-
PDF
- Split View
-
Views
-
Cite
Cite
Atsushi Kagimoto, Yasuhiro Tsutani, Yoshihisa Shimada, Takahiro Mimae, Yoshihiro Miyata, Hiroyuki Ito, Haruhiko Nakayama, Norihiko Ikeda, Morihito Okada, Oncological outcome of segmentectomy for early-stage non-small-cell lung cancer with invasive characteristics: a multicentre study, European Journal of Cardio-Thoracic Surgery, Volume 62, Issue 2, August 2022, ezac055, https://doi.org/10.1093/ejcts/ezac055
Close - Share Icon Share
Abstract
Segmentectomy can provide oncologically acceptable results for small-sized non-small-cell lung cancer (NSCLC). However, in cases of NSCLC with pathological invasive characteristics such as lymphatic invasion (LY), vascular invasion (V), pleural invasion (PL) and/or lymph node metastasis, the feasibility of segmentectomy is not known.
The patients included in the study (i) underwent lobectomy or segmentectomy for NSCLC with invasive characteristics such as LY, V, PL or pathological lymph node metastasis; (ii) presented with a node-negative, solid component-predominant tumour (consolidation tumour ratio >50%) on preoperative computed tomography; (iii) had a whole-tumour size of 2 cm or less; and (iv) presented between January 2010 and December 2019 to one of the 3 institutions. Cumulative incidences of recurrence (CIRs) after segmentectomy and lobectomy were compared.
A total of 321 patients were included. Segmentectomy and lobectomy were performed in 80 (24.9%) and 241 (75.1%) patients, respectively. There was no significant difference in CIR between segmentectomy (5-year CIR rate, 17.2%) and lobectomy patients (5-year CIR rate, 27.8%, P = 0.135). In the propensity score-matched cohort, there was no significant difference in CIR between segmentectomy (5-year CIR rate, 19.1%) and lobectomy patients (5-year CIR rate, 19.2%; P = 0.650). In the multivariable analysis using inverse probability of treatment weighting and surgical method, segmentectomy was not a significant predictor of worse CIR (P = 0.920).
Segmentectomy is feasible for clinically early-stage NSCLC irrespective of the presence of LY, V, PL or lymph node metastasis.
INTRODUCTION
Given the recent improvements in imaging modalities, such as high-resolution computed tomography (CT) and [18F]-fluoro-2-deoxy-D-glucose (FDG) positron emission tomography (PET)/CT, early-stage lung cancer is more frequently detected [1]. Although the standard procedure for treating non-small-cell lung cancer (NSCLC) is lobectomy, according to the results of a randomized trial [2], many studies since the 2000s have reported that segmentectomy can be safe and provide oncologically acceptable results for small-sized NSCLC [3–5]. A randomized trial (JCOG0802/WJOG4607L) investigating the feasibility of segmentectomy for NSCLC with maximum tumour size ≤2 cm and consolidation tumour ratio (CTR) >51% was carried out [6] and showed that the overall survival (OS) of patients who underwent segmentectomy was significantly better than those who underwent lobectomy [7]. Segmentectomy has therefore become a standard procedure for treating early-stage NSCLC.
Lymphatic invasion (LY) [8, 9], vascular invasion (V) [8, 9], pleural invasion (PL) [10] and lymph node metastasis [11] are factors known to predict significantly worse outcomes. Several studies have demonstrated the feasibility of segmentectomy for early-stage NSCLC with clinically invasive characteristics, i.e., high CTR and maximum standardized uptake value (SUVmax) on FDG-PET/CT [12–14]. However, to our knowledge, no study has investigated the feasibility of segmentectomy from pathological aspects, and it is not yet known whether segmentectomy is feasible for early-stage NSCLC with invasive characteristics such as LY, V, PL and lymph node metastasis. This study investigated prognosis after segmentectomy for early-stage NSCLC with invasive characteristics.
PATIENTS AND METHODS
Ethics statement
Ethical Committee for Epidemiology of Hiroshima University (E1216), Kanagawa Cancer Center Institutional Review Board (2012-EKI-54) and Institutional Review Board of Tokyo Medical University (2017-263) approved this retrospective study using a prospective database and the requirement for informed consent from individual patients was waived because it was a retrospective study.
Patients
Patients were included who (i) underwent curative-intent lobectomy or segmentectomy for NSCLC with invasive characteristics such as LY, V, PL or pN; (ii) presented with a node-negative (cN0), solid component-predominant tumour (CTR >50%) on preoperative CT; (iii) had a whole-tumour size of 2 cm or less; and (iv) presented between January 2010 and December 2019 to Hiroshima University Hospital, Kanagawa Cancer Center or Tokyo Medical University. Patients who underwent wedge resection were excluded from this study. Prognoses after segmentectomy and lobectomy were compared between all included patients and propensity score-matched patients. A flow chart for inclusion and exclusion criteria for this study is shown in Fig. 1.
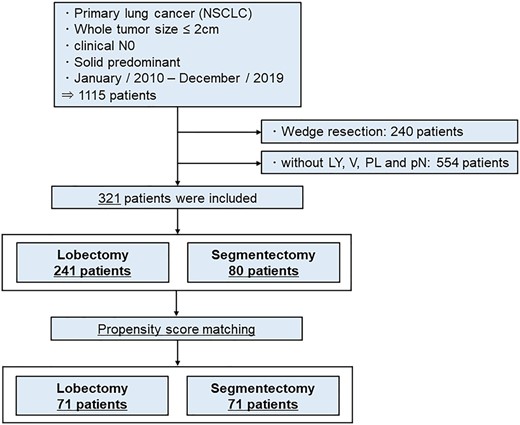
Flow chart of patient selection. Patients who underwent curative-intent lobectomy or segmentectomy for non-small-cell lung cancer with invasive characteristics, such as lymphatic invasion, vascular invasion or pleural invasion; and presented with node-negative, solid component-predominant tumour (consolidation tumour ratio >50%) on preoperative computed tomography, with a whole-tumour size of 2 cm or less, were included in this study.
Preoperative examination
Preoperative evaluations, including chest CT, whole-body FDG-PET/CT and brain magnetic resonance imaging, were performed to determine clinical stage and treatment strategies.
However, because FDG-PET/CT was performed according to each institution’s protocol and SUVmax varied among institutions, SUVmax was excluded from the analysis.
Staging and pathological evaluations
The clinical and pathological stage were determined based on the 8th edition of the TNM classification of malignant tumours [15]. The histologic subtype was determined based on World Health Organization classification [16]. The diagnosis of LY was based on pathological examination using immunostaining for D2-40 to clarify the locations of lymphatic ducts. The presence of V and PL was evaluated using elastic van Gieson staining to determine tumour invasion above the elastic layer of the vessels and the visceral pleura.
