-
PDF
- Split View
-
Views
-
Cite
Cite
Alvise Guariento, Chiara A Schiena, Claudia Cattapan, Martina Avesani, Ilias P Doulamis, Massimo A Padalino, Biagio Castaldi, Giovanni di Salvo, Vladimiro Vida, Pulmonary valve preservation during tetralogy of Fallot repair: midterm functional outcomes and risk factors for pulmonary regurgitation, European Journal of Cardio-Thoracic Surgery, Volume 62, Issue 2, August 2022, ezac365, https://doi.org/10.1093/ejcts/ezac365
Close - Share Icon Share
Abstract
Many centres have recently adopted pulmonary valve (PV) preservation (PVP) during tetralogy of Fallot (ToF) repair. We sought to identify the midterm functional outcomes and risk factors for pulmonary regurgitation after this procedure.
All patients undergoing PVP during transatrial–transpulmonary repair for ToF with PV stenosis at our institution between January 2007 and December 2020 were reviewed.
Overall, 73 patients were included. At the index surgery, the body surface area was 0.31 ± 0.04 m2, the age was 4.9 ± 2.9 months and the preoperative PV z-score was -3.02 ± 1.11. At a mean follow-up of 5.3 ± 2.7 years, the fractional area change of the right ventricle (RV) was 47.1 ± 5.2%, and the tricuspid annular plane systolic excursion z-score was -3.31 ± 1.89%. The 5-year freedom from moderate/severe PV regurgitation was 61.3% [95% confidence interval (CI): 48, 73%]. There was a significant correlation between RV function and moderate/severe PR at follow-up (R2: 0.08; P = 0.03). A comparison with a group of patients undergoing a transannular patch procedure (N = 33) showed superior outcomes for patients with PVP. The preoperative PV z-score and the degree of PR at discharge were risk factors for the early development of moderate/severe PR at follow-up [hazard ratio (HR): 0.64; 95% CI: 0.48, 0.86, P = 0.01 and HR: 2.31; 95% CI: 1.00, 5.36, P = 0.04, respectively]. A preoperative PV annulus z-score ≤ -2.85 was found to be predictive for moderate/severe PR at 5 years after PVP (HR: 2.56; 95% CI: 1.31, 5.01, P = 0.002).
A pulmonary valve preservation strategy during tetralogy of Fallot repair should always be attempted. However, a preoperative PV annulus z-score < -2.85 and moderate/severe regurgitation upon discharge are risk factors for midterm pulmonary regurgitation.
INTRODUCTION
Tetralogy of Fallot (TOF) is the most common cyanotic heart malformation in newborns, with an estimated frequency of 0.24 per thousand live births [1]. Its aetiology is unknown, and the disease appears to be largely sporadic and multifactorial. In TOF, misalignment of the infundibular septum is responsible for both the presence of an interventricular septal defect, aortic overriding and pulmonary stenosis [2]. Depending on the degree of dextroposition of the infundibular septum, there may be different degrees of pulmonary obstruction. The pulmonary valve (PV) is also often involved in this process and can exhibit varying degrees of dysplasia. This valve is traditionally sacrificed with a transannular patch (TAP) during TOF surgical correction in order to relieve the obstruction of the right ventricular (RV) outflow tract (RVOT).
There has been increasing evidence regarding the deleterious effect of chronic pulmonary regurgitation (PR) after surgical correction, particularly on RV function [3–5]. As a result, several PV preservation (PVP) strategies have been proposed [6]. For more than 10 years, our centre has adopted and modified techniques involving an intraoperative balloon dilation of the PV or minimal transannular incision with simultaneous preservation of the native PV apparatus, as widely described in our previous studies [7–12]. The goal of these new surgical strategies is to preserve the PV as much as possible, without the placement of a TAP. Although PR has been associated with poor RV function, few studies have investigated the potential benefit of PVP strategies in this setting [4, 13–16], mainly due to the scarcity of centres performing these procedures and the relatively short follow-up periods of these case series.
The goal of this study was to evaluate the midterm outcomes of a PVP approach, with particular attention to RV and PV function at follow-up, and to identify the risk factors for PR at follow-up.
PATIENTS AND METHODS
Ethical statement
A single-centre review of consecutive patients undergoing complete repair of TOF at our centre between January 2007 and December 2020 was approved by the University of Padua institutional review board for clinical investigations, with a waiver of consent (Protocol n. 3254/AO/14).
Study design and definitions
All patients with TOF and RVOT obstruction undergoing TOF repair were enrolled. Excluded from the study were patients with mild forms of TOF that did not require PV manipulation; TOF with severe RVOT obstruction and with hypoplasia of the pulmonary branches with aortopulmonary collaterals treated with the interposition of a conduit between the right ventricle and the pulmonary artery; TOF with absent or atretic PV; double-outlet right ventricle; TOF with concomitant atrioventricular defect; and adults undergoing surgical correction of TOF.
The PV was assessed for each patient with preoperative, intraoperative and postoperative echocardiography and with direct measurements intraoperatively. PV dimensions were divided into the “real PV annulus”, i.e. the orifice generated after commissurotomy (fusion of the commissures is almost always present in the case of dysplastic valves), and the “effective PV orifice”, i.e. the orifice of the PV measured with a Hegar dilator immediately after valve exposure.
A total of 73 patients underwent a successful PVP. A separate cohort of 33 patients undergoing TOF repair with TAP interposition and monocusp reconstruction in the same period was used for comparison of midterm outcomes [8].
Echocardiographic measurements
All echocardiographic studies were performed using a Philips EpiQ 7 ultrasound machine (Koninklijke Philips N.V., Amsterdam, Netherlands) and a GE E95 ultrasound machine (GE Healthcare, Wauwatosa, WI, USA).
Data were digitally stored and then analysed offline by echocardiographers experienced in paediatric cardiology and blind to the clinical and surgical data (MA, BC, GDS). Chamber quantification measurements were evaluated according to current guidelines from the European Association of Echocardiography [17]. From the apical window, RV end-diastolic and end-systolic areas were measured and normalized for the body surface area (BSA). The tricuspid annular plane systolic excursion (TAPSE) and the fractional area change of the RV were also measured and expressed as percentages. The PV z-score was calculated by measuring the BSA (body surface index) and the real PV annulus by 2-dimensional echocardiography.
PR was assessed from the parasternal short-axis view using colour flow Doppler, as previously described [18]. PR was defined as grade 1 (mild) in the presence of reverse diastolic flow at the level of the PV annulus, as grade 2 (moderate) when the jet reached the main pulmonary artery and as grade 3 (severe) in the presence of reverse diastolic flow entering the pulmonary branches. The colour scale was set at a Nyquist limit of 50–60 cm/s. The pressure half time was assessed by continuous-wave Doppler. When the pressure half time was < 100 ms, PR was considered to be at least moderate [19]. The ratio between the duration of PR in diastole and the entire duration of diastole (the PR index) was also calculated and a PR index value < 0.77 was used to identify severe PR [20]. The transpulmonary velocity flow curve evaluated with continuous-wave Doppler was used to estimate the maximum and mean systolic pressure gradients, according to the simplified Bernoulli equation (DP = 4V2). PV stenosis was defined as severe with a peak gradient > 64 mmHg. Antegrade systolic and retrograde diastolic time velocity integrals were measured separately. Echocardiographic data were evaluated preoperatively, at discharge and at the last available follow-up.
