ischemia during thoracic endovascular aortic repair is not supportive, European Journal of Cardio-Thoracic Surgery, Volume 62, Issue 6, December 2022, ezac441, https://doi.org/10.1093/ejcts/ezac441
Close - Share Icon Share
Abstract
We investigated whether prophylactic preoperative cerebrospinal fluid drainage (CSFD) was effective in preventing spinal cord ischemia (SCI) during thoracic endovascular aortic repair of degenerative descending thoracic aortic aneurysms, excluding dissecting aneurysms.
We retrospectively reviewed the medical records of patients who underwent thoracic endovascular aortic repair involving proximal landing zones 3 and 4 between 2009 and 2020.
Eighty-nine patients with preemptive CSFD [68 men; median (range) age, 76.0 (71.0–81.0) years] and 115 patients without CSFD [89 men; median (range) age, 77.0 (74.0–81.5) years] were included in this study. Among them, 59 from each group were matched based on propensity scores to regulate for differences in backgrounds. The incidence rate of SCI was similar: 8/89 (9.0%) in the CSFD group and 6/115 (5.2%) in the non-CSFD group (P = 0.403). Shaggy aorta (odds ratio, 5.13; P = 0.004) and iliac artery access (odds ratio, 5.04; P = 0.005) were identified as positive predictors of SCI. Other clinically important confounders included Adamkiewicz artery coverage (odds ratio, 2.53; P = 0.108) and extensive stent graft coverage (>8 vertebrae) (odds ratio, 1.41; P = 0.541) were not statistically significant. Propensity score matching yielded similar incidence of SCI: 4/59 (6.8%) in the CSFD group and 3/59 (5.1%) in the non-CSFD group (P = 0.697).
Aggressive use of prophylactic CSFD was not supportive in patients without complex risks of SCI.
INTRODUCTION
Spinal cord ischemia (SCI) after thoracic endovascular aortic repair (TEVAR) for degenerative descending thoracic aortic aneurysms (TAA) still occurs at a significant rate [1, 2]. After initiating the TEVAR program to treat descending TAA, we have routinely applied preoperative cerebrospinal fluid drainage (CSFD) as prophylaxis in patients at high risk of SCI, including those with shaggy aorta, long-segment aortic coverage, bilateral hypogastric occlusion, left subclavian artery (LSCA) occlusion and Adamkiewicz artery (AKA) coverage [1, 3–5]. However, owing to the multifactorial pathophysiology along with the collateral blood supply network theory [6, 7] as well as the invasiveness of the procedure, the role of CSFD as a preventive measure for SCI remains controversial [8–10]. The goal of this study was to investigate the effectiveness of prophylactic CSFD in the prevention of SCI during TEVAR in patients with degenerative descending TAA.
MATERIALS AND METHODS
Ethics statement
This study was approved by the institutional review board at the National Cerebral and Cardiovascular Center (M30-036). Individual oral and written informed consent was not required because of its retrospective design.
Study design and study population
This study was an observational, retrospective cohort study and was reported in keeping with the STROBE guidelines [11]. We retrospectively reviewed the medical records of 468 patients who underwent TEVAR involving proximal landing zones 3 and 4 for various descending TAA between January 2009 and December 2020. To refine the target cohort, we excluded 120 patients with aortic dissection, 46 with redo TEVAR for any type of endoleak, 33 with anastomotic pseudoaneurysms, 24 with aortic rupture, 5 with thoracoabdominal aortic aneurysms (TAAA) and 36 whose AKA was not evaluated before the operation. As a result, emergency and urgent TEVARs were excluded. Dissection-related TEVARs were excluded to avoid the effects of malperfusion syndrome on SCI. TAAAs were excluded concidering influences of additional debranching procedures (Fig. 1).
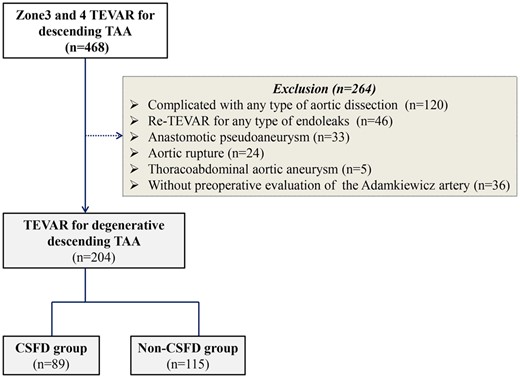
Flow chart of the study population and method. CSFD: cerebrospinal fluid drainage; TAA: thoracic aortic aneurysm; TEVAR: thoracic endovascular aortic repair.
Indications for preemptive cerebrospinal fluid drainage
Antiplatelets and anticoagulants were discontinued when we were considering the prophylactic insertion of a CSFD tube. On the day of the operation, the patient had a CSFD tube inserted before TEVAR, which was performed in a hybrid operating room. Prophylactic CSFD was indicated if a patient was considered to have an increased risk of SCI, including planned coverage of the intercostal arteries (ICA) branching from the AKA (ICA-AKA) in combination with other risk factors, including extensive aortic coverage (>8 thoracic vertebrae), previous aortic repair of the downstream aorta and a shaggy aorta. Previous studies reported the high incidence of SCI due to embolic events from a shaggy aorta [12, 13]. However, the trend was for more aggressive CSFD tube insertion early in the introduction of TEVAR. Moreover, in an actual setting, insertion of this tube was influenced by other circumstances (including a predisposition to bleeding, vertebral deformity and the surgeon’s preference) in addition to strict indications.
CSFD was started immediately at 12 cm H2O after deployment of the stent graft. The drainage gradient was changed every few hours in the range of 10 to 15 cm H2O to keep drainage volume at less than 20 ml per h to prevent haemorrhagic events. CSFD was stopped 6 h after TEVAR if neither the symptoms of SCI nor the ischaemic findings of motor-evoked potentials (MEPs) were observed by the anaesthesiologists and surgeons. Otherwise, CSFD was continued for 24–48 h.
Thoracic endovascular aortic repair techniques
Both cardiovascular surgeons and interventional radiologists performed TEVAR in patients who were under general anaesthesia. A stent graft was 10%–20% oversized in relation to the landing zone of a healthy aorta according to the instructions for use of each device. To obtain the appropriate distal landing zone, the coeliac artery (CA) was closed without revascularization if the CA was patent preoperatively [14]. The iliac artery approach was considered in patients with a thin diameter of the external iliac artery of less than 6–7 mm and a history of bifurcated graft replacement of an abdominal aortic aneurysm.
Other methods to prevent spinal cord ischemia
After deployment of the stent graft, all patients were monitored to preserve spinal cord function based on transcranial MEPs of the anterior tibial muscle and the thenar muscles. A significant change in MEPs was defined as a reduction in MEPs to 25% or less of the baseline value [15]. If MEPs were lost or the amplitude significantly declined, the patients were managed aggressively with catecholamines to control mean blood pressure (BP) to >90 mmHg. This BP control was continued for 48 h after TEVAR. Even if no symptoms of SCI were present in high-risk patients, the mean BP was controlled to above 80 mmHg for 48 h after deployment of the endograft [16].