Follow-up evaluation
Postoperative follow-up procedures, including physical examination and chest CT scans every 6 months, were performed for 5 or 10 years after surgical resection. Recurrence was determined via radiographic features or histologic evidence. Recurrence pattern was classified as either loco-regional (recurrence within the preserved lung and ipsilateral hilum or mediastinal lymph node metastasis) or distant.
Statistical analysis
Results were presented as median and interquartile range for continuous variables and n (%) for categorical variables. Categorical variables were compared using the chi-squared test. Continuous variables that were normally distributed were analysed using Student’s t-test. Continuous variables that were non-normally distributed were analysed using the Wilcoxon’s rank-sum test. Prognoses were analysed using competing risk analysis. The risk of recurrence [defined as cumulative incidence of recurrence (CIR)], which was defined as the period from surgery to recurrence, was a main outcome of this study and was estimated using a cumulative incidence function that accounted for mortality without recurrence as a competing event. The risk of lung cancer-specific death [defined as cumulative incidence of lung cancer-specific death (CILSD), defined as the period from surgery to death from lung cancer] was estimated using a cumulative incidence function that accounted for death from other than lung cancer as a competing event. The risk of death without recurrence [defined as cumulative incidence of death without recurrence (CIDWR), defined as the period from surgery to death without recurrence] was also estimated using a cumulative incidence function that accounted for recurrence as a competing event. In the analysis of CIR and CIDWR, patients were censored if they were alive and without recurrence at the time of the most recent follow-up. In the analysis of CILSD, patients were censored if they were alive with or without recurrence at the time of the most recent follow-up. In the analysis of CIR, CILSD and CIDWR, differences between groups were assessed using the methods of Gray. The cumulative incidence of all death (CIAD), recurrence-free survival (RFS; the period from surgery to recurrence, death or most recent follow-up visit), OS (the period from surgery until death due to any cause or most recent follow-up visit), incidence of loco-regional recurrence (the period from date of surgery until recurrence for patients with detected loco-regional recurrence as an initial recurrent site), incidence of distant recurrence (the period from date of surgery to recurrence for patients with detected distant recurrence as an initial recurrent site) and all recurrence (the period from date of surgery to recurrence for all patients with recurrence) were calculated using the Kaplan–Meier method and compared using the log rank test.
Propensity scores were calculated to balance potential differences in the baseline characteristics of a tumour between patients who underwent lobectomy and segmentectomy. Propensity scores were estimated using a logistic regression model that included age, sex, solid component size, histological subtype, LY, V, PL and lymph node metastasis. Matching cohorts were formed using this propensity score; segmentectomy and lobectomy group pairs with equivalent propensity scores were selected by a 1-to-1 match with a caliper width of 0.2 of 1 standard deviation.
In the figures showing CIR, CILSD and CIDWR of matched cohort, P-values and hazard ratios (HRs) of Fine and Gray models were shown in figures. In the figures showing CIAD, RFS, OS, incidence of loco-regional recurrence, incidence of distant recurrence and all recurrence of the matched cohort, the P-values and HRs of the log rank test stratified by pairs were shown in figures.
Inverse probability of treatment weighting (IPTW) based on propensity scores was also used to adjust for differences in covariates between both groups and multivariable analysis for CIR was performed using the IPTW and surgical method to investigate whether the surgical procedure affected prognosis using the Fine and Gray models.
All statistical analyses were performed with EZR version 1.51 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [17], which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Three hundred twenty-one patients were included in this study. The median follow-up period was 32 months (interquartile range, 14–58 months). The distribution of the patients is shown in Supplementary Material, Fig. S1. Segmentectomy and lobectomy were performed in 80 (24.9%) and 241 (75.1%) patients, respectively. The patient characteristics and details of the surgical procedure are shown in Table 1 and Supplementary Material, Table S1, respectively. Whole-tumour size, solid component size and CTR were higher in patients who underwent lobectomy. The pathological stage was higher in patients who underwent lobectomy. There was no significant difference in CIR between patients who underwent segmentectomy [5-year CIR rate, 17.2%; 95% confidence interval (CI), 8.4–28.7%] and those who underwent lobectomy (5-year CIR rate, 27.8%; 95% CI, 20.6–35.3%; P = 0.135; Fig. 2A). Recurrence pattern did not differ between patients who underwent segmentectomy and lobectomy (P = 0.222, Table 1). There was no significant difference in CILSD between patients who underwent segmentectomy (5-year CILSD rate, 12.1%; 95% CI, 4.1–24.6%) and lobectomy (5-year CILSD rate, 8.8%: 95% CI, 4.5–14.8%; P = 0.908; Fig. 2B). There was no significant difference in CIDWR between patients who underwent segmentectomy (5-yr CIDWR rate, 6.7%; 95% CI, 2.5–14.0%) and those who underwent lobectomy (5-year CIDWR rate, 5.0%; 95% CI, 2.5–8.7%; P = 0.442; Fig. 2C). There was also no significant difference in CIAD between patients who underwent segmentectomy (5-year CIAD rate, 18.7%; 95% CI, 10.0–33.5%) and lobectomy (5-year CIAD rate 15.3%; 95% CI, 10.2–22.7%; P = 0.796; Fig. 2D). There were no significant differences in RFS (P = 0.372, Supplementary Material, Fig. S2A), OS (P = 0.796, Supplementary Material, Fig. S2B), incidence of local recurrence (P = 0.808, Supplementary Material, Fig. S2C), distant recurrence (P = 0.301, Supplementary Material, Fig. S2D) and all recurrence (P = 0.162, Supplementary Material, Fig. S2E) between patients who underwent segmentectomy and lobectomy.
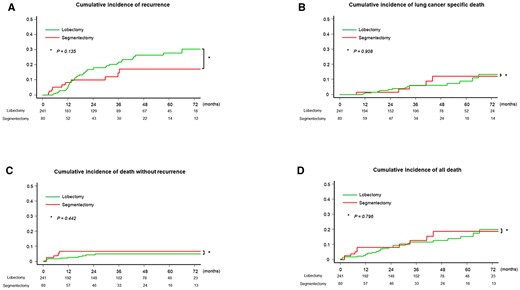
Cumulative incidence of recurrence, cumulative incidence of lung cancer-specific death, cumulative incidence of death without recurrence and cumulative incidence of all death of all included patients. (A) There was no significant difference in cumulative incidence of recurrence between patients who underwent segmentectomy vs. lobectomy (P = 0.135). (B) There was no significant difference in cumulative incidence of lung cancer-specific death between patients who underwent segmentectomy vs. lobectomy (P = 0.908). (C) There was no significant difference in cumulative incidence of death without recurrence between patients who underwent segmentectomy vs. lobectomy (P = 0.442). (D) There was also no significant difference in cumulative incidence of all death between patients who underwent segmentectomy vs. lobectomy (P = 0.796).
