-
PDF
- Split View
-
Views
-
Cite
Cite
Iuliana Coti, Paul Werner, Alexandra Kaider, Markus Mach, Alfred Kocher, Guenther Laufer, Martin Andreas, Rapid-deployment aortic valve replacement for patients with bicuspid aortic valve: a single-centre experience, European Journal of Cardio-Thoracic Surgery, Volume 62, Issue 4, October 2022, ezac017, https://doi.org/10.1093/ejcts/ezac017
Close - Share Icon Share
Abstract
The benefit of rapid-deployment aortic valve replacement (RD-AVR) in patients with a bicuspid aortic valve (BAV) is controversial due to aortic root asymmetry and potential increased risk for valve dislocation and paravalvular leak. This study aimed to analyse the outcomes of surgical aortic valve replacement with a rapid-deployment bioprosthesis in patients with a BAV.
Between May 2010 and December 2020, all consecutive patients who underwent RD-AVR at the Medical University of Vienna were included in our institutional database. Assessment of preoperative characteristics, operative outcomes, long-term survival and clinical events was performed. The outcomes of patients presenting with a native BAV were compared with a control group of patients with native tricuspid valve (TAV); reoperative aortic valve replacements were excluded.
Out of 816 patients, who underwent RD-AVR at our institution, 107 patients with a BAV, mean age 68 (standard deviation: 8) years, were compared with a control group of 690 patients with a TAV, mean age 74 (standard deviation: 7) years; patients presenting with a BAV were significantly younger than patients with a TAV (P < 0.001). Concomitant procedures were performed in 44 (41.1%) patients in the BAV group and in 339 (49.1%) patients in the TAV group (P = 0.123); surgery of the ascending aorta was necessary in 24 (22.4%) in the BAV group, compared with 29 (4.2%) in the control group (P < 0.001). The 5-year cumulative incidence of moderate-to-severe paravalvular regurgitation in the BAV group was 10.7% [95% confidence interval (CI): 4.2–20.7%] and 3.9% (95% CI: 2.4–6.1%) in the TAV group (P = 0.057). Reoperation with valve explantation due to non-structural valve dysfunction at 5 years was 2.8% (95% CI: 0.5–8.8%) in the BAV group, compared to 1.9% (95% CI: 1.0–3.2%) in the TAV cohort (P = 0.89). The overall long-term survival rate in the BAV group was 92% (95% CI: 81–97%) at 5 years and 88% (95% CI: 73–95%), at 10 years, significantly better compared to the TAV group (log-rank test P = 0.002).
RD-AVR can be performed in patients with a BAV with convincingly medical outcomes. However, a trend to increased frequency of moderate–severe paravalvular regurgitation was observed at long-term follow-up. Consequently, a different surgical approach, compared to tricuspid valves, with distinctly specific technical- and anatomical considerations and requirements, is recommended.
INTRODUCTION
Bicuspid aortic valve (BAV) is the most common congenital heart valve disease, described in 1–2% of the general population [1]. Previous reports suggested that more than half of the patients requiring aortic valve surgery present with congenitally malformed valves in unicuspid or bicuspid conformation [2, 3]. To facilitate scientific reporting of the different phenotypes of bicuspid valves, Sievers and Schmidtke [4] proposed a systematic classification in accordance with the number of raphes (type 0—no raphe, type 1—1 raphe, type 2—2 raphes), the orientation of the raphes and cusps and the functional status of the valve.
The experience with rapid-deployment (RD) and sutureless surgical valves in bicuspid anatomy is currently limited to a few reports [5, 6] and the level of evidence is low. Therefore, there are no strong recommendations available for this specific patient population (level C, class IIA). Currently, the implantation of RD and sutureless valves is not recommended in patients with a Sievers 0 anatomy, when the coronary ostia have a 180° orientation. However, rapid-deployment aortic valve replacement (RD-AVR) might be considered in other bicuspid valve anatomies with a round aortic annulus and uniform height of the commissures [7]. In clinical practice, these ideal conditions are sometimes difficult to assess. We aimed to report our single-centre, real-world experience with RD-AVR in all bicuspid valve anatomies and describe our surgical techniques and pitfalls, which may contribute to a successful implantation and reduce the risk of valve dislocation and paravalvular leak in this particular patient population.
MATERIALS AND METHODS
Ethics statement
The Institutional Ethic Review Board of the Medical University of Vienna approved this study registry (Ethics No 1861/2016) and all patients who underwent study-related procedures signed a written informed consent.
Study population
Between May 2010 and December 2020, all consecutive patients who underwent a successful aortic valve replacement (AVR) with an Edwards Intuity RD aortic valve system (Edwards Lifesciences LLC, Irvine, CA, USA) at the Department of Cardiac Surgery, Medical University of Vienna, were included in a prospective, ongoing database with longitudinal end point assessment, as previously reported [8]. The baseline characteristics, operative and postoperative outcomes of the bicuspid anatomy collective (BAV) were assessed and compared with a control group, including patients with native tricuspid valves who underwent RD-AVR; patients who presented with previous AVR (artificial prostheses) were excluded from the study.
The safety and clinical course following RD-AVR were assessed according to the guidelines for reporting mortality and morbidity after heart valve surgery [9]. Patients underwent a clinical follow-up at discharge, 3–6 months and 1, 3, 5 and 10 years; the clinical status and the occurrence of any adverse events, the haemodynamic performance by means of a transthoracic echocardiography and the occurrence of any conduction disturbances or arrythmias were assessed during the follow-up visits. Between the clinical visits, a telephone follow-up interview was performed annually. The database was locked at the end of February 2021.
Study end points
The primary end points of this study were overall mortality, occurrence of moderate-to-severe paravalvular regurgitation and valve explantation due to non-structural valve dysfunction (NSVD). The overall mortality included all deaths after valve implantation regardless of the cause. Early mortality was defined as mortality during the ≤30 postoperative day, in-hospital mortality was defined as any event occurring between surgery and first discharge and perioperative mortality was calculated as composite of early and in-hospital mortality. The risk of early postoperative mortality was assessed by means of European System for Cardiac Operative Risk Evaluation (EuroSCORE) II and Society of Thoracic Surgeons score.
As secondary end points, we assessed the occurrence of other clinical events: the rate of reoperations with valve explantations or valve-in-valve procedures reported as composite end point, which includes reoperations due to NSVD, structural valve degeneration and endocarditis. Another secondary end point was the occurrence of thromboembolic and major bleeding events, defined as composite end point, which included the overall strokes, ischaemic transient attacks, peripheral embolism, valve thrombosis and major bleeding events (not including surgical re-explorations for bleeding). Furthermore, we assessed postoperative permanent pacemaker implantations (PPIs) and the valve implantation success defined as correct positioning within the aortic annulus of a single prosthesis, without the need for repositioning; herein, we also reported the number of failed implantations, which required intraoperative switch to a conventional sutured prosthesis, even though these patients were not further analysed in the registry. Moreover, the haemodynamic valve performance by means of TTE and other postoperative events were reported according to the guidelines [9].
Statistical analysis
Continuous variables are presented as mean and standard deviation (SD) or median and interquartile ranges (IQR: 25th–75th interval). Categorical outcomes are reported as total numbers and proportions (in %). Statistical calculations comparing continuous variables between 2 groups were made with the Student’s t-test (normally distributed variables) or Mann–Whitney U-test (not normally distributed variables). Comparisons of categorical variables were made using the chi-squared test or Fisher’s exact test. Normality of continuous variables was checked using graphical methods (box plots and histograms).
Repeated measures analysis of variance models were used to compare echocardiographic findings in the follow-up between the 2 valve anatomy groups. Due to their skewed distributions, log2-transformed mean and peak gradient values were used for statistical analyses. The inverse Kaplan–Meier method [10] was used to calculate the median follow-up time. The Kaplan–Meier method was performed to assess survival probabilities and the log-rank test was used to test for statistical differences between the curves. Univariate Cox regression models were used to evaluate potential risk factors for long-term survival and a multivariable Cox regression model was used to perform an adjusted comparison of the 2 patient cohorts (BAV versus TAV), including all components of the EuroSCORE II in addition to the variables concomitant procedures and surgery of the ascending aorta as potential confounding factors. EuroSCORE II components that were found in <5% of the patients were not considered in the multivariable regression model. The resulting estimated hazard ratios with 95% confidence intervals (CIs) are provided. The patients with missing data were excluded (BAV: 3 observations, TAV: 8 observations, with a total of 786 analysed patients and 142 observed events). Furthermore, following the request of a reviewer, a propensity score analysis was performed by considering the same potential confounding factors as explanatory variables in a multivariable logistic regression model with the valve anatomy (BAV versus TAV) as outcome variable. The resulting probabilities (propensity scores) were then used to apply the inverse probability of received treatment weighting method [11] for comparison of the 2 valve anatomy cohorts with respect to long-term survival.