Primary and secondary outcomes
The primary outcome was SCI after TEVAR. It was defined as any transient or permanent neurological dysfunction in the lower extremities, including both monoplegia and paraplegia as well as bladder or bowel dysfunctions. SCI symptoms were independently evaluated by a neurologist. MRI or other imaging studies were not mandatory because CSFD was a priority. Pre- and intraoperative variables were assessed using univariable analysis to recognize the association for SCI. The secondary outcomes were CSFD-related complications.
Statistical analyses
Statistical analyses were conducted using SPSS Statistics for Windows, version 24.0 (SPSS Inc., Chicago, IL, USA). The primary and secondary outcomes were reassessed by propensity score matching (PSM). A logistic regression analysis was performed to calculate propensity scores for patients with SCI using their baseline characteristics. We performed 1:1 PSM based on the nearest-neighbour matching algorithm with replacement. The caliper width was set at 20% of the standard deviation of the propensity scores. Balances in baseline variables were also evaluated using standardized differences. Absolute values of less than 10% were considered balanced [17, 18]. In both overall and matched cohorts, categorical data were compared using Fisheŕs exact test, and continuous variables were expressed as median and interquartile range in case of results with non-normal distribution assessed with the Shapiro–Wilk test. The difference was considered statistically significant if the P value was less than 0.05. Univariable analysis was performed using logistic regression analysis to assess the effects of the covariables, including age, male sex, history of cerebral infarction, low ejection fraction (<50%), respiratory disorder, chronic kidney disease, antiplatelet therapy, anticoagulant therapy, shaggy aorta, occluded LSCA, occluded unilateral hypogastric artery, prior descending aorta graft replacement, prior intervention on an abdominal aortic aneurysm (including both graft replacement and endovascular repair), iliac access, zones covered by the stent graft, extensive stent graft coverage of more than 8 vertebrae, coverage of the ICA-AKA and coverage of the CA. Shaggy aorta was defined as an atheroma thickness greater than 5 mm with intimal irregularity [9]. Zones covered by TEVAR as a treatment length were calculated by the number of covered thoracic vertebrae to enhance the number of covered ICAs [19].
RESULTS
Patients
Of the 204 patients included in this study, 89 were treated prophylactically with CSFD and were assigned to the CSFD group [68 men and 21 women; median age (range), 76.0 (71.0–81.0) years], and the other 115 were assigned to the non-CSFD group [89 men and 26 women; median age (range), 77.0 [74.0–81.5] years] (Fig. 1).
Baseline demographic and aneurysm characteristics
The preoperative patient characteristics of both groups are listed in Table 1. In the overall cohort, patients in the CSFD group had a significantly lower frequency of old cerebral infarctions (22.4% in CSFD vs 35.2% in non-CSFD; P = 0.043), anticoagulant therapy (5.6% vs 15.6%; P = 0.017) and prior total arch replacements (24.7% vs 39.3%; P = 0.012), and a significant number of them required iliac access (37.1% vs 21.3%; P = 0.029) and coverage of the ICA-AKA (60.1% vs 27.9%; P < 0.001) than those in the non-CSFD group (Table 1). The AKA was identified in 128 patients (94.1%) by computed tomography angiography with a 16-channel multidetector-row helical computed tomography scanner.
| . | Overall . | Matched . | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable . | CSFD (89) . | Non-CSFD (115) . | P-value . | ASD . | CSFD (59) . | Non-CSFD (59) . | P-value . | ASD . |
| Mean age (years) | 76.0 (71.0-81.0) | 77.0 (74.0-81.5) | 0.106 | −0.231 | 76.0 (72.0-82.0) | 77.0 (73.0-79.0) | 0.697 | 0.072 |
| Male sex | 68 (76.4%) | 89 (77.4%) | 0.869 | 0.023 | 45 (76.0%) | 47 (80.0%) | 0.657 | 0.081 |
| Old cerebral infarction | 20 (22.4%) | 43 (37.4%) | 0.043 | −0.286 | 18 (30.5%) | 20 (33.9%) | 0.694 | −0.072 |
| Low ejection fraction (<50%) | 3 (3.4%) | 6 (5.2%) | 0.734 | −0.091 | 1 (1.7%) | 2 (3.4%) | 0.559 | −0.107 |
| Respiratory disorder | 9 (10.1%) | 14 (12.2%) | 0.824 | −0.065 | 6 (10.1%) | 5 (8.5%) | 0.752 | 0.058 |
| Chronic kidney disease (Cr > 1.5) | 21 (23.6%) | 25 (21.7%) | 0.866 | 0.044 | 13 (22.0%) | 14 (23.7%) | 0.827 | −0.040 |
| Antiplatelet therapy | 31 (34.8%) | 55 (47.8%) | 0.065 | −0.265 | 24 (40.8%) | 21 (35.6%) | 0.570 | 0.104 |