| Variables . | Segmentectomy, n = 80 (24.9%) . | Lobectomy, n = 241 (75.1%) . | P-Value . |
|---|---|---|---|
| Age, median (IQR) | 69 (66–76) | 67 (61–74) | 0.039 |
| Sex, n (%) | 0.696 | ||
| Male | 55 (68.8) | 160 (66.4) | |
| Female | 25 (31.3) | 81 (33.6) | |
| Tumour size | |||
| Whole-tumour size (mm) (median) (IQR) | 13 (12–17) | 16 (14–19) | <0.001 |
| Solid component size (mm) (median) (IQR) | 13 (12–16) | 16 (13–18) | <0.001 |
| CTR (median) (IQR) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.264 |
| Pure solid (CTR, 1.0), n (%) | 71 (88.8) | 205 (85.1) | 0.401 |
| Clinical stage n (%) | 0.007 | ||
| IA1 | 15 (18.8) | 18 (7.5) | |
| IA2 | 65 (81.3) | 223 (92.5) | |
| Lymph node dissection, n (%) | <0.001 | ||
| 0 | 4 (5.0) | 2 (0.8) | |
| ND 1b | 32 (40.0) | 26 (10.8) | |
| ND 2a-1 | 43 (53.8) | 166 (68.9) | |
| ND 2a-2 | 1 (1.3) | 47 (19.5) | |
| Number of resected lymph nodes, median (IQR) | 8 (4–11) | 15 (11–22) | <0.001 |
| Histological subtype n (%) | 0.424 | ||
| Adenocarcinoma | 52 (65.0) | 183 (75.9) | |
| Squamous cell carcinoma | 19 (23.8) | 36 (14.9) | |
| Adenosquamous cell carcinoma | 2 (2.5) | 6 (2.5) | |
| Sarcomatoid | 1 (1.3) | 3 (1.2) | |
| LCNEC | 5 (6.3) | 8 (3.3) | |
| Other subtypes of NSCLC | 1 (1.3) | 5 (2.1) | |
| LY, n (%) | 37 (46.3) | 138 (57.3) | 0.087 |
| V, n (%) | 54 (67.5) | 154 (63.9) | 0.558 |
| PL, n (%) | 22 (27.5) | 98 (40.7) | 0.032 |
| Pathological stage n (%) | 0.004 | ||
| IA1 | 12 (15.0) | 11 (4.6) | |
| IA2 | 27 (33.8) | 75 (31.1) | |
| IA3 | 8 (10.0) | 11 (4.6) | |
| IB | 16 (20.0) | 72 (29.9) | |
| IIA | 1 (1.3) | 0 (0) | |
| IIB | 13 (16.3) | 44 (18.3) | |
| IIIA | 3 (3.8) | 25 (10.4) | |
| IIIB | 0 (0) | 3 (1.2) | |
| Lymph node metastasis, n (%) | 12 (15.0) | 60 (24.9) | 0.058 |
| Adjuvant therapy, n (%) | 12 (15.0) | 71 (29.5) | 0.008 |
| Prognosis, n (%) | |||
| Recurrence | 10 (12.5) | 48 (19.9) | 0.123 |
| Recurrence pattern | 0.222 | ||
| Locoregional | 3 (30.0) | 17 (35.4) | |
| Distant | 4 (40.0) | 27 (56.3) | |
| Locoregional + distant | 3 (30.0) | 4 (8.3) | |
| Death from any cause | 10 (12.5) | 27 (11.2) | 0.755 |
| Lung cancer-specific death | 5 (6.3) | 15 (6.2) | 0.993 |
| Variables . | Segmentectomy, n = 80 (24.9%) . | Lobectomy, n = 241 (75.1%) . | P-Value . |
|---|---|---|---|
| Age, median (IQR) | 69 (66–76) | 67 (61–74) | 0.039 |
| Sex, n (%) | 0.696 | ||
| Male | 55 (68.8) | 160 (66.4) | |
| Female | 25 (31.3) | 81 (33.6) | |
| Tumour size | |||
| Whole-tumour size (mm) (median) (IQR) | 13 (12–17) | 16 (14–19) | <0.001 |
| Solid component size (mm) (median) (IQR) | 13 (12–16) | 16 (13–18) | <0.001 |
| CTR (median) (IQR) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.264 |
| Pure solid (CTR, 1.0), n (%) | 71 (88.8) | 205 (85.1) | 0.401 |
| Clinical stage n (%) | 0.007 | ||
| IA1 | 15 (18.8) | 18 (7.5) | |
| IA2 | 65 (81.3) | 223 (92.5) | |
| Lymph node dissection, n (%) | <0.001 | ||
| 0 | 4 (5.0) | 2 (0.8) | |
| ND 1b | 32 (40.0) | 26 (10.8) | |
| ND 2a-1 | 43 (53.8) | 166 (68.9) | |
| ND 2a-2 | 1 (1.3) | 47 (19.5) | |
| Number of resected lymph nodes, median (IQR) | 8 (4–11) | 15 (11–22) | <0.001 |
| Histological subtype n (%) | 0.424 | ||
| Adenocarcinoma | 52 (65.0) | 183 (75.9) | |
| Squamous cell carcinoma | 19 (23.8) | 36 (14.9) | |
| Adenosquamous cell carcinoma | 2 (2.5) | 6 (2.5) | |
| Sarcomatoid | 1 (1.3) | 3 (1.2) | |
| LCNEC | 5 (6.3) | 8 (3.3) | |
| Other subtypes of NSCLC | 1 (1.3) | 5 (2.1) | |
| LY, n (%) | 37 (46.3) | 138 (57.3) | 0.087 |
| V, n (%) | 54 (67.5) | 154 (63.9) | 0.558 |
| PL, n (%) | 22 (27.5) | 98 (40.7) | 0.032 |
| Pathological stage n (%) | 0.004 | ||
| IA1 | 12 (15.0) | 11 (4.6) | |
| IA2 | 27 (33.8) | 75 (31.1) | |
| IA3 | 8 (10.0) | 11 (4.6) | |
| IB | 16 (20.0) | 72 (29.9) | |
| IIA | 1 (1.3) | 0 (0) | |
| IIB | 13 (16.3) | 44 (18.3) | |
| IIIA | 3 (3.8) | 25 (10.4) | |
| IIIB | 0 (0) | 3 (1.2) | |
| Lymph node metastasis, n (%) | 12 (15.0) | 60 (24.9) | 0.058 |
| Adjuvant therapy, n (%) | 12 (15.0) | 71 (29.5) | 0.008 |
| Prognosis, n (%) | |||
| Recurrence | 10 (12.5) | 48 (19.9) | 0.123 |
| Recurrence pattern | 0.222 | ||
| Locoregional | 3 (30.0) | 17 (35.4) | |
| Distant | 4 (40.0) | 27 (56.3) | |
| Locoregional + distant | 3 (30.0) | 4 (8.3) | |
| Death from any cause | 10 (12.5) | 27 (11.2) | 0.755 |
| Lung cancer-specific death | 5 (6.3) | 15 (6.2) | 0.993 |
CTR: consolidation tumour ratio; IQR: interquartile range; LCNEC: large cell neuroendocrine carcinoma; LY: lymphatic invasion; NSCLC: non-small-cell lung cancer; PL: pleural invasion; V: vascular invasion.