Statistical analyses
Distributions of the quantitative variables were evaluated for normality using the Shapiro-Wilk test. Continuous variables following normal distribution are expressed as mean ± standard deviation. Categorical data are presented using frequencies and percentages (Tables 1–4). Normality was assessed by the Shapiro-Wilk test as well as graphically by Q-Q plots. The non-parametric Kruskal-Wallis signed-rank test was used for non-normal distributions. An unpaired t-test was used in case of normalized data distribution. The χ2 test was used for categorical data. Overall, 5-year or 3-year freedom from moderate/severe PV regurgitation was analysed using Kaplan–Meier curves with 95% CIs (Supplemental Table 1) estimated using Greenwood’s formula. Curves were truncated at the time point when < 10 patients were at risk. The log-rank test was used to generate a P-value when performing between-group comparisons. Pearson’s R2 was calculated to determine the correlation between echocardiographic data and the length of freedom from moderate/severe PR. (Only data from patients who did not have moderate/severe PV regurgitation at follow-up were included in the correlation analysis.) Univariate and multivariable Cox proportional hazards regression modelling analyses were used to analyse risk factors for moderate/severe PR while adjusting for baseline covariates, with the results presented as HRs with 95% CIs and P-values. The proportional hazards assumption was tested on the basis of Schoenfeld residuals. A priori selected preoperative and intraoperative variables were included in the models. Variables with P < 0.05 on univariate analysis were included in the multivariable regression modelling. Receiver operating characteristic curves were plotted to identify the optimal PV z-score threshold for predicting 5-year freedom from moderate/severe PR. Stata software version 16.0 (Stata Corp LLC, College Station, TX, USA) and GraphPad Prism 9.0 (MacOS, GraphPad Software, La Jolla, CA, USA) were used for statistical analyses.
| . | Total (N = 73) . | Z-score ≤ -2.85 (N = 37) . | Z-score > -2.85 (N = 36) . | P-value . |
|---|---|---|---|---|
| Demographics | ||||
| Male gender | 34 (47) | 15 (41) | 19 (53) | 0.3 |
| Weight (kg) | 6.0 ± 1.2 | 6.1 ± 1.5 | 5.9 ± 0.8 | 0.4 |
| Height (cm) | 63.3 ± 5.3 | 63.6 ± 6.8 | 63.0 ± 3.2 | 0.6 |
| BSA (m2) | 0.31 ± 0.04 | 0.32 ± 0.05 | 0.31 ± 0.03 | 0.3 |
| SpO2 (%) | 92 ± 7 | 89 ± 8 | 94 ± 5 | 0.04 |
| Cyanotic spell | 4 (5) | 3 (8) | 1 (3) | 0.3 |
| Coronary anomalies | 2 (3) | 2 (5) | 0 (0) | 0.2 |
| Preoperative data | ||||
| Numbers of PV cusps | 0.3 | |||
| Unicuspid | 2 (3) | 2 (5) | 0 (0) | |
| Bicuspid | 58 (79) | 30 (81) | 28 (78) | |
| Tricuspid | 13 (18) | 5 (14) | 8 (22) | |
| Types of PV leaflets | 0.2 | |||
| Normal | 31 (42) | 13 (35) | 18 (50) | |
| Dysplastic | 42 (58) | 24 (65) | 18 (50) | |
| Max RVOT gradient preoperatively (mmHg) | 70.0 ± 16.8 | 68.8 ± 14.2 | 71.3 ± 19.2 | 0.5 |
| PV z-score | −3.02 ± 1.11 | −3.86 ± 0.84 | −2.17 ± 0.54 | <0.001 |
| . | Total (N = 73) . | Z-score ≤ -2.85 (N = 37) . | Z-score > -2.85 (N = 36) . | P-value . |
|---|---|---|---|---|
| Demographics | ||||
| Male gender | 34 (47) | 15 (41) | 19 (53) | 0.3 |
| Weight (kg) | 6.0 ± 1.2 | 6.1 ± 1.5 | 5.9 ± 0.8 | 0.4 |
| Height (cm) | 63.3 ± 5.3 | 63.6 ± 6.8 | 63.0 ± 3.2 | 0.6 |
| BSA (m2) | 0.31 ± 0.04 | 0.32 ± 0.05 | 0.31 ± 0.03 | 0.3 |
| SpO2 (%) | 92 ± 7 | 89 ± 8 | 94 ± 5 | 0.04 |
| Cyanotic spell | 4 (5) | 3 (8) | 1 (3) | 0.3 |
| Coronary anomalies | 2 (3) | 2 (5) | 0 (0) | 0.2 |
| Preoperative data | ||||
| Numbers of PV cusps | 0.3 | |||
| Unicuspid | 2 (3) | 2 (5) | 0 (0) | |
| Bicuspid | 58 (79) | 30 (81) | 28 (78) | |
| Tricuspid | 13 (18) | 5 (14) | 8 (22) | |
| Types of PV leaflets | 0.2 | |||
| Normal | 31 (42) | 13 (35) | 18 (50) | |
| Dysplastic | 42 (58) | 24 (65) | 18 (50) | |
| Max RVOT gradient preoperatively (mmHg) | 70.0 ± 16.8 | 68.8 ± 14.2 | 71.3 ± 19.2 | 0.5 |
| PV z-score | −3.02 ± 1.11 | −3.86 ± 0.84 | −2.17 ± 0.54 | <0.001 |
BSA: body surface area; PV: pulmonary valve; RVOT; right ventricular outflow tract; SpO2: oxygen saturation.
| . | Total (N = 73) . | Z-score ≤ -2.85 (N = 37) . | Z-score > -2.85 (N = 36) . | P-value . |
|---|---|---|---|---|
| Demographics | ||||
| Male gender | 34 (47) | 15 (41) | 19 (53) | 0.3 |
| Weight (kg) | 6.0 ± 1.2 | 6.1 ± 1.5 | 5.9 ± 0.8 | 0.4 |
| Height (cm) | 63.3 ± 5.3 | 63.6 ± 6.8 | 63.0 ± 3.2 | 0.6 |
| BSA (m2) | 0.31 ± 0.04 | 0.32 ± 0.05 | 0.31 ± 0.03 | 0.3 |
| SpO2 (%) | 92 ± 7 | 89 ± 8 | 94 ± 5 | 0.04 |
| Cyanotic spell | 4 (5) | 3 (8) | 1 (3) | 0.3 |
| Coronary anomalies | 2 (3) | 2 (5) | 0 (0) | 0.2 |
| Preoperative data | ||||
| Numbers of PV cusps | 0.3 | |||
| Unicuspid | 2 (3) | 2 (5) | 0 (0) | |
| Bicuspid | 58 (79) | 30 (81) | 28 (78) | |
| Tricuspid | 13 (18) | 5 (14) | 8 (22) | |
| Types of PV leaflets | 0.2 | |||
| Normal | 31 (42) | 13 (35) | 18 (50) | |
| Dysplastic | 42 (58) | 24 (65) | 18 (50) | |
| Max RVOT gradient preoperatively (mmHg) | 70.0 ± 16.8 | 68.8 ± 14.2 | 71.3 ± 19.2 | 0.5 |
| PV z-score | −3.02 ± 1.11 | −3.86 ± 0.84 | −2.17 ± 0.54 | <0.001 |
| . | Total (N = 73) . | Z-score ≤ -2.85 (N = 37) . | Z-score > -2.85 (N = 36) . | P-value . |
|---|---|---|---|---|
| Demographics | ||||
| Male gender | 34 (47) | 15 (41) | 19 (53) | 0.3 |
| Weight (kg) | 6.0 ± 1.2 | 6.1 ± 1.5 | 5.9 ± 0.8 | 0.4 |
| Height (cm) | 63.3 ± 5.3 | 63.6 ± 6.8 | 63.0 ± 3.2 | 0.6 |
| BSA (m2) | 0.31 ± 0.04 | 0.32 ± 0.05 | 0.31 ± 0.03 | 0.3 |
| SpO2 (%) | 92 ± 7 | 89 ± 8 | 94 ± 5 | 0.04 |
| Cyanotic spell | 4 (5) | 3 (8) | 1 (3) | 0.3 |
| Coronary anomalies | 2 (3) | 2 (5) | 0 (0) | 0.2 |
| Preoperative data | ||||
| Numbers of PV cusps | 0.3 | |||
| Unicuspid | 2 (3) | 2 (5) | 0 (0) | |
| Bicuspid | 58 (79) | 30 (81) | 28 (78) | |
| Tricuspid | 13 (18) | 5 (14) | 8 (22) | |
| Types of PV leaflets | 0.2 | |||
| Normal | 31 (42) | 13 (35) | 18 (50) | |
| Dysplastic | 42 (58) | 24 (65) | 18 (50) | |
| Max RVOT gradient preoperatively (mmHg) | 70.0 ± 16.8 | 68.8 ± 14.2 | 71.3 ± 19.2 | 0.5 |
| PV z-score | −3.02 ± 1.11 | −3.86 ± 0.84 | −2.17 ± 0.54 | <0.001 |
BSA: body surface area; PV: pulmonary valve; RVOT; right ventricular outflow tract; SpO2: oxygen saturation.