| Anticoagulant therapy | 5 (5.6%) | 19 (16.5%) | 0.017 | −0.351 | 5 (8.5%) | 4 (6.8%) | 0.729 | 0.063 |
| Smoking history | 41 (20.7%) | 45 (39.1%) | 0.391 | 0.140 | 28 (47.5%) | 27 (45.8%) | 0.854 | 0.034 |
| Shaggy aorta | 19 (21.3%) | 19 (16.5%) | 0.469 | 0.123 | 12 (20.3%) | 14 (23.7%) | 0.657 | −0.081 |
| Occluded LSCA | 0 | 3 (2.7%) | 0.259 | −0.230 | 0 | 0 | – | – |
| Occluded unilateral HA | 9 (10.1%) | 12 (10.4%) | 0.940 | −0.011 | 7 (11.9%) | 5 (8.5%) | 0.542 | 0.111 |
| Prior descending replacement | 6 (6.7%) | 1 (0.9%) | 0.012 | 0.309 | 0 | 1 (1.7%) | 0.315 | −0.184 |
| Prior intervention on AAA | 26 (29.2%) | 29 (25.2%) | 0.529 | 0.089 | 18 (30.5%) | 15 (25.4%) | 0.538 | 0.113 |
| Iliac access | 33 (37.1%) | 26 (22.6%) | 0.029 | 0.319 | 20 (33.9%) | 19 (32.2%) | 0.845 | 0.036 |
| Zones covered by TEVAR | 7 (6-8) | 7 (5-8) | 0.275 | 0.201 | 7 (6-8) | 6 (5-8) | 0.432 | 0.145 |
| Coverage of the ICA-AKA | 54 (60.1%) | 34 (29.6%) | <0.001 | 0.655 | 26 (44.1%) | 25 (42.4%) | 0.347 | 0.172 |
| Coverage of the coeliac trunk | 6 (6.7%) | 4 (3.5%) | 0.337 | 0.148 | 3 (5.1%) | 3 (5.1%) | 1.000 | −0.000 |
| . | Overall . | Matched . | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable . | CSFD (89) . | Non-CSFD (115) . | P-value . | ASD . | CSFD (59) . | Non-CSFD (59) . | P-value . | ASD . |
| Mean age (years) | 76.0 (71.0-81.0) | 77.0 (74.0-81.5) | 0.106 | −0.231 | 76.0 (72.0-82.0) | 77.0 (73.0-79.0) | 0.697 | 0.072 |
| Male sex | 68 (76.4%) | 89 (77.4%) | 0.869 | 0.023 | 45 (76.0%) | 47 (80.0%) | 0.657 | 0.081 |
| Old cerebral infarction | 20 (22.4%) | 43 (37.4%) | 0.043 | −0.286 | 18 (30.5%) | 20 (33.9%) | 0.694 | −0.072 |
| Low ejection fraction (<50%) | 3 (3.4%) | 6 (5.2%) | 0.734 | −0.091 | 1 (1.7%) | 2 (3.4%) | 0.559 | −0.107 |
| Respiratory disorder | 9 (10.1%) | 14 (12.2%) | 0.824 | −0.065 | 6 (10.1%) | 5 (8.5%) | 0.752 | 0.058 |
| Chronic kidney disease (Cr > 1.5) | 21 (23.6%) | 25 (21.7%) | 0.866 | 0.044 | 13 (22.0%) | 14 (23.7%) | 0.827 | −0.040 |
| Antiplatelet therapy | 31 (34.8%) | 55 (47.8%) | 0.065 | −0.265 | 24 (40.8%) | 21 (35.6%) | 0.570 | 0.104 |
| Anticoagulant therapy | 5 (5.6%) | 19 (16.5%) | 0.017 | −0.351 | 5 (8.5%) | 4 (6.8%) | 0.729 | 0.063 |
| Smoking history | 41 (20.7%) | 45 (39.1%) | 0.391 | 0.140 | 28 (47.5%) | 27 (45.8%) | 0.854 | 0.034 |
| Shaggy aorta | 19 (21.3%) | 19 (16.5%) | 0.469 | 0.123 | 12 (20.3%) | 14 (23.7%) | 0.657 | −0.081 |
| Occluded LSCA | 0 | 3 (2.7%) | 0.259 | −0.230 | 0 | 0 | – | – |
| Occluded unilateral HA | 9 (10.1%) | 12 (10.4%) | 0.940 | −0.011 | 7 (11.9%) | 5 (8.5%) | 0.542 | 0.111 |
| Prior descending replacement | 6 (6.7%) | 1 (0.9%) | 0.012 | 0.309 | 0 | 1 (1.7%) | 0.315 | −0.184 |
| Prior intervention on AAA | 26 (29.2%) | 29 (25.2%) | 0.529 | 0.089 | 18 (30.5%) | 15 (25.4%) | 0.538 | 0.113 |
| Iliac access | 33 (37.1%) | 26 (22.6%) | 0.029 | 0.319 | 20 (33.9%) | 19 (32.2%) | 0.845 | 0.036 |
| Zones covered by TEVAR | 7 (6-8) | 7 (5-8) | 0.275 | 0.201 | 7 (6-8) | 6 (5-8) | 0.432 | 0.145 |
| Coverage of the ICA-AKA | 54 (60.1%) | 34 (29.6%) | <0.001 | 0.655 | 26 (44.1%) | 25 (42.4%) | 0.347 | 0.172 |
| Coverage of the coeliac trunk | 6 (6.7%) | 4 (3.5%) | 0.337 | 0.148 | 3 (5.1%) | 3 (5.1%) | 1.000 | −0.000 |
AAA: abdominal aortic aneurysm; AKA: Adamkiewicz artery; ASD: absolute standardized difference; CSFD: cerebrospinal fluid drainage; Cr: creatinine; HA: hypogastric artery; ICA: intercostal artery; LSCA: left subclavian artery; TEVAR: thoracic endovascular aortic repair.
| . | Overall . | Matched . | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable . | CSFD (89) . | Non-CSFD (115) . | P-value . | ASD . | CSFD (59) . | Non-CSFD (59) . | P-value . | ASD . |
| Mean age (years) | 76.0 (71.0-81.0) | 77.0 (74.0-81.5) | 0.106 | −0.231 | 76.0 (72.0-82.0) | 77.0 (73.0-79.0) | 0.697 | 0.072 |
| Male sex | 68 (76.4%) | 89 (77.4%) | 0.869 | 0.023 | 45 (76.0%) | 47 (80.0%) | 0.657 | 0.081 |
| Old cerebral infarction | 20 (22.4%) | 43 (37.4%) | 0.043 | −0.286 | 18 (30.5%) | 20 (33.9%) | 0.694 | −0.072 |
| Low ejection fraction (<50%) | 3 (3.4%) | 6 (5.2%) | 0.734 | −0.091 | 1 (1.7%) | 2 (3.4%) | 0.559 | −0.107 |
| Respiratory disorder | 9 (10.1%) | 14 (12.2%) | 0.824 | −0.065 | 6 (10.1%) | 5 (8.5%) | 0.752 | 0.058 |
| Chronic kidney disease (Cr > 1.5) | 21 (23.6%) | 25 (21.7%) | 0.866 | 0.044 | 13 (22.0%) | 14 (23.7%) | 0.827 | −0.040 |
| Antiplatelet therapy | 31 (34.8%) | 55 (47.8%) | 0.065 | −0.265 | 24 (40.8%) | 21 (35.6%) | 0.570 | 0.104 |
| Anticoagulant therapy | 5 (5.6%) | 19 (16.5%) | 0.017 | −0.351 | 5 (8.5%) | 4 (6.8%) | 0.729 | 0.063 |