| Variables . | Segmentectomy, n = 80 (24.9%) . | Lobectomy, n = 241 (75.1%) . | P-Value . |
|---|---|---|---|
| Age, median (IQR) | 69 (66–76) | 67 (61–74) | 0.039 |
| Sex, n (%) | 0.696 | ||
| Male | 55 (68.8) | 160 (66.4) | |
| Female | 25 (31.3) | 81 (33.6) | |
| Tumour size | |||
| Whole-tumour size (mm) (median) (IQR) | 13 (12–17) | 16 (14–19) | <0.001 |
| Solid component size (mm) (median) (IQR) | 13 (12–16) | 16 (13–18) | <0.001 |
| CTR (median) (IQR) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.264 |
| Pure solid (CTR, 1.0), n (%) | 71 (88.8) | 205 (85.1) | 0.401 |
| Clinical stage n (%) | 0.007 | ||
| IA1 | 15 (18.8) | 18 (7.5) | |
| IA2 | 65 (81.3) | 223 (92.5) | |
| Lymph node dissection, n (%) | <0.001 | ||
| 0 | 4 (5.0) | 2 (0.8) | |
| ND 1b | 32 (40.0) | 26 (10.8) | |
| ND 2a-1 | 43 (53.8) | 166 (68.9) | |
| ND 2a-2 | 1 (1.3) | 47 (19.5) | |
| Number of resected lymph nodes, median (IQR) | 8 (4–11) | 15 (11–22) | <0.001 |
| Histological subtype n (%) | 0.424 | ||
| Adenocarcinoma | 52 (65.0) | 183 (75.9) | |
| Squamous cell carcinoma | 19 (23.8) | 36 (14.9) | |
| Adenosquamous cell carcinoma | 2 (2.5) | 6 (2.5) | |
| Sarcomatoid | 1 (1.3) | 3 (1.2) | |
| LCNEC | 5 (6.3) | 8 (3.3) | |
| Other subtypes of NSCLC | 1 (1.3) | 5 (2.1) | |
| LY, n (%) | 37 (46.3) | 138 (57.3) | 0.087 |
| V, n (%) | 54 (67.5) | 154 (63.9) | 0.558 |
| PL, n (%) | 22 (27.5) | 98 (40.7) | 0.032 |
| Pathological stage n (%) | 0.004 | ||
| IA1 | 12 (15.0) | 11 (4.6) | |
| IA2 | 27 (33.8) | 75 (31.1) | |
| IA3 | 8 (10.0) | 11 (4.6) | |
| IB | 16 (20.0) | 72 (29.9) | |
| IIA | 1 (1.3) | 0 (0) | |
| IIB | 13 (16.3) | 44 (18.3) | |
| IIIA | 3 (3.8) | 25 (10.4) | |
| IIIB | 0 (0) | 3 (1.2) | |
| Lymph node metastasis, n (%) | 12 (15.0) | 60 (24.9) | 0.058 |
| Adjuvant therapy, n (%) | 12 (15.0) | 71 (29.5) | 0.008 |
| Prognosis, n (%) | |||
| Recurrence | 10 (12.5) | 48 (19.9) | 0.123 |
| Recurrence pattern | 0.222 | ||
| Locoregional | 3 (30.0) | 17 (35.4) | |
| Distant | 4 (40.0) | 27 (56.3) | |
| Locoregional + distant | 3 (30.0) | 4 (8.3) | |
| Death from any cause | 10 (12.5) | 27 (11.2) | 0.755 |
| Lung cancer-specific death | 5 (6.3) | 15 (6.2) | 0.993 |
| Variables . | Segmentectomy, n = 80 (24.9%) . | Lobectomy, n = 241 (75.1%) . | P-Value . |
|---|---|---|---|
| Age, median (IQR) | 69 (66–76) | 67 (61–74) | 0.039 |
| Sex, n (%) | 0.696 | ||
| Male | 55 (68.8) | 160 (66.4) | |
| Female | 25 (31.3) | 81 (33.6) | |
| Tumour size | |||
| Whole-tumour size (mm) (median) (IQR) | 13 (12–17) | 16 (14–19) | <0.001 |
| Solid component size (mm) (median) (IQR) | 13 (12–16) | 16 (13–18) | <0.001 |
| CTR (median) (IQR) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.264 |
| Pure solid (CTR, 1.0), n (%) | 71 (88.8) | 205 (85.1) | 0.401 |
| Clinical stage n (%) | 0.007 | ||
| IA1 | 15 (18.8) | 18 (7.5) | |
| IA2 | 65 (81.3) | 223 (92.5) | |
| Lymph node dissection, n (%) | <0.001 | ||
| 0 | 4 (5.0) | 2 (0.8) | |
| ND 1b | 32 (40.0) | 26 (10.8) | |
| ND 2a-1 | 43 (53.8) | 166 (68.9) | |
| ND 2a-2 | 1 (1.3) | 47 (19.5) | |
| Number of resected lymph nodes, median (IQR) | 8 (4–11) | 15 (11–22) | <0.001 |
| Histological subtype n (%) | 0.424 | ||
| Adenocarcinoma | 52 (65.0) | 183 (75.9) | |
| Squamous cell carcinoma | 19 (23.8) | 36 (14.9) | |
| Adenosquamous cell carcinoma | 2 (2.5) | 6 (2.5) | |
| Sarcomatoid | 1 (1.3) | 3 (1.2) | |
| LCNEC | 5 (6.3) | 8 (3.3) | |
| Other subtypes of NSCLC | 1 (1.3) | 5 (2.1) | |
| LY, n (%) | 37 (46.3) | 138 (57.3) | 0.087 |
| V, n (%) | 54 (67.5) | 154 (63.9) | 0.558 |
| PL, n (%) | 22 (27.5) | 98 (40.7) | 0.032 |
| Pathological stage n (%) | 0.004 | ||
| IA1 | 12 (15.0) | 11 (4.6) | |
| IA2 | 27 (33.8) | 75 (31.1) | |
| IA3 | 8 (10.0) | 11 (4.6) | |
| IB | 16 (20.0) | 72 (29.9) | |
| IIA | 1 (1.3) | 0 (0) | |
| IIB | 13 (16.3) | 44 (18.3) | |
| IIIA | 3 (3.8) | 25 (10.4) | |
| IIIB | 0 (0) | 3 (1.2) | |
| Lymph node metastasis, n (%) | 12 (15.0) | 60 (24.9) | 0.058 |
| Adjuvant therapy, n (%) | 12 (15.0) | 71 (29.5) | 0.008 |
| Prognosis, n (%) | |||
| Recurrence | 10 (12.5) | 48 (19.9) | 0.123 |
| Recurrence pattern | 0.222 | ||
| Locoregional | 3 (30.0) | 17 (35.4) | |
| Distant | 4 (40.0) | 27 (56.3) | |
| Locoregional + distant | 3 (30.0) | 4 (8.3) | |
| Death from any cause | 10 (12.5) | 27 (11.2) | 0.755 |
| Lung cancer-specific death | 5 (6.3) | 15 (6.2) | 0.993 |
CTR: consolidation tumour ratio; IQR: interquartile range; LCNEC: large cell neuroendocrine carcinoma; LY: lymphatic invasion; NSCLC: non-small-cell lung cancer; PL: pleural invasion; V: vascular invasion.