| . | Total (N = 73) . | Z-score ≤ -2.85 (N = 37) . | Z-score > -2.85 (N = 36) . | P-value . |
|---|---|---|---|---|
| Operative data | ||||
| Age at surgery (months) | 4.8 ± 2.9 | 5.5 ± 3.8 | 4.2 ± 1.4 | 0.3 |
| Effective PV orifice precommissurotomy | 5.1 ± 1.2 | 4.4 ± 0.9 | 5.9 ± 1.0 | <0.001 |
| Real PV annulus after the commissurotomy | 7.0 ± 1.0 | 6.3 ± 0.7 | 7.6 ± 0.7 | <0.001 |
| RVSP (% of systemic) | 0.01 | |||
| 1/3 | 23 (32) | 9 (24) | 14 (39) | |
| 1/2 | 31 (42) | 13 (35) | 18 (50) | |
| 2/3 | 19 (26) | 15 (41) | 4 (11) | |
| CPB time (min) | 139 ± 31 | 148 ± 29 | 130 ± 31 | 0.008 |
| AOX time (min) | 86 ± 22 | 94 ± 16 | 79 ± 25 | 0.001 |
| Postoperative outcomes | ||||
| ICU stay (days) | 3.8 ± 2.6 | 4.3 ± 3.3 | 3.3 ± 1.5 | 0.2 |
| Length of stay (days) | 13.2 ± 7.8 | 13.8 ± 8.0 | 12.7 ± 7.5 | 0.5 |
| Postoperative complications | 18 (25) | 11 (30) | 7 (19) | 0.4 |
| Arrhythmia | 8 (11) | 4 (11) | 4 (11) | 0.8 |
| Permanent arrhythmia | 2 (3) | 2 (5) | 0 (0) | |
| Temporary arrhythmia | 6 (8) | 2 (5) | 4 (11) | |
| LCOS | 3 (4) | 2 (5) | 1 (3) | 0.5 |
| Other complications | 7 (10) | 5 (14) | 3 (8) | |
| Max RVOT gradient at D/C (mmHg) | 29.8 ± 8.6 | 30.5 ± 9.1 | 29.2 ± 8.3 | 0.7 |
| PR grade at D/C | 0.04 | |||
| Grade 1 (mild) | 66 (90) | 31 (84) | 35 (97) | |
| Grade 2 (moderate) | 7 (10) | 6 (16) | 1 (3) | |
| Grade 3 (severe) | 0 (0) | 0 (0) | 0 (0) | |
| . | Total (N = 73) . | Z-score ≤ -2.85 (N = 37) . | Z-score > -2.85 (N = 36) . | P-value . |
|---|---|---|---|---|
| Operative data | ||||
| Age at surgery (months) | 4.8 ± 2.9 | 5.5 ± 3.8 | 4.2 ± 1.4 | 0.3 |
| Effective PV orifice precommissurotomy | 5.1 ± 1.2 | 4.4 ± 0.9 | 5.9 ± 1.0 | <0.001 |
| Real PV annulus after the commissurotomy | 7.0 ± 1.0 | 6.3 ± 0.7 | 7.6 ± 0.7 | <0.001 |
| RVSP (% of systemic) | 0.01 | |||
| 1/3 | 23 (32) | 9 (24) | 14 (39) | |
| 1/2 | 31 (42) | 13 (35) | 18 (50) | |
| 2/3 | 19 (26) | 15 (41) | 4 (11) | |
| CPB time (min) | 139 ± 31 | 148 ± 29 | 130 ± 31 | 0.008 |
| AOX time (min) | 86 ± 22 | 94 ± 16 | 79 ± 25 | 0.001 |
| Postoperative outcomes | ||||
| ICU stay (days) | 3.8 ± 2.6 | 4.3 ± 3.3 | 3.3 ± 1.5 | 0.2 |
| Length of stay (days) | 13.2 ± 7.8 | 13.8 ± 8.0 | 12.7 ± 7.5 | 0.5 |
| Postoperative complications | 18 (25) | 11 (30) | 7 (19) | 0.4 |
| Arrhythmia | 8 (11) | 4 (11) | 4 (11) | 0.8 |
| Permanent arrhythmia | 2 (3) | 2 (5) | 0 (0) | |
| Temporary arrhythmia | 6 (8) | 2 (5) | 4 (11) | |
| LCOS | 3 (4) | 2 (5) | 1 (3) | 0.5 |
| Other complications | 7 (10) | 5 (14) | 3 (8) | |
| Max RVOT gradient at D/C (mmHg) | 29.8 ± 8.6 | 30.5 ± 9.1 | 29.2 ± 8.3 | 0.7 |
| PR grade at D/C | 0.04 | |||
| Grade 1 (mild) | 66 (90) | 31 (84) | 35 (97) | |
| Grade 2 (moderate) | 7 (10) | 6 (16) | 1 (3) | |
| Grade 3 (severe) | 0 (0) | 0 (0) | 0 (0) | |
AOX: aortic cross-clamp; CPB: cardiopulmonary bypass; D/C: discharge; ICU: intensive care unit; LCOS: low cardiac output syndrome; PR: pulmonary valve regurgitation; PV: pulmonary valve; RVOT; right ventricular outflow tract; RVSP: right ventricular systolic pressure.
| . | Total (N = 73) . | Z-score ≤ -2.85 (N = 37) . | Z-score > -2.85 (N = 36) . | P-value . |
|---|---|---|---|---|
| Operative data | ||||
| Age at surgery (months) | 4.8 ± 2.9 | 5.5 ± 3.8 | 4.2 ± 1.4 | 0.3 |
| Effective PV orifice precommissurotomy | 5.1 ± 1.2 | 4.4 ± 0.9 | 5.9 ± 1.0 | <0.001 |
| Real PV annulus after the commissurotomy | 7.0 ± 1.0 | 6.3 ± 0.7 | 7.6 ± 0.7 | <0.001 |
| RVSP (% of systemic) | 0.01 | |||
| 1/3 | 23 (32) | 9 (24) | 14 (39) | |
| 1/2 | 31 (42) | 13 (35) | 18 (50) | |
| 2/3 | 19 (26) | 15 (41) | 4 (11) | |
| CPB time (min) | 139 ± 31 | 148 ± 29 | 130 ± 31 | 0.008 |
| AOX time (min) | 86 ± 22 | 94 ± 16 | 79 ± 25 | 0.001 |
| Postoperative outcomes | ||||
| ICU stay (days) | 3.8 ± 2.6 | 4.3 ± 3.3 | 3.3 ± 1.5 | 0.2 |
| Length of stay (days) | 13.2 ± 7.8 | 13.8 ± 8.0 | 12.7 ± 7.5 | 0.5 |
| Postoperative complications | 18 (25) | 11 (30) | 7 (19) | 0.4 |
| Arrhythmia | 8 (11) | 4 (11) | 4 (11) | 0.8 |
| Permanent arrhythmia | 2 (3) | 2 (5) | 0 (0) | |
| Temporary arrhythmia | 6 (8) | 2 (5) | 4 (11) | |
| LCOS | 3 (4) | 2 (5) | 1 (3) | 0.5 |
| Other complications | 7 (10) | 5 (14) | 3 (8) | |
| Max RVOT gradient at D/C (mmHg) | 29.8 ± 8.6 | 30.5 ± 9.1 | 29.2 ± 8.3 | 0.7 |
| PR grade at D/C | 0.04 | |||
| Grade 1 (mild) | 66 (90) | 31 (84) | 35 (97) | |
| Grade 2 (moderate) | 7 (10) | 6 (16) | 1 (3) | |
| Grade 3 (severe) | 0 (0) | 0 (0) | 0 (0) | |
| . | Total (N = 73) . | Z-score ≤ -2.85 (N = 37) . | Z-score > -2.85 (N = 36) . | P-value . |
|---|---|---|---|---|
| Operative data | ||||
| Age at surgery (months) | 4.8 ± 2.9 | 5.5 ± 3.8 | 4.2 ± 1.4 | 0.3 |
| Effective PV orifice precommissurotomy | 5.1 ± 1.2 | 4.4 ± 0.9 | 5.9 ± 1.0 | <0.001 |
| Real PV annulus after the commissurotomy | 7.0 ± 1.0 | 6.3 ± 0.7 | 7.6 ± 0.7 | <0.001 |
| RVSP (% of systemic) | 0.01 | |||
| 1/3 | 23 (32) | 9 (24) | 14 (39) | |
| 1/2 | 31 (42) | 13 (35) | 18 (50) | |
| 2/3 | 19 (26) | 15 (41) | 4 (11) | |
| CPB time (min) | 139 ± 31 | 148 ± 29 | 130 ± 31 | 0.008 |
| AOX time (min) | 86 ± 22 | 94 ± 16 | 79 ± 25 | 0.001 |
| Postoperative outcomes | ||||
| ICU stay (days) | 3.8 ± 2.6 | 4.3 ± 3.3 | 3.3 ± 1.5 | 0.2 |
| Length of stay (days) | 13.2 ± 7.8 | 13.8 ± 8.0 | 12.7 ± 7.5 | 0.5 |
| Postoperative complications | 18 (25) | 11 (30) | 7 (19) | 0.4 |
| Arrhythmia | 8 (11) | 4 (11) | 4 (11) | 0.8 |
| Permanent arrhythmia | 2 (3) | 2 (5) | 0 (0) | |
| Temporary arrhythmia | 6 (8) | 2 (5) | 4 (11) | |
| LCOS | 3 (4) | 2 (5) | 1 (3) | 0.5 |
| Other complications | 7 (10) | 5 (14) | 3 (8) | |
| Max RVOT gradient at D/C (mmHg) | 29.8 ± 8.6 | 30.5 ± 9.1 | 29.2 ± 8.3 | 0.7 |
| PR grade at D/C | 0.04 | |||
| Grade 1 (mild) | 66 (90) | 31 (84) | 35 (97) | |
| Grade 2 (moderate) | 7 (10) | 6 (16) | 1 (3) | |
| Grade 3 (severe) | 0 (0) | 0 (0) | 0 (0) | |
AOX: aortic cross-clamp; CPB: cardiopulmonary bypass; D/C: discharge; ICU: intensive care unit; LCOS: low cardiac output syndrome; PR: pulmonary valve regurgitation; PV: pulmonary valve; RVOT; right ventricular outflow tract; RVSP: right ventricular systolic pressure.