| Smoking history | 41 (20.7%) | 45 (39.1%) | 0.391 | 0.140 | 28 (47.5%) | 27 (45.8%) | 0.854 | 0.034 |
| Shaggy aorta | 19 (21.3%) | 19 (16.5%) | 0.469 | 0.123 | 12 (20.3%) | 14 (23.7%) | 0.657 | −0.081 |
| Occluded LSCA | 0 | 3 (2.7%) | 0.259 | −0.230 | 0 | 0 | – | – |
| Occluded unilateral HA | 9 (10.1%) | 12 (10.4%) | 0.940 | −0.011 | 7 (11.9%) | 5 (8.5%) | 0.542 | 0.111 |
| Prior descending replacement | 6 (6.7%) | 1 (0.9%) | 0.012 | 0.309 | 0 | 1 (1.7%) | 0.315 | −0.184 |
| Prior intervention on AAA | 26 (29.2%) | 29 (25.2%) | 0.529 | 0.089 | 18 (30.5%) | 15 (25.4%) | 0.538 | 0.113 |
| Iliac access | 33 (37.1%) | 26 (22.6%) | 0.029 | 0.319 | 20 (33.9%) | 19 (32.2%) | 0.845 | 0.036 |
| Zones covered by TEVAR | 7 (6-8) | 7 (5-8) | 0.275 | 0.201 | 7 (6-8) | 6 (5-8) | 0.432 | 0.145 |
| Coverage of the ICA-AKA | 54 (60.1%) | 34 (29.6%) | <0.001 | 0.655 | 26 (44.1%) | 25 (42.4%) | 0.347 | 0.172 |
| Coverage of the coeliac trunk | 6 (6.7%) | 4 (3.5%) | 0.337 | 0.148 | 3 (5.1%) | 3 (5.1%) | 1.000 | −0.000 |
| . | Overall . | Matched . | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable . | CSFD (89) . | Non-CSFD (115) . | P-value . | ASD . | CSFD (59) . | Non-CSFD (59) . | P-value . | ASD . |
| Mean age (years) | 76.0 (71.0-81.0) | 77.0 (74.0-81.5) | 0.106 | −0.231 | 76.0 (72.0-82.0) | 77.0 (73.0-79.0) | 0.697 | 0.072 |
| Male sex | 68 (76.4%) | 89 (77.4%) | 0.869 | 0.023 | 45 (76.0%) | 47 (80.0%) | 0.657 | 0.081 |
| Old cerebral infarction | 20 (22.4%) | 43 (37.4%) | 0.043 | −0.286 | 18 (30.5%) | 20 (33.9%) | 0.694 | −0.072 |
| Low ejection fraction (<50%) | 3 (3.4%) | 6 (5.2%) | 0.734 | −0.091 | 1 (1.7%) | 2 (3.4%) | 0.559 | −0.107 |
| Respiratory disorder | 9 (10.1%) | 14 (12.2%) | 0.824 | −0.065 | 6 (10.1%) | 5 (8.5%) | 0.752 | 0.058 |
| Chronic kidney disease (Cr > 1.5) | 21 (23.6%) | 25 (21.7%) | 0.866 | 0.044 | 13 (22.0%) | 14 (23.7%) | 0.827 | −0.040 |
| Antiplatelet therapy | 31 (34.8%) | 55 (47.8%) | 0.065 | −0.265 | 24 (40.8%) | 21 (35.6%) | 0.570 | 0.104 |
| Anticoagulant therapy | 5 (5.6%) | 19 (16.5%) | 0.017 | −0.351 | 5 (8.5%) | 4 (6.8%) | 0.729 | 0.063 |
| Smoking history | 41 (20.7%) | 45 (39.1%) | 0.391 | 0.140 | 28 (47.5%) | 27 (45.8%) | 0.854 | 0.034 |
| Shaggy aorta | 19 (21.3%) | 19 (16.5%) | 0.469 | 0.123 | 12 (20.3%) | 14 (23.7%) | 0.657 | −0.081 |
| Occluded LSCA | 0 | 3 (2.7%) | 0.259 | −0.230 | 0 | 0 | – | – |
| Occluded unilateral HA | 9 (10.1%) | 12 (10.4%) | 0.940 | −0.011 | 7 (11.9%) | 5 (8.5%) | 0.542 | 0.111 |
| Prior descending replacement | 6 (6.7%) | 1 (0.9%) | 0.012 | 0.309 | 0 | 1 (1.7%) | 0.315 | −0.184 |
| Prior intervention on AAA | 26 (29.2%) | 29 (25.2%) | 0.529 | 0.089 | 18 (30.5%) | 15 (25.4%) | 0.538 | 0.113 |
| Iliac access | 33 (37.1%) | 26 (22.6%) | 0.029 | 0.319 | 20 (33.9%) | 19 (32.2%) | 0.845 | 0.036 |
| Zones covered by TEVAR | 7 (6-8) | 7 (5-8) | 0.275 | 0.201 | 7 (6-8) | 6 (5-8) | 0.432 | 0.145 |
| Coverage of the ICA-AKA | 54 (60.1%) | 34 (29.6%) | <0.001 | 0.655 | 26 (44.1%) | 25 (42.4%) | 0.347 | 0.172 |
| Coverage of the coeliac trunk | 6 (6.7%) | 4 (3.5%) | 0.337 | 0.148 | 3 (5.1%) | 3 (5.1%) | 1.000 | −0.000 |
AAA: abdominal aortic aneurysm; AKA: Adamkiewicz artery; ASD: absolute standardized difference; CSFD: cerebrospinal fluid drainage; Cr: creatinine; HA: hypogastric artery; ICA: intercostal artery; LSCA: left subclavian artery; TEVAR: thoracic endovascular aortic repair.
Early outcomes in the overall cohort
A comparison of intra- and postoperative findings is presented in Table 2. Procedure time did not differ between the 2 groups [111 (91–153) min in the CSFD group vs 110 (93–148) in the non-CSFD group; P = 0.504]. The median (interquartile range) intraoperative blood loss was significantly greater in the CSFD group than in the non-CSFD group [170 (70–46) mL vs 50 (10–175) mL; P = 0.012]. Selection of the stent graft was made individually and was based on anatomical features and the surgeon’s preference without a specific protocol. No significant between-group difference was observed in the use of Gore TAG or cTAG (W. L. Gore and Associates Inc, Flagstaff, AZ, USA) (46% in the CSFD group vs 44% in the non-CSFD group; P = 0.887). There were significant differences between the CSFD and non-CSFD groups in the use of the Talent (Medtronic Inc, Minneapolis, MN, USA) (12% vs 1.7%; P = 0.003), Valiant (10% vs 23%; P = 0.024) and Zenith TX2 or Alpha grafts (Cook Inc, Bloomington, IN, USA) (11% vs 9.6%; P = 0.027) . The RELAY stent (Bolton Medical Inc, Sunrise, FL, USA) was used less frequently in the CSFD group than in the non-CSFD group (10% vs 20%; P = 0.027).