The patient characteristics and details of the surgical procedure after propensity score matching are shown in Table 2 and Supplementary Material, Table S2, respectively. There were no significant differences in whole-tumour size, solid component size, CTR or other pathological characteristics. Among the matched patients, the 5-year CIR rate was 19.1% (95% CI, 9.4–31.5%) with segmentectomy and 19.2% (95% CI, 8.8–32.6%) with lobectomy (Fig. 3A). The recurrence pattern did not differ between patients who underwent segmentectomy and lobectomy (P = 0.707, Table 3). The 5-year CILSD rate was 13.5% (95% CI, 4.6–27.1%) with segmentectomy and 9.5% (95% CI, 2.2–23.4%) with lobectomy (Fig. 3B). The 5-year CIDWR rate was 7.6% (95% CI, 2.8–15.7%) with segmentectomy and 5.0% (95% CI, 1.3–12.8%) with lobectomy (Fig. 3C). The 5-year CIAD rate was 21.1% (95% CI, 11.4–27.2%) with segmentectomy and 14.5% (95% CI, 6.4–31.2%) with segmentectomy (Fig. 3D). Among the matched patients, RFS (Supplementary Material, Fig. S3A), OS (Supplementary Material, Fig. S3B), incidence of local recurrence (Supplementary Material, Fig. S3C), distant recurrence (Supplementary Material, Fig. S3D) and all recurrence (Supplementary Material, Fig. S3E) were similar between patients who underwent segmentectomy and lobectomy.
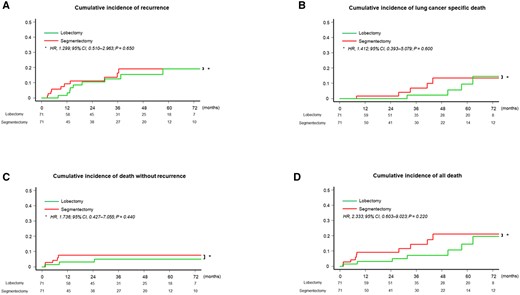
Cumulative incidence of recurrence, cumulative incidence of lung cancer-specific death, cumulative incidence of death without recurrence and cumulative incidence of all death of matched patients. (A) The 5-year cumulative incidence of recurrence rate was 19.1% (95% confidence interval, 9.4–31.5%) with segmentectomy and 19.2% (95% confidence interval, 8.8–32.6%) with lobectomy. The difference in cumulative incidence of recurrence between patients who underwent segmentectomy and lobectomy was not significant (hazard ratio, 1.299; 95% confidence interval, 0.510–2.963; P = 0.650). (B) The 5-year cumulative incidence of lung cancer-specific death rate was 13.5% (95% confidence interval, 4.6–27.1%) with segmentectomy and 9.5% (95% confidence interval, 2.2–23.4%) with lobectomy. The difference in cumulative incidence of lung cancer-specific death between patients who underwent segmentectomy and lobectomy was not significant (hazard ratio, 1.412; 95% confidence interval, 0.393–5.079; P = 0.600). (C) The 5-year cumulative incidence of death without recurrence rate was 7.6% (95% confidence interval, 2.8–15.7%) with segmentectomy and 5.0% (95% confidence interval, 1.3–12.8%) with lobectomy. The difference in cumulative incidence of death without recurrence between patients who underwent segmentectomy and lobectomy was not significant (hazard ratio, 1.736; 95% confidence interval, 0.427–7.055; P = 0.440). (D) The 5-year cumulative incidence of all death rate was 21.1% (95% confidence interval, 11.4–27.2%) with segmentectomy and 14.5% (95% confidence interval, 6.4–31.2%) with segmentectomy. The difference in CIAD between patients who underwent segmentectomy and lobectomy was not significant (hazard ratio, 2.333; 95% confidence interval, 0.603–9.023; P = 0.220).