Trial safety end points are reported as early (≤30 postoperative day) or late (>30 postoperative day) events. Early events are reported as numbers and percentages. The probabilities of adverse events (primary and secondary end points) during the follow-up period were estimated using the cumulative incidence functions accounting for the competing events death (in case of all adverse event outcomes) and valve explantation (in case of the outcomes moderate-to-severe PVL, and thromboembolic and major bleeding events, respectively). The Gray’s test was used to test for differences in the cumulative incidence curves.
The statistical analysis was performed using SPSS software version 26.0 (SPSS, Inc., Armonk, New York) and SAS software (SAS Institute Inc., Cary, NC, USA). A two-sided P-value of <0.05 was considered to be statistically significant.
RESULTS
Baseline characteristics
A total of 816 consecutive patients underwent surgical aortic valve replacement (SAVR) with an RD bioprosthesis at between May 2010 and December 2020 and were included in a prospective, ongoing registry. Out of these, 107 (13.1%) presented with a BAV. After excluding patients with previous AVRs (n = 19), the outcomes in patients with bicuspid anatomy were assessed and compared with a control group, including patients with native tricuspid valve (TAV) who underwent RD-AVR (n = 690 patients). Follow-up time reached up to 11 years, with a median of 44 (0–128) months. The mean age in the BAV group was 68 (SD: 8) years and 54% were male. In the group with TAV, the mean age was 74 (SD: 7) years and 54% were male (p < 0.001 for age). The median EuroSCORE II was 1.6 (IQR: 1.0–2.6) in the BAV group and 2.6 (IQR: 1.4–4.7) in the control group (P < 0.001). Other baseline characteristics are summarized in Table 1.
| Preoperative patient characteristics . | BAV, N = 107 . | TAV, N = 690 . | P-Value . |
|---|---|---|---|
| Age (years), mean (SD) | 67.8 (8.4) | 74.4 (7.0) | <0.001a |
| Male, n (%) | 58 (54.2) | 371 (53.8) | 0.933c |
| BMI (kg/m2), mean (SD) | 27.5 (5.2) | 28.2 (4.8) | 0.180a |
| BSA (m2), mean (SD) | 1.9 (0.2) | 1.9 (0.2) | 0.844a |
| EuroSCORE II (%), median (IQR) | 1.6 (1.0–2.6) | 2.6 (1.4–4.7) | <0.001b |
| STS score (%), median (IQR) | 1.4 (1.0–1.8) | 2.1 (1.4–3.2) | <0.001b |
| NYHA class III/IV, n (%) | 65 (61.3) | 483 (70.4) | 0.059c |
| Arterial hypertension, n (%) | 76 (71.7) | 620 (89.9) | <0.001c |
| Dyslipidaemia, n (%) | 46 (43) | 455 (66.0) | <0.001c |
| Creatinine, median (IQR) | 0.9 (0.8–1.0) | 1.0 (0.8–1.2) | <0.001b |
| Coronary artery disease, n (%) | 19 (17.9) | 308 (44.8) | <0.001c |
| Cerebrovascular disease, n (%) | 9 (8.6) | 136 (19.7) | 0.006c |
| Peripheral vascular disease, n (%) | 2 (1.9) | 62 (9) | 0.013c |
| Chronic lung disease, n (%) | 25 (23.4) | 128 (18.6) | 0.239c |
| Prior atrial fibrillation, n (%) | 15 (14.3) | 147 (21.5) | 0.088c |
| Previous pacemaker, n (%) | 2 (1.9) | 38 (5.5) | 0.108c |
| Previous cardiovascular interventions, n (%) | 6 (5.8) | 99 (14.4) | 0.016c |
| EF < 40%, n (%) | 14 (13.1) | 76 (11) | 0.533c |
| Mean transvalvular gradient (mmHg), mean (SD) | 59.1 (19.0) | 53.1 (18.5) | 0.004a |
| Preoperative patient characteristics . | BAV, N = 107 . | TAV, N = 690 . | P-Value . |
|---|---|---|---|
| Age (years), mean (SD) | 67.8 (8.4) | 74.4 (7.0) | <0.001a |
| Male, n (%) | 58 (54.2) | 371 (53.8) | 0.933c |
| BMI (kg/m2), mean (SD) | 27.5 (5.2) | 28.2 (4.8) | 0.180a |
| BSA (m2), mean (SD) | 1.9 (0.2) | 1.9 (0.2) | 0.844a |
| EuroSCORE II (%), median (IQR) | 1.6 (1.0–2.6) | 2.6 (1.4–4.7) | <0.001b |
| STS score (%), median (IQR) | 1.4 (1.0–1.8) | 2.1 (1.4–3.2) | <0.001b |
| NYHA class III/IV, n (%) | 65 (61.3) | 483 (70.4) | 0.059c |
| Arterial hypertension, n (%) | 76 (71.7) | 620 (89.9) | <0.001c |
| Dyslipidaemia, n (%) | 46 (43) | 455 (66.0) | <0.001c |
| Creatinine, median (IQR) | 0.9 (0.8–1.0) | 1.0 (0.8–1.2) | <0.001b |
| Coronary artery disease, n (%) | 19 (17.9) | 308 (44.8) | <0.001c |
| Cerebrovascular disease, n (%) | 9 (8.6) | 136 (19.7) | 0.006c |
| Peripheral vascular disease, n (%) | 2 (1.9) | 62 (9) | 0.013c |
| Chronic lung disease, n (%) | 25 (23.4) | 128 (18.6) | 0.239c |
| Prior atrial fibrillation, n (%) | 15 (14.3) | 147 (21.5) | 0.088c |
| Previous pacemaker, n (%) | 2 (1.9) | 38 (5.5) | 0.108c |
| Previous cardiovascular interventions, n (%) | 6 (5.8) | 99 (14.4) | 0.016c |
| EF < 40%, n (%) | 14 (13.1) | 76 (11) | 0.533c |
| Mean transvalvular gradient (mmHg), mean (SD) | 59.1 (19.0) | 53.1 (18.5) | 0.004a |
Two-sample t-test.
Mann–Whitney test.
Chi-squared test.
BAV: bicuspid aortic valve; EuroSCORE: European System for Cardiac Operative Risk Evaluation; IQR: interquartile range; SD: standard deviation; STS: Society of Thoracic Surgeons; TAV: tricuspid aortic valve.