| . | Total (N = 73) . | Z-score ≤ -2.85 (N = 37) . | Z-score > -2.85 (N = 36) . | P-value . |
|---|---|---|---|---|
| Type of sparing procedure | <0.001 | |||
| Balloon only | 20 (27) | 3 (8) | 17 (47) | |
| Resuspension only | 7 (10) | 3 (8) | 4 (11) | |
| Delamination and resuspension | 21 (29) | 12 (32) | 9 (25) | |
| Leaflet patch augmentation | 7 (10) | 3 (8) | 4 (11) | |
| Minitransannular incision | 18 (25) | 16 (43) | 2 (6) | |
| Simple sparing procedure | 20 (27) | 3 (8) | 17 (47) | <0.001 |
| Complex sparing procedure | 53 (73) | 34 (92) | 19 (53) | <0.001 |
| Maximum balloon size (mm) | 0.2 | |||
| 9 | 12 (16) | 5 (14) | 7 (19) | |
| 10 | 42 (58) | 14 (38) | 28 (78) | |
| 11 | 17 (23) | 16 (43) | 1 (3) | |
| 12 | 2 (3) | 2 (5) | 0 (0) | |
| Series balloon dilation | 20 (27) | 10 (27) | 10 (28) | 0.2 |
| PV effective orifice after balloon dilation | 9.5 ± 0.7 | 10.7 ± 0.9 | 9.5 ± 0.6 | 0.3 |
| . | Total (N = 73) . | Z-score ≤ -2.85 (N = 37) . | Z-score > -2.85 (N = 36) . | P-value . |
|---|---|---|---|---|
| Type of sparing procedure | <0.001 | |||
| Balloon only | 20 (27) | 3 (8) | 17 (47) | |
| Resuspension only | 7 (10) | 3 (8) | 4 (11) | |
| Delamination and resuspension | 21 (29) | 12 (32) | 9 (25) | |
| Leaflet patch augmentation | 7 (10) | 3 (8) | 4 (11) | |
| Minitransannular incision | 18 (25) | 16 (43) | 2 (6) | |
| Simple sparing procedure | 20 (27) | 3 (8) | 17 (47) | <0.001 |
| Complex sparing procedure | 53 (73) | 34 (92) | 19 (53) | <0.001 |
| Maximum balloon size (mm) | 0.2 | |||
| 9 | 12 (16) | 5 (14) | 7 (19) | |
| 10 | 42 (58) | 14 (38) | 28 (78) | |
| 11 | 17 (23) | 16 (43) | 1 (3) | |
| 12 | 2 (3) | 2 (5) | 0 (0) | |
| Series balloon dilation | 20 (27) | 10 (27) | 10 (28) | 0.2 |
| PV effective orifice after balloon dilation | 9.5 ± 0.7 | 10.7 ± 0.9 | 9.5 ± 0.6 | 0.3 |
PV: pulmonary valve.
| . | Total (N = 73) . | Z-score ≤ -2.85 (N = 37) . | Z-score > -2.85 (N = 36) . | P-value . |
|---|---|---|---|---|
| Type of sparing procedure | <0.001 | |||
| Balloon only | 20 (27) | 3 (8) | 17 (47) | |
| Resuspension only | 7 (10) | 3 (8) | 4 (11) | |
| Delamination and resuspension | 21 (29) | 12 (32) | 9 (25) | |
| Leaflet patch augmentation | 7 (10) | 3 (8) | 4 (11) | |
| Minitransannular incision | 18 (25) | 16 (43) | 2 (6) | |
| Simple sparing procedure | 20 (27) | 3 (8) | 17 (47) | <0.001 |
| Complex sparing procedure | 53 (73) | 34 (92) | 19 (53) | <0.001 |
| Maximum balloon size (mm) | 0.2 | |||
| 9 | 12 (16) | 5 (14) | 7 (19) | |
| 10 | 42 (58) | 14 (38) | 28 (78) | |
| 11 | 17 (23) | 16 (43) | 1 (3) | |
| 12 | 2 (3) | 2 (5) | 0 (0) | |
| Series balloon dilation | 20 (27) | 10 (27) | 10 (28) | 0.2 |
| PV effective orifice after balloon dilation | 9.5 ± 0.7 | 10.7 ± 0.9 | 9.5 ± 0.6 | 0.3 |
| . | Total (N = 73) . | Z-score ≤ -2.85 (N = 37) . | Z-score > -2.85 (N = 36) . | P-value . |
|---|---|---|---|---|
| Type of sparing procedure | <0.001 | |||
| Balloon only | 20 (27) | 3 (8) | 17 (47) | |
| Resuspension only | 7 (10) | 3 (8) | 4 (11) | |
| Delamination and resuspension | 21 (29) | 12 (32) | 9 (25) | |
| Leaflet patch augmentation | 7 (10) | 3 (8) | 4 (11) | |
| Minitransannular incision | 18 (25) | 16 (43) | 2 (6) | |
| Simple sparing procedure | 20 (27) | 3 (8) | 17 (47) | <0.001 |
| Complex sparing procedure | 53 (73) | 34 (92) | 19 (53) | <0.001 |
| Maximum balloon size (mm) | 0.2 | |||
| 9 | 12 (16) | 5 (14) | 7 (19) | |
| 10 | 42 (58) | 14 (38) | 28 (78) | |
| 11 | 17 (23) | 16 (43) | 1 (3) | |
| 12 | 2 (3) | 2 (5) | 0 (0) | |
| Series balloon dilation | 20 (27) | 10 (27) | 10 (28) | 0.2 |
| PV effective orifice after balloon dilation | 9.5 ± 0.7 | 10.7 ± 0.9 | 9.5 ± 0.6 | 0.3 |
PV: pulmonary valve.