| Variables . | Segmentectomy, n = 71 . | Lobectomy, n = 71 . | P-Value . | SMD . |
|---|---|---|---|---|
| Age (median) (IQR) | 69 (66–75) | 69 (62–75) | 0.434 | 0.132 |
| Sex, n (%) | 1.000 | 0 | ||
| Male | 51 (71.8) | 51 (71.8) | ||
| Female | 20 (28.2) | 20 (28.2) | ||
| Tumour size | ||||
| Whole-tumour size (mm), median (IQR) | 14 (13–17) | 14 (12–17) | 0.462 | |
| Solid component size (mm), median (IQR) | 14 (12–17) | 14 (12–16) | 0.846 | −0.033 |
| CTR, median (IQR) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.236 | |
| Pure solid (CTR, 1.0), n (%) | 63 (88.7) | 62 (87.3) | 0.356 | |
| Clinical stage n (%) | 0.336 | |||
| IA1 | 8 (11.3) | 6 (8.5) | ||
| IA2 | 63 (88.7) | 65 (91.2) | ||
| Lymph node dissection, n (%) | < 0.001 | |||
| 0 | 4 (5.6) | 1 (1.4) | ||
| ND 1b | 27 (38.0) | 11 (15.5) | ||
| ND 2a-1 | 39 (54.9) | 46 (64.8) | ||
| ND 2a-2 | 1 (1.4) | 13 (18.3) | ||
| Number of resected lymph nodes, median (IQR) | 8 (5–13) | 13 (10–24) | < 0.001 | |
| Histological subtype n (%) | 0.161 | 0.042 | ||
| Adenocarcinoma | 45 (63.4) | 54 (76.1) | ||
| Squamous cell carcinoma | 18 (25.4) | 10 (14.1) | ||
| Adenosquamous cell carcinoma | 2 (2.8) | 1 (1.4) | ||
| Sarcomatoid | 1 (1.4) | 2 (2.8) | ||
| LCNEC | 5 (7.0) | 2 (2.8) | ||
| Other subtype of NSCLC | 0 (0) | 2 (2.8) | ||
| LY, n (%) | 33 (46.5) | 31 (43.7) | 0.736 | 0.057 |
| V, n (%) | 50 (70.4) | 49 (69.0) | 0.855 | 0.031 |
| PL, n (%) | 19 (26.8) | 18 (25.4) | 0.848 | 0.032 |
| Pathological stage n (%) | 0.616 | |||
| IA1 | 9 (12.7) | 5 (7.0) | ||
| IA2 | 26 (36.6) | 31 (43.7) | ||
| IA3 | 7 (9.9) | 5 (7.0) | ||
| IB | 14 (19.7) | 18 (25.4) | ||
| IIA | 1 (1.4) | 0 (0) | ||
| IIB | 12 (16.9) | 9 (12.7) | ||
| IIIA | 2 (2.8) | 3 (4.2) | ||
| Lymph node metastasis, n (%) | 11 (15.5) | 11 (15.5) | 1.000 | 0 |
| Adjuvant therapy, n (%) | 12 (15.6) | 20 (26.0) | 0.111 | |
| Prognosis, n (%) | ||||
| Recurrence | 10 (14.1) | 9 (12.7) | 0.805 | |
| Recurrence pattern | 0.707 | |||
| Locoregional | 3 (4.2) | 5 (7.0) | ||
| Distant | 4 (5.6) | 2 (2.8) | ||
| Locoregional + distant | 3 (4.2) | 2 (2.8) | ||
| Death from any cause | 10 (14.1) | 7 (9.9) | 0.437 | |
| Lung cancer-specific death | 5 (7.0) | 4 (5.6) | 0.730 |
| Variables . | Segmentectomy, n = 71 . | Lobectomy, n = 71 . | P-Value . | SMD . |
|---|---|---|---|---|
| Age (median) (IQR) | 69 (66–75) | 69 (62–75) | 0.434 | 0.132 |
| Sex, n (%) | 1.000 | 0 | ||
| Male | 51 (71.8) | 51 (71.8) | ||
| Female | 20 (28.2) | 20 (28.2) | ||
| Tumour size | ||||
| Whole-tumour size (mm), median (IQR) | 14 (13–17) | 14 (12–17) | 0.462 | |
| Solid component size (mm), median (IQR) | 14 (12–17) | 14 (12–16) | 0.846 | −0.033 |
| CTR, median (IQR) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.236 | |
| Pure solid (CTR, 1.0), n (%) | 63 (88.7) | 62 (87.3) | 0.356 | |
| Clinical stage n (%) | 0.336 | |||
| IA1 | 8 (11.3) | 6 (8.5) | ||
| IA2 | 63 (88.7) | 65 (91.2) | ||
| Lymph node dissection, n (%) | < 0.001 | |||
| 0 | 4 (5.6) | 1 (1.4) | ||
| ND 1b | 27 (38.0) | 11 (15.5) | ||
| ND 2a-1 | 39 (54.9) | 46 (64.8) | ||
| ND 2a-2 | 1 (1.4) | 13 (18.3) | ||
| Number of resected lymph nodes, median (IQR) | 8 (5–13) | 13 (10–24) | < 0.001 | |
| Histological subtype n (%) | 0.161 | 0.042 | ||
| Adenocarcinoma | 45 (63.4) | 54 (76.1) | ||
| Squamous cell carcinoma | 18 (25.4) | 10 (14.1) | ||
| Adenosquamous cell carcinoma | 2 (2.8) | 1 (1.4) | ||
| Sarcomatoid | 1 (1.4) | 2 (2.8) | ||
| LCNEC | 5 (7.0) | 2 (2.8) | ||
| Other subtype of NSCLC | 0 (0) | 2 (2.8) | ||
| LY, n (%) | 33 (46.5) | 31 (43.7) | 0.736 | 0.057 |
| V, n (%) | 50 (70.4) | 49 (69.0) | 0.855 | 0.031 |
| PL, n (%) | 19 (26.8) | 18 (25.4) | 0.848 | 0.032 |
| Pathological stage n (%) | 0.616 | |||
| IA1 | 9 (12.7) | 5 (7.0) | ||
| IA2 | 26 (36.6) | 31 (43.7) | ||
| IA3 | 7 (9.9) | 5 (7.0) | ||
| IB | 14 (19.7) | 18 (25.4) | ||
| IIA | 1 (1.4) | 0 (0) | ||
| IIB | 12 (16.9) | 9 (12.7) | ||
| IIIA | 2 (2.8) | 3 (4.2) | ||
| Lymph node metastasis, n (%) | 11 (15.5) | 11 (15.5) | 1.000 | 0 |
| Adjuvant therapy, n (%) | 12 (15.6) | 20 (26.0) | 0.111 | |
| Prognosis, n (%) | ||||
| Recurrence | 10 (14.1) | 9 (12.7) | 0.805 | |
| Recurrence pattern | 0.707 | |||
| Locoregional | 3 (4.2) | 5 (7.0) | ||
| Distant | 4 (5.6) | 2 (2.8) | ||
| Locoregional + distant | 3 (4.2) | 2 (2.8) | ||
| Death from any cause | 10 (14.1) | 7 (9.9) | 0.437 | |
| Lung cancer-specific death | 5 (7.0) | 4 (5.6) | 0.730 |
CTR: consolidation tumour ratio; IQR: interquartile range; LCNEC: large cell neuroendocrine carcinoma; LY: lymphatic invasion; NSCLC: non-small-cell lung cancer; PL: pleural invasion; SMD: standardized mean difference; V: vascular invasion.