| Preoperative patient characteristics . | BAV, N = 107 . | TAV, N = 690 . | P-Value . |
|---|---|---|---|
| Age (years), mean (SD) | 67.8 (8.4) | 74.4 (7.0) | <0.001a |
| Male, n (%) | 58 (54.2) | 371 (53.8) | 0.933c |
| BMI (kg/m2), mean (SD) | 27.5 (5.2) | 28.2 (4.8) | 0.180a |
| BSA (m2), mean (SD) | 1.9 (0.2) | 1.9 (0.2) | 0.844a |
| EuroSCORE II (%), median (IQR) | 1.6 (1.0–2.6) | 2.6 (1.4–4.7) | <0.001b |
| STS score (%), median (IQR) | 1.4 (1.0–1.8) | 2.1 (1.4–3.2) | <0.001b |
| NYHA class III/IV, n (%) | 65 (61.3) | 483 (70.4) | 0.059c |
| Arterial hypertension, n (%) | 76 (71.7) | 620 (89.9) | <0.001c |
| Dyslipidaemia, n (%) | 46 (43) | 455 (66.0) | <0.001c |
| Creatinine, median (IQR) | 0.9 (0.8–1.0) | 1.0 (0.8–1.2) | <0.001b |
| Coronary artery disease, n (%) | 19 (17.9) | 308 (44.8) | <0.001c |
| Cerebrovascular disease, n (%) | 9 (8.6) | 136 (19.7) | 0.006c |
| Peripheral vascular disease, n (%) | 2 (1.9) | 62 (9) | 0.013c |
| Chronic lung disease, n (%) | 25 (23.4) | 128 (18.6) | 0.239c |
| Prior atrial fibrillation, n (%) | 15 (14.3) | 147 (21.5) | 0.088c |
| Previous pacemaker, n (%) | 2 (1.9) | 38 (5.5) | 0.108c |
| Previous cardiovascular interventions, n (%) | 6 (5.8) | 99 (14.4) | 0.016c |
| EF < 40%, n (%) | 14 (13.1) | 76 (11) | 0.533c |
| Mean transvalvular gradient (mmHg), mean (SD) | 59.1 (19.0) | 53.1 (18.5) | 0.004a |
| Preoperative patient characteristics . | BAV, N = 107 . | TAV, N = 690 . | P-Value . |
|---|---|---|---|
| Age (years), mean (SD) | 67.8 (8.4) | 74.4 (7.0) | <0.001a |
| Male, n (%) | 58 (54.2) | 371 (53.8) | 0.933c |
| BMI (kg/m2), mean (SD) | 27.5 (5.2) | 28.2 (4.8) | 0.180a |
| BSA (m2), mean (SD) | 1.9 (0.2) | 1.9 (0.2) | 0.844a |
| EuroSCORE II (%), median (IQR) | 1.6 (1.0–2.6) | 2.6 (1.4–4.7) | <0.001b |
| STS score (%), median (IQR) | 1.4 (1.0–1.8) | 2.1 (1.4–3.2) | <0.001b |
| NYHA class III/IV, n (%) | 65 (61.3) | 483 (70.4) | 0.059c |
| Arterial hypertension, n (%) | 76 (71.7) | 620 (89.9) | <0.001c |
| Dyslipidaemia, n (%) | 46 (43) | 455 (66.0) | <0.001c |
| Creatinine, median (IQR) | 0.9 (0.8–1.0) | 1.0 (0.8–1.2) | <0.001b |
| Coronary artery disease, n (%) | 19 (17.9) | 308 (44.8) | <0.001c |
| Cerebrovascular disease, n (%) | 9 (8.6) | 136 (19.7) | 0.006c |
| Peripheral vascular disease, n (%) | 2 (1.9) | 62 (9) | 0.013c |
| Chronic lung disease, n (%) | 25 (23.4) | 128 (18.6) | 0.239c |
| Prior atrial fibrillation, n (%) | 15 (14.3) | 147 (21.5) | 0.088c |
| Previous pacemaker, n (%) | 2 (1.9) | 38 (5.5) | 0.108c |
| Previous cardiovascular interventions, n (%) | 6 (5.8) | 99 (14.4) | 0.016c |
| EF < 40%, n (%) | 14 (13.1) | 76 (11) | 0.533c |
| Mean transvalvular gradient (mmHg), mean (SD) | 59.1 (19.0) | 53.1 (18.5) | 0.004a |
Two-sample t-test.
Mann–Whitney test.
Chi-squared test.
BAV: bicuspid aortic valve; EuroSCORE: European System for Cardiac Operative Risk Evaluation; IQR: interquartile range; SD: standard deviation; STS: Society of Thoracic Surgeons; TAV: tricuspid aortic valve.
A precise description of the bicuspid phenotypes according to the Sievers classification, taking into account the number of raphes (type 0—no raphe, type 1—1 raphe, type 2—2 raphes), was available and retrievable in 70.9% of the bicuspid anatomies with 9 (8.4%) patients with a true bicuspid, Sievers 0 phenotype, 62 (57.9%) with Sievers 1 phenotype, of which the majority 47 (43.9%) with a raphe between the left and right commissure, 12 (11.2%) a right-non-coronary commissure raphe and 3 (2.8%) a non-left coronary raphe; a Sievers 2 phenotype was described in 4 (3.7%) patients; the remaining 32 (29.9%) bicuspid phenotypes were undefined (see Table 2).
| Variables . | BAV, N = 107 . | TAV, N = 690 . | P-Value . |
|---|---|---|---|
| BAV Phenotype Sievers 1, n (%) | 62 (57.9) | – | |
| LR | 47 (43.9) | – | |
| RN | 12 (11.2) | – | |
| LN | 3 (2.8) | – | |
| Sievers 0, n (%) | 9 (8.4) | – | |
| Sievers 2, n (%) | 4 (3.7) | – | |
| Undetermined, n (%) | 32 (29.9) | – | |
| Elective procedure, n (%) | 105 (98.1) | 628 (91.4) | 0.015b |
| Concomitant procedures, n (%) | 44 (41.1) | 339 (49.1) | 0.123b |
| Aortic surgery, n (%) | 24 (22.4) | 29 (4.2) | <0.001b |
| CABG, n (%) | 14 (13.1) | 232 (33.6) | <0.001b |
| Mitral valve surgery, n (%) | 1 (0.9) | 47 (6.8) | 0.017b |
| Tricuspid valve surgery, n (%) | 1 (0.9) | 33 (4.8) | 0.072c |
| Atrial fibrillation surgery, n (%) | 4 (3.7) | 33 (4.8) | 0.807c |
| Access, n (%) | <0.001b | ||
| Full sternotomy | 29 (27.1) | 371 (53.8) | |
| Upper sternotomy | 36 (33.6) | 158 (22.9) | |
| Thoracotomy | 42 (39.3) | 161 (23.3) | |
| CPB time (min), median (IQR) | 106 (86–136) | 110 (91–137) | 0.079a |
| XCT time (min), median (IQR) | 73 (57–92) | 76 (60–97) | 0.181a |
| >1 attempt valve positioning, n (%) | 2 (1.9) | 17 (2.5) | 1.00c |
| Revision for bleeding, n (%) | 2 (1.9) | 26 (3.8) | 0.569c |
| ECMO, n (%) | 0 (0) | 11 (1.6) | 0.377c |
| Dialysis, n (%) | 0 (0) | 14 (2.0) | 0.236c |
| Early PPI (14 days), n (%) | 12 (11.2) | 63 (9.1) | 0.492b |
| Perioperative AF, n (%) | 31 (29.3) | 178 (25.9) | 0.463b |
| Perioperative neurological event, n (%) | 1 (0.9) | 23 (3.3) | 0.234c |
| Variables . | BAV, N = 107 . | TAV, N = 690 . | P-Value . |
|---|---|---|---|
| BAV Phenotype Sievers 1, n (%) | 62 (57.9) | – | |
| LR | 47 (43.9) | – | |
| RN | 12 (11.2) | – | |
| LN | 3 (2.8) | – | |
| Sievers 0, n (%) | 9 (8.4) | – | |
| Sievers 2, n (%) | 4 (3.7) | – | |
| Undetermined, n (%) | 32 (29.9) | – | |
| Elective procedure, n (%) | 105 (98.1) | 628 (91.4) | 0.015b |
| Concomitant procedures, n (%) | 44 (41.1) | 339 (49.1) | 0.123b |
| Aortic surgery, n (%) | 24 (22.4) | 29 (4.2) | <0.001b |
| CABG, n (%) | 14 (13.1) | 232 (33.6) | <0.001b |
| Mitral valve surgery, n (%) | 1 (0.9) | 47 (6.8) | 0.017b |
| Tricuspid valve surgery, n (%) | 1 (0.9) | 33 (4.8) | 0.072c |
| Atrial fibrillation surgery, n (%) | 4 (3.7) | 33 (4.8) | 0.807c |
| Access, n (%) | <0.001b | ||
| Full sternotomy | 29 (27.1) | 371 (53.8) | |
| Upper sternotomy | 36 (33.6) | 158 (22.9) | |
| Thoracotomy | 42 (39.3) | 161 (23.3) | |
| CPB time (min), median (IQR) | 106 (86–136) | 110 (91–137) | 0.079a |
| XCT time (min), median (IQR) | 73 (57–92) | 76 (60–97) | 0.181a |
| >1 attempt valve positioning, n (%) | 2 (1.9) | 17 (2.5) | 1.00c |
| Revision for bleeding, n (%) | 2 (1.9) | 26 (3.8) | 0.569c |
| ECMO, n (%) | 0 (0) | 11 (1.6) | 0.377c |
| Dialysis, n (%) | 0 (0) | 14 (2.0) | 0.236c |
| Early PPI (14 days), n (%) | 12 (11.2) | 63 (9.1) | 0.492b |
| Perioperative AF, n (%) | 31 (29.3) | 178 (25.9) | 0.463b |
| Perioperative neurological event, n (%) | 1 (0.9) | 23 (3.3) | 0.234c |
Mann–Whitney test.
Chi-squared test.
Fisher’s exact test.