Comparison of intra- and postoperative findings and complications (entire and matched cohorts)
| . | Overall . | Matched . | ||||
|---|---|---|---|---|---|---|
| Variable . | CSFD (89) . | Non-CSFD (115) . | P-value . | CSFD (59) . | Non-CSFD (59) . | P-value . |
| Intraoperative findings | ||||||
| Procedure time, min | 111 (91-153) | 110 (93-148) | 0.504 | 109 (88-146) | 120 (99-159) | 0.197 |
| Intraoperative blood loss, ml | 170 (70-460) | 50 (10-175) | 0.012 | 170 (50-420) | 100 (10-325) | 0.139 |
| Postoperative complications | ||||||
| Stroke | 0 | 1 (0.8%) | 0.898 | 0 | 1 (1.7%) | 0.315 |
| Pneumonia | 0 | 5 (4.1%) | 0.070 | 0 | 4 (6.8%) | 0.042 |
| Continuous haemodiafiltration | 0 | 1 (0.8%) | 0.898 | 0 | 1 (1.7%) | 0.315 |
| Gastrointestinal disorders | 1 (1.1%) | 2 (1.6%) | 0.717 | 1 (1.7%) | 1 (1.7%) | 1.000 |
| Access route injury | 3 (3.4%) | 3 (2.5%) | 0.749 | 3 (5.1%) | 3 (5.1%) | 1.000 |
| Wound problems | 2 (2.2%) | 1 (0.8%) | 0.418 | 0 | 0 | – |
| Infectious diseases | 1 (1.1%) | 1 (0.8%) | 0.855 | 1 (1.7%) | 1 (1.8%) | 1.000 |
| CSFD-related findings | ||||||
| SCI | 8 (9.0%) | 6 (4.9%) | 0.403 | 3 (5.1%) | 4 (7.3%) | 0.697 |
| Transient paraparesis | 6 (6.7%) | 3 (2.5%) | 0.182 | 3 (5.1%) | 2 (3.4%) | 0.648 |
| Permanent paraplegia | 2 (2.2%) | 3 (2.5%) | 0.868 | 0 | 2 (3.4%) | 0.157 |
| Intracranial haemorrhage | 1 (1.1%) | 0 | 0.436 | 0 | 0 | – |
| Postoperative therapeutic CSFD | 0 | 3 (2.5%) | 0.259 | 0 | 1 (1.7%) | 0.315 |
| . | Overall . | Matched . | ||||
|---|---|---|---|---|---|---|
| Variable . | CSFD (89) . | Non-CSFD (115) . | P-value . | CSFD (59) . | Non-CSFD (59) . | P-value . |
| Intraoperative findings | ||||||
| Procedure time, min | 111 (91-153) | 110 (93-148) | 0.504 | 109 (88-146) | 120 (99-159) | 0.197 |
| Intraoperative blood loss, ml | 170 (70-460) | 50 (10-175) | 0.012 | 170 (50-420) | 100 (10-325) | 0.139 |
| Postoperative complications | ||||||
| Stroke | 0 | 1 (0.8%) | 0.898 | 0 | 1 (1.7%) | 0.315 |
| Pneumonia | 0 | 5 (4.1%) | 0.070 | 0 | 4 (6.8%) | 0.042 |
| Continuous haemodiafiltration | 0 | 1 (0.8%) | 0.898 | 0 | 1 (1.7%) | 0.315 |
| Gastrointestinal disorders | 1 (1.1%) | 2 (1.6%) | 0.717 | 1 (1.7%) | 1 (1.7%) | 1.000 |
| Access route injury | 3 (3.4%) | 3 (2.5%) | 0.749 | 3 (5.1%) | 3 (5.1%) | 1.000 |
| Wound problems | 2 (2.2%) | 1 (0.8%) | 0.418 | 0 | 0 | – |
| Infectious diseases | 1 (1.1%) | 1 (0.8%) | 0.855 | 1 (1.7%) | 1 (1.8%) | 1.000 |
| CSFD-related findings | ||||||
| SCI | 8 (9.0%) | 6 (4.9%) | 0.403 | 3 (5.1%) | 4 (7.3%) | 0.697 |
| Transient paraparesis | 6 (6.7%) | 3 (2.5%) | 0.182 | 3 (5.1%) | 2 (3.4%) | 0.648 |
| Permanent paraplegia | 2 (2.2%) | 3 (2.5%) | 0.868 | 0 | 2 (3.4%) | 0.157 |
| Intracranial haemorrhage | 1 (1.1%) | 0 | 0.436 | 0 | 0 | – |
| Postoperative therapeutic CSFD | 0 | 3 (2.5%) | 0.259 | 0 | 1 (1.7%) | 0.315 |
CSFD: cerebrospinal fluid drainage; SCI: spinal cord ischemia.
Comparison of intra- and postoperative findings and complications (entire and matched cohorts)
| . | Overall . | Matched . | ||||
|---|---|---|---|---|---|---|
| Variable . | CSFD (89) . | Non-CSFD (115) . | P-value . | CSFD (59) . | Non-CSFD (59) . | P-value . |
| Intraoperative findings | ||||||
| Procedure time, min | 111 (91-153) | 110 (93-148) | 0.504 | 109 (88-146) | 120 (99-159) | 0.197 |
| Intraoperative blood loss, ml | 170 (70-460) | 50 (10-175) | 0.012 | 170 (50-420) | 100 (10-325) | 0.139 |
| Postoperative complications | ||||||
| Stroke | 0 | 1 (0.8%) | 0.898 | 0 | 1 (1.7%) | 0.315 |
| Pneumonia | 0 | 5 (4.1%) | 0.070 | 0 | 4 (6.8%) | 0.042 |
| Continuous haemodiafiltration | 0 | 1 (0.8%) | 0.898 | 0 | 1 (1.7%) | 0.315 |
| Gastrointestinal disorders | 1 (1.1%) | 2 (1.6%) | 0.717 | 1 (1.7%) | 1 (1.7%) | 1.000 |
| Access route injury | 3 (3.4%) | 3 (2.5%) | 0.749 | 3 (5.1%) | 3 (5.1%) | 1.000 |
| Wound problems | 2 (2.2%) | 1 (0.8%) | 0.418 | 0 | 0 | – |
| Infectious diseases | 1 (1.1%) | 1 (0.8%) | 0.855 | 1 (1.7%) | 1 (1.8%) | 1.000 |
| CSFD-related findings | ||||||
| SCI | 8 (9.0%) | 6 (4.9%) | 0.403 | 3 (5.1%) | 4 (7.3%) | 0.697 |
| Transient paraparesis | 6 (6.7%) | 3 (2.5%) | 0.182 | 3 (5.1%) | 2 (3.4%) | 0.648 |
| Permanent paraplegia | 2 (2.2%) | 3 (2.5%) | 0.868 | 0 | 2 (3.4%) | 0.157 |
| Intracranial haemorrhage | 1 (1.1%) | 0 | 0.436 | 0 | 0 | – |
| Postoperative therapeutic CSFD | 0 | 3 (2.5%) | 0.259 | 0 | 1 (1.7%) | 0.315 |
| . | Overall . | Matched . | ||||
|---|---|---|---|---|---|---|
| Variable . | CSFD (89) . | Non-CSFD (115) . | P-value . | CSFD (59) . | Non-CSFD (59) . | P-value . |
| Intraoperative findings | ||||||
| Procedure time, min | 111 (91-153) | 110 (93-148) | 0.504 | 109 (88-146) | 120 (99-159) | 0.197 |
| Intraoperative blood loss, ml | 170 (70-460) | 50 (10-175) | 0.012 | 170 (50-420) | 100 (10-325) | 0.139 |
| Postoperative complications | ||||||
| Stroke | 0 | 1 (0.8%) | 0.898 | 0 | 1 (1.7%) | 0.315 |
| Pneumonia | 0 | 5 (4.1%) | 0.070 | 0 | 4 (6.8%) | 0.042 |
| Continuous haemodiafiltration | 0 | 1 (0.8%) | 0.898 | 0 | 1 (1.7%) | 0.315 |
| Gastrointestinal disorders | 1 (1.1%) | 2 (1.6%) | 0.717 | 1 (1.7%) | 1 (1.7%) | 1.000 |
| Access route injury | 3 (3.4%) | 3 (2.5%) | 0.749 | 3 (5.1%) | 3 (5.1%) | 1.000 |
| Wound problems | 2 (2.2%) | 1 (0.8%) | 0.418 | 0 | 0 | – |
| Infectious diseases | 1 (1.1%) | 1 (0.8%) | 0.855 | 1 (1.7%) | 1 (1.8%) | 1.000 |
| CSFD-related findings | ||||||
| SCI | 8 (9.0%) | 6 (4.9%) | 0.403 | 3 (5.1%) | 4 (7.3%) | 0.697 |
| Transient paraparesis | 6 (6.7%) | 3 (2.5%) | 0.182 | 3 (5.1%) | 2 (3.4%) | 0.648 |
| Permanent paraplegia | 2 (2.2%) | 3 (2.5%) | 0.868 | 0 | 2 (3.4%) | 0.157 |
| Intracranial haemorrhage | 1 (1.1%) | 0 | 0.436 | 0 | 0 | – |
| Postoperative therapeutic CSFD | 0 | 3 (2.5%) | 0.259 | 0 | 1 (1.7%) | 0.315 |
CSFD: cerebrospinal fluid drainage; SCI: spinal cord ischemia.