| Variables . | Segmentectomy, n = 71 . | Lobectomy, n = 71 . | P-Value . | SMD . |
|---|---|---|---|---|
| Age (median) (IQR) | 69 (66–75) | 69 (62–75) | 0.434 | 0.132 |
| Sex, n (%) | 1.000 | 0 | ||
| Male | 51 (71.8) | 51 (71.8) | ||
| Female | 20 (28.2) | 20 (28.2) | ||
| Tumour size | ||||
| Whole-tumour size (mm), median (IQR) | 14 (13–17) | 14 (12–17) | 0.462 | |
| Solid component size (mm), median (IQR) | 14 (12–17) | 14 (12–16) | 0.846 | −0.033 |
| CTR, median (IQR) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.236 | |
| Pure solid (CTR, 1.0), n (%) | 63 (88.7) | 62 (87.3) | 0.356 | |
| Clinical stage n (%) | 0.336 | |||
| IA1 | 8 (11.3) | 6 (8.5) | ||
| IA2 | 63 (88.7) | 65 (91.2) | ||
| Lymph node dissection, n (%) | < 0.001 | |||
| 0 | 4 (5.6) | 1 (1.4) | ||
| ND 1b | 27 (38.0) | 11 (15.5) | ||
| ND 2a-1 | 39 (54.9) | 46 (64.8) | ||
| ND 2a-2 | 1 (1.4) | 13 (18.3) | ||
| Number of resected lymph nodes, median (IQR) | 8 (5–13) | 13 (10–24) | < 0.001 | |
| Histological subtype n (%) | 0.161 | 0.042 | ||
| Adenocarcinoma | 45 (63.4) | 54 (76.1) | ||
| Squamous cell carcinoma | 18 (25.4) | 10 (14.1) | ||
| Adenosquamous cell carcinoma | 2 (2.8) | 1 (1.4) | ||
| Sarcomatoid | 1 (1.4) | 2 (2.8) | ||
| LCNEC | 5 (7.0) | 2 (2.8) | ||
| Other subtype of NSCLC | 0 (0) | 2 (2.8) | ||
| LY, n (%) | 33 (46.5) | 31 (43.7) | 0.736 | 0.057 |
| V, n (%) | 50 (70.4) | 49 (69.0) | 0.855 | 0.031 |
| PL, n (%) | 19 (26.8) | 18 (25.4) | 0.848 | 0.032 |
| Pathological stage n (%) | 0.616 | |||
| IA1 | 9 (12.7) | 5 (7.0) | ||
| IA2 | 26 (36.6) | 31 (43.7) | ||
| IA3 | 7 (9.9) | 5 (7.0) | ||
| IB | 14 (19.7) | 18 (25.4) | ||
| IIA | 1 (1.4) | 0 (0) | ||
| IIB | 12 (16.9) | 9 (12.7) | ||
| IIIA | 2 (2.8) | 3 (4.2) | ||
| Lymph node metastasis, n (%) | 11 (15.5) | 11 (15.5) | 1.000 | 0 |
| Adjuvant therapy, n (%) | 12 (15.6) | 20 (26.0) | 0.111 | |
| Prognosis, n (%) | ||||
| Recurrence | 10 (14.1) | 9 (12.7) | 0.805 | |
| Recurrence pattern | 0.707 | |||
| Locoregional | 3 (4.2) | 5 (7.0) | ||
| Distant | 4 (5.6) | 2 (2.8) | ||
| Locoregional + distant | 3 (4.2) | 2 (2.8) | ||
| Death from any cause | 10 (14.1) | 7 (9.9) | 0.437 | |
| Lung cancer-specific death | 5 (7.0) | 4 (5.6) | 0.730 |
| Variables . | Segmentectomy, n = 71 . | Lobectomy, n = 71 . | P-Value . | SMD . |
|---|---|---|---|---|
| Age (median) (IQR) | 69 (66–75) | 69 (62–75) | 0.434 | 0.132 |
| Sex, n (%) | 1.000 | 0 | ||
| Male | 51 (71.8) | 51 (71.8) | ||
| Female | 20 (28.2) | 20 (28.2) | ||
| Tumour size | ||||
| Whole-tumour size (mm), median (IQR) | 14 (13–17) | 14 (12–17) | 0.462 | |
| Solid component size (mm), median (IQR) | 14 (12–17) | 14 (12–16) | 0.846 | −0.033 |
| CTR, median (IQR) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.236 | |
| Pure solid (CTR, 1.0), n (%) | 63 (88.7) | 62 (87.3) | 0.356 | |
| Clinical stage n (%) | 0.336 | |||
| IA1 | 8 (11.3) | 6 (8.5) | ||
| IA2 | 63 (88.7) | 65 (91.2) | ||
| Lymph node dissection, n (%) | < 0.001 | |||
| 0 | 4 (5.6) | 1 (1.4) | ||
| ND 1b | 27 (38.0) | 11 (15.5) | ||
| ND 2a-1 | 39 (54.9) | 46 (64.8) | ||
| ND 2a-2 | 1 (1.4) | 13 (18.3) | ||
| Number of resected lymph nodes, median (IQR) | 8 (5–13) | 13 (10–24) | < 0.001 | |
| Histological subtype n (%) | 0.161 | 0.042 | ||
| Adenocarcinoma | 45 (63.4) | 54 (76.1) | ||
| Squamous cell carcinoma | 18 (25.4) | 10 (14.1) | ||
| Adenosquamous cell carcinoma | 2 (2.8) | 1 (1.4) | ||
| Sarcomatoid | 1 (1.4) | 2 (2.8) | ||
| LCNEC | 5 (7.0) | 2 (2.8) | ||
| Other subtype of NSCLC | 0 (0) | 2 (2.8) | ||
| LY, n (%) | 33 (46.5) | 31 (43.7) | 0.736 | 0.057 |
| V, n (%) | 50 (70.4) | 49 (69.0) | 0.855 | 0.031 |
| PL, n (%) | 19 (26.8) | 18 (25.4) | 0.848 | 0.032 |
| Pathological stage n (%) | 0.616 | |||
| IA1 | 9 (12.7) | 5 (7.0) | ||
| IA2 | 26 (36.6) | 31 (43.7) | ||
| IA3 | 7 (9.9) | 5 (7.0) | ||
| IB | 14 (19.7) | 18 (25.4) | ||
| IIA | 1 (1.4) | 0 (0) | ||
| IIB | 12 (16.9) | 9 (12.7) | ||
| IIIA | 2 (2.8) | 3 (4.2) | ||
| Lymph node metastasis, n (%) | 11 (15.5) | 11 (15.5) | 1.000 | 0 |
| Adjuvant therapy, n (%) | 12 (15.6) | 20 (26.0) | 0.111 | |
| Prognosis, n (%) | ||||
| Recurrence | 10 (14.1) | 9 (12.7) | 0.805 | |
| Recurrence pattern | 0.707 | |||
| Locoregional | 3 (4.2) | 5 (7.0) | ||
| Distant | 4 (5.6) | 2 (2.8) | ||
| Locoregional + distant | 3 (4.2) | 2 (2.8) | ||
| Death from any cause | 10 (14.1) | 7 (9.9) | 0.437 | |
| Lung cancer-specific death | 5 (7.0) | 4 (5.6) | 0.730 |
CTR: consolidation tumour ratio; IQR: interquartile range; LCNEC: large cell neuroendocrine carcinoma; LY: lymphatic invasion; NSCLC: non-small-cell lung cancer; PL: pleural invasion; SMD: standardized mean difference; V: vascular invasion.