AF: atrial fibrillation; BAV: bicuspid aortic valve; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; IQR: interquartile range; LN: left-non-coronary leaflets; LR: raphe left–right leaflets; PPI: permanent pacemaker implantation; RN: raphe right-non-coronary leaflets; TAV: tricuspid aortic valve; XCT: cross-clamp time.
| Variables . | BAV, N = 107 . | TAV, N = 690 . | P-Value . |
|---|---|---|---|
| BAV Phenotype Sievers 1, n (%) | 62 (57.9) | – | |
| LR | 47 (43.9) | – | |
| RN | 12 (11.2) | – | |
| LN | 3 (2.8) | – | |
| Sievers 0, n (%) | 9 (8.4) | – | |
| Sievers 2, n (%) | 4 (3.7) | – | |
| Undetermined, n (%) | 32 (29.9) | – | |
| Elective procedure, n (%) | 105 (98.1) | 628 (91.4) | 0.015b |
| Concomitant procedures, n (%) | 44 (41.1) | 339 (49.1) | 0.123b |
| Aortic surgery, n (%) | 24 (22.4) | 29 (4.2) | <0.001b |
| CABG, n (%) | 14 (13.1) | 232 (33.6) | <0.001b |
| Mitral valve surgery, n (%) | 1 (0.9) | 47 (6.8) | 0.017b |
| Tricuspid valve surgery, n (%) | 1 (0.9) | 33 (4.8) | 0.072c |
| Atrial fibrillation surgery, n (%) | 4 (3.7) | 33 (4.8) | 0.807c |
| Access, n (%) | <0.001b | ||
| Full sternotomy | 29 (27.1) | 371 (53.8) | |
| Upper sternotomy | 36 (33.6) | 158 (22.9) | |
| Thoracotomy | 42 (39.3) | 161 (23.3) | |
| CPB time (min), median (IQR) | 106 (86–136) | 110 (91–137) | 0.079a |
| XCT time (min), median (IQR) | 73 (57–92) | 76 (60–97) | 0.181a |
| >1 attempt valve positioning, n (%) | 2 (1.9) | 17 (2.5) | 1.00c |
| Revision for bleeding, n (%) | 2 (1.9) | 26 (3.8) | 0.569c |
| ECMO, n (%) | 0 (0) | 11 (1.6) | 0.377c |
| Dialysis, n (%) | 0 (0) | 14 (2.0) | 0.236c |
| Early PPI (14 days), n (%) | 12 (11.2) | 63 (9.1) | 0.492b |
| Perioperative AF, n (%) | 31 (29.3) | 178 (25.9) | 0.463b |
| Perioperative neurological event, n (%) | 1 (0.9) | 23 (3.3) | 0.234c |
| Variables . | BAV, N = 107 . | TAV, N = 690 . | P-Value . |
|---|---|---|---|
| BAV Phenotype Sievers 1, n (%) | 62 (57.9) | – | |
| LR | 47 (43.9) | – | |
| RN | 12 (11.2) | – | |
| LN | 3 (2.8) | – | |
| Sievers 0, n (%) | 9 (8.4) | – | |
| Sievers 2, n (%) | 4 (3.7) | – | |
| Undetermined, n (%) | 32 (29.9) | – | |
| Elective procedure, n (%) | 105 (98.1) | 628 (91.4) | 0.015b |
| Concomitant procedures, n (%) | 44 (41.1) | 339 (49.1) | 0.123b |
| Aortic surgery, n (%) | 24 (22.4) | 29 (4.2) | <0.001b |
| CABG, n (%) | 14 (13.1) | 232 (33.6) | <0.001b |
| Mitral valve surgery, n (%) | 1 (0.9) | 47 (6.8) | 0.017b |
| Tricuspid valve surgery, n (%) | 1 (0.9) | 33 (4.8) | 0.072c |
| Atrial fibrillation surgery, n (%) | 4 (3.7) | 33 (4.8) | 0.807c |
| Access, n (%) | <0.001b | ||
| Full sternotomy | 29 (27.1) | 371 (53.8) | |
| Upper sternotomy | 36 (33.6) | 158 (22.9) | |
| Thoracotomy | 42 (39.3) | 161 (23.3) | |
| CPB time (min), median (IQR) | 106 (86–136) | 110 (91–137) | 0.079a |
| XCT time (min), median (IQR) | 73 (57–92) | 76 (60–97) | 0.181a |
| >1 attempt valve positioning, n (%) | 2 (1.9) | 17 (2.5) | 1.00c |
| Revision for bleeding, n (%) | 2 (1.9) | 26 (3.8) | 0.569c |
| ECMO, n (%) | 0 (0) | 11 (1.6) | 0.377c |
| Dialysis, n (%) | 0 (0) | 14 (2.0) | 0.236c |
| Early PPI (14 days), n (%) | 12 (11.2) | 63 (9.1) | 0.492b |
| Perioperative AF, n (%) | 31 (29.3) | 178 (25.9) | 0.463b |
| Perioperative neurological event, n (%) | 1 (0.9) | 23 (3.3) | 0.234c |
Mann–Whitney test.
Chi-squared test.
Fisher’s exact test.
AF: atrial fibrillation; BAV: bicuspid aortic valve; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; IQR: interquartile range; LN: left-non-coronary leaflets; LR: raphe left–right leaflets; PPI: permanent pacemaker implantation; RN: raphe right-non-coronary leaflets; TAV: tricuspid aortic valve; XCT: cross-clamp time.
Concomitant procedures were performed in 44 (41.1%) patients in the BAV group and in 339 (49.1%) patients in the control group (P = 0.123); surgery of the ascending aorta (AA) due to dilation or aneurysm was necessary in 24 (22.4%) in the BAV group, compared with 29 (4.2%) in the control group (P < 0.001). However, coronary artery disease occurred more often in the control group; coronary bypass grafting was performed in 14 (13.1%) of the 107 patients with bicuspid valve at the time of the AVR and in 232 (33.6%) of the patients with tricuspid valve (P < 0.001).
More than 1 attempt for valve deployment to secure the prosthesis in the correct annular position was necessary in 2 (1.9%) cases in the BAV group and in 17 (2.5%) cases in the control group (P = 1.0). In addition, 16 patients (2 in the BAV group) underwent SAVR with a conventional sutured bioprosthesis following 1 or more failed RD-AVR implantation attempts. Other operative data and perioperative outcomes are found in Table 2.
Primary end points
Survival
No 30-day mortality was observed in the BAV group; in the control group, 4 (0.6%) patients died (P = 1.0). The overall long-term survival was excellent in the BAV group, with a cumulative survival rate at 5 years of 92% (95% CI 81–97%) and at 10 years of 88% (95% CI 73–95%), and significantly better than in the control group (log-rank test P = 0.002), see Figure 1.
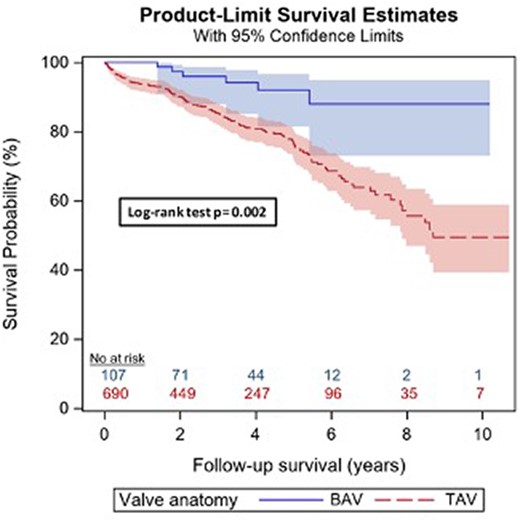
Kaplan–Meier survival analysis, log-rank test. Blue: bicuspid anatomy group; red: tricuspid group. The survival in patients with a bicuspid anatomy is significantly higher than in patients with a native tricuspid valve—log-rank P = 0.002.