The overall hospital mortality rate was 2.0% (4/204); in the CSFD group, it was 1.1% (1/89), and in the non-CSFD group, it was 2.6% (3/115) (P = 0.633). Infective endocarditis was the cause of in-hospital death in 1 patient from the CSFD group. In the non-CSFD group, 2 patients died of pneumonia and 1 patient died of pan-peritonitis. Among postoperative complications, pneumonia was less prevalent in the CSFD group than in the non-CSFD group [0/89 (0%) vs 5/115 (4.1%); P = 0.070]. The study groups did not differ with regard to the incidence of other perioperative complications including stroke, acute kidney injury requiring temporary dialysis, gastrointestinal disorders and access route injury (Table 2). Moreover, the study groups did not differ with regard to postoperative type I endoleak [3/89 (3.4%) vs 4/115 (3.5%); P = 0.967], type II [8/89 (9.0%) vs 9/115 (7.8%); P = 0.766] and type III [1/89 (1.1%) vs 1/115 (0.9%); P = 0.855].
Findings related to cerebrospinal fluid drainage in the overall cohort
Overall, SCI was observed in 14 patients (6.9%). The incidence of SCI was similar between the study groups [8/89 (9.0%) in the CSFD group and 6/115 (5.2%) in the non-CSFD group; P = 0.403]. The incidences of transient paraparesis [6/89 (6.7%) vs 3/115 (2.5%); P = 0.182] and permanent paraplegia [2/89 (2.2%) vs 3/115 (2.5%); P = 0.868] were similar between the CSFD and non-CSFD groups. A major CSFD-related complication occurred in 1 patient from the CSFD group (1.1%, 1/89) (Fig. 2). There were no CSFD-related infections and CSFD fractures requiring surgical intervention. Postoperative therapeutic CSFD was performed in 3 patients (2.5%) in the non-CSFD group (Table 2).
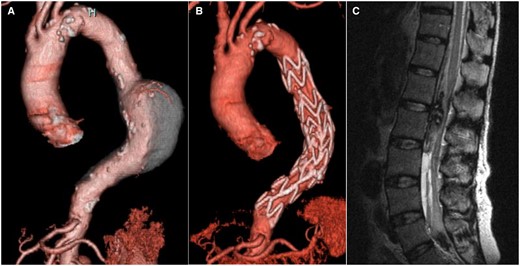
Spinal subdural haematoma. The case of a spinal subdural haematoma is exhibited. (A) Preoperative computed tomography demonstrates the descending thoracic aortic aneurysm. (B) Postoperative computed tomography demonstrates excluded descending thoracic aortic aneurysm with no endoleaks. (C) On magnetic resonance imaging, a spinal subdural haematoma was detected after removal of the cerebrospinal fluid drainage tube 2 days after thoracic endovascular aortic repair.
Factors associated with spinal cord ischemia
The univariable analysis showed that shaggy aorta [odds ratio (OR), 5.13; P = 0.004] and iliac artery access (OR, 5.04; P = 0.005) were identified as positive predictors of SCI. Other clinically important confounders including CA coverage (OR, 3.79; P = 0.115), occluded unilateral hypogastric artery (OR, 2.61; P = 0.169), AKA coverage (OR, 2.53; P = 0.108), history of descending replacement (OR, 2.36; P = 0.443) and extensive stent graft coverage (>8 vertebrae) (OR, 1.41; P = 0.541) were not statistically significant (Table 3).
| Covariate . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| Univariable | |||
| Mean age (years) | 1.05 | 0.97-1.14 | 0.258 |
| Female sex | 1.37 | 0.41-4.58 | 0.612 |
| Preoperative factor | |||
| Old cerebral infarction | 0.85 | 0.26-2.76 | 0.789 |
| Low ejection fraction (<50%) | 0.00 | 0.00- | 0.999 |
| Respiratory disorder | 1.34 | 0.28-6.41 | 0.713 |
| Chronic kidney disease (Cr > 1.5) | 0.93 | 0.25-3.49 | 0.917 |
| Preoperative CSFD | 1.79 | 0.60-5.37 | 0.296 |
| Antiplatelet therapy | 0.53 | 0.16-1.74 | 0.293 |
| Anticoagulant therapy | 1.27 | 0.27-6.07 | 0.762 |
| Smoking history | 1.92 | 0.64-5.74 | 0.246 |
| Shaggy aorta | 5.13 | 1.68-15.7 | 0.004 |
| Occluded LSCA | 0.00 | 0.00- | 0.999 |
| Occluded hypogastric artery | 2.61 | 0.67-10.2 | 0.169 |
| Prior intervention on the descending aorta | 2.36 | 0.26-21.1 | 0.443 |
| Prior intervention on the abdominal aorta | 0.43 | 0.09-1.99 | 0.281 |
| Intraoperative factor | |||
| Iliac artery access | 5.04 | 1.62-15.8 | 0.005 |
| Distal landing zone | 1.36 | 0.92-1.99 | 0.120 |
| Zones covered by TEVAR | 1.07 | 0.79-1.44 | 0.661 |
| Extensive stent graft coverage (more than 8 vertebrae) | 1.41 | 0.47-4.23 | 0.541 |
| Coverage of the ICA-AKA | 2.53 | 0.82-7.83 | 0.108 |
| Coverage of the coeliac trunk | 3.79 | 0.72-19.9 | 0.115 |
| Covariate . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| Univariable | |||
| Mean age (years) | 1.05 | 0.97-1.14 | 0.258 |
| Female sex | 1.37 | 0.41-4.58 | 0.612 |
| Preoperative factor | |||
| Old cerebral infarction | 0.85 | 0.26-2.76 | 0.789 |
| Low ejection fraction (<50%) | 0.00 | 0.00- | 0.999 |
| Respiratory disorder | 1.34 | 0.28-6.41 | 0.713 |
| Chronic kidney disease (Cr > 1.5) | 0.93 | 0.25-3.49 | 0.917 |
| Preoperative CSFD | 1.79 | 0.60-5.37 | 0.296 |
| Antiplatelet therapy | 0.53 | 0.16-1.74 | 0.293 |
| Anticoagulant therapy | 1.27 | 0.27-6.07 | 0.762 |
| Smoking history | 1.92 | 0.64-5.74 | 0.246 |
| Shaggy aorta | 5.13 | 1.68-15.7 | 0.004 |
| Occluded LSCA | 0.00 | 0.00- | 0.999 |
| Occluded hypogastric artery | 2.61 | 0.67-10.2 | 0.169 |
| Prior intervention on the descending aorta | 2.36 | 0.26-21.1 | 0.443 |
| Prior intervention on the abdominal aorta | 0.43 | 0.09-1.99 | 0.281 |
| Intraoperative factor | |||
| Iliac artery access | 5.04 | 1.62-15.8 | 0.005 |
| Distal landing zone | 1.36 | 0.92-1.99 | 0.120 |
| Zones covered by TEVAR | 1.07 | 0.79-1.44 | 0.661 |
| Extensive stent graft coverage (more than 8 vertebrae) | 1.41 | 0.47-4.23 | 0.541 |
| Coverage of the ICA-AKA | 2.53 | 0.82-7.83 | 0.108 |
| Coverage of the coeliac trunk | 3.79 | 0.72-19.9 | 0.115 |
AKA: Adamkiewicz artery; CI: confidence interval; Cr: creatinine; CSFD: cerebrospinal fluid drainage; ICA: intercostal artery; LSCA: left subclavian artery; OR: odds ratio; TEVAR: thoracic endovascular aortic repair.