Inverse probability of treatment weighting-adjusted multivariable analysis for cumulative incidence of recurrence
| . | HR (95% CI) . | P-Value . |
|---|---|---|
| Procedure (segmentectomy/lobectomy) | 1.045 (0.439–2.488) | 0.920 |
| . | HR (95% CI) . | P-Value . |
|---|---|---|
| Procedure (segmentectomy/lobectomy) | 1.045 (0.439–2.488) | 0.920 |
CI: confidence interval; HR: hazard ratio.
Inverse probability of treatment weighting-adjusted multivariable analysis for cumulative incidence of recurrence
| . | HR (95% CI) . | P-Value . |
|---|---|---|
| Procedure (segmentectomy/lobectomy) | 1.045 (0.439–2.488) | 0.920 |
| . | HR (95% CI) . | P-Value . |
|---|---|---|
| Procedure (segmentectomy/lobectomy) | 1.045 (0.439–2.488) | 0.920 |
CI: confidence interval; HR: hazard ratio.
In the multivariable analysis using IPTW and surgical method (characteristics of cohort are shown in Supplementary Material, Table S3), segmentectomy was not a significant predictor of CIR (HR, 1.045; 95% CI, 0.439–2.488; P = 0.920, Table 3).
DISCUSSION
In the present study, there was no difference in prognosis between segmentectomy and lobectomy for clinically early-stage NSCLC with LY, V, PL or lymph node metastasis. Similar results were obtained after propensity score matching. This result shows that segmentectomy is feasible for small-sized, solid-predominant tumour regardless of invasive characteristics.
Although the efficacy of segmentectomy was demonstrated in JCOG0802/WJOG4607L, there are no data for pathological invasiveness of resected tumour. An accurate incidence of LY, V, PL and lymph node metastasis is not known. In JCOG0201, a prospective study investigating radiologic findings of preoperative CT predicting non-invasive NSCLC with a whole-tumour size of 3 cm or less, the incidence of LY, V and lymph node metastasis was 26.4% (113/428 patients), 23.1% (100/433 patients) and 9.4% (47/498 patients), respectively [18]. These incidences are almost same as those identified in retrospective studies from our institutions [19–21] and are large percentages. However, no study has investigated prognosis after segmentectomy for clinically early-stage but pathologically invasive NSCLC. Results of the present study mean that segmentectomy is feasible if candidates are clinically selected appropriately, regardless of the presence of invasive characteristics.
CTR and FDG-PET/CT are useful in predicting invasive characteristics and prognosis of early-stage NSCLC [18, 22, 23]. We have previously shown that prognosis after segmentectomy is comparable to that after lobectomy, even in patients with radiologically aggressive (i.e. high CTR and SUVmax) early-stage lung cancer [21–23]. The results of the present study probably reflect the findings of the previous studies. However, to our knowledge, no study has investigated the feasibility of segmentectomy with regard to pathological characteristics. Because the patients included in this study were chosen based on pathological findings of resected tumour, it is difficult directly to adapt the results of our study to selection of a surgical procedure. However, we believe that this study is important in terms of supporting the results of JCOG0802/WJOG4607L and previous retrospective studies showing the effectiveness of segmentectomy.
Limitations
There are several limitations in this study. First, although multiple institutions are included, this was a retrospective study and the follow-up period was too short to evaluate prognosis of patients with early-stage lung cancer. Second, there were differences in the characteristics of patients who underwent segmentectomy and lobectomy. For instance, the number of patients who underwent adjuvant therapy was higher among patients who underwent lobectomy. However, we believe that these differences were minimized by propensity score matching. Comparisons of RFS and OS using the Kaplan–Meier method are often used as an end point in such types of retrospective studies. Recurrence and death from other than lung cancer are treated as events in the analysis using RFS. In analysis using OS, all deaths are treated equally as events regardless of cause. However, data on comorbid conditions and lung function were not available. We were also unable to assess a detailed cause of death in patients who died of causes other than lung cancer. Because information about whether segmentectomy was performed with curative or compromised intent were also not obtained, we set CIR as a primary end point and used competing risk analysis. In our study, there was no difference in CIDWR between patients who underwent segmentectomy and those who underwent lobectomy. This may indicate that there are no differences in background factors other than oncologic factors between patients who underwent segmentectomy and those who underwent lobectomy that would affect survival. In addition, patients who were switched to lobectomy were evaluated together with patients in the lobectomy group; there are no data on the patients that were switched to lobectomy. To overcome the above-mentioned limitations of our study, a prospective study should be carried out. However, the results of this study are meaningful and will help determine treatment strategies in patients with early-stage NSCLC.
CONCLUSION
There was no difference in prognosis between segmentectomy and lobectomy for node-negative, solid component-predominant tumour (CTR >50% on preoperative CT), ≤2-cm-whole-tumour-sized NSCLC with LY, V, PL or lymph node metastasis. Segmentectomy can be considered a standard treatment strategy for early-stage NSCLC regardless of the presence of invasive characteristics.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Funding
This work was supported by Japan Society for the Promotion of Science KAKENHI (Grant Number JP20K17749).
ACKNOWLEDGEMENT
The authors would like to thank Enago (www.enago.jp) for the English language review.
Conflict of interest: none declared.
Data Availability Statement
The data underlying this study are available in this article and in the online supplementary material.
Author contributions
Atsushi Kagimoto: Data curation; Formal analysis; Writing—original draft. Yasuhiro Tsutani: Conceptualization; Data curation; Supervision; Writing—review & editing. Yoshihisa Shimada: Data curation; Writing—review & editing. Takahiro Mimae: Data curation; Writing—review & editing. Yoshihiro Miyata: Supervision. Hiroyuki Ito: Data curation; Supervision. Haruhiko Nakayama: Data curation; Supervision. Norihiko Ikeda: Data curation; Supervision. Morihito Okada: Supervision.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Emmanouil Ioannis Kapetanakis, Henning A. Gaissert, Eugenio Pompeo and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
ABBREVIATIONS
- CI
Confidence interval
- CIAD
Cumulative incidence of all death
- CIDWR
Cumulative incidence of death without recurrence
- CILSD
Cumulative incidence of lung cancer-specific death
- CIR
Cumulative incidence of recurrence
- CT
Computed tomography
- CTR
Consolidation tumor ratio
- FDG
[18F]-fluoro-2-deoxy-d-glucose
- HRs
Hazard ratios
- IPTW
Inverse probability of treatment weighting
- LCNEC
Large cell neuroendocrine carcinoma
- LY
Lymphatic invasion
- NSCLC
Non-small-cell lung cancer
- OS
Overall survival
- PET
Positron emission tomography
- PL
Pleural invasion
- RFS
Recurrence-free survival
- SUVmax
Maximum standardized uptake value
- V
Vascular invasion