Univariate Cox regression models were used to evaluate potential risk factors for long-term survival. Considering results of the multivariable model, the effect of valve anatomy diminishes notably after adjustment for these confounding factors (Table 3). Based upon the request of a reviewer, we also performed an inverse probability of received treatment weighting analysis in which the BAV and TAV cohorts were reweighted such that they correspond to a hypothetical pseudo-population with, counter to the fact, equal distributions of the confounding factors in these 2 groups. The resulting hazard ratio (TAV versus BAV) is 0.97 (95% CI: 0.39–2.38, P = 0.94).
| Prognostic factors . | Univariate analysis . | Multivariable analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-Value . | HR (95% CI) . | P-Value . | |
| Valve anatomy (TAV) | 3.25 (1.43–7.37) | 0.005 | 1.92 (0.80–4.60) | 0.144 |
| Age (years)a | 1.06 (1.04–1.09) | <0.001 | 1.05 (1.03–1.08) | <0.001 |
| Gender | 1.12 (0.80–1.56) | 0.501 | 0.90 (0.62–1.31) | 0.596 |
| Renal impairment | 2.64 (1.80–3.88) | <0.001 | 1.76 (1.09–2.83) | 0.020 |
| Creatinineb | 1.69 (1.36–2.11) | <0.001 | 1.33 (0.95–1.87) | 0.097 |
| Extracardiac arteriopathy | 1.37 (0.93–2.01) | 0.113 | 1.03 (0.69–1.56) | 0.879 |
| COPD | 1.39 (0.96–2.01) | 0.082 | 1.41 (0.96–2.06) | 0.081 |
| Diabetes (insulin) | 1.89 (1.09–3.29) | 0.024 | 1.61 (0.91–2.85) | 0.101 |
| Emergency status | 2.76 (1.82–4.21) | <0.001 | 2.42 (1.50–3.90) | <0.001 |
| NYHA class | 1.30 (0.98–1.72) | 0.065 | 0.84 (0.62–1.12) | 0.229 |
| Angina pectoris CCS IV | 1.57 (0.89–2.78) | 0.123 | 1.07 (0.57–2.01) | 0.830 |
| LV function | 1.38 (1.04–1.81) | 0.024 | 1.10 (0.81–1.50) | 0.542 |
| Pulmonary hypertension | 2.11 (1.37–3.24) | <0.001 | 1.50 (0.95–2.38) | 0.080 |
| Weight of intervention | 1.51 (1.20–1.89) | <0.001 | 0.88 (0.54–1.44) | 0.610 |
| Concomitant procedures | 1.97 (1.40–2.77) | <0.001 | 2.33 (1.14–4.76) | 0.020 |
| Aortic surgery | 0.60 (0.28–1.29) | 0.193 | 0.68 (0.30–1.53) | 0.347 |
| Prognostic factors . | Univariate analysis . | Multivariable analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-Value . | HR (95% CI) . | P-Value . | |
| Valve anatomy (TAV) | 3.25 (1.43–7.37) | 0.005 | 1.92 (0.80–4.60) | 0.144 |
| Age (years)a | 1.06 (1.04–1.09) | <0.001 | 1.05 (1.03–1.08) | <0.001 |
| Gender | 1.12 (0.80–1.56) | 0.501 | 0.90 (0.62–1.31) | 0.596 |
| Renal impairment | 2.64 (1.80–3.88) | <0.001 | 1.76 (1.09–2.83) | 0.020 |
| Creatinineb | 1.69 (1.36–2.11) | <0.001 | 1.33 (0.95–1.87) | 0.097 |
| Extracardiac arteriopathy | 1.37 (0.93–2.01) | 0.113 | 1.03 (0.69–1.56) | 0.879 |
| COPD | 1.39 (0.96–2.01) | 0.082 | 1.41 (0.96–2.06) | 0.081 |
| Diabetes (insulin) | 1.89 (1.09–3.29) | 0.024 | 1.61 (0.91–2.85) | 0.101 |
| Emergency status | 2.76 (1.82–4.21) | <0.001 | 2.42 (1.50–3.90) | <0.001 |
| NYHA class | 1.30 (0.98–1.72) | 0.065 | 0.84 (0.62–1.12) | 0.229 |
| Angina pectoris CCS IV | 1.57 (0.89–2.78) | 0.123 | 1.07 (0.57–2.01) | 0.830 |
| LV function | 1.38 (1.04–1.81) | 0.024 | 1.10 (0.81–1.50) | 0.542 |
| Pulmonary hypertension | 2.11 (1.37–3.24) | <0.001 | 1.50 (0.95–2.38) | 0.080 |
| Weight of intervention | 1.51 (1.20–1.89) | <0.001 | 0.88 (0.54–1.44) | 0.610 |
| Concomitant procedures | 1.97 (1.40–2.77) | <0.001 | 2.33 (1.14–4.76) | 0.020 |
| Aortic surgery | 0.60 (0.28–1.29) | 0.193 | 0.68 (0.30–1.53) | 0.347 |
Univariate and multivariable Cox regression for survival.
Age unit = 10.
Creatine was converted to log2. Weight of intervention describes the number of procedures.
CI: confidence interval; COPD: chronic obstructive pulmonary disease; LV: left ventricle; TAV: tricuspid aortic valve.
| Prognostic factors . | Univariate analysis . | Multivariable analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-Value . | HR (95% CI) . | P-Value . | |
| Valve anatomy (TAV) | 3.25 (1.43–7.37) | 0.005 | 1.92 (0.80–4.60) | 0.144 |
| Age (years)a | 1.06 (1.04–1.09) | <0.001 | 1.05 (1.03–1.08) | <0.001 |
| Gender | 1.12 (0.80–1.56) | 0.501 | 0.90 (0.62–1.31) | 0.596 |
| Renal impairment | 2.64 (1.80–3.88) | <0.001 | 1.76 (1.09–2.83) | 0.020 |
| Creatinineb | 1.69 (1.36–2.11) | <0.001 | 1.33 (0.95–1.87) | 0.097 |
| Extracardiac arteriopathy | 1.37 (0.93–2.01) | 0.113 | 1.03 (0.69–1.56) | 0.879 |
| COPD | 1.39 (0.96–2.01) | 0.082 | 1.41 (0.96–2.06) | 0.081 |
| Diabetes (insulin) | 1.89 (1.09–3.29) | 0.024 | 1.61 (0.91–2.85) | 0.101 |
| Emergency status | 2.76 (1.82–4.21) | <0.001 | 2.42 (1.50–3.90) | <0.001 |
| NYHA class | 1.30 (0.98–1.72) | 0.065 | 0.84 (0.62–1.12) | 0.229 |
| Angina pectoris CCS IV | 1.57 (0.89–2.78) | 0.123 | 1.07 (0.57–2.01) | 0.830 |
| LV function | 1.38 (1.04–1.81) | 0.024 | 1.10 (0.81–1.50) | 0.542 |
| Pulmonary hypertension | 2.11 (1.37–3.24) | <0.001 | 1.50 (0.95–2.38) | 0.080 |
| Weight of intervention | 1.51 (1.20–1.89) | <0.001 | 0.88 (0.54–1.44) | 0.610 |
| Concomitant procedures | 1.97 (1.40–2.77) | <0.001 | 2.33 (1.14–4.76) | 0.020 |
| Aortic surgery | 0.60 (0.28–1.29) | 0.193 | 0.68 (0.30–1.53) | 0.347 |
| Prognostic factors . | Univariate analysis . | Multivariable analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-Value . | HR (95% CI) . | P-Value . | |
| Valve anatomy (TAV) | 3.25 (1.43–7.37) | 0.005 | 1.92 (0.80–4.60) | 0.144 |
| Age (years)a | 1.06 (1.04–1.09) | <0.001 | 1.05 (1.03–1.08) | <0.001 |
| Gender | 1.12 (0.80–1.56) | 0.501 | 0.90 (0.62–1.31) | 0.596 |
| Renal impairment | 2.64 (1.80–3.88) | <0.001 | 1.76 (1.09–2.83) | 0.020 |
| Creatinineb | 1.69 (1.36–2.11) | <0.001 | 1.33 (0.95–1.87) | 0.097 |
| Extracardiac arteriopathy | 1.37 (0.93–2.01) | 0.113 | 1.03 (0.69–1.56) | 0.879 |
| COPD | 1.39 (0.96–2.01) | 0.082 | 1.41 (0.96–2.06) | 0.081 |
| Diabetes (insulin) | 1.89 (1.09–3.29) | 0.024 | 1.61 (0.91–2.85) | 0.101 |
| Emergency status | 2.76 (1.82–4.21) | <0.001 | 2.42 (1.50–3.90) | <0.001 |
| NYHA class | 1.30 (0.98–1.72) | 0.065 | 0.84 (0.62–1.12) | 0.229 |
| Angina pectoris CCS IV | 1.57 (0.89–2.78) | 0.123 | 1.07 (0.57–2.01) | 0.830 |
| LV function | 1.38 (1.04–1.81) | 0.024 | 1.10 (0.81–1.50) | 0.542 |
| Pulmonary hypertension | 2.11 (1.37–3.24) | <0.001 | 1.50 (0.95–2.38) | 0.080 |
| Weight of intervention | 1.51 (1.20–1.89) | <0.001 | 0.88 (0.54–1.44) | 0.610 |
| Concomitant procedures | 1.97 (1.40–2.77) | <0.001 | 2.33 (1.14–4.76) | 0.020 |
| Aortic surgery | 0.60 (0.28–1.29) | 0.193 | 0.68 (0.30–1.53) | 0.347 |
Univariate and multivariable Cox regression for survival.
Age unit = 10.
Creatine was converted to log2. Weight of intervention describes the number of procedures.
CI: confidence interval; COPD: chronic obstructive pulmonary disease; LV: left ventricle; TAV: tricuspid aortic valve.