| Covariate . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| Univariable | |||
| Mean age (years) | 1.05 | 0.97-1.14 | 0.258 |
| Female sex | 1.37 | 0.41-4.58 | 0.612 |
| Preoperative factor | |||
| Old cerebral infarction | 0.85 | 0.26-2.76 | 0.789 |
| Low ejection fraction (<50%) | 0.00 | 0.00- | 0.999 |
| Respiratory disorder | 1.34 | 0.28-6.41 | 0.713 |
| Chronic kidney disease (Cr > 1.5) | 0.93 | 0.25-3.49 | 0.917 |
| Preoperative CSFD | 1.79 | 0.60-5.37 | 0.296 |
| Antiplatelet therapy | 0.53 | 0.16-1.74 | 0.293 |
| Anticoagulant therapy | 1.27 | 0.27-6.07 | 0.762 |
| Smoking history | 1.92 | 0.64-5.74 | 0.246 |
| Shaggy aorta | 5.13 | 1.68-15.7 | 0.004 |
| Occluded LSCA | 0.00 | 0.00- | 0.999 |
| Occluded hypogastric artery | 2.61 | 0.67-10.2 | 0.169 |
| Prior intervention on the descending aorta | 2.36 | 0.26-21.1 | 0.443 |
| Prior intervention on the abdominal aorta | 0.43 | 0.09-1.99 | 0.281 |
| Intraoperative factor | |||
| Iliac artery access | 5.04 | 1.62-15.8 | 0.005 |
| Distal landing zone | 1.36 | 0.92-1.99 | 0.120 |
| Zones covered by TEVAR | 1.07 | 0.79-1.44 | 0.661 |
| Extensive stent graft coverage (more than 8 vertebrae) | 1.41 | 0.47-4.23 | 0.541 |
| Coverage of the ICA-AKA | 2.53 | 0.82-7.83 | 0.108 |
| Coverage of the coeliac trunk | 3.79 | 0.72-19.9 | 0.115 |
| Covariate . | OR . | 95% CI . | P-value . |
|---|---|---|---|
| Univariable | |||
| Mean age (years) | 1.05 | 0.97-1.14 | 0.258 |
| Female sex | 1.37 | 0.41-4.58 | 0.612 |
| Preoperative factor | |||
| Old cerebral infarction | 0.85 | 0.26-2.76 | 0.789 |
| Low ejection fraction (<50%) | 0.00 | 0.00- | 0.999 |
| Respiratory disorder | 1.34 | 0.28-6.41 | 0.713 |
| Chronic kidney disease (Cr > 1.5) | 0.93 | 0.25-3.49 | 0.917 |
| Preoperative CSFD | 1.79 | 0.60-5.37 | 0.296 |
| Antiplatelet therapy | 0.53 | 0.16-1.74 | 0.293 |
| Anticoagulant therapy | 1.27 | 0.27-6.07 | 0.762 |
| Smoking history | 1.92 | 0.64-5.74 | 0.246 |
| Shaggy aorta | 5.13 | 1.68-15.7 | 0.004 |
| Occluded LSCA | 0.00 | 0.00- | 0.999 |
| Occluded hypogastric artery | 2.61 | 0.67-10.2 | 0.169 |
| Prior intervention on the descending aorta | 2.36 | 0.26-21.1 | 0.443 |
| Prior intervention on the abdominal aorta | 0.43 | 0.09-1.99 | 0.281 |
| Intraoperative factor | |||
| Iliac artery access | 5.04 | 1.62-15.8 | 0.005 |
| Distal landing zone | 1.36 | 0.92-1.99 | 0.120 |
| Zones covered by TEVAR | 1.07 | 0.79-1.44 | 0.661 |
| Extensive stent graft coverage (more than 8 vertebrae) | 1.41 | 0.47-4.23 | 0.541 |
| Coverage of the ICA-AKA | 2.53 | 0.82-7.83 | 0.108 |
| Coverage of the coeliac trunk | 3.79 | 0.72-19.9 | 0.115 |
AKA: Adamkiewicz artery; CI: confidence interval; Cr: creatinine; CSFD: cerebrospinal fluid drainage; ICA: intercostal artery; LSCA: left subclavian artery; OR: odds ratio; TEVAR: thoracic endovascular aortic repair.
Analysis of matched cohorts by propensity score matching
After matching, 59 patients were selected from each group using the propensity scores to adjust for the risks for SCI including the frequency of coverage of the ICA-AKA by a stent graft and the variables related to CSFD (Table 1). PSM yielded a similar incidence of SCI: 4 patients (6.8%) from the CSFD group and 3 (5.1%) from the non-CSFD group (P = 0.697). The overall hospital mortality rate [2 (3.4%) vs 0; P = 0.496] and the incidences of other perioperative complications (including stroke, acute kidney injury, gastrointestinal disorders and access route injury) did not differ between the study groups. Postoperative pneumonia was the only complication that occurred significantly more frequently in the non-CSFD group than in the CSFD group [4 (6.8%) vs 0; P = 0.042] (Table 2).