| . | Total (N = 73) . | Z-score ≤ -2.85 (N = 37) . | Z-score > -2.85 (N = 36) . | P-value . |
|---|---|---|---|---|
| Length of follow-up (years) | 5.3 ± 2.7 | 4.3 ± 2.4 | 6.3 ± 2.7 | 0.003 |
| Weight (kg) | 19.9 ± 9.4 | 17.0 ± 7.1 | 22.5 ± 10.6 | 0.04 |
| Height (cm) | 111.3 ± 22.4 | 104.4 ± 21.5 | 117.6 ± 21.6 | 0.07 |
| BSA (m2) | 0.76 ± 0.26 | 0.69 ± 0.23 | 0.83 ± 0.27 | 0.07 |
| Indexed EDA (cm2/m2) | 18.9 ± 5.4 | 19.6 ± 4.9 | 18.3 ± 5.7 | 0.4 |
| Indexed ESA (cm2/m2) | 10.4 ± 3.1 | 10.6 ± 2.8 | 10.3 ± 3.3 | 0.7 |
| Indexed RA (cm2/m2) | 10.8 ± 2.7 | 11.5 ± 2.6 | 10.2 ± 2.7 | 0.1 |
| RV FAC (%) | 47.1 ± 5.2 | 47.9 ± 3.4 | 46.4 ± 5.1 | 0.3 |
| TAPSE (mm) | 13.8 ± 2.6 | 13.0 ± 3.4 | 15.0 ± 3.2 | 0.04 |
| TR velocity (m/s) | 2.5 ± 0.5 | 2.6 ± 0.5 | 2.3 ± 0.5 | 0.06 |
| Vmax PV (m/s) | 2.2 ± 0.6 | 2.3 ± 0.5 | 2.1 ± 0.6 | 0.2 |
| Max gradient (mmHg) | 20.5 ± 10.6 | 21.6 ± 9.0 | 19.5 ± 11.9 | 0.4 |
| Mean gradient (mmHg) | 12.0 ± 6.6 | 12.7 ± 5.7 | 11.3 ± 7.3 | 0.4 |
| TVI antegrade (mm) | 46.8 ± 14.3 | 48.8 ± 12.1 | 44.7 ± 16.1 | 0.3 |
| TVI retrograde (mm) | 32.9 ± 9.8 | 31.5 ± 7.3 | 33.9 ± 11.3 | 0.4 |
| PR grade | 0.03 | |||
| Grade 1 (mild) | 36 (49) | 16 (43) | 20 (56) | |
| Grade 2 (moderate) | 30 (41) | 16 (43) | 14 (39) | |
| Grade 3 (severe) | 7 (10) | 5 (14) | 2 (5) | |
| PHT (ms) | 105.1 ± 42.2 | 106.5 ± 45.9 | 103.9 ± 39.5 | 0.8 |
| PR Index | 88.6 ± 8.6 | 88.7 ± 9.4 | 88.5 ± 8.0 | 0.9 |
| Indexed LV EDV (mL/m2) | 44.4 ± 12.6 | 44.7 ± 12.7 | 44.2 ± 12.7 | 0.9 |
| Indexed LV ESV (mL/m2) | 16.8 ± 5.4 | 16.8 ± 4.7 | 16.8 ± 6.0 | 0.9 |
| LVEF | 62 ± 6 | 63 ± 7 | 61 ± 5 | 0.4 |
| . | Total (N = 73) . | Z-score ≤ -2.85 (N = 37) . | Z-score > -2.85 (N = 36) . | P-value . |
|---|---|---|---|---|
| Length of follow-up (years) | 5.3 ± 2.7 | 4.3 ± 2.4 | 6.3 ± 2.7 | 0.003 |
| Weight (kg) | 19.9 ± 9.4 | 17.0 ± 7.1 | 22.5 ± 10.6 | 0.04 |
| Height (cm) | 111.3 ± 22.4 | 104.4 ± 21.5 | 117.6 ± 21.6 | 0.07 |
| BSA (m2) | 0.76 ± 0.26 | 0.69 ± 0.23 | 0.83 ± 0.27 | 0.07 |
| Indexed EDA (cm2/m2) | 18.9 ± 5.4 | 19.6 ± 4.9 | 18.3 ± 5.7 | 0.4 |
| Indexed ESA (cm2/m2) | 10.4 ± 3.1 | 10.6 ± 2.8 | 10.3 ± 3.3 | 0.7 |
| Indexed RA (cm2/m2) | 10.8 ± 2.7 | 11.5 ± 2.6 | 10.2 ± 2.7 | 0.1 |
| RV FAC (%) | 47.1 ± 5.2 | 47.9 ± 3.4 | 46.4 ± 5.1 | 0.3 |
| TAPSE (mm) | 13.8 ± 2.6 | 13.0 ± 3.4 | 15.0 ± 3.2 | 0.04 |
| TR velocity (m/s) | 2.5 ± 0.5 | 2.6 ± 0.5 | 2.3 ± 0.5 | 0.06 |
| Vmax PV (m/s) | 2.2 ± 0.6 | 2.3 ± 0.5 | 2.1 ± 0.6 | 0.2 |
| Max gradient (mmHg) | 20.5 ± 10.6 | 21.6 ± 9.0 | 19.5 ± 11.9 | 0.4 |
| Mean gradient (mmHg) | 12.0 ± 6.6 | 12.7 ± 5.7 | 11.3 ± 7.3 | 0.4 |
| TVI antegrade (mm) | 46.8 ± 14.3 | 48.8 ± 12.1 | 44.7 ± 16.1 | 0.3 |
| TVI retrograde (mm) | 32.9 ± 9.8 | 31.5 ± 7.3 | 33.9 ± 11.3 | 0.4 |
| PR grade | 0.03 | |||
| Grade 1 (mild) | 36 (49) | 16 (43) | 20 (56) | |
| Grade 2 (moderate) | 30 (41) | 16 (43) | 14 (39) | |
| Grade 3 (severe) | 7 (10) | 5 (14) | 2 (5) | |
| PHT (ms) | 105.1 ± 42.2 | 106.5 ± 45.9 | 103.9 ± 39.5 | 0.8 |
| PR Index | 88.6 ± 8.6 | 88.7 ± 9.4 | 88.5 ± 8.0 | 0.9 |
| Indexed LV EDV (mL/m2) | 44.4 ± 12.6 | 44.7 ± 12.7 | 44.2 ± 12.7 | 0.9 |
| Indexed LV ESV (mL/m2) | 16.8 ± 5.4 | 16.8 ± 4.7 | 16.8 ± 6.0 | 0.9 |
| LVEF | 62 ± 6 | 63 ± 7 | 61 ± 5 | 0.4 |
BSA: body surface area; EDA: end-diastolic area; EDV: end-diastolic volume; ESA: end-systolic area; ESV: end-systolic volume; FAC: fractional area change; LV: left ventricle; LVEF: left ventricular ejection fraction; PHT: pressure half time; PR: pulmonary valve regurgitation; PV: pulmonary valve; RA: right atrium area; RV: right ventricle; TAPSE: tricuspid annular plane systolic excursion; TR: tricuspid valve regurgitation; TVI: time velocity integrals.
| . | Total (N = 73) . | Z-score ≤ -2.85 (N = 37) . | Z-score > -2.85 (N = 36) . | P-value . |
|---|---|---|---|---|
| Length of follow-up (years) | 5.3 ± 2.7 | 4.3 ± 2.4 | 6.3 ± 2.7 | 0.003 |
| Weight (kg) | 19.9 ± 9.4 | 17.0 ± 7.1 | 22.5 ± 10.6 | 0.04 |
| Height (cm) | 111.3 ± 22.4 | 104.4 ± 21.5 | 117.6 ± 21.6 | 0.07 |
| BSA (m2) | 0.76 ± 0.26 | 0.69 ± 0.23 | 0.83 ± 0.27 | 0.07 |
| Indexed EDA (cm2/m2) | 18.9 ± 5.4 | 19.6 ± 4.9 | 18.3 ± 5.7 | 0.4 |
| Indexed ESA (cm2/m2) | 10.4 ± 3.1 | 10.6 ± 2.8 | 10.3 ± 3.3 | 0.7 |
| Indexed RA (cm2/m2) | 10.8 ± 2.7 | 11.5 ± 2.6 | 10.2 ± 2.7 | 0.1 |
| RV FAC (%) | 47.1 ± 5.2 | 47.9 ± 3.4 | 46.4 ± 5.1 | 0.3 |
| TAPSE (mm) | 13.8 ± 2.6 | 13.0 ± 3.4 | 15.0 ± 3.2 | 0.04 |
| TR velocity (m/s) | 2.5 ± 0.5 | 2.6 ± 0.5 | 2.3 ± 0.5 | 0.06 |
| Vmax PV (m/s) | 2.2 ± 0.6 | 2.3 ± 0.5 | 2.1 ± 0.6 | 0.2 |
| Max gradient (mmHg) | 20.5 ± 10.6 | 21.6 ± 9.0 | 19.5 ± 11.9 | 0.4 |
| Mean gradient (mmHg) | 12.0 ± 6.6 | 12.7 ± 5.7 | 11.3 ± 7.3 | 0.4 |
| TVI antegrade (mm) | 46.8 ± 14.3 | 48.8 ± 12.1 | 44.7 ± 16.1 | 0.3 |
| TVI retrograde (mm) | 32.9 ± 9.8 | 31.5 ± 7.3 | 33.9 ± 11.3 | 0.4 |
| PR grade | 0.03 | |||
| Grade 1 (mild) | 36 (49) | 16 (43) | 20 (56) | |
| Grade 2 (moderate) | 30 (41) | 16 (43) | 14 (39) | |
| Grade 3 (severe) | 7 (10) | 5 (14) | 2 (5) | |
| PHT (ms) | 105.1 ± 42.2 | 106.5 ± 45.9 | 103.9 ± 39.5 | 0.8 |
| PR Index | 88.6 ± 8.6 | 88.7 ± 9.4 | 88.5 ± 8.0 | 0.9 |
| Indexed LV EDV (mL/m2) | 44.4 ± 12.6 | 44.7 ± 12.7 | 44.2 ± 12.7 | 0.9 |
| Indexed LV ESV (mL/m2) | 16.8 ± 5.4 | 16.8 ± 4.7 | 16.8 ± 6.0 | 0.9 |
| LVEF | 62 ± 6 | 63 ± 7 | 61 ± 5 | 0.4 |
| . | Total (N = 73) . | Z-score ≤ -2.85 (N = 37) . | Z-score > -2.85 (N = 36) . | P-value . |
|---|---|---|---|---|
| Length of follow-up (years) | 5.3 ± 2.7 | 4.3 ± 2.4 | 6.3 ± 2.7 | 0.003 |
| Weight (kg) | 19.9 ± 9.4 | 17.0 ± 7.1 | 22.5 ± 10.6 | 0.04 |