Non-structural valve dysfunction
The 1- and 5-year cumulative incidence of moderate-to-severe paravalvular regurgitation in the BAV group was 3.3% (95% CI: 0.9–8.6%) and 10.7% (95% CI: 4.2–20.7%), compared to 2.4% (95% CI: 1.4–3.8%) and 3.9% (95% CI: 2.4–6.1%) in the TAV group (P = 0.057). Cumulative incidence of reoperations with valve explantation due to NSVD at 1 and 5 years was 1.2% (95% CI: 0.1–5.7%) and 2.8% (95% CI: 0.5–8.8%) in the BAV group and 1.9% (95% CI: 1.0–3.2%) in the TAV group, which remained constant at 5 years with no further events after 1-year follow-up (P = 0.89, Table 3). Figures 2 and 3 demonstrate the long-term cumulative incidence of reoperation with valve explantation due to NSVD and the occurrence of moderate–severe paravalvular leak with or without reoperation (Table 4).
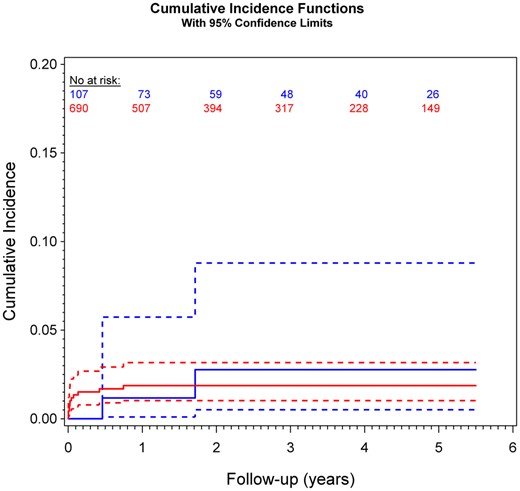
Cumulative incidence of reoperation with valve explantation due to non-structural valve dysfunction in bicuspid (bicuspid aortic valve: blue) compared with tricuspid (tricuspid aortic valve: red) valve anatomies, P = 0.89. Competing risk analysis was performed to estimate the cumulative incidence of re-do due to non-structural valve dysfunction after rapid-deployment aortic valve replacement considering death as competing event.

Cumulative incidence of moderate–severe paravalvular aortic regurgitation in bicuspid (bicuspid aortic valve: blue) compared with tricuspid (tricuspid aortic valve: red) valve anatomies, P = 0.06. Competing risk analysis was performed to estimate the cumulative incidence of moderate–severe paravalvular leakage after rapid-deployment aortic valve replacement considering death as competing event.
| Variables . | BAV, N = 107 . | TAV, N = 690 . | P-Value . |
|---|---|---|---|
| Moderate–severe PVL (95% CI) | 10.7% (4.2–20.7%) | 3.9% (2.4–6.1%) | 0.06 |
| Reoperation due to NSVD (95% CI) | 2.8% (0.5–8.8%) | 1.9% (1.0–3.2%) | 0.89 |
| Composite aortic valve reop (95% CI) | 4.9% (1.2–12.6%) | 3.3% (1.9–5.1%) | 0.58 |
| Pacemaker implantation (95% CI) | 11.4 (6.2–18.3%) | 14.8% (12.0–18.0%) | 0.75 |
| Composite thrombembolic—major bleeding event (95% CI) | 2.1% (0.4–6.7%) | 8.9% (6.5–11.8%) | 0.04 |
| Variables . | BAV, N = 107 . | TAV, N = 690 . | P-Value . |
|---|---|---|---|
| Moderate–severe PVL (95% CI) | 10.7% (4.2–20.7%) | 3.9% (2.4–6.1%) | 0.06 |
| Reoperation due to NSVD (95% CI) | 2.8% (0.5–8.8%) | 1.9% (1.0–3.2%) | 0.89 |
| Composite aortic valve reop (95% CI) | 4.9% (1.2–12.6%) | 3.3% (1.9–5.1%) | 0.58 |
| Pacemaker implantation (95% CI) | 11.4 (6.2–18.3%) | 14.8% (12.0–18.0%) | 0.75 |
| Composite thrombembolic—major bleeding event (95% CI) | 2.1% (0.4–6.7%) | 8.9% (6.5–11.8%) | 0.04 |
Cumulative incidence rates at 5 years; composite aortic valve reop: composite end point reoperation and valve explantation or valve-in-valve due to structural valve degeneration, prosthesis endocarditis or NSVD; composite thromboembolic major bleeding event: composite end point stroke, transient ischaemic attack, peripheral embolism, valve thrombosis, major bleeding events. The cumulative incidence rates at 5 years are estimated accounting for competing events and the P-values are results of the Gray’s test.
BAV: bicuspid aortic valve; CI: confidence interval; NSVD: non-structural valve dysfunction; PVL: paravalvular leak; TAV: tricuspid aortic valve.
| Variables . | BAV, N = 107 . | TAV, N = 690 . | P-Value . |
|---|---|---|---|
| Moderate–severe PVL (95% CI) | 10.7% (4.2–20.7%) | 3.9% (2.4–6.1%) | 0.06 |
| Reoperation due to NSVD (95% CI) | 2.8% (0.5–8.8%) | 1.9% (1.0–3.2%) | 0.89 |
| Composite aortic valve reop (95% CI) | 4.9% (1.2–12.6%) | 3.3% (1.9–5.1%) | 0.58 |
| Pacemaker implantation (95% CI) | 11.4 (6.2–18.3%) | 14.8% (12.0–18.0%) | 0.75 |
| Composite thrombembolic—major bleeding event (95% CI) | 2.1% (0.4–6.7%) | 8.9% (6.5–11.8%) | 0.04 |
| Variables . | BAV, N = 107 . | TAV, N = 690 . | P-Value . |
|---|---|---|---|
| Moderate–severe PVL (95% CI) | 10.7% (4.2–20.7%) | 3.9% (2.4–6.1%) | 0.06 |
| Reoperation due to NSVD (95% CI) | 2.8% (0.5–8.8%) | 1.9% (1.0–3.2%) | 0.89 |
| Composite aortic valve reop (95% CI) | 4.9% (1.2–12.6%) | 3.3% (1.9–5.1%) | 0.58 |
| Pacemaker implantation (95% CI) | 11.4 (6.2–18.3%) | 14.8% (12.0–18.0%) | 0.75 |
| Composite thrombembolic—major bleeding event (95% CI) | 2.1% (0.4–6.7%) | 8.9% (6.5–11.8%) | 0.04 |
Cumulative incidence rates at 5 years; composite aortic valve reop: composite end point reoperation and valve explantation or valve-in-valve due to structural valve degeneration, prosthesis endocarditis or NSVD; composite thromboembolic major bleeding event: composite end point stroke, transient ischaemic attack, peripheral embolism, valve thrombosis, major bleeding events. The cumulative incidence rates at 5 years are estimated accounting for competing events and the P-values are results of the Gray’s test.
BAV: bicuspid aortic valve; CI: confidence interval; NSVD: non-structural valve dysfunction; PVL: paravalvular leak; TAV: tricuspid aortic valve.
Secondary end points
Permanent pacemaker implantation
The cumulative incidence of PPI at 3 months was 11.4% (95% CI: 6.2–18.3%) and remained unchanged at 5 years, reflecting the perioperative, procedure-related need for PPI before discharge after the index-valve procedure, due to complete atrio-ventricular block (AVB) in almost all patients (11/12). In the tricuspid group, the cumulative incidence of PPI increased from 11.8% (95% CI: 9.5–14.4%) at 3 months to 14.8% (95% CI: 12.0–18.0%) at 5 years follow-up (P = 0.75). In contrary to the bicuspid cohort, the TAV patients presented both in the perioperative predischarge (18/70 patients) and at follow-up (8/20 events) with other causes than complete AVB (2nd degree AVB, bradycardia, sick sinus syndrome, different high-degree blocks following MAZE procedure).
Composite end point thrombembolic and major bleeding events
The cumulative incidence of thromboembolic and major bleeding events at 1-year follow-up was 2.1% (95% CI: 0.4–6.7%) and remained constant until 5 years with no further events in the BAV group, compared with 5.0% (95% CI: 3.5–6.8%) and 8.9% (95% CI: 6.5–11.8%) at 1 and 5 years in the control group (P = 0.04).