DISCUSSION
If not contraindicated, prophylactic CSFD in patients at high risk of SCI who are to undergo TEVAR is recommended (level B and class I) by the Society for Vascular Surgery Practice Guidelines applicable to TEVAR for descending TAA [20]. In these guidelines, the following are considered risk factors of SCI: long coverage of the descending aorta including previous surgical aortic coverage, occluded hypogastric arteries, occluded vertebral arteries and planned LSCA coverage [21, 22]. As a preventive measure, we have also usually applied CSFD in patients who were considered at high risk of SCI. We have additionally considered coverage of the ICA-AKA to be an important risk factor that is not included in the Society of Vascular Surgery guidelines [5].
Nevertheless, the ability of prophylactic CSFD to prevent SCI during TEVAR is still controversial, with studies showing conflicting results. Wong et al., in their systematic review of the literature on SCI and CSFD available in 2012, could not establish the role of prophylactic CSFD [23]. They argued that a high-quality study, such as an adequately powered clinical trial in patients stratified for pre-existing risk factors, would be required to validate the efficacy of prophylactic CSFD. In the most recent systematic review and meta-analysis on prophylactic CSFD for TEVAR published in 2021, Zhang et al. concluded that there was no significant relationship between the incidence of SCI and routine or selective use of prophylactic CSFD in patients undergoing TEVAR for degenerative TAA [23]. However, this study was not performed in patients with comparable risk factors for SCI, so that conclusion is still debatable.
To strengthen comparisons in patients with or without prophylactic CSFD, our study cohort was limited to patients who were treated for degradative TAA, excluding those with aortic dissection, pseudoaneurysm, aortic rupture, TAAA and a history of TEVAR. In addition, we applied PSM to match patients of various backgrounds including clinically significant variables related to SCI based on previous research and experience. Consequently, our matched cohort analysis showed no advantage of prophylactic CSFD to prevent SCI during TEVAR for degenerative descending TAA.
In Japan, Yoshitani et al. recently indicated that prophylactic CSFD might not be effective for postoperative motor deficits at discharge in a large-scale study including reviewed data from 1214 patients [open surgery, 601 (49.5%); endovascular repair, 613 (50.5%)]. Interestingly, in their study, a PSM of 700 patients was conducted, and logistic regression analysis showed that CSFD was associated with postoperative motor deficits at discharge (OR, 3.87; 95% CI, 2.30–6.51). This result, based on a significant number of cases, supports our outcomes.
Another key issue related to using prophylactic CSFD for degenerative descending TAA is its invasiveness. The significance of complications related to CSFD has been reported previously [24–26]. Estrera et al. reported that the CSFD-related complications occurred in 1.5% (17 of 1107) of patients with no identified specific risk factors for TAAA repair [24]. The most devastating complication associated with CSFD after TEVAR for TAA is spinal subdural haematoma, which has a prevalence of about 0.5% [24–26]. In such cases, some patients could experience neurological deficits due to SCI and might need an intervention to remove the haematoma. In our study, fortunately, the patient with a spinal subdural haematoma received successful medical treatment. However, the necessity for prophylactic CSFD should be carefully considered, an issue that we raised in the central image, given the potential for these complications and considering the relatively low possibility to prevent SCI after TEVAR [1–3].
As another measure to prevent SCI, we have generally aggressively applied catecholamines to increase blood supply through collateral networks to the spinal cord [27]. In their study, Weissler et al. included 223 patients who had descending and/or arch TEVAR during a 6.5-year period and found a 0% incidence of SCI by their restrictive CSFD algorithm, including permissive hypertension with LSCA revascularization [10]. They set the systolic BP at more than 120 mmHg and the mean BP at more than 70 mmHg. Similarly, in our institute, mean BP is controlled to above 80 mmHg for 48 h after deployment of the endograft in high-risk patients or in patients with abnormal intraoperative MEPs. In fact, we had patients with SCI whose symptoms improved only with elevated BP; thus, management of BP is of high importance.
Furthermore, if the SCI occurred because of an embolism, prophylactic CSFD can be therapeutic but will have no prophylactic effect. In addition, TEVAR for descending TAA is generally not time-consuming, and we think that it is not too late to perform CSFD after the anaesthesia is withdrawn and actual symptoms are confirmed. Based on these considerations, we believe that it is preferable to selectively plan prophylactic CSFD in a limited number of patients with complex risks, taking into account the risks and benefits, rather than using prophylactic CSFD for a large number of patients on a preventive basis. For the combined risk, shaggy aorta and iliac artery access were indicated by univariable analysis in this study. Based on previous studies, the following are also considered important risk factors: coverage of the ICA-AKA, extensive stent graft coverage, occluded hypogastric artery and occluded LSCA [3–5, 10, 21, 28].
Limitations
Our study has some limitations. It was a single-centre, retrospective observational study on a specific cohort of patients limited by sample size. Additional investigations and follow-ups are required to confirm our results. Improved TEVAR techniques including devices that have been introduced during the period covered by our study might affect the effectiveness of prophylactic CSFD. We stated that the indications for prophylactic CSFD were several risk factors or a combination of several risk factors. However, placement of a CSFD could not follow strict rules in an actual setting due to patient background including bleeding predisposition, vertebral deformity and the surgeon’s preference. Therefore, this factor was a major source of heterogeneity that could not be controlled using statistical methods including PSM. A larger number of TEVAR patients including patients with SCI would be needed to power this study to identify important differences in outcomes between the patients with or without prophylactic CSFD. Finally, SCI was diagnosed clinically without evidence obtained using magnetic resonance imaging due to technical issues in some patients.
CONCLUSIONS
We have demonstrated that prophylactic CSFD did not have a significant effect on the prevention of SCI after TEVAR for degenerative descending TAA. The aggressive use of prophylactic CSFD was not supportive in patients without complex risks for SCI. In this patient population, it might be acceptable to consider CSFD mainly in case of postoperative symptoms.
FUNDING
There was no funding provided.
Conflicts of interest statement: The authors have nothing to disclose regarding commercial support.
Data availability statement
The data underlying this paper cannot be shared publicly because of relevant data protection regulations. However, the data will be shared on reasonable request to the corresponding author with permission from the ethics committee.
Author contributions
Yoshimasa Seike: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Visualization; Writing—original draft; Writing—review & editing. Tetsuya Fukuda: Writing—review & editing. Koki Yokawa: Writing—review & editing. Shigeki Koizumi: Writing—review & editing. Kenta Masada: Writing—review & editing. Yosuke Inoue: Writing—review & editing. Hitoshi Matsuda: Conceptualization; Methodology; Supervision; Visualization; Writing—original draft; Writing—review & editing.
REFERENCES
Abbreviations and Acronyms
- AKA
Adamkiewicz artery
- BP
blood pressure
- CA
coeliac artery
- CSFD
cerebrospinal fluid drainage
- ICA
intercostal artery
- LSCA
left subclavian artery
- MEPs
motor-evoked potentials
- OR
odds ratio
- PSM
propensity score matching
- SCI
spinal cord ischemia
- TAA
thoracic aortic aneurysm
- TEVAR
thoracic endovascular aortic repair