| Height (cm) | 111.3 ± 22.4 | 104.4 ± 21.5 | 117.6 ± 21.6 | 0.07 |
| BSA (m2) | 0.76 ± 0.26 | 0.69 ± 0.23 | 0.83 ± 0.27 | 0.07 |
| Indexed EDA (cm2/m2) | 18.9 ± 5.4 | 19.6 ± 4.9 | 18.3 ± 5.7 | 0.4 |
| Indexed ESA (cm2/m2) | 10.4 ± 3.1 | 10.6 ± 2.8 | 10.3 ± 3.3 | 0.7 |
| Indexed RA (cm2/m2) | 10.8 ± 2.7 | 11.5 ± 2.6 | 10.2 ± 2.7 | 0.1 |
| RV FAC (%) | 47.1 ± 5.2 | 47.9 ± 3.4 | 46.4 ± 5.1 | 0.3 |
| TAPSE (mm) | 13.8 ± 2.6 | 13.0 ± 3.4 | 15.0 ± 3.2 | 0.04 |
| TR velocity (m/s) | 2.5 ± 0.5 | 2.6 ± 0.5 | 2.3 ± 0.5 | 0.06 |
| Vmax PV (m/s) | 2.2 ± 0.6 | 2.3 ± 0.5 | 2.1 ± 0.6 | 0.2 |
| Max gradient (mmHg) | 20.5 ± 10.6 | 21.6 ± 9.0 | 19.5 ± 11.9 | 0.4 |
| Mean gradient (mmHg) | 12.0 ± 6.6 | 12.7 ± 5.7 | 11.3 ± 7.3 | 0.4 |
| TVI antegrade (mm) | 46.8 ± 14.3 | 48.8 ± 12.1 | 44.7 ± 16.1 | 0.3 |
| TVI retrograde (mm) | 32.9 ± 9.8 | 31.5 ± 7.3 | 33.9 ± 11.3 | 0.4 |
| PR grade | 0.03 | |||
| Grade 1 (mild) | 36 (49) | 16 (43) | 20 (56) | |
| Grade 2 (moderate) | 30 (41) | 16 (43) | 14 (39) | |
| Grade 3 (severe) | 7 (10) | 5 (14) | 2 (5) | |
| PHT (ms) | 105.1 ± 42.2 | 106.5 ± 45.9 | 103.9 ± 39.5 | 0.8 |
| PR Index | 88.6 ± 8.6 | 88.7 ± 9.4 | 88.5 ± 8.0 | 0.9 |
| Indexed LV EDV (mL/m2) | 44.4 ± 12.6 | 44.7 ± 12.7 | 44.2 ± 12.7 | 0.9 |
| Indexed LV ESV (mL/m2) | 16.8 ± 5.4 | 16.8 ± 4.7 | 16.8 ± 6.0 | 0.9 |
| LVEF | 62 ± 6 | 63 ± 7 | 61 ± 5 | 0.4 |
BSA: body surface area; EDA: end-diastolic area; EDV: end-diastolic volume; ESA: end-systolic area; ESV: end-systolic volume; FAC: fractional area change; LV: left ventricle; LVEF: left ventricular ejection fraction; PHT: pressure half time; PR: pulmonary valve regurgitation; PV: pulmonary valve; RA: right atrium area; RV: right ventricle; TAPSE: tricuspid annular plane systolic excursion; TR: tricuspid valve regurgitation; TVI: time velocity integrals.
RESULTS
Demographic and preoperative data
Among 78 patients undergoing successful PVP techniques, 5 patients (6.4%) were excluded from the study for inconsistent data at follow-up: 2 patients because they were followed by centres abroad, 3 because they were recently operated on and therefore had a too-short follow-up period (< 1 year) (Supplemental Fig. 1). Therefore, the total population of the study comprised 73 patients.
At the index surgeries, the mean weight was 6.0 ± 1.2 kg; height, 63.3 ± 5.3 cm; BSA, 0.31 ± 0.04 m2; and age, 4.8 ± 2.9 months. Preoperatively, the mean saturation was 92 ± 7%, with an incidence of cyanotic spells of 5%. PV was predominantly bicuspid (79%), more rarely tricuspid (18%) and unicuspid (3%). Approximately half of the patients showed PV dysplasia (58%). The maximum preoperative RVOT gradient was 70.0 ± 16.8 mmHg; the PV z-score was -3.02 ± 1.11 mmHg.
Operative data and postoperative outcomes
The mean effective PV orifice precommissurotomy was 5.1 ± 1.2 mm and increased to a real PV annulus of 7.0 ± 1.0 mm. Twenty (27%) patients underwent only simple balloon dilation plasty; 53 (73%) underwent complex plasty (dilation and subsequent PV plasty, Table 3). Of these 53 patients, 18 (17%) underwent a minitransannular incision (5 mm) with subsequent PV reconstruction with native tissue (Supplemental Methods) (Supplemental Fig. 2). Specific operative details of the type of PVP strategy are described in Table 3.
The mean cardiopulmonary bypass (CPB) time was 139 ± 31 min, whereas aortic cross-clamp time was 86 ± 22 min. RV systolic pressure at the end of the procedure was predominantly half of the systemic pressure (Table 2).
The mean hospitalization time in the intensive care unit was 3.8 ± 2.6 days, and the mean length of stay was 13.2 ± 7.8 days. After surgery, 18 patients (25%) had postoperative complications, mainly arrhythmias (8 patients, 11%). Of these, most were temporary arrhythmias (supraventricular tachycardia and junctional ectopic tachycardia) (6 patients, 8%).
At discharge, the maximum RVOT gradient was 29.8 ± 8.6 mmHg. The degree of PR was mild in 90% of patients, and moderate in the remaining.
Follow-up echocardiographic data
The mean interval between the index procedure and the follow-up was 5.3 ± 2.7 years. Indexed end-diastolic and end-systolic areas of the RV were 18.9 ± 5.4 cm2/m2 and 10.3 ± 3.1 cm2/m2, respectively. RV function as assessed by the RV fractional area change was 47.1 ± 5.2%, whereas TAPSE was 13.8 ± 2.6 mm. There was a significant correlation between the RV fractional area change and the duration of moderate/severe PR at follow-up (R2: 0.08; P = 0.03) (Supplemental Fig. 4).
The maximum RVOT gradient was 20.5 ± 10.6 mmHg. The degree of PR remained mild (49%) and moderate (41%) in the majority of the patients, but 7 patients (10%) developed severe regurgitation. This assessment was confirmed by the PR index, which had a mean of 88.6 ± 8.6 at the last follow-up. Five-year freedom from moderate/severe PV regurgitation was 61.3% (95% CI: 48, 73%) (Fig. 1).
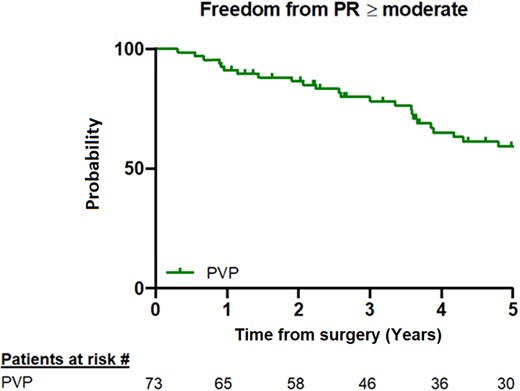
Kaplan–Meier curve at the 5-year follow-up showing freedom from moderate/severe pulmonary valve regurgitation. The numbers at risk are shown at the bottom of the panel. PR: pulmonary regurgitation; PVP: pulmonary valve preservation.
Comparison with a group of patients undergoing transannular patch interposition
A group of 33 patients undergoing a surgical correction with TAP placement and monocusp reconstruction was used to compare midterm outcomes. In this cohort, 7 patients underwent an attempt of PVP, but this failed due to leaflet rupture in 5 (15%) or a too severe z-score in 2 (6%) (PV z-score < -5).