Composite end point reoperation with valve explantation or valve-in-valve procedures
The overall cumulative incidence for reoperations including NSVD, endocarditis and structural valve degeneration at 1-, 5- and 10-year follow-up was 1.2% (95% CI: 0.1–5.7%), 4.9% (95% CI: 1.2–12.6%) and 12.1% (95% CI: 2.2–31.2%) in the bicuspid group; in the control group, the cumulative incidence of reoperations on the study valve at 1 year was 2.4% (95% CI: 1.4–3.9%), followed by 3.3% (95% CI: 1.9–5.1%) at 5 years and 5.2% (95% CI: 2.7–8.9%) at 10 years (P = 0.58).
Other postoperative outcomes
Echocardiographic findings
Repeated measures analysis of variance models were used to compare echocardiographic findings in the follow-up between the 2 valve anatomy groups. No significant difference in the transvalvular gradients and velocities was observed between BAV and TAV groups considering measurements 1, 3 and 5 years after AVR: mean AV gradients (P = 0.370), peak AV gradients (P = 0.444), AV max. velocity (P = 0.239, see Table 5). The effective orifice area and indexed EOA at discharge were 1.97 (SD: 0.58) cm2 and 1.01 (SD: 0.25) cm/m2, respectively, in the bicuspid group and 1.89 (SD: 0.56) cm2 and 0.99 (SD: 0.28) cm/m2, respectively, in the control group.
Repeated measures analysis of variance models of haemodynamic valve performance over time
| Aortic valve parameters . | FU time . | BAV . | TAV . | P-Value . |
|---|---|---|---|---|
| Mean grad., median (IQR) | 1-Year FU | (N = 51) 8 [10–12] | (N = 346) 8 [10–13] | 0.370 |
| 3-Year FU | (N = 18) 9.5 [8–11] | (N = 133) 8 [10–13] | ||
| 5-Year FU | (N = 13) 9 [11–16] | (N = 81) 8 [10–14] | ||
| Peak grad., median (IQR) | 1-Year FU | (N = 51) 19 [14–21] | (N = 346) 19 [15–23] | 0.444 |
| 3-Year FU | (N = 18) 17 [15–20] | (N = 133) 19 [15–24] | ||
| 5-Year FU | (N = 13) 18 [17–29] | (N = 81) 18 [15–25] | ||
| Velocity, mean (SD) | 1-Year FU | (N = 51) 2.1(0.4) | (N = 347) 2.2(0.4) | 0.239 |
| 3-Year FU | (N = 18) 2.1(0.3) | (N = 133) 2.2(0.4) | ||
| 5-Year FU | (N = 12) 2.3(0.5) | (N = 81) 2.3(0.6) |
| Aortic valve parameters . | FU time . | BAV . | TAV . | P-Value . |
|---|---|---|---|---|
| Mean grad., median (IQR) | 1-Year FU | (N = 51) 8 [10–12] | (N = 346) 8 [10–13] | 0.370 |
| 3-Year FU | (N = 18) 9.5 [8–11] | (N = 133) 8 [10–13] | ||
| 5-Year FU | (N = 13) 9 [11–16] | (N = 81) 8 [10–14] | ||
| Peak grad., median (IQR) | 1-Year FU | (N = 51) 19 [14–21] | (N = 346) 19 [15–23] | 0.444 |
| 3-Year FU | (N = 18) 17 [15–20] | (N = 133) 19 [15–24] | ||
| 5-Year FU | (N = 13) 18 [17–29] | (N = 81) 18 [15–25] | ||
| Velocity, mean (SD) | 1-Year FU | (N = 51) 2.1(0.4) | (N = 347) 2.2(0.4) | 0.239 |
| 3-Year FU | (N = 18) 2.1(0.3) | (N = 133) 2.2(0.4) | ||
| 5-Year FU | (N = 12) 2.3(0.5) | (N = 81) 2.3(0.6) |
Repeated measures analysis of variance models were used to compare echocardiographic findings in the follow-up between the 2 valve anatomy groups. Mean and peak transvalvular aortic gradients are expressed as median and interquartile range, and velocity is expressed as mean and standard deviation. Due to their skewed distributions, log2-transformed mean and peak gradient values were used for statistical analysis.
BAV: bicuspid aortic valve; FU: follow up; IQR: interquartile; SD: standard deviation; TAV: tricuspid aortic valve.
Repeated measures analysis of variance models of haemodynamic valve performance over time
| Aortic valve parameters . | FU time . | BAV . | TAV . | P-Value . |
|---|---|---|---|---|
| Mean grad., median (IQR) | 1-Year FU | (N = 51) 8 [10–12] | (N = 346) 8 [10–13] | 0.370 |
| 3-Year FU | (N = 18) 9.5 [8–11] | (N = 133) 8 [10–13] | ||
| 5-Year FU | (N = 13) 9 [11–16] | (N = 81) 8 [10–14] | ||
| Peak grad., median (IQR) | 1-Year FU | (N = 51) 19 [14–21] | (N = 346) 19 [15–23] | 0.444 |
| 3-Year FU | (N = 18) 17 [15–20] | (N = 133) 19 [15–24] | ||
| 5-Year FU | (N = 13) 18 [17–29] | (N = 81) 18 [15–25] | ||
| Velocity, mean (SD) | 1-Year FU | (N = 51) 2.1(0.4) | (N = 347) 2.2(0.4) | 0.239 |
| 3-Year FU | (N = 18) 2.1(0.3) | (N = 133) 2.2(0.4) | ||
| 5-Year FU | (N = 12) 2.3(0.5) | (N = 81) 2.3(0.6) |
| Aortic valve parameters . | FU time . | BAV . | TAV . | P-Value . |
|---|---|---|---|---|
| Mean grad., median (IQR) | 1-Year FU | (N = 51) 8 [10–12] | (N = 346) 8 [10–13] | 0.370 |
| 3-Year FU | (N = 18) 9.5 [8–11] | (N = 133) 8 [10–13] | ||
| 5-Year FU | (N = 13) 9 [11–16] | (N = 81) 8 [10–14] | ||
| Peak grad., median (IQR) | 1-Year FU | (N = 51) 19 [14–21] | (N = 346) 19 [15–23] | 0.444 |
| 3-Year FU | (N = 18) 17 [15–20] | (N = 133) 19 [15–24] | ||
| 5-Year FU | (N = 13) 18 [17–29] | (N = 81) 18 [15–25] | ||
| Velocity, mean (SD) | 1-Year FU | (N = 51) 2.1(0.4) | (N = 347) 2.2(0.4) | 0.239 |
| 3-Year FU | (N = 18) 2.1(0.3) | (N = 133) 2.2(0.4) | ||
| 5-Year FU | (N = 12) 2.3(0.5) | (N = 81) 2.3(0.6) |
Repeated measures analysis of variance models were used to compare echocardiographic findings in the follow-up between the 2 valve anatomy groups. Mean and peak transvalvular aortic gradients are expressed as median and interquartile range, and velocity is expressed as mean and standard deviation. Due to their skewed distributions, log2-transformed mean and peak gradient values were used for statistical analysis.
BAV: bicuspid aortic valve; FU: follow up; IQR: interquartile; SD: standard deviation; TAV: tricuspid aortic valve.
DISCUSSION
Bicuspid aortic morphology is a common congenital valve anomaly which was distinguished in approximately half of the patients requiring aortic valve surgery in different series [2]. Roberts and Ko [2] classified the frequency of aortic valve morphologies by decades in patients requiring AVR. In this collective, the mean age in the bicuspid group was 67 (SD: 11) years, compared with 74 (SD: 8) years in the tricuspid group (P < 0.001), very similar with our study collective with a mean age of 68 (SD: 8) years and the tricuspid control group of 74 (SD: 7) years (P < 0.001).
More than 25% of BAV patients develop significant aortic dilation over 45 mm at 25 years from the first diagnosis of BAV [3] with a risk of aneurysm development up to 80-fold higher than in the general population [12]. In our cohort, 22.4% of the patients with a BAV underwent surgery of the ascending aorta, compared with 4.2% in the TAV group (P < 0.001).
In the transcatheter aortic valve implantation (TAVI) era, this patient population gained more attention, especially with the newer-generation devices and the general trend towards treating younger and lower-risk patients. Because of the concerns regarding paravalvular leakage due to elliptical deployment instead of circular deployment with incomplete prosthesis expansion, the asymmetric calcification and suboptimal alignment especially in non-raphe Sievers Type 0 BAVs, the most transcatheter randomized control trials excluded bicuspid valve phenotypes [13]. A recent systematic-review and meta-analysis, which compared the TAVI outcomes with first- and new-generation in 3741 BAV vs 7291 TAV patients, revealed significant higher risk of conversion to surgery (OR = 2.35; 95% CI 1.30–4.23, P = 0.005), device failure (OR = 1.26; 95% CI 1.02–1.56; P = 0.04), implantation of a second valve (OR = 2.06; 95% CI 1.31–3.25; P = 0.002) or moderate–severe paravalvular regurgitation (OR = 1.67; 95% CI 1.29–2.17; P = 0.0001) in the BAV group [14]. These results indicate that bicuspid valve anatomy remains a matter of concern when considering TAVI for younger patients among whom the bicuspid valve morphology is more likely to be present. SAVR with an RD valve allows complete annular and subannular decalcification, which is an advantage compared with TAVI.
The first-generation TAVI was also related with a higher incidence of new PPI in BAV patients, assumingly due to the asymmetric deployment and compression towards the atrioventricular node and His bundle [15]. In our study cohort, the rate of pacemaker implantation is high due to the radial forces and compression exerted by the subannular stent frame in the LVOT, similar to transcatheter valves. In our collective, no significant difference between bicuspid and tricuspid groups was observed (P = 0.75), with a cumulative incidence of 11.4% (95% CI: 6.2–18.3%) in the BAV, compared to 11.8% (95% CI: 9.5–14.4%) in the TAV group at 3 months. However, the cumulative incidence of PPI at long-term 5-year follow-up remained stable at 11.4 (95% CI: 6.2–18.3%) in the BAV group and slightly increased in the TAV group to 14.8% (95% CI: 12.0–18.0%), which reflected the older population and other indications not related to complete AVB (sick sinus syndrome, bradycardia) for late PPI. Our previous analysis revealed that baseline conduction disturbances as RBBB, advanced age and overall combined procedures were an independent predictor for the occurrence of PPI [16].
Last but not least long-term data regarding durability of TAVI technologies in bicuspid patients is missing. The risk for leaflet thickening associated with subclinical valve thrombosis might be higher in these patients due to incomplete prosthesis expansion and could be associated with accelerated structural valve degeneration [17]. We previously showed a very low rate of structural valve degeneration with the Edwards Intuity Valve at long-term follow-up [18]. Moreover, no cases of valve thrombosis were observed in our cohort.
Miceli et al. reported the early results from the SURD registry regarding 191 AVRs in a bicuspid valve anatomy with either the sutureless Perceval Valve or the RD Edwards Intuity Valve System. The 30-day mortality was 1.6% and the in-hospital outcomes revealed the occurrence of moderate–severe aortic valve regurgitation in 3.7% of the cases; however, the origin of the regurgitation, valvular or paravalvular could not be discriminated from this retrospective analysis [5]. In our study, we analysed 107 patients with a native bicuspid valve implanted with the RD Intuity Valve and compared the outcomes with a control group of 690 patients with a tricuspid valve. At 1 year, the cumulative incidence of moderate-to-severe paravalvular regurgitation in the BAV group was 3.3% (95% CI: 0.9–8.6%) and progressed to 10.7% (95% CI: 4.2–20.7%) at 5 years, compared with 2.4% (95% CI: 1.4–3.8%) and 3.9% (95% CI: 2.4–6.1%) in the TAV group. Moreover, the cumulative incidence rate of reoperation with valve explantation due to NSVD at 1 and 5 years was 1.2% (95% CI: 0.1–5.7%) and 2.8% (95% CI: 0.5–8.8%) in the BAV group, and 1.9% (95% CI: 1.0–3.2%), which remained constant at 5 years in the TAV group, with no further events after 1-year follow-up, also without any significant difference between groups (P = 0.89) (Central Image). However, these findings confirm a trend to increased paravalvular leak progression in the BAV group and despite the non-significant statistical difference these findings are clinically relevant. Given our institutional experience, we consider that RD AVR should be performed with caution in bicuspid valve anatomies, taking into account several considerations. Correct sizing is crucial when implanting rapid-deployment valves and undersizing should be avoided. In bicuspid valves with asymmetric annular shapes and calcifications, where the annulus is in between 2 sizes, extensive decalcification and choosing of a larger prosthesis, may prevent asymmetric expansion of the subannular stent frame and consequently avoid paravalvular regurgitation. This fact may be advantageous in small bicuspid aortic roots with annular sizes varying from 18 to 22 mm. In these cases, we recommend the use of an RD valve in favour of sutured valve implantation. This rationale is supported by the excellent haemodynamics and reduced incidence of prosthesis–patient mismatch previously described [19]. However, snug-fit sizing might come with the price of a new onset of a high-degree block and the need of PPI. These advantages and disadvantages of RD prosthesis must be weighted to take the best therapeutic decision for our patients. A further technical consideration is the asymmetry of the commissures, often observed in bicuspid anatomies, may determine the need of creating neo-commissures and placing the nadir guiding sutures according to that; this step has to be performed with caution in order to avoid coronary obstruction through one of the prosthesis commissural posts. Nguyen et al. [6] reported no valve malpositioning or moderate–severe aortic regurgitation after 25 sutureless AVRs in bicuspid valve patients and suggested positioning the replica of the commercial sizer into the annulus for a better orientation at 120° of the 3 guiding sutures. In case of Sievers 0 with 2 sinuses and anterior–posterior cusp orientation with abnormal origin of both coronaries from 1 cusp (predominantly the anterior one), the risk of coronary obstruction is increased and the implantation of the Intuity Valve should be avoided. Another deviation from the standard implantation protocol, which we applied in some selected cases, might be an extra guiding suture placed in the larger sinus, mostly in the non-coronary sinus. Last but not least, we do not recommend the use of the Intuity Valve in bicuspid anatomy for surgeons with a low case load and limited experience with the Intuity Valve; we recommend at least 30–50 Intuity Valve implantations in tricuspid anatomy before considering RD-AVR in bicuspid valves. RD-AVR is feasible in patients with bicuspid valve after careful pre- and intraoperative evaluation in high-volume centres.
Limitations
Our study has the limitations of an observational study, without centre-independent assessment of the adverse events and an independent core laboratory to assess the paravalvular regurgitation severity. Part of the outcomes, as Society of Thoracic Surgeons score or EuroSCORE, obtained retrospectively in case of the early generation of implanted valves. Device selection was at the operator’s discretion and the operator experience as well as patient selection might have led to intraoperative decision to select a conventional sutured valve due to Sievers type 0 phenotype, visible elliptical annulus or annulus injury by extensive decalcification. From our experience, the most trained surgeons at our institution, with a considerable number of RD-AVR, implanted the valve in all anatomy variations. Moreover, the failed implantations were described but per study protocol excluded from further analyses, which might have slightly influenced the outcomes.
CONCLUSIONS
RD-AVR can be performed in patients with a BAV without significantly increasing the risk valve explantation for NSVD compared with tricuspid anatomy. However, a trend to increased frequency of moderate–severe paravalvular regurgitation was observed at long-term follow-up. When performing RD-SAVR in bicuspid anatomies, specific technical considerations and precautions have to be taken into account to avoid valve dislocation and paravalvular regurgitation.
Funding
The patients from multicentre clinical trials funded by Edwards Lifesciences (Irvine, CA, USA) were included in this analysis. Our institution receives financial support from the same company to conduct a long-term follow-up after Intuity Valve implantation.
Conflict of interest: Martin Andreas has received institutional research funding (Edwards, Abbott, Medtronic, LSI) and has served as a proctor/speaker/consultant (Edwards, Abbott, Medtronic). Guenther Laufer has received consulting fees from Edwards Lifesciences. Alfred Kocher has received speaking honoraria from Edwards Lifesciences. All other authors have no conflicts of interest to declare.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (Iuliana Coti), upon reasonable request.
Author contributions
Iuliana Coti: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Validation; Visualization; Writing—original draft; Writing—review & editing. Paul Werner: Formal analysis; Validation; Visualization; Writing—review & editing. Alexandra Kaider: Data curation; Formal analysis; Methodology; Software; Validation; Visualization; Writing—review & editing. Markus Mach: Validation; Visualization; Writing—review & editing. Alfred Kocher: Funding acquisition; Resources; Supervision; Validation; Visualization; Writing—review & editing. Guenther Laufer: Formal analysis; Funding acquisition; Resources; Supervision; Validation; Visualization; Writing—review & editing. Martin Andreas: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Borut Gersak, Andrew E. Newcomb and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
ABBREVIATIONS
- AVB
Atrio-ventricular block
- AVR
Aortic valve replacement
- BAV
Bicuspid aortic valve
- CI
Confidence interval
- NSVD
Non-structural valve dysfunction
- PPI
Permanent pacemaker implantation
- RD
Rapid deployment
- RD-AVR
Rapid-deployment aortic valve replacement
- SAVR
Surgical aortic valve replacement
- SD
Standard deviation
- TAVI
Transcatheter aortic valve implantation