At the index surgery, weight (P = 0.6), height (P = 0.1), BSA (P = 0.3) and age (P = 0.5) did not differ between the groups (Supplemental Table 2). Preoperatively, patients in the PVP group had higher saturation (P = 0.04) but the same incidence of cyanotic spells (P = 0.3). The PVs had similar rates of dysplasia (P = 0.9), but a difference distribution of the number of PV cusps (P = 0.001). The maximum preoperative RVOT gradient (P = 0.07) and PV z-score (P = 0.1) did not differ between groups. The mean effective PV orifice precommissurotomy had a higher increase in the PVP group (P < 0.001) (Supplemental Table 3).
The mean CPB time was longer in the TAP group (P = 0.007), whereas aortic cross-clamp time was increased in the PVP group (P = 0.02). The RV systolic pressure/systemic pressure ratio at the end of the operative procedure was not different (P = 0.4) (Supplemental Table 3).
The mean hospitalization time in the intensive care unit and the mean length of stay were also not statistically different (P = 0.9 and P = 0.5, respectively); the same was true for the postoperative complications (P = 0.8). At discharge, the mean RVOT gradient was not significantly different between the groups (P = 0.01). However, the degree of PR was worse in the TAP group compared to the PVP group (P < 0.001).
The mean interval between the index procedure and the follow-up was longer in the TAP group (P < 0.001) (Supplemental Table 4). The indexed areas of the RV were not different between groups. However, RV function as assessed by RV fractional area change was reduced in the TAP group compared to the PVP group (P = 0.01). This reduced function was confirmed by the TAPSE z-score, which was more impaired in the TAP group (P = 0.003).
At a mean follow-up of 6.3 ± 3.1 years, there was no difference between the groups in terms of RVOT gradients (P = 0.3). The TAP group showed more severe PR than the PVP group (P < 0.001). This finding was confirmed by the PR index (P = 0.01). Five-year freedom from moderate/severe PV regurgitation was significantly higher in the PVP group than in the TAP [61.3% (95% CI: 48, 73%) vs 25.9% (95% CI: 12, 43%), respectively; P = 0.002]. Patients undergoing TAP had an increased risk for developing moderate/severe PR at follow-up compared to PVP (HR: 2.01; 95% CI: 1.11, 3.61, P = 0.02) (Fig. 2).
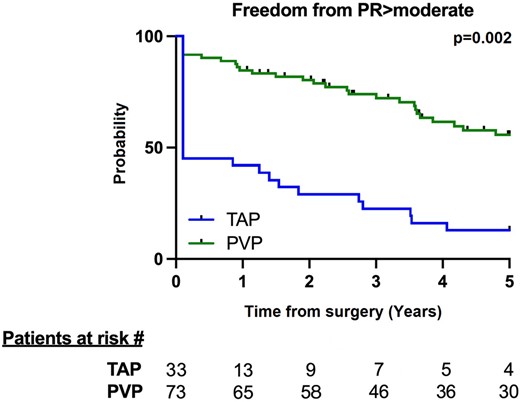
Kaplan–Meier curve at the 5-year follow-up showing freedom from moderate/severe pulmonary regurgitation. The numbers at risk in each group are shown at the bottom of the panel. PR: pulmonary regurgitation; PVP: pulmonary valve preservation; TAP: transannular patch.
Risk factors for moderate/severe pulmonary regurgitation
A sensitivity analysis was performed in the group of patients undergoing a PVP strategy. A preoperative PV annulus z-score ≤ -2.85 was found to be predictive for moderate/severe PR at follow-up (area under the curve: 0.76; sensitivity: 73% and specificity: 62%) (Supplemental Fig. 5). Indeed, an increased risk for moderate/severe PR at a follow-up of 5 years was noted in patients with a PV z-score ≤ -2.85 (HR: 2.56; 95% CI: 1.31, 5.01, P = 0.002) (Fig. 3A). There was no difference between the type of PVP strategy (HR: 1.92; 95% CI: 0.90, 4.09, P = 0.1), dysplastic or normal PV (HR: 1.61; 95% CI: 0.85, 3.06, P = 0.1) and bicuspid and tricuspid PV, although the latter was marginal (HR: 3, 52; 95% CI: 0.95, 12.98, P = 0.06) at 5 and 3 years, respectively (Fig. 3 B-D).
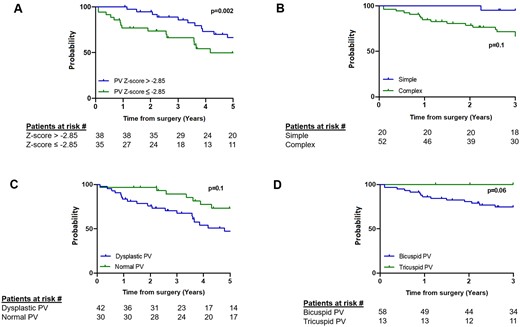
Kaplan–Meier curves showing freedom from moderate/severe pulmonary valve regurgitation for pulmonary valve z-score < -2.85 vs ≥ -2.85 (A), simple versus complex preservation strategies (B), dysplastic versus normal pulmonary valve (C) and bicuspid versus tricuspid pulmonary valve (D). The numbers at risk in each group are shown at the bottom of the panel. PV: pulmonary valve. Curves are truncated at the time point when < 10 patients were at risk.
Univariate regression analysis identified the preoperative PV z-score and the degree of PR at discharge as risk factors for the early development of moderate/severe PR at follow-up (HR: 0.62; 95% CI: 0.47, 0.83, P = 0.007 and HR: 3.66; 95% CI: 0.26, 10.64, P = 0.01, respectively). In the multivariable analysis, this finding was confirmed (adjusted HR: 0.64; 95% CI: 0.48, 0.86, P = 0.01 and HR: 2.31; 95% CI: 1.00, 5.36, P = 0.04, respectively) (Table 5).
Univariate analysis of freedom from moderate/severe pulmonary regurgitation
| Variable . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| Gender | 0.91 | 0.47, 1.79 | 0.8 |
| Preoperative BSA | 1.49 | 0.16, 1.7 | 0.2 |
| Number of PV cusps | 0.44 | 0.18, 1.06 | 0.1 |
| PV cusp dysplasia | 0.57 | 0.29, 1.14 | 0.1 |
| Preoperative RVOT gradient | 0.99 | 0.98, 1.01 | 0.7 |
| Cyanotic spells | 1.38 | 0.18, 10.22 | 0.8 |
| Preoperative PV z-score | 0.62 | 0.47, 0.83 | 0.007 |
| Age at surgery | 1.04 | 0.95, 1.14 | 0.4 |
| Complex vs simple PVP | 1.92 | 0.90, 4.09 | 0.1 |
| RVOT gradient at D/C | 1.01 | 0.97, 1.05 | 0.77 |
| Postoperative RVSP (% of systemic) | 1.02 | 0.99, 1.04 | 0.1 |
| Degree of PR at D/C | 3.66 | 1.26, 10.64 | 0.01 |
| Multivariable analysis of freedom from moderate/severe PR in patients undergoing PVP | |||
| Preoperative PV z-score | 0.64 | 0.48, 0.86 | 0.01 |
| Degree of PR at D/C | 2.31 | 1.00, 5.36 | 0.04 |
| Variable . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| Gender | 0.91 | 0.47, 1.79 | 0.8 |
| Preoperative BSA | 1.49 | 0.16, 1.7 | 0.2 |
| Number of PV cusps | 0.44 | 0.18, 1.06 | 0.1 |
| PV cusp dysplasia | 0.57 | 0.29, 1.14 | 0.1 |
| Preoperative RVOT gradient | 0.99 | 0.98, 1.01 | 0.7 |
| Cyanotic spells | 1.38 | 0.18, 10.22 | 0.8 |
| Preoperative PV z-score | 0.62 | 0.47, 0.83 | 0.007 |
| Age at surgery | 1.04 | 0.95, 1.14 | 0.4 |
| Complex vs simple PVP | 1.92 | 0.90, 4.09 | 0.1 |
| RVOT gradient at D/C | 1.01 | 0.97, 1.05 | 0.77 |
| Postoperative RVSP (% of systemic) | 1.02 | 0.99, 1.04 | 0.1 |
| Degree of PR at D/C | 3.66 | 1.26, 10.64 | 0.01 |
| Multivariable analysis of freedom from moderate/severe PR in patients undergoing PVP | |||
| Preoperative PV z-score | 0.64 | 0.48, 0.86 | 0.01 |
| Degree of PR at D/C | 2.31 | 1.00, 5.36 | 0.04 |
Variables with P<0.05 on univariable analysis were included in the multivariable model.
P<.05.
BSA: body surface area; D/C: discharge; PR: pulmonary valve regurgitation; PV: pulmonary valve; PVP: pulmonary valve preservation; RVOT: right ventricular outflow tract; RVSP: right ventricular systolic pressure.
Univariate analysis of freedom from moderate/severe pulmonary regurgitation
| Variable . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| Gender | 0.91 | 0.47, 1.79 | 0.8 |
| Preoperative BSA | 1.49 | 0.16, 1.7 | 0.2 |
| Number of PV cusps | 0.44 | 0.18, 1.06 | 0.1 |
| PV cusp dysplasia | 0.57 | 0.29, 1.14 | 0.1 |
| Preoperative RVOT gradient | 0.99 | 0.98, 1.01 | 0.7 |
| Cyanotic spells | 1.38 | 0.18, 10.22 | 0.8 |
| Preoperative PV z-score | 0.62 | 0.47, 0.83 | 0.007 |
| Age at surgery | 1.04 | 0.95, 1.14 | 0.4 |
| Complex vs simple PVP | 1.92 | 0.90, 4.09 | 0.1 |
| RVOT gradient at D/C | 1.01 | 0.97, 1.05 | 0.77 |
| Postoperative RVSP (% of systemic) | 1.02 | 0.99, 1.04 | 0.1 |
| Degree of PR at D/C | 3.66 | 1.26, 10.64 | 0.01 |
| Multivariable analysis of freedom from moderate/severe PR in patients undergoing PVP | |||
| Preoperative PV z-score | 0.64 | 0.48, 0.86 | 0.01 |
| Degree of PR at D/C | 2.31 | 1.00, 5.36 | 0.04 |
| Variable . | HR . | 95% CI . | P-value . |
|---|---|---|---|
| Gender | 0.91 | 0.47, 1.79 | 0.8 |
| Preoperative BSA | 1.49 | 0.16, 1.7 | 0.2 |
| Number of PV cusps | 0.44 | 0.18, 1.06 | 0.1 |
| PV cusp dysplasia | 0.57 | 0.29, 1.14 | 0.1 |
| Preoperative RVOT gradient | 0.99 | 0.98, 1.01 | 0.7 |
| Cyanotic spells | 1.38 | 0.18, 10.22 | 0.8 |
| Preoperative PV z-score | 0.62 | 0.47, 0.83 | 0.007 |
| Age at surgery | 1.04 | 0.95, 1.14 | 0.4 |
| Complex vs simple PVP | 1.92 | 0.90, 4.09 | 0.1 |
| RVOT gradient at D/C | 1.01 | 0.97, 1.05 | 0.77 |
| Postoperative RVSP (% of systemic) | 1.02 | 0.99, 1.04 | 0.1 |
| Degree of PR at D/C | 3.66 | 1.26, 10.64 | 0.01 |
| Multivariable analysis of freedom from moderate/severe PR in patients undergoing PVP | |||
| Preoperative PV z-score | 0.64 | 0.48, 0.86 | 0.01 |
| Degree of PR at D/C | 2.31 | 1.00, 5.36 | 0.04 |
Variables with P<0.05 on univariable analysis were included in the multivariable model.
P<.05.
BSA: body surface area; D/C: discharge; PR: pulmonary valve regurgitation; PV: pulmonary valve; PVP: pulmonary valve preservation; RVOT: right ventricular outflow tract; RVSP: right ventricular systolic pressure.
Patient demographics and preoperative, operative and follow-up data were analysed according to the PV z-score (value ≤ or > 2.85) (Tables 1–3). Patients in the PV z-score ≤ 2.85 group had lower saturation (89 ± 8% vs 94 ± 5%, P = 0.04) but did not have an increase in cyanotic spells (P = 0.3). As expected, the mean effective PV orifice precommissurotomy (4.4 ± 0.9 mm vs 5.9 ± 1.0 mm) (P < 0.001) and the mean real PV annulus (6.3 ± 0.7 mm vs 7.6 ± 0.7 mm) (P < 0.001) were also lower in the group with a more severe PV z-score. CPB time (148 ± 29 min vs 130 ± 31 min) (P = 0.008) and aortic cross-clamp time (94 ± 16 min vs 79 ± 25 min) (P = 0.001) were longer in the PV z-score ≤ 2.85 group. These patients showed more severe PR at discharge (P = 0.04) and follow-up (P = 0.03) and more severe TAPSE at follow-up (13.0 ± 3.4 mm vs 15.0 ± 3.2 mm) (P = 0.04). All other variables were comparable in the 2 groups.
DISCUSSION
Continuous improvements in surgical techniques and postoperative management have made it possible to achieve complete surgical repair of TOF with relatively low risks. Although long-term complications such as PR and RV dysfunction are reported, a transannular patch (TAP) still remains the most performed type of surgery worldwide [21]. Because of these complications, many surgeons have begun to develop new PVP surgical techniques with the aim of limiting PR in order to minimize late RV dilatation and ventricular arrhythmias [10, 22, 23].
Since 2007, our centre has adopted and developed new PVP strategies that combine the classic transatrial/transpulmonary approach with PV plasty techniques. These include simple techniques (intraoperative PV dilatation) and complex reconstructions (Supplemental Methods) [7–10, 12]. We have previously demonstrated that PV annulus and function can be preserved in selected patients [7–10, 12]. However, our follow-up period was still relatively short and restricted to a limited assessment of the PV and RV functions.
The goal of this study was to evaluate functional outcomes in patients undergoing PVP at the mid- and long-term follow-ups. Among the 73 patients examined, preoperative and intraoperative data were similar. No patients required reoperations and no patients died, results that differed from the results of other reports [24]. At a mean follow-up of 5 years, nearly 90% of patients with PVP showed freedom from severe PR and 60% were predicted to have minimal PR.
Early in our experience, PVP was indicated only in patients with a valvular z-score ≥ 3 and was limited to a simple plasty strategy. The indications were restricted because, following balloon dilatation alone, valves with a z-score < -3 often experienced damage of the leaflets and commissures. To solve this problem, additional surgical manoeuveurs have been developed over the years to re-establish the coaptation surface of the PV native leaflets [25]. Consequently, we could enroll patients with a PV z-score ≤ -3, which limited even more the need for a TAP, which is currently reserved only for patients with a severe PV z-score (< -5) and highly dysplastic valves.
A group of patients undergoing TOF repair with TAP placement and monocusp reconstruction was used for comparison of midterm outcomes. At follow-up, all PV function indexes were significantly superior in the PVP group, with similar RVOT gradients between the groups. In the TAP group, 75% of patients had severe PR at the last follow-up, as shown in our previous studies [7, 8].
In this study, we took this investigation to the next level by identifying a PV z-score < -2.85 as the threshold for predicting midterm pulmonary regurgitation. We believe that this value can be of great benefit in the decision-making process at centres that are planning to initiate a PVP program or for surgical indication in centres that are already using this strategy.
In addition, we demonstrated once again that PR function strictly determines RV function at follow-up. Indeed, RV fractional area change and TAPSE depend on the duration of PV competence (Supplemental Fig. 4). A large amount of data shows that longitudinal systolic function is impaired in adults and children with repaired TOF [26]. In parallel, a prominent middle layer with circumferentially oriented myofibers is found in these patients, suggesting the relative dominance of radial RV function [27]. This might explain the reason why, in our population, functional area change and TAPSE are reduced in children with a larger amount of PR.
Limitations
This study has several limitations. First, it is a retrospective examination of a cohort of patients from a single centre. Furthermore, the study population still has a midterm follow-up. The question of the potential growth of reconstructed valves remains unanswered at the moment. No patient needed a reoperation; therefore, it was difficult for us to comment on the quality of the reconstructed leaflets. A higher-quality echocardiographic assessment will likely be possible as the PV reconstruction population grows. Also, it must be considered that patients undergoing classical TOF correction and TAP had a longer follow-up than patients with PVP. Finally, the restrictive physiology of TOF post-repair may minimize the midterm degree of PR.
CONCLUSIONS
A PVP strategy during TOF repair should always be attempted. However, a preoperative PV annulus z-score < -2.85 and moderate/severe regurgitation upon discharge are risk factors for midterm pulmonary regurgitation.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of interest: None declared.
Data availability statement
Data supporting the findings of this study are available upon request from the corresponding author. The data are not publicly available due to restrictions imposed because the information may compromise the privacy of research participants.
AUTHOR CONTRIBUTIONS
Dr. Guariento and Dr. Vida conceived the concept of the present study, defined analysis methods and drafted the paper. Dr. Schiena, Dr. Cattapan, Dr. Avesani and Dr. Padalino contributed with data collection. Dr. Castaldi and Dr. Di Salvo supported the echocardiographic imaging assessment. Dr. Doulamis contributed to study design and statistical analysis. All authors took part in both data interpretation and critically revision of the manuscript.





